Abstract
Borneol is a traditional Chinese medicine that can promote drug absorption from the gastrointestinal tract and distribution to the brain. However, stomach irritation may occur when high doses of borneol are used. In the present work, gastrodin, the main bioactive ingredient of the traditional Chinese drug “Tianma” (Rhizoma Gastrodiae) was used as a model drug to explore reasonable application of borneol. Sustained-release solid dispersions (SRSDs) for co-loading gastrodin and borneol were prepared using ethylcellulose as a sustained release matrix and hydroxy-propyl methylcellulose as a retarder. The dispersion state of drug within the SRSDs was analyzed by using scanning electron microscopy, differential scanning calorimetry, and powder X-ray diffractometry. The results indicated that both gastrodin and borneol were molecularly dispersed in an amorphous form. Assay of in vitro drug release demonstrated that the dissolution profiles of gastrodin and borneol from the SRSDs both fitted the Higuchi model. Subsequently, gastric mucosa irritation and the brain targeting of the SRSDs were evaluated. Compared with the free mixture of gastrodin and borneol, brain targeting of SRSDs was slightly weaker (brain targeting index: 1.83 vs. 2.09), but stomach irritation obviously reduced. Sustained-release technology can be used to reduce stomach irritation caused by borneol while preserving sufficient transport capacity for oral brain-targeting drug delivery.
KEY WORDS: Borneol, Gastrodin, Oral drug delivery, Brain-targeting, Gastric mucosa irritation, Sustained-release
Graphical abstract
Although the brain targeting of the sustained-release solid dispersions was slightly weaker (brain targeting index: 1.83 vs. 2.09) compared with the free mixture of gastrodin and borneol, stomach irritation caused by borneol obviously reduced. Sustained-release technology can be used to reduce stomach irritation by borneol while preserving sufficient transport capacity for oral brain-targeting drug delivery.
1. Introduction
In many compounds employed in traditional Chinese medicine (TCM), a so-called messenger drug is usually included. It is capable of introducing the main effective drugs in the prescription to the target site to increase therapeutic efficacy1–3. The bicyclic monoterpene borneol (Fig. 1A) is one such messenger drug that is frequently used in the treatment of encephalopathy like stroke, epilepsy and headache. Studies have shown that borneol can promote drug absorption from the gastrointestinal tract and facilitate distribution to the brain4–8. However, stomach irritation may occur when a high dose of borneol is used9,10, which limits the clinical application of borneol as an oral brain-targeting enhancer.
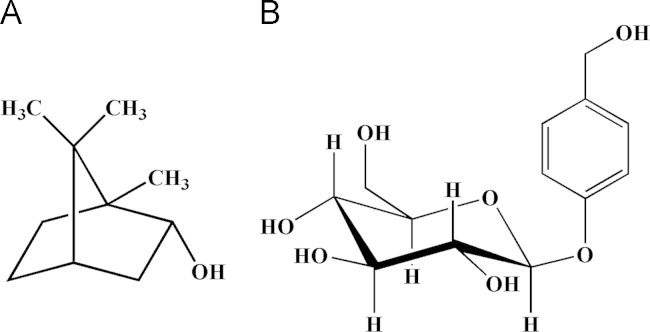
Figure 1.
Chemical structures of borneol (A) and gastrodin (B).
Gastrodin (p-hydroxymethylphenyl-β-d-glucopyranoside, Fig. 1B), the main bioactive ingredient of the traditional Chinese drug “Tianma” (Rhizoma Gastrodiae), is used in the treatment of some central nervous system disorders, such as vertigo, headache, insomnia, neuralgia, neurasthenia and epilepsy in TCM11–13. Gastrodin has attracted increasing attention because of its low toxicity and therapeutic efficacy. Gastrodin acts on the brain to produce central inhibitory effects, but it does not easily pass through the blood–brain barrier (BBB) due to its hydrophilicity. In fact, the drug concentration in brain after administration is far lower than in other tissues14,15. Enhancing the delivery of gastrodin to the brain could improve therapeutic efficacy.
Our previous work indicated that 400 mg/kg of borneol can accelerate the absorption of gastrodin (200 mg/kg) in mice gastrointestinal tract and promote its distribution to the brain4. In this study, gastrodin was still used as a model drug to explore reasonable application of borneol as an oral brain-targeting enhancer. Sustained-release technology is one of the efficient methods for decreasing stomach irritation by drugs16,17. The solid dispersion technique using water-insoluble carriers can be used to sustain the drug release and reduce drug side effects18. A sustained-release solid dispersion (SRSD) medium was designed for co-loading gastrodin with borneol. The SRSDs were prepared using ethylcellulose (EC) as a sustained-release matrix and hydroxypropyl methylcellulose (HPMC) as a retarder. The physicochemical characterization, levels of gastric mucosa irritation, and brain targeting effect of borneol on gastrodin within the SRSDs was evaluated.
2. Materials and methods
2.1. Materials and animals
Synthetic borneol was purchased from Guangzhou Chemical Industrial Co. (Guangzhou, China). Gastrodin (purity 99.7%) was synthesized and analyzed for purity by Kunming Pharmaceutical Co. (Kunming, China). Ethylcellulose (EC, 100 cp) and hydroxy-propyl methylcellulose (HPMC, K100M) were kindly provided by Shanghai Colorcon Coating Technology Co., Ltd. (Shanghai, China). Gastrodigenin (purity 99.5%) was purchased from Dingxin Chemical Industrial Co. (Yizheng, China). Hydroquinone (purity 99.4%) was purchased from Sanjili Chemical Industrial Co. (Lianyungang, China) and used as the internal standard solution (100 μg/mL in methanol). Water was deionized and double distilled. Other chemical reagents were of chromatographic or analytical grade and commercially available.
Healthy male Sprague-Dawley rats (230–270 g) and male Kunming strain mice (18–22 g) were obtained from the Laboratory Animal Center of Southern Medical University (SCXK 2006-0015, China). Prior to experimentation, animals were acclimated for at least 1 week to a 12 h light/dark cycle with free access to standard chow and water. The animals were fasted overnight but supplied with water ad libitum before the experiments. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Southern Medical University.
2.2. Preparation of the SRSDs
The SRSDs were prepared by conventional solvent evaporation method. Gastrodin, borneol, EC and HPMC at a weight ratio of 1:2:4:1 were mixed and dissolved in a minimum volume of ethanol in a beaker. The solvent was removed at 30 °C in a vacuum oven until there was no alcohol smell. The dried dispersion was kept in refrigerator (−20 °C) for 2 days to harden. The resultant solid dispersions were pulverized with a mortar and pestle, sieved to obtain 40–60 mesh particles, and then stored in a desiccator at room temperature.
2.3. Scanning electron microscopy
Representative scanning electron microscope (SEM) images of the samples, including the physical mixture of drugs with excipients and the SRSDs, were taken using a JSM-5900LV SEM (Jeol, Tokyo, Japan) at 20 kV acceleration voltage without Au or Pt coating. For the SEM observations, each sample was fixed on an aluminum sample holder using double-side carbon tape.
2.4. Differential scanning calorimetry
The thermal behavior of drug formulations are important in pharmaceutical technologies, since the obtained information such as melting, recrystallization, decomposition, or a change in heat capacity could help to ascertain the physicochemical status of the entrapped drug inside the excipient. Differential scanning calorimetry (DSC) measurements were performed using a DSC-Q100 (TA instruments, New Castle, DE, USA). The DSC thermograms were collected from a 3 mg sample held in a sealed aluminum pan. The thermograms were generated at a heating rate of 20 °C/min from 0 to 270 °C with nitrogen purge at 50 mL/min. An empty aluminum pan was used as reference.
2.5. Powder X-ray diffractometry
In order to determine whether the drugs were amorphous or crystalline within the formulation, powder X-ray diffractometry (PXRD) study was conducted for the pure drug, the polymers, the physical mixture of drugs and polymers and the SRSDs. The PXRD patterns were obtained by using an X-ray diffractometer (D/Max-rA, Rigaku, Tokyo, Japan) with Nickel filtered Cu-Kα line as the source of radiation, operated at 40 kV voltage and a current of 100 mA. The samples were analyzed in the 2θ angle range of 0–50° at a step size of 0.02° and scanning speed of 4°/min.
2.6. In vitro drug release test
The drug release tests of the SRSDs were carried out by the paddle method specified in the Chinese Pharmacopoeia at a paddle rotation speed of 100 rpm in 900 mL distilled water at 37±0.5 °C. The SRSDs were weighed so as to contain 20 mg of gastrodin. At the specified time (0.5, 1, 2, 4, 6 and 8 h), 8 mL of the dissolution medium was sampled, and fresh medium (8 mL) prewarmed at 37 °C was simultaneously added to the dissolution medium to maintain a constant volume throughout the test. The sample was filtered with a membrane filter (0.45 μm). Aliquots of 2.5 mL were withdrawn and assayed for borneol content by gas chromatograph (GC-122, Factory of Analytical Apparatus, Shanghai, China)19. The remaining filtrate was assayed for gastrodin content by measuring the absorbance at 221 nm on a UV–visible spectrophotometer (UV2201, Shimadzu, Japan).
To analyze the in vitro release data, various kinetic models including the zero-order model (Eq. (1)), the first-order model (Eq. (2)) and the Higuchi model (Eq. (3)) were used to describe the release kinetics20.
| (1) |
| (2) |
| (3) |
In these equations, R and UR are the released and unreleased percentages at time (t) and k is the rate constant.
2.7. Gastric mucosa irritation test
Twelve Sprague-Dawley male rats were divided randomly into 4 groups, 3 rats per group, and intragastrically administered various drug combinations and doses twice a day for 4 days. Group 1 was administered 20 mL/kg physiological saline, group 2 was administered 300 mg/kg gastrodin, group 3 was administered 300 mg/kg of gastrodin and 600 mg/kg borneol, and group 4 was administered SRSDs containing 300 mg/kg of gastrodin and 600 mg/kg of borneol. Drug solutions and suspensions were prepared with 1% CMC-Na solution and given in equal volume of 20 mL/kg.
On the fifth day, the animals were anaesthetized with ether and the stomach was removed. The resected stomach was opened along the lesser curvature and a mucosal specimen (about 0.5 cm2) was obtained. The specimen was rinsed in physiological saline and then fixed overnight in 2.5% glutaraldehyde. Subsequently, the specimen was dehydrated through an ethanol series (30%–100%), substituted with isoamyl acetate, critical point dried with CO2, and sputter coated with gold. The prepared specimens were observed with a scanning electron microscope (Amray 1000B, Bedford, MA, USA). The rest of the stomach tissue was fixed in 4% paraformaldehyde after being washed in physiological saline. A strip of stomach sample containing sinus ventriculi and corpus gastricum was cut from the lesser curvature, and embedded in paraffin. Sections with the thickness of 5 μm were cut for light microscopic evaluation.
2.8. In vivo pharmacokinetics study
All mice were divided randomly into 3 groups, 40 mice each group, and intragastrically administered as follows: gastrodin group, administered 200 mg/kg of gastrodin; physical mixture group, administered 200 mg/kg gastrodin with 400 mg/kg borneol; SRSDs group, administered SRSDs containing 200 mg/kg of gastrodin and 400 mg/kg borneol. Drug solutions and suspensions were prepared with 1% CMC-Na solution in equal volume.
At the indicated time points (2, 5, 15, 30, 60, 120, 180 and 300 min) following intragastric administration, 5 mice were sampled from each group. After anaesthetization, blood was collected from orbital venous plexus, drawn into a heparinized centrifuge tube and centrifuged at 500×g for 10 min. About 200–300 μL plasma was obtained. After blood collection, the animals were euthanized by cervical dislocation. Brain tissue was immediately collected, washed with physiological saline and dried with filter paper. The plasma and brain tissue samples were stored at −20 °C until analysis.
The brain tissue samples were weighed and homogenized in physiological saline (0.5 g/mL). Brain homogenate (500 μL) was mixed with the internal standard solution (10 μL) in a centrifuge tube. Gastrodigenin and the internal standard were extracted in 3 mL of ethyl acetate by vortex mixing for 3 min. After centrifugation at 1000×g for 5 min, 2.5 mL of supernatant was obtained and evaporated under air stream in a water bath at 50 °C. The residue was reconstructed in 200 μL of methanol. After centrifuged at 8000×g for 10 min, a 20 μL aliquot of supernatant was injected into the HPLC system for analysis.
The concentrations of gastrodin in the plasma and gastrodigenin in the brain were determined according to a previously described method4. The analysis was performed on an Agilent 1100 series HPLC system (Agilent Technologies, USA) equipped with a quaternary pump, a vacuum degasser, a column thermostat, a 20 μL injector loop, a UV detector and HP ChemStation software. The separation was carried out using a Diamonsil C18 column (250 mm×4.6 mm, i.d., 5.0 μm, Dikma Technologies, China) under the following chromatographic conditions: column temperature, 25 °C; sample injection volume, 20 μL; flow rate, 1.0 mL/min; detection wavelength, 221 nm; mobile phase, acetonitrile–water. The contents of acetonitrile in mobile phase were 6.5% (v/v) for gastrodin and 10.5% for gastrodigenin. Data were acquired and processed by HP ChemStation software, and the each analyte was quantified by the ratio of the analyte peak area to that of the internal standard. The method used for analyses were validated over a concentration range 1.5–500 μg/mL for gastrodin in plasma, and a concentration range 0.05–1.5 μg/mL for gastrodigein in brain. Intra- and inter-day precision (%RSD) were within 5% and the accuracy ranged 92%–104%.
The pharmacokinetic parameters were estimated by non-compartmental methods using 3P97 software. The area under the concentration–time curve (AUC) was calculated up to the last data point using trapezoidal method without extrapolation to infinity. The standard deviation of AUC was calculated as described21. The mean residence time (MRT) was calculated as follows: MRT=AUMC/AUC where AUMC is the area under the first moment–time curve. Peak concentration (Cmax) and peak time (Tmax) were derived directly from the original measured values.
The relative bioavailabilities (F) of gastrodin in the plasma and gastrodigenin in the brain were calculated as the ratio of AUCG+B/AUCG (“G+B” meant administration of gastrodin combined with borneol and “G” meant administration of gastrodin alone). The brain targeting efficiency was evaluated using brain targeting index (BTI) described as follows: BTI=(AUCBr(G+B)/AUCP(G+B))/(AUCBr(G)/AUCp(G)) (“Br” meant brain and “P” meant plasma), and the value of BTI>1 was considered brain targeting distribution.
2.9. Statistical analysis
The data in this paper were presented as mean±SD, if not otherwise specified. Significant differences were assessed by using one-way ANOVA. A probability value of P<0.05 was considered to be statistically significant.
3. Results and discussion
3.1. Preparation of the SRSDs
Ethylcellulose has been widely used in the production of sustained-release matrices, because it is insoluble in water and well tolerated by patients. Conversely, HPMC is a hydrophilic polymer that can retard drug release by swelling in water. In our previous work, we found that the optimal ratio of gastrodin and borneol was 1:24, and it was reported that the optimal ratio of drug to polymer in solid dispersions was usually in the range of 1:1–1:320. Therefore, the formula of the SRSDs was designed and optimized as follows: gastrodin: borneol: EC: HPMC at a ratio (w/w) of 1:2:4:1. Both gastrodin and borneol are readily soluble in ethanol, as is EC. So it was suitable to prepare the SRSDs by solvent evaporation method. Drug release rate became slower when grain size of the SRSDs was increased. However, big particles were difficult to be administered to mice or rats. Therefore, the SRSDs were sieved to obtain 40–60 mesh particles.
3.2. Physicochemical characterization of the SRSDs
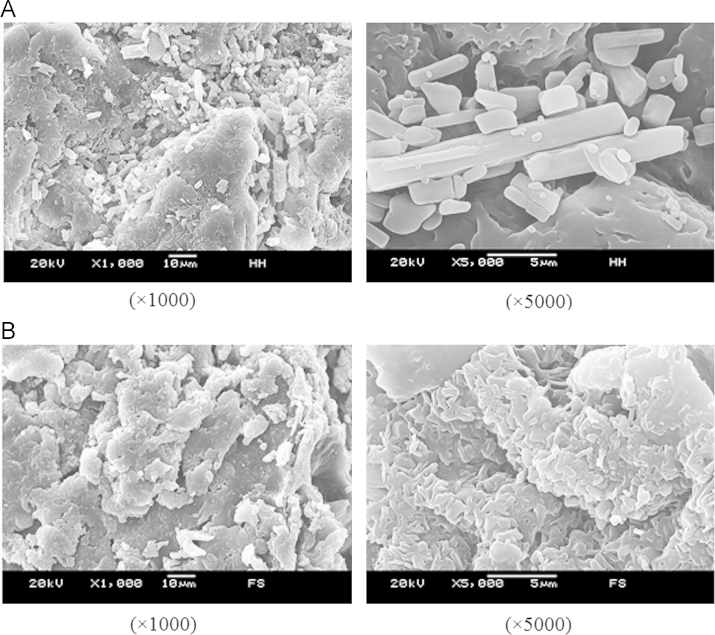
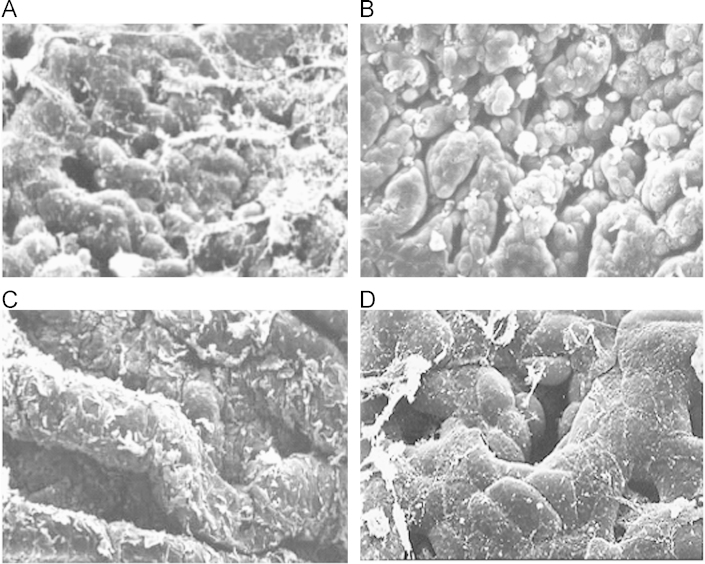
Scanning electron micrographs revealed clear changes in the morphology of the drugs and excipients in different formulations. In the physical mixture, separate gostrodin, borneol and polymers crystals were clearly seen (Fig. 2A), whereas the solid dispersions exhibited the appearance of typical flaky material (Fig. 2B).
Figure 2.
Scanning electron microphotographs of physical mixture of drugs with EC and HPMC (A) and the SRSDs (B).
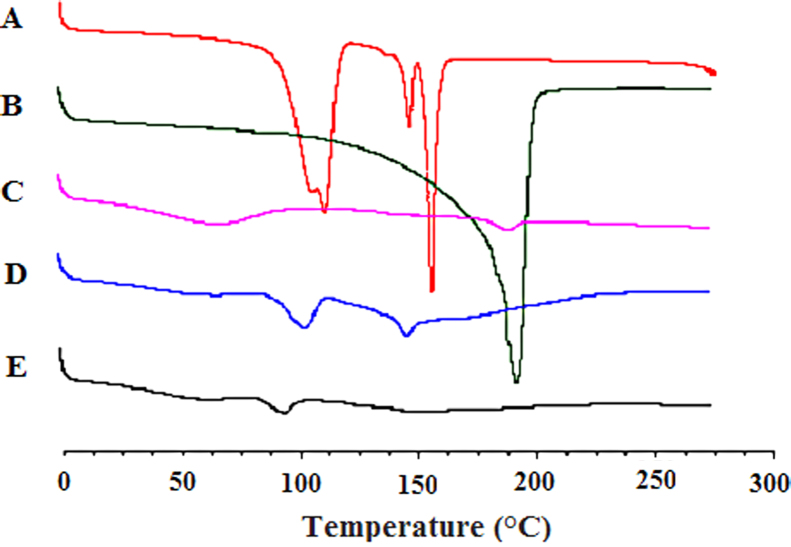
The physical state of drugs in the SRSDs was further evaluated by DSC and PXRD. The DSC thermograms are shown in Fig. 3. Gastrodin produced a large melting endotherm at 157.9 °C. However, the SRSDs did not display any thermal event around this temperature. This indicated that gastrodin was present in an amorphous state. The DSC thermogram of the excipients showed a broad peak probably due to presence of water. The dispersion state of borneol in the SRSDs could not be evaluated by DSC due to its volatility.
Figure 3.
Differential scanning calorimetry thermograms of gastrodin (A), borneol (B), EC+HPMC (C), physical mixture of drugs with EC and HPMC (D) and the SRSDs (E).
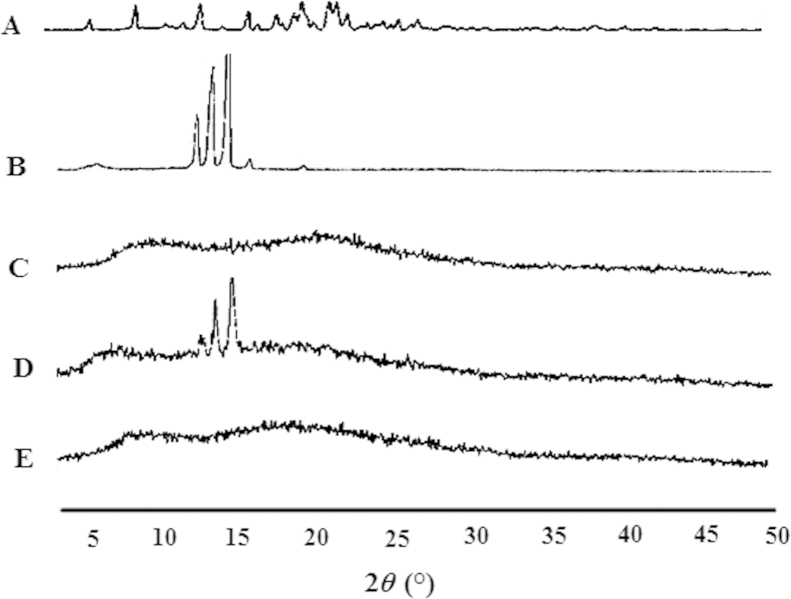
The PXRD pattern of pure borneol indicated that borneol was crystalline as demonstrated by several distinctive peaks observed at 2θ of 13.92°, 15.11°, and 16.18° (Fig. 4B). Moreover, the physical mixture of drugs and excipients showed the same series of three peaks. In contrast, all these characteristic peaks of borneol were absent in the diffraction spectrum of the SRSDs, suggesting that borneol was not in crystalline form. These findings demonstrated that both gastrodin and borneol in the SRSDs were molecularly dispersed in an amorphous form, thus confirming the formation of solid dispersions.
Figure 4.
Powder X-ray patterns of gastrodin (A), borneol (B), EC+HPMC (C), physical mixture of drugs with EC and HPMC (D) and the SRSDs (E).
3.3. Drug release behavior of the SRSDs
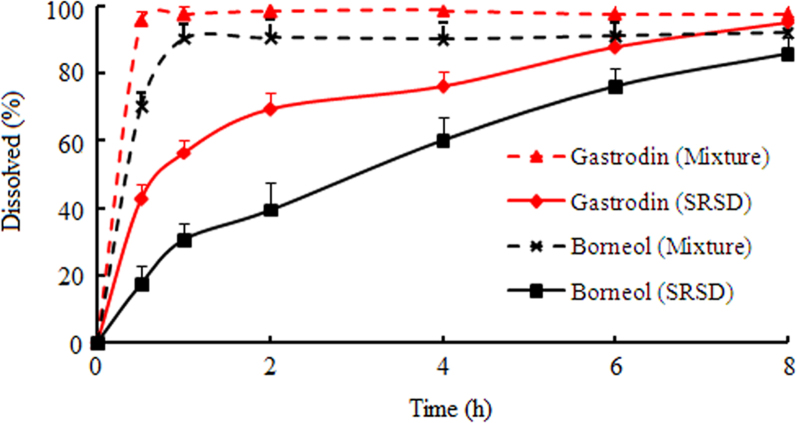
The mean cumulative release of gastrodin and borneol with time is shown in Fig. 5. The SRSDs were able to sustain drugs release for 8 h. The release rate of gastrodin was higher than that of borneol, because gastrodin is readily soluble in water. Indeed, more than 60% of gastrodin was released within 2 h. These SRSDs in powder show significant sustained-release effect compared to crude drugs. They could be reformulated for oral dosage (tablets, capsules and pills) that might provide better control of drug release rate.
Figure 5.
In vitro dissolution profiles of gastrodin and borneol from the physical mixture of drugs with excipients and the SRSDs (n=6).
Table 1 lists the regression parameters obtained after fitting the different release kinetic models to the in vitro dissolution data. The goodness of fit for various models was investigated for gastrodin and borneol. In vitro release data of gastrodin and borneol best fitted the first-order model. It was demonstrated that the SRSDs possessed matrix structure, from which the dissolved drug diffused into the dissolution medium.
Table 1.
Regression parameters obtained from fit of release data to various kinetic models.
| Kinetic model | Gastrodin |
Borneol |
||
|---|---|---|---|---|
| k | R | k | R | |
| Zero-order model | 7.921 | 0.8439 | 9.361 | 0.9673 |
| First-order model | 2.916 | 0.9782 | 4.244 | 0.9975 |
| Higuchi model | 2.970 | 0.9558 | 3.198 | 0.9982 |
3.4. Gastric mucosa irritation of the SRSDs
The scanning electron micrographs of gastric mucosa are shown in Fig. 6. There were no abnormalities found in the gastrodin and SRSDs groups compared with the physiological saline group. However, visible gastric mucosa stripping was observed in the group treated with the physical mixture of gastrodin and borneol. The acute toxicity test dose of borneol used in this study was nearly 100 times higher than that for clinical use. In fact, the clinical gastric irritation reaction was frequently met, but not as serious as was observed in this study.
Figure 6.
Scanning electron microphotographs (500×) of gastric mucosa of rats after intragastric administration of physiological saline (A), gastrodin (B), physical mixture of gastrodin and borneol (C) and the SRSDs (D).
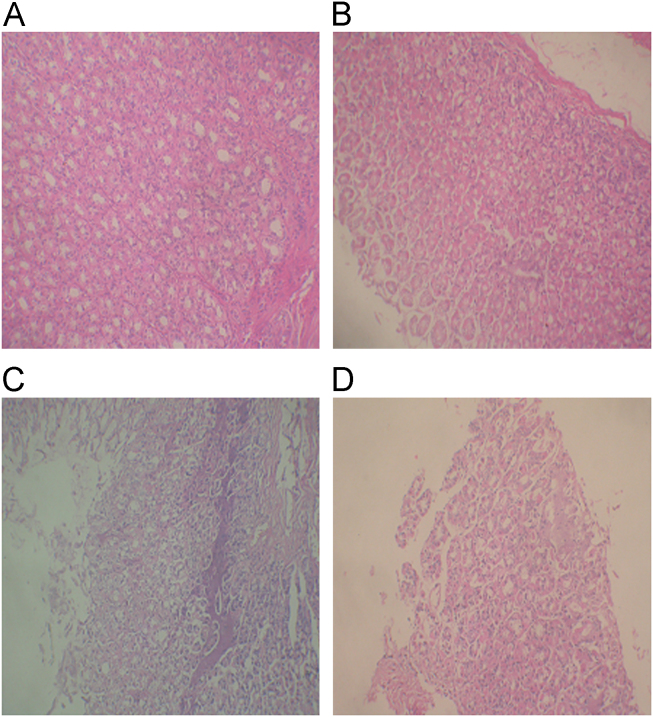
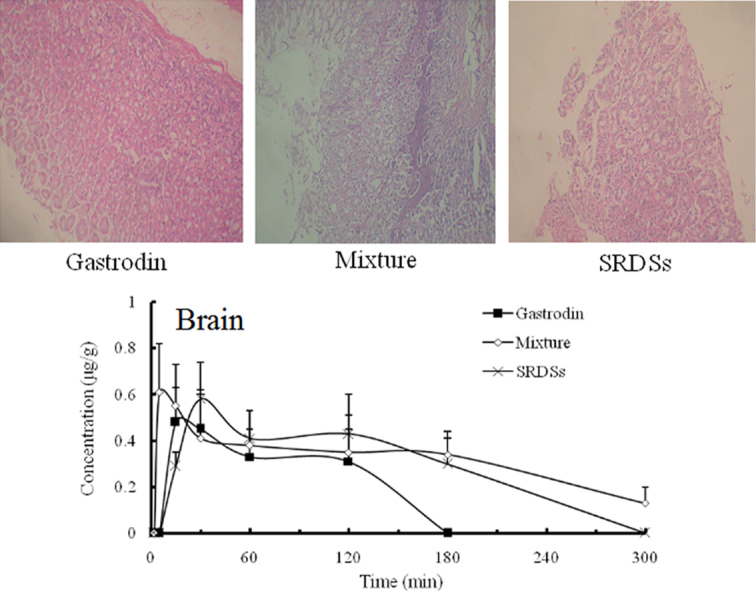
Histopathological examination of the stomach (Fig. 7) did not reveal any abnormalities in the physiological saline or gastrodin group. However, there was obvious multifocal necrosis in the mixture group, supporting the result from SEM analysis. In addition, a slight focal necrosis was recognized in the SRSDs group, which was not observed in the scanning electron micrograph. These results indicated that high dose of borneol had significant toxicity to gastric mucosa and the irritation was reduced when borneol was entrapped in the sustained-release matrix.
Figure 7.
Pathological microphotographs (400×) of gastric mucosa of rats after intragastric administration of physiological saline (A), gastrodin (B), physical mixture of gastrodin and borneol (C) and the SRSDs (D).
3.5. Brain-targeting effect of the SRSDs
Gastrodin cannot bind to benzodiazepine receptors directly. In brain, gastrodin is rapidly metabolized into gastrodigenin (p-hydroxybenzylalcohol) which can bind to benzodiazepine receptors in vivo and induce central inhibitory effects22–24. Therefore, the gastrodin concentrations in the plasma and gastrodigenin concentrations in the brain were determined to evaluate the distribution of gastrodin in mice.
Both borneol and gastrodin are low toxic drugs. The oral LD50 of borneol for mice is 3830 mg/kg25, and the oral LD50 of gastrodin is higher than 5000 mg/kg. Therefore, co-administration of 200 mg/kg gastrodin and 400 mg/kg borneol is safe for mice in the pharmacokinetics study.
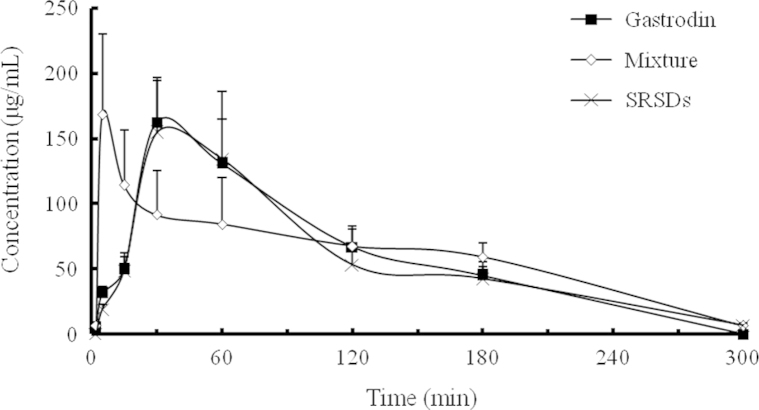
The concentration–time profiles of gastrodin in the plasma and gastrodigenin in brain after intragastric administration of gastrodin alone, physical mixture of gastrodin and borneol, and the SRSDs are shown in Figs. 8 and 9. The pharmacokinetic parameters calculated by statistical moment analysis are shown in Tables 2–4.
Figure 8.
The concentration–time profiles of gastrodin in mice plasma after intragastric administration of gastrodin alone, physical mixture of gastrodin and borneol, and the SRSDs, respectively (n=5).
Figure 9.
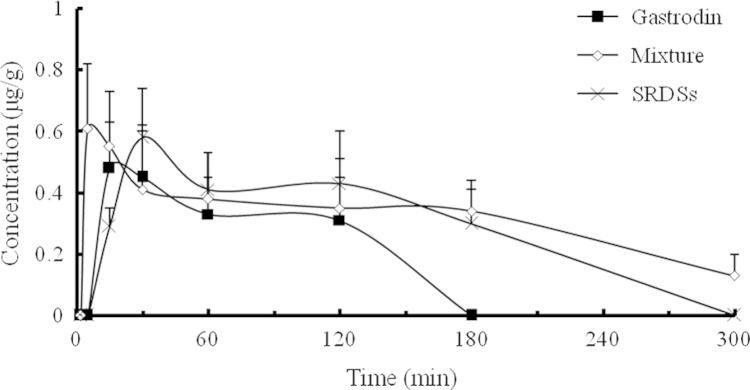
The concentration–time profiles of gastrodigenin in mice brain after intragastric administration of gastrodin alone, physical mixture of gastrodin and borneol, and the SRSDs, respectively (n=5).
Table 2.
Pharmacokinetic parameters of gastrodin in mice plasma after intragastric administration of gastrodin alone, physical mixture of gastrodin and borneol, and the SRSDs, respectively (n=5).
| Parameter | Gastrodin group | Physical mixture group | SRSDs group |
|---|---|---|---|
| Cmax (μg/mL) | 162.39±31.85 | 168.54±61.84 | 154.43±41.89 |
| Tmax (min) | 30 | 5 | 30 |
| AUC (μg·min/mL) | 18,464±2028 | 181,745±2273 | 17,504±2881 |
| MRT (min) | 104.4 | 115.5 | 107.1 |
Table 3.
Pharmacokinetic parameters of gastrodigenin in mice brain after intragastric administration of gastrodin alone, physical mixture of gastrodin and borneol, and the SRSDs, respectively (n=5).
| Parameter | Gastrodin group | Physical mixture group | SRSDs group |
|---|---|---|---|
| Cmax (μg/mL) | 0.48±0.15 | 0.61±0.21 | 0.58±0.16 |
| Tmax (min) | 15 | 5 | 30 |
| AUC (μg·min/mL) | 49.58±10.86 | 101.96±16.17⁎⁎ | 86.12±15.64⁎⁎ |
| MRT (min) | 77.27 | 136.29 | 123.48 |
Compared with gastrodin group: P<0.01.
Table 4.
The relative bioavailabilities and brain targeting indexes for oral administration of gastrodin alone, physical mixture of gastrodin and borneol, and the SRSDs, respectively (n=5).
| Parameter | Gastrodin group | Physical mixture group | SRSDs group |
|---|---|---|---|
| F (gastrodin in plasma, %) | 100.0 | 98.4 | 94.8 |
| F (gastrodigenin in brain, %) | 100.0 | 205.6 | 173.7 |
| BTI | 1.00 | 2.09 | 1.83 |
The pharmacokinetic parameters of gastrodin group (negative control) and physical mixture group (positive control) were similar to those found in previous study4. Compared to administration of gastrodin alone, gastrodin co-administrated with borneol was rapidly absorbed from the gastrointestinal tract. The Tmax of gastrodin in plasma reduced to 5 min from 30 min, while the AUC of gastrodigenin in mouse brain was increased by 105.6%. Thus, an obvious brain targeting effect was observed. Because the absolute oral bioavailability of gastrodin is as high as 80%26, there was no significant difference in Cmax and AUC for gastrodin in the plasma between gastrodin administered alone and co-administrated with borneol.
Compared to gastrodin group, relative bioavailability of the SRSDs was 94.8%, indicating that gastrodin in the SRSDs was released and absorbed almost completely. Gastrodin is easily soluble in water, and it can be absorbed fast in the intestinal tract. Burst effect might occur, so Tmax showed no difference between gastroin group and the SRSD group. Compared with the mixture group, the Tmax of gastrodin in plasma and gastrodigenin in mouse brain were delayed 25 min due to sustained-release of borneol, but there was no significant difference in AUC and BTI between the two groups. The results indicated that the SRSDs preserved sufficient transport capacity for oral brain-targeting drug delivery.
Borneol likely promotes drug transport through biological membranes such as gastrointestinal mucosa and the blood–brain barrier by transiently loosening intercellular tight junctions of the membranes27,28. This could cause an irritation reaction, when a large amount of borneol acts on the membrane simultaneously. Therefore, sustained-release of borneol might avoid irreversibly altering the structure of multicellular membranes.
4. Conclusions
Borneol can accelerate the absorption of gastrodin through the gastrointestinal mucosa and promote its transport across the BBB into the brain. However, gastric mucosa irritation results from high doses of borneol. Stomach irritation can be reduced by using solid dispersion techniques to promote sustain release of borneol. The brain targeting effect of borneol was not significantly changed, although it is incorporated in the solid dispersions. In conclusion, sustained-release technology can be used to reduce stomach irritation caused by borneol, making it more suitable for practical application in oral brain-targeting drug delivery. This study provides a strategy for preparation and application of those traditional Chinese medicines with slight irritation or toxicity.
Acknowledgments
This study was supported by the grants of National Natural Science Foundation of China (30902009) and the Key Scientific and Technological Innovation Programs of Higher Education Institutions in Guangdong (CXZD1121).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Wei T, Li Y. The appliance of disease position syndrome differentiation and messenger drug in clinic. J Liaoning Univ Tradit Chin Med. 2009;11:23–24. [Google Scholar]
- 2.Yu WH. Exploration on the guiding chemical constituents in the medicinal guides of traditional Chinese medicinal herbs. Shizhen J Tradit Med Res. 2007;18:2549–2551. [Google Scholar]
- 3.Zhao R, Liu SJ. TCM medicinal guide theory and target-tropism administration. J Tradit Chin Med. 2005;46:643–645. [Google Scholar]
- 4.Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16:178–184. doi: 10.1080/10611860701794395. [DOI] [PubMed] [Google Scholar]
- 5.Gao C, Li X, Li Y, Wang L, Xue M. Pharmacokinetic interaction between puerarin and edaravone, and effect of borneol on the brain distribution kinetics of puerarin in rats. J Pharm Pharmacol. 2010;62:360–367. doi: 10.1211/jpp.62.03.0011. [DOI] [PubMed] [Google Scholar]
- 6.Xiao YY, Ping QN, Chen ZP. The enhancing effect of synthetical borneol on the absorption of tetramethylpyrazine phosphate in mouse. Int J Pharm. 2007;337:74–79. doi: 10.1016/j.ijpharm.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Han L, Qin J, Lu W, Wang J. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG-PLGA nanoparticles to the brain. Pharm Res. 2013;30:2560–2572. doi: 10.1007/s11095-013-1055-y. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Liao Q, Yao M, Xu X, Zhou Y, Hou X. Effect of natural borneol on the pharmacokinetics and distribution of nimodipine in mice. Eur J Drug Metab Pharmacokinet. 2013 doi: 10.1007/s13318-013-0135-z. May 15. Available form: 10.1007/s13318-013-0135-z. [DOI] [PubMed] [Google Scholar]
- 9.Liu YH, Shao F, Wang Y, Zheng X, Liu ZF, Rong ZD. The damage of borneol and its preparation on gastric mucosa in rats. Qilu Pharm Aff. 2010;29:232–233. [Google Scholar]
- 10.Zhong YZ, Liu J, Wang JY. Erosive gastritis induced by compound Danshen dripping pills: two cases report. Chin J Pharmacoepidemiol. 2006;15:316. [Google Scholar]
- 11.Zeng X, Zhang Y, Zhang S, Zheng X. A microdialysis study of effects of gastrodin on neurochemical changes in the ischemic/reperfused rat cerebral hippocampus. Biol Pharm Bull. 2007;30:801–804. doi: 10.1248/bpb.30.801. [DOI] [PubMed] [Google Scholar]
- 12.Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA. Tianma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006;8:376–383. doi: 10.1016/j.yebeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson's disease model. Evid Based Complement Alternat Med. 2013;2013:514095. doi: 10.1155/2013/514095. Available from: 10.1155/2013/514095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin LC, Chen YF, Tsai TR, Tsai TH. Analysis of brain distribution and biliary excretion of a nutrient supplement, gastrodin, in rat. Anal Chim Acta. 2007;590:173–179. doi: 10.1016/j.aca.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Liu KX, Su CY, Han GZ, Zhang YL, Tang NY, Chen YR. Studies on physiologic disposition of gastrodin in rats. J Dalian Med Univ. 1988;10:62–66. [Google Scholar]
- 16.Mori M, Nakamura Y, Shirai Y, Seto Y, Nakamura H, Makita H. Prolongation of antipyretic action and reduction of gastric ulcerogenicity in the rat by controlled-release granules of bermoprofen, a new nonsteroidal anti-inflammatory drug. J Pharm Sci. 1991;80:876–880. doi: 10.1002/jps.2600800915. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, Wen R, Yang H, He Y. Sustained-release tablets of indomethacin-loaded microcapsules: preparation, in vitro and in vivo characterization. Int J Pharm. 2007;333:87–94. doi: 10.1016/j.ijpharm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersions: a review. Pak J Pharm Sci. 2009;22:234–246. [PubMed] [Google Scholar]
- 19.Guo T, Song HT, Zhao MH, Ma Y, Zhang RH. Studies on preparation and dissolution of Shexiang Baoxin dispersible tablets. Chin Pharm J. 2002;37:836–841. [Google Scholar]
- 20.Sahoo J, Murthy PN, Biswal S, Manik. Formulation of sustained-release dosage form of verapamil hydrochloride by solid dispersion technique using Eudragit RLPO or Kollidon SR. AAPS PharmSciTech. 2009;10:27–33. doi: 10.1208/s12249-008-9175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16:303–309. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z, Tan T, Zhong Y, Wu C. Study of the mechanism of gastrodin and derivatives of gastrodigenin. J West China Univ Med Sci. 1991;22:79–82. [PubMed] [Google Scholar]
- 23.Tan TZ, Kuang AR, Liang ZL, Zhong YG, Chai HX, Zhang JL. The distribution of 3H-alpha-sec-butyl-p-hydroxybenzyl alcohol in mice. J West China Univ Med Sci. 1989;20:58–61. [PubMed] [Google Scholar]
- 24.You J, Tan T, Kuang A, Zhong Y, He S. Biodistribution and metabolism of 3H-gastrodigenin and 3H-gastrodin in mice. J West China Univ Med Sci. 1994;25:325–328. [PubMed] [Google Scholar]
- 25.Wu SR, Cheng G, Feng Y. Progress in studies on pharmacology of borneol. Chin Tradit Herb Drugs. 2001;32:1143–1145. [Google Scholar]
- 26.Cheng G, Hao XH, Liu GL, Zou MJ, Cui FD. Pharmacokinectics of gastrodin in rats. Chin Pharm J. 2003;38:127–129. [Google Scholar]
- 27.Chen ZZ, Lu Y, Du SY, Shang KX, Cai CB. Influence of borneol and muscone on geniposide transport through MDCK and MDCK-MDR1 cells as blood–brain barrier in vitro model. Int J Pharm. 2013;456:73–79. doi: 10.1016/j.ijpharm.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Jin D, Wang F, Qu L, Li Z, Jin L, Liu P. The distribution and expression of claudin-5 and occludin at the rat blood-optic nerve barrier after borneol treatment. Mol Biol Rep. 2011;38:913–920. doi: 10.1007/s11033-010-0184-1. [DOI] [PubMed] [Google Scholar]