Abstract
The purpose of this study was to compare the pharmacokinetic profiles of tetramethylpyrazine phosphate (TMPP) in plasma and extracellular fluid of the cerebral cortex of rats via three delivery routes: intranasal (i.n.), intragastric (i.g.) and intravenous (i.v.) administration. After i.n., i.g. and i.v. administration of a single-dose at 10 mg/kg, cerebral cortex dialysates and plasma samples drawn from the carotid artery were collected at timed intervals. The concentration of TMPP in the samples was analyzed by HPLC. The area under the concentration–time curve (AUC) and the ratio of the AUCbrain to the AUCplasma (drug targeting efficiency, DTE) was calculated to evaluate the brain targeting efficiency of the drug via these different routes of administration. After i.n. administration, TMPP was rapidly absorbed to reach its peak plasma concentration within 5 min and showed a delayed uptake into cerebral cortex (tmax=15 min). The ratio of the AUCbrain dialysates value between i.n. route and i.v. injection was 0.68, which was greater than that obtained after i.g. administration (0.43). The systemic bioavailability obtained with i.n. administration was greater than that obtained by the i.g. route (86.33% vs. 50.39%), whereas the DTE of the nasal route was 78.89%, close to that of oral administration (85.69%). These results indicate that TMPP is rapidly absorbed from the nasal mucosa into the systemic circulation, and then crosses the blood–brain barrier (BBB) to reach the cerebral cortex. Intranasal administration of TMPP could be a promising alternative to intravenous and oral approaches.
Key words: Tetramethylpyrazine phosphate, Intranasal delivery, Pharmacokinetics, Microdialysis, Rat
Graphical abstract
Pharmacokinetic analysis of tetramethylpyrazine phosphate (TMPP) in plasma and extracellular fluid in cerebral cortex of rats after intranasal, intragastric and intravenous administration was carried out with microdialysis sampling and HPLC-UV analysis. The results indicated that TMPP was rapidly absorbed from the nasal mucosa into the systemic circulation and then across the blood–brain barrier to reach the cerebral cortex. Intranasal administration of TMPP may be a promising alternative to intravenous and oral approaches.
1. Introduction
2,3,5,6-Tetramethylpyrazine (ligustrazine, TMP) is a major biologically active compound isolated from the Chinese herbal medicine Ligusticum wallichii Franch. TMP possesses antiplatelet activities and has been widely used in China for the treatment of patients with vascular disorders such as myocardial and cerebral infarction1. It has been reported that TMP may provide antithrombotic effects and neuroprotection against ischemic brain injury through suppression of inflammation, blocking of calcium channels, inhibiting the formation of free radicals, and reducing the bioactivity of platelets2. It has also been proposed to increase cerebral blood flow during ischemic brain infarction3.
The most widely used salt form of TMP in clinical therapy is TMP phosphate (TMPP). However, the absorption of TMPP after oral administration is variable and incomplete, with low bioavailability of 10%–30%4. Intravenous infusion every 4–6 h produces a superior pharmacodynamic effect but brings poor patient compliance. Therefore, an alternative route of administration is greatly needed.
In the recent years, systemic drug delivery through the nasal route has received considerable attention5. Intranasal (i.n.) administration offers some advantages including rapid absorption, avoidance of hepatic first-pass metabolism, and preferential drug delivery to brain via the olfactory region6,7. Hence i.n. delivery could be especially important in the management of crisis situations such as cerebral infarction8. The nasal delivery of TMPP may provide a better alternative to intragastric (i.g.) and intravenous (i.v.) administration.
Microdialysis is a continuous sampling technique to study unbound drug disposition and metabolism in blood and tissues9. Using this technique, TMP was reported to have appreciable blood–brain barrier (BBB) penetrability after i.v. administration10. The pharmacokinetics of TMP hydrochloride following i.n. and i.v. administration also has been investigated using the brain microdialysis technique in free-moving rats11. However, there is a limitation associated with i.n. administration in awake rats: TMPP administered nasally can be cleared from the nasal cavity into the gastrointestinal tract. Therefore, to investigate the brain pharmacokinetics of TMPP along with the plasma pharmacokinetics following i.n., i.g. and i.v. administration in parallel, our research was carried out using brain microdialysis in anaesthetized rats with a tracheotomy. These studies tested the efficacy of the nasal route of delivery, and could indicate whether there exists a direct nose-to-brain transport of TMPP. In addition, the brain pharmacokinetics of TMPP following oral administration was investigated.
2. Materials and methods
2.1. Chemicals, reagents and animals
Tetramethylpyrazine phosphate (TMPP) was purchased from Limin Pharmaceutical Company (Guangdong, China). Carbamazepine (internal standard, I.S.) was supplied by Hengyi Pharmaceutical Co., Ltd. (Tianjin, China). Methanol (HPLC grade) was purchased from Hanbon Sci. & Tech. Co., Ltd. (Jiangsu, China). Water was prepared in a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other chemicals and reagents used were of analytical grade.
The brain microdialysis system was obtained from BAS bioanalytical systems, Inc. (Indiana, USA), and concentric microdialysis probes (MD-2204, membrane length: 4 mm, cutoff: 30 kDa) were used in this study. The artificial cerebrospinal fluid (ACSF) buffer (147 mmol/L NaCl, 4 mmol/L KCl, 2.3 mmol/L CaCl2) was prepared weekly, filtered, degassed and used as the perfusate12.
Male Sprague–Dawley rats weighing 200–250 g were obtained from the Experimental Animal Center of Sun Yat-sen University, and maintained on a light/dark cycle. Temperature and relative humidity were maintained at 25 °C and 50%, respectively. All care and handling of animals were approved by the Animal Ethics Committee of Sun Yat-sen University. The rats were fasted overnight (approximately 12 h) before each experiment.
2.2. Preparation of TMPP solution for administration
Dosing solution of 25 mg/mL was prepared by dissolving TMPP powder in physiological saline for the i.n. and i.v. administration, and dosing solution of 5 mg/mL was prepared by dissolving TMPP powder in distilled water for i.g. route. The preparations were made immediately prior to drug administration.
2.3. Animal experiment
2.3.1. Experiment design
Thirty-six Sprague–Dawley rats randomly divided into six groups were used in this study (n=6), three groups for plasma PK study following i.n., i.g. or i.v. administration and the other three groups for brain microdialysis analysis of the above three delivery routes. TMPP solution was administered at a single dose of 10 mg/kg body weight (BW) to each rat.
2.3.2. Blood sampling and treatment for PK studies
The rats were anesthetized with an intraperitoneal injection of urethane (1.0 g/kg), after which tracheotomy and cannulation of the carotid artery was performed. For i.v. injection, dosing solutions were delivered using a 1 mL syringe into the femoral vein (a cannula was inserted for injection). For i.n. delivery, the esophagus and the passage of the nasopalatine duct were first occluded to prevent drug being cleared into the gastrointestinal tract12. Then preparations were given via a cannula inserted 7 mm into the left cavity. Oral gavage of TMPP was performed by attaching a stainless steel feeding needle to a syringe containing the oral formulation.
A volume of 0.25 mL of blood was collected pre-dose and at time 0.033, 0.083, 0.25, 0.5, 1, 1.5, 2, 3 and 5 h post-dosing and transferred into heparinized polystyrene tubes. Plasma was separated by centrifugation at 10,000 rpm for 10 min and kept frozen at −20 °C prior to analysis.
Plasma samples were processed with the following steps: a volume of 100 μL plasma was pipetted into polystyrene tubes and 20 μL of I.S. working solution (40 μg/mL) and 1 mL water were added. The mixture was vortex mixed for 1 min and loaded onto a SPE cartridge (60 mg/3 mL, Strata-X, Phenomenex, USA), which had been conditioned by washing with methanol (1 mL) followed by water (1 mL). The sample-loaded SPE cartridge was further washed with water (1 mL) and TMPP ws eluted with 1 mL of methanol. A 20 μL aliquot of this extract was subjected to HPLC separation.
2.3.3. Microdialysis procedure
Rats were anesthetized and mounted on a stereotaxic frame, the skull was exposed and a small hole was drilled (+2.0 mm lateral to the midsagittal suture and 2.0 mm anterior to bregma). The dural and arachnoid membranes were removed to avoid damage to the microdialysis probes during their insertion into the brain. An intracerebral guide cannula was implanted, secured by screw and cement. A microdialysis probe was stereotaxically inserted via the guide cannula into the cerebral cortex, identically to a depth of 4.0 mm ventrally from the dura, according to the atlas of Paxinos and Watson13.
To avoid the possible influence of tissue trauma resulting from insertion of the probe on the microdialysis results, the probe was perfused with ACSF at a flow rate of 2 μL/min for 1 h to stabilize solute levels around the dialysis membrane14. A 1 mL microsyringe was fitted to a precision pump (MD-1001) and connected to the tubing to provide the perfusate solution. Outflow from probe (dialysate) was connected to a refrigerated fraction collector (MD-1201). The rats were then held in supine position, and tracheotomy, cannulation and other procedures were completed before drug administration.
After the stabilization period, TMPP was administered at a single dose of 10 mg/kg to each rat and dialysates were collected every 10 min within 2 h and then every 20 min thereafter. 10 μL of the brain dialysate was directly injected into HPLC system and immediately analyzed after collection. Throughout the experiment, the rats were placed on heating pads to maintain body temperature at 36–37 °C. The position of the probe was verified by standard histological procedures at the end of the experiment.
2.4. Analytical method
Plasma and dialysate concentrations of TMPP were measured by an Agilent 1100 HPLC system equipped with a UV detector (Agilent technologies, USA). Separations were carried out on an Elipse XDB C18 column (150 mm×4.6 mm, 5 μm, Agilent, USA) with a C18 guard column (Security Guard, Phenomenex, USA). The mobile phase consisted of water-methanol (40:60, v/v) at a flow-rate of 0.7 mL/min and the detector wavelength was set at 295 nm. The retention times of TMPP and I.S. were 3.6 min and 5.5 min, respectively. For plasma analysis, the lower limit of quantification (LLOQ) for TMPP was 20 ng/mL and the linear range was 0.08–40.96 μg/mL in rat plasma. The LLOQ value of the dialyste was 10 ng/mL and the method was linear over the concentration range from 0.02 to 1.6 μg/mL.
With the premise that in vitro recoveries by gain and loss are equal, in vivo extracellular drug concentration can be recalculated with in vivo probe recovery by loss15. In the present study, in vitro recoveries of TMPP by gain and loss evaluated at 2.0 μL/min flow-rate with four concentration levels (0.1, 0.2, 0.4 and 0.8 μg/mL) were 30.4±2.6% and 45.1±3.0%, respectively, and were significant different (P<0.05). As the recoveries of the microdialysis probes were constant, the concentrations of TMPP in dialysate measured in vivo were analyzed in parallel and the data were not recalculated with in vivo recovery to avoid deviation from actual data.
Before and at the end of the in vivo experiments, the in vitro recovery by gain of TMPP for each probe was determined by continuing the perfusion at the same settings in a calibration solution. Change in the range of 5% was accepted.
2.5. Data analysis
Results obtained from the HPLC analyses were plotted as concentration–time curves for plasma or brain dialysate. As the in vitro recoveries were similar between probes, the concentration of TMPP in cerebral cortex after different routes of administration can be compared from the drug concentration in dialysate. PK analysis was performed using the KINETICA 4.4 software. The mean area under the curve (AUC) was calculated by the trapezoidal method. The maximum concentration (Cmax) and the time to reach peak concentration (tmax) were the observed values. Results are presented as mean values±S.D.
The degree of TMPP targeting to brain after i.n. and i.g. administration can be evaluated by the drug targeting efficiency (DTE), The higher the DTE is, the further degree of TMPP targeting to brain can be expected. DTE that represents the time average partitioning ratio was calculated as follows16–18:
3. Results
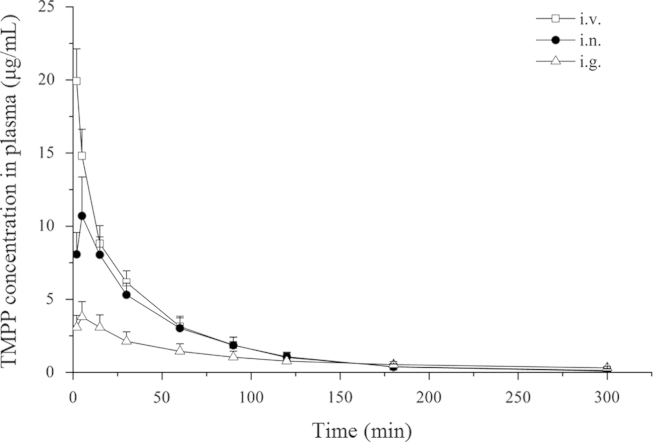
The mean plasma levels of TMPP following a single dose of i.n., i.g. and i.v. administration are shown in Fig. 1. The plasma drug concentration data were best fitted to a two-compartment open model, and the PK parameters are presented in Table 1. The plasma Cmax following i.n. administration was 2.8 times higher compared to that after oral delivery and the nasal bioavailability was 86.33%, 1.7 times higher than oral administration.
Figure 1.
Average TMPP concentration in plasma as a function of time after intravenous, intranasal and oral administration of TMPP at a dose of 10 mg/kg (mean±S.D., n=6).
Table 1.
Plasma pharmacokinetic parameters of TMPP after intravenous, intranasal and oral administration at a dose of 10 mg/kg (mean±S.D., n=6).
| Parameter | i.n. | i.g. | i.v. |
|---|---|---|---|
| tmax (min) | 5.00 | 11.67±5.16 | – |
| Cmax (μg/mL) | 10.70±2.67a | 3.79±1.05a | 19.92±2.21 |
| t1/2α (min) | 6.60±1.80a | 13.68±3.90a | 3.12±0.60 |
| t1/2β (min) | 41.16±8.16 | 92.16±22.56a | 33.84±7.68 |
| MRT (min) | 54.02±15.03 | 115.20±34.22a | 44.41±9.03 |
| AUC0−t (μg·min/mL) | 530.43±130.20 | 309.62±84.01a | 614.40±96.61 |
| Bioavailability (%) | 86.33 | 50.39 | – |
Significantly different from i.v. group, P<0.05.
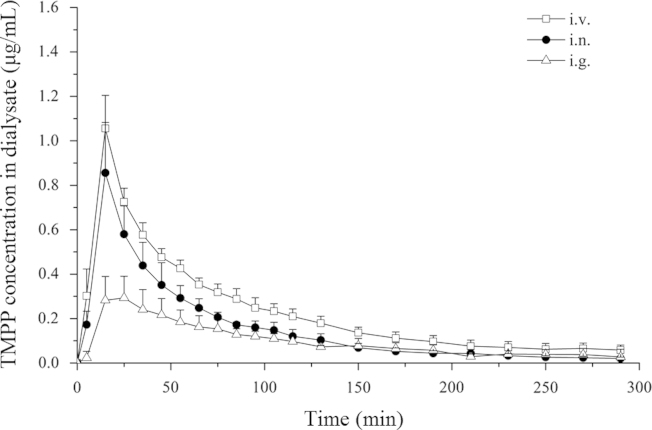
The unbound TMPP in the brain dialysate concentration–time profiles following i.n., i.g. and i.v. application, respectively, are presented in Fig. 2. Drug concentrations were plotted at the mid-time point of each single collection interval. Similar to other reports11, the TMPP concentration reached Cmax at 15 min after i.n. and i.v. routes, faster than i.g. route (20.00±5.48 min). The brain dialysate drug concentration data were best fitted to a non-compartment open model, and selected PK parameters are presented in Table 2. The ratio of the AUCbrain dialysates value between i.n. route and i.v. injection was 0.68, which was greater than that obtained after i.g. administration (0.43). The DTE of nasal route was 78.89%, close to that of i.g. route (85.69%).
Figure 2.
Average TMPP concentration in brain dialysate as a function of time after intravenous, intranasal and oral administration of TMPP at a dose of 10 mg/kg (mean±S.D., n=6).
Table 2.
Selected pharmacokinetic measures of TMPP in brain dialysate after intravenous, intranasal and oral administration at a dose of 10 mg/kg (mean±S.D., n=6).
| Parameter | i.n. | i.g. | i.v. |
|---|---|---|---|
| tmax (min) | 15.00 | 20.00±5.48 | 15.00 |
| Cmax (μg/mL) | 0.86±0.23 | 0.29±0.10a | 1.06±0.15 |
| MRT (min) | 82.60±19.51 | 110.42±30.30 | 101.93±24.02 |
| AUC0−t (μg·min/mL) | 45.12±7.34a | 28.61±6.28a | 66.25±6.69 |
| DTE (%) | 78.89 | 85.69 | — |
Significantly different from i.v. group, P<0.05.
4. Discussion
For the nasal administration groups the esophagus and the passage of the nasopalatine duct were occluded under anesthesia to prevent drug from being cleared into the gastrointestinal tract and subsequently absorbed. There have been numerous pharmacokinetics studies using microdialysis sampling in anaesthetized rats19–21, so we adopted a brain microdialysis technique in anesthetic rats for our studies.
In this study, the brain dialysate was sampled by the microdialysis method with time intervals of 10 min within 2 h and then every 20 min, thereafter enabling the calculation of AUC values with sufficient time points. The tubes connecting the pump, the dialysis probe and the fraction collector were as short as possible in order to reduce the effect of the void volume on sampling time.
There have been different opinions on the damage to the BBB after insertion of a microdialysis probe. Recent publications described a biphasic response in increased BBB permeability with a prompt increase immediately after probe insertion, followed by a second increase 1–2 days after insertion22. In this study the animals were allowed to stabilize for 1 h after probe implantation, and then each formulation was administered.
In a preliminary experiment we investigated the TMPP concentration in different parts of the rat brain using the homogenate method, and there was no significant difference between the concentration TMPP in the olfactory, cerebellum and cerebral cortex at 5 and 15 min. This was consistent with the conclusion of the study by Liang et al.23, so we chose the cerebral cortex to sample in the microdialysis experiment.
From the profiles in Figs. 1 and 2, comparison of i.n. and i.v. delivery of TMPP demonstrated that i.n. administration of TMPP achieved a much higher degree of drug delivery to brain tissue. The ratio of the AUCbrain dialysates value between i.n. route and i.v. injection was 0.68 and the bioavailability of i.n. administration was 86.33%, while the DTE was 78.89%, indicating that TMPP could be efficiently absorbed through the nasal mucosa into the systemic circulation and then delivered to the brain in rats.
The mean residence time (MRT) calculation shows that the decrease of TMPP in brain tissue was slower than that in the plasma, which was the same as the results reported by Feng et al.11
The oral bioavailability of TMPP was lower than the nasal bioavailability (50.39% vs. 86.33%). This suggests that nasal absorption can circumvent the gastrointestinal tract, and may be of practical value to avoid first-pass effect frequently associated with oral administration of TMPP.
The drug uptake into the brain from the nasal mucosa can occur via three different pathways5. One is that drug may be absorbed into the systemic circulation and subsequently reaches the brain by crossing the BBB. The others are the olfactory pathway and the trigeminal neural pathway by which the drug may permeate the brain directly. The extent and the route of drug delivery to the brain mainly depends on characteristics that include lipophilicity and molecular weight (MW). For a small molecular weight lipophilic drug, the rate of transport into the brain via the systemic pathway is rapid with the tmax usually ranging from 1 to 20 min post-dosing24,25. While small molecular weight hydrophilic drugs can be delivered into the brain via the olfactory pathway, with a tmax usually ranging from 30 to 60 min post-dosing and with relatively high brain bioavailability26. Hydrophilic drugs do not easily pass the BBB from the systemic circulation after i.v. administration.
After nasal administration, TMPP reached a Cmax at 15 min in brain, the DTE to the brain was 78.89%, and there was a time delay between tmax in plasma and brain. Considering the physicochemical characterization of TMPP with a MW of 252.2 and a log P=0.294 according to our preliminary experiment, it is probable that TMPP was mainly delivered from the nasal cavity into the systemic circulation and then taken up via the BBB into the brain. The potential of nasal transport can be increased with the use of absorption enhancers and more effective formulations, and a controlled release nasal formulation is under development in our lab. Intranasal administration of TMPP may be a promising alternative to traditional routes.
Acknowledgment
The authors gratefully acknowledge the financial support by Department of Science and Technology, Guangdong, China (2010B03070009).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yuehong Xu, Email: lssxyh@mail.sysu.edu.cn.
Chuanbin Wu, Email: chuanbin_wu@126.com.
References
- 1.Guo SK, Chen KJ, Qian ZH, Weng WL, Qian MY. Tetramethylpyrazine in the treatment of cardiovascular and cerebrovascular diseases. Planta Med. 1983;47:89. [PubMed] [Google Scholar]
- 2.Sheu JR, Kan YC, Hung WC, Ko WC, Yen MH. Mechanisms involved in the antiplatelet activity of tetramethylpyrazine in human platelets. Thromb Res. 1997;88:259–270. doi: 10.1016/s0049-3848(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 3.Fan LH, Wang KZ, Cheng B, Wang CS, Dang XQ. Anti-apoptotic and neuroprotective effects of tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci. 2006;7:48. doi: 10.1186/1471-2202-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai W, Dong SN, Lou YQ. HPLC determination of tetramethylpyrazine in human serum and its pharmacokinetic parameters. Acta Pharm Sin. 1989;24:881–886. [PubMed] [Google Scholar]
- 5.Illum L. Nasal drug delivery–possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 6.Chow HH, Anavy N, Villalobos A. Direct nose-brain transport of benzoylecgonine following intranasal administration in rats. J Pharm Sci. 2001;90:1729–1735. doi: 10.1002/jps.1122. [DOI] [PubMed] [Google Scholar]
- 7.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94:1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 9.Plock N, Kloft C. Microdialysis-theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25:1–24. doi: 10.1016/j.ejps.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Tsai TH, Liang CC. Pharmacokinetics of tetramethylpyrazine in rat blood and brain using microdialysis. Int J Pharm. 2001;216:61–66. doi: 10.1016/s0378-5173(01)00572-5. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Li FZ, Zhao YM, Feng YR, Abe Y. Brain pharmacokinetics of tetramethylpyrazine after intranasal and intravenous administration in awake rats. Int J Pharm. 2009;375:55–60. doi: 10.1016/j.ijpharm.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Wang XM, Wang J, Liu GL, Tang X. Evaluation of brain-targeting for the nasal delivery of ergoloid mesylate by the microdialysis method in rats. Eur J Pharm Biopharm. 2008;68:694–700. doi: 10.1016/j.ejpb.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. 3rd Ed. Academic Press; Sydney: 1997. The rat brain in stereotaxic coordinates. [Google Scholar]
- 14.Okura T, Komiyama N, Morita Y, Kimura M, Deguchi Y, Yamada S. Comparative measurement of spinal CSF microdialysate concentrations and concomitant antinociception of morphine and morphine-6β-glucuronide in rats. Life Sci. 2007;80:1319–1326. doi: 10.1016/j.lfs.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Sasongko L, Williams KM, Ramzan I, McLachlan AJ. Assessment of in vitro and in vivo recovery of gallamine using microdialysis. J Pharmacol Toxicol Methods. 2000;44:519–525. doi: 10.1016/s1056-8719(00)00117-9. [DOI] [PubMed] [Google Scholar]
- 16.Kumar M, Misra A, Mishra A.K, Mishra P, Pathak K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Targeting. 2008;16:806–814. doi: 10.1080/10611860802476504. [DOI] [PubMed] [Google Scholar]
- 17.Salama HA, Mahmoud AA, Kamel AO, Hady MA, Awad GAS. Phospholipid based colloidal poloxamer-nanocubic vesicles for brain targeting via nasal route. Colloids Surface B Biointerfaces. 2012;100:146–154. doi: 10.1016/j.colsurfb.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni JK. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47:6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Wu JW, Shih HH, Wang SC, Tsai TH. Determination and pharmacokinetic profile of pyrazinamide in rat blood, brain and bile using microdialysis coupled with high-performance liquid chromatography and verified by tandem mass spectrometry. Anal Chim Acta. 2004;522:231–239. [Google Scholar]
- 20.Zhang YJ, Wu L, Zhang QL, Li J, Yin FX, Yuan Y. Pharmacokinetics of phenoic compounds of Danshen extract in rat blood and brain by microdialysis sampling. J Ethnopharmacol. 2011;136:129–136. doi: 10.1016/j.jep.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Tang Z, Wang Q, Xu HY, Zhang WG. Microdialysis sampling for inverstigation of tetramethylpyrazine following transdermal and intraperitoneal administration. Eur J Pharm Sci. 2012;50:454–458. doi: 10.1016/j.ejps.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Groothuis DR, Ward S, Schlageter KE, Itskovich AC, Schwerin SC, Allen CV. Changes in blood-brain barrier permeability associated with insertion of brain cannulas and microdialysis probes. Brain Res. 1998;803:218–230. doi: 10.1016/s0006-8993(98)00572-1. [DOI] [PubMed] [Google Scholar]
- 23.Liang CC, Hong CY, Chen CF, Tsai TH. Measurement and pharmacokinetic study of tetramethylpyrazine in rat blood and its regional brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;724:303–309. doi: 10.1016/s0378-4347(99)00010-9. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Chen GS. Pharmacokinetic behavior of huperzine A in plasma and cerebrospinal fluid after intranasal administration in rats. Biopharm Drug Dispos. 2009;30:551–555. doi: 10.1002/bdd.686. [DOI] [PubMed] [Google Scholar]
- 25.Chou KJ, Donovan MD. Lidocaine distribution into the CNS following nasal and arterial delivery: a comparison of local sampling and microdialysis techniques. Int J Pharm. 1998;171:53–61. [Google Scholar]
- 26.Bagger MA, Bechgaard E. The potential of nasal application for delivery to the central brain—a microdialysis study of fluorescein in rats. Eur J Pharm Sci. 2004;21:235–242. doi: 10.1016/j.ejps.2003.10.012. [DOI] [PubMed] [Google Scholar]