Abstract
Kidney-targeted drug delivery systems represent a promising technology to improve drug efficacy and safety in the treatment of renal diseases. In this review, we summarize the strategies that have been employed to develop kidney-targeted drug delivery systems. We also describe how macromolecular carriers and prodrugs play crucial roles in targeting drugs to particular target cells in the kidney. New technologies render it possible to create renal targeting conjugates and other delivery systems including nanoparticles and liposomes present promising strategies to achieve the goal of targeting drugs to the kidney.
KEY WORDS: Kidney-targeted, Prodrug, Macromolecular carrier, Nanoparticles, Liposomes
Graphical abstract
In this review, the strategies that have been employed to develop kidney-targeted drug delivery systems were summarized and how macromolecular carriers and prodrugs play crucial roles in targeting drugs to particular target cells in the kidney was described.
1. Introduction
The kidney is a key organ serving several essential functions. It is responsible for the formation of urine and performs a homeostatic function in maintaining acid–base balance and regulating electrolytes and water to maintain blood pressure. Any disorder in renal physiological function can lead to serious problems such as infections of the urinary tract, inflammation and hypertension. Renal disease and systemic disease caused by renal disease occur throughout the world and are generally difficult to treat.
In the past 10 years, nephropathy has received increasing attention in developing countries. According to a cross-sectional survey of 47,204 people, it was found that the overall prevalence of chronic kidney disease was 10.8%, indicating there could be as many as 119.5 million people in China with renal disease1. These patients often incur a considerable financial burden since renal disease can require long-term medication and in some cases expensive dialysis (¥50,000 per year) or even kidney transplantation (¥100,000) to prolong life.
Treatment of nephrotic syndrome with drugs such as hormones, antibiotics and antihypertensive agents often requires high drug concentrations in the kidney. Unfortunately, such high concentrations are often associated with adverse effects particularly for drugs such as non-steroidal anti-inflammatory drugs (NSAIDs). In addition, high renal drug concentrations may not translate into high concentrations in the target cell and, in pathological conditions such as abnormal glomerular filtration, tubular secretion or proteinuria, the distribution of drugs to the kidney may be affected. Therefore, kidney-targeted drug delivery is of great significance to overcome these problems and improve the therapeutic index of drugs used in treating renal disease.
2. Strategies
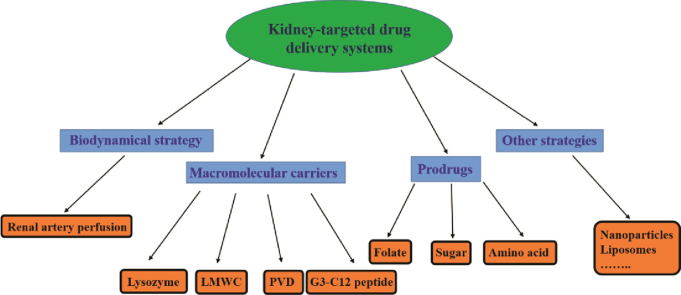
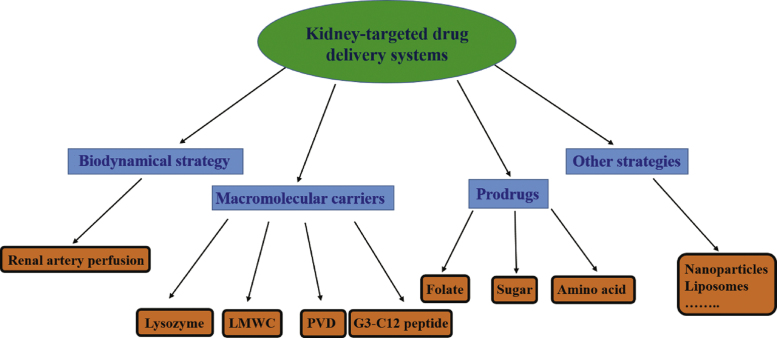
To achieve the goal of kidney-targeted drug delivery, prodrugs2 and macromolecular carriers3 have been the most common strategies employed (Fig. 1). Among the macromolecular carriers, proteins, peptides, antibodies and viruses have been utilized. Prodrugs capable of selectively targeting the kidney generally release the active drug through the action of renal enzymes.
Figure 1.
The strategies of kidney-targeted drug delivery systems.
Glomerular mesangial cells and proximal tubular cells play an important role in the etiology of many renal diseases. However, reports of strategies to target glomerular mesangial cells are very few and delivery systems are mostly targeted to proximal tubular cells. In this review, we mainly discuss the targeting of proximal tubular cells.
Proximal tubular epithelial cells are involved in many aspects of tubular interstitial damage which can contribute to the decline of renal function. They also promote the formation and development of interstitial inflammation and fibrosis by means of chemotaxis, antigen-presenting and the action of autocrine and paracrine cytokines. Proximal tubular cells induced by pathological factors excessively produce and activate complement (C3) which is an essential part of the immune response to injury. Blocking this process can protect renal function and reduce ongoing renal disease4. Therefore, drug delivery to the proximal tubules can improve the treatment of kidney disease and reduce adverse effects on other organs and tissues.
Targeting anti-inflammatory and anti-fibrotic drugs to proximal tubular cells may prevent systemic infection and renal tubular inflammation. In addition, kidney-targeted drug delivery is of great significance in shock, kidney transplantation, ureteral obstruction, diabetes, proteinuria, and in some diseases involving changes in renal tubular function such as Fanconi and Bartter's syndrome. Targeting proximal tubular cells may provide new ways to treat renal disease whilst reducing drug toxicity5–9.
2.1. Macromolecular carriers
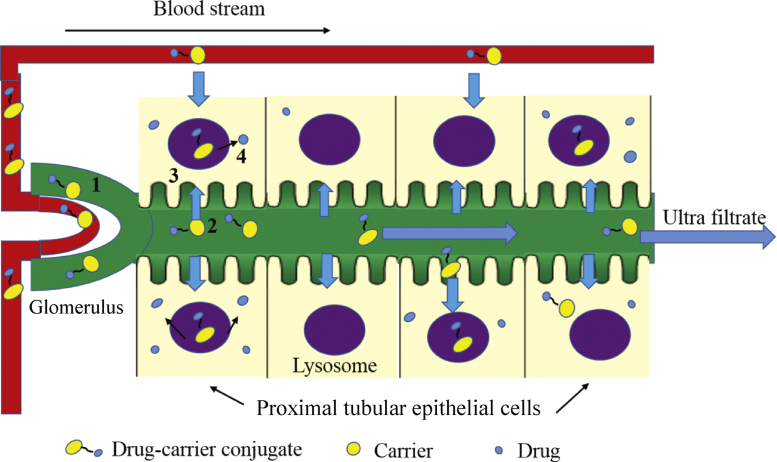
Macromolecular carriers are very useful for targeting drugs to the kidney, in particular low molecular weight glomerular proteins (LMWP) which can selectively accumulate in the kidneys. They are generally small molecular weight (MW<30,000 Da), biologically active proteins in the circulatory system which include enzymes (such as lysozyme), immune proteins (such as light chain immunoglobulin) and peptide hormones (such as insulin). In general, the molecular weight of the macromolecular carrier is larger than that of the drug and the kinetics of the protein carrier overrule the intrinsic kinetics of the drug. LMWP can be filtered at the glomerulus and reabsorbed in the renal tubules (Fig. 2). Of particular importance is that LMWP are non-immunogenic and contain functional groups to which drugs can be linked. Ideally, a drug-macromolecular carrier conjugate is rapidly removed from the circulation and undergoes drug release and activation in lysosomes. Normally, distribution of drug released in the kidney is relatively slow allowing the concentration of drug in the circulatory system to remain below the level associated with extra-renal effects10–12.
Figure 2.
The uptake route of kidney-targeted delivery of drug–carrier conjugates. 1: The drug–carrier conjugates enter rapidly into the renal tubule in the way of glomerular filtration; 2: the drug–carrier conjugates are reabsorbed by the renal tubule; 3: the drug–carrier conjugates enter into renal proximal tubular cells; 4: the drugs release and activation in lysosomes.
LMWP can be conjugated to drugs in a variety of ways including through the formation of peptide, ester, amide and disulfide bonds. The drug can then be released from the conjugate by enzymatic or chemical hydrolysis. The synthesis of a particular drug–LMWP conjugate requires careful design and a high degree of skill partly because LMWP have many active groups and are very vulnerable to self-aggregation. In fact it is often necessary to introduce protecting groups and use carefully controlled reaction conditions to avoid LMWP degeneration.
2.1.1. Lysozyme
The most studied LMWP is lysozyme, a low molecular weight endogenous protein with a molecular weight of 14 kDa. Lysozyme has a number of advantages as a macromolecular carrier including specific renal uptake, biodegradability and ease of conjugation with drugs. One of the most extensively studied kidney-targeted conjugates is with naproxen13–15 where the drug is linked to lysozyme by a peptide bond. The naproxen–lysozyme conjugate is converted into an active metabolite, naproxen–lysine, which can inhibit cyclooxygenase to the same extent as naproxen itself. After its uptake by renal proximal tubular cells, naproxen is gradually released and produces renal naproxen concentrations some 70 times greater than naproxen itself indicating the naproxen–lysozyme conjugate significantly improves the renal targeting of naproxen.
Another well studied kidney-targeted conjugate is with triptolide. Triptolide has been extensively utilized to treat renal diseases because of its potent anti-inflammatory and immunosuppressive properties but it has both limited water solubility and exerts toxic effects on the digestive, urogenital, circulatory and reproductive systems. Zheng and co-workers16,17 synthesized a triptolide–lysozyme (TPS–LZM) 1:1 conjugate with an ester linkage and found that the renal concentration of conjugate was 20-fold higher than from an equivalent dose of drug 30 min after intravenous (i.v.) injection. The targeting efficiency of TPS–LZM was enhanced from 11.7% to 95.5% and its mean residence time was moderately prolonged from 3.08 h to 4.10 h. In addition, the TPS–LZM conjugate showed 22% less hepatotoxicity than triptolide and produced no adverse effects on the immune system. The internalization of the TPS–LZM conjugate was mediated by a 600 kDa glycoprotein called megalin, a multi-ligand endocytic receptor highly expressed in the endocytic pathway of renal proximal tubules.
The ACE inhibitor captopril has also been conjugated with lysozyme via a disulfide bond and using succinimidyloxycarbonyl-α-methyl-α-(2-pyridyldithio) toluene (SMPT) as a linker18,19. Research showed captopril could be released from the conjugate either enzymatically by β-lyase and/or non-enzymatically by thiol-disulfide exchange with endogenous thiols. The renal concentration of captopril was increased 6-fold in male Wistar rats and, compared with the non-targeted drug, the captopril-lysozyme conjugate reduced proteinuria with no systemic effects on blood pressure.
According to a recent report, a sunitinib analog named 17864 was linked to lysozyme via a platinum-based linker20. The 17864-lysozyme conjugate strongly inhibited tyrosine kinase activity and, after i.v. administration, rapidly accumulated in the kidneys and gave a 29-fold increase in renal AUC. It also produced sustained renal drug levels for up to 3 days and showed no anti-fibrotic effects in normal male C57BI/6 J mice.
A number of other drugs have been linked to lysozyme in various ways, for example sulfamethoxazole and doxorubicin both via cis-aconitic anhydride and SB202190 via the platinum (II)-based Universal Linkage System™ (ULS)21.
2.1.2. Low molecular weight chitosan
Another carrier that has been successfully used for kidney-targeted is acetylated low molecular weight chitosan (LMWC). Chitosan is a natural copolymer of glucosamine and N-acetyl-glucosamine derived from chitin. Because of its excellent biocompatibility and biodegradability, chitosan has been widely used in drug delivery systems. In investigating a kidney-targeted conjugate with prednisone, researchers used 50% acetylated LMWC with different molecular weights22–24 and found that the distribution to the kidney could be increased 13-fold compared to prednisolone alone with greatly reduced toxicity. LMWC is specifically taken up by renal tubular cells probably via megalin and, compared to lysozyme, is cleared from the kidneys more rapidly. These findings indicate that LMWCs with molecular weights of 19 and 31 kDa are useful drug carriers with a high degree of safety.
2.1.3. Poly(vinylpyrrolidone-co-dimethyl maleic acid)
Yamamoto and co-workers25–27 prepared PVD and investigated how molecular weight and charge affected its distribution in mouse. Their results showed that PVD with a molecular weight in the range 6–8 kDa produced the best renal targeting with about 80% of an administered dose being distributed to the kidneys. The molecular charge also affected the distribution where it was found that the more negatively charged PVD derivatives were eliminated more slowly from the kidneys. Using a superoxide dismutase conjugate (SOD–PVD), it was shown that the conjugate exerted a good therapeutic effect in a mouse model of acute renal failure. However, the mechanism of absorption of PVD remained unclear and the biocompatibility and immunogenicity of conjugates requires further study.
2.1.4. G3-C12 peptide
A peptide specific to the galectin-3 carbohydrate recognition domain (G3-C12) (ANTPCG-PYTHDCPVKR), identified using a combinatorial phase display technique, was shown to specifically accumulate in mouse kidneys after i.v. injection28–30. This was done by preparing an FITC-labeled G3-C12 peptide (G3-C12-FITC) and showing that it selectively accumulated in the kidneys soon after injection, probably due to reabsorption of the peptide by proximal renal tubular cells. Taking advantage of this, Geng et al.31 linked G3-C12 to captopril to produce a kidney-targeted delivery system for the drug. They showed the conjugate gave a 2.7-fold increase in renal AUC compared with the drug alone and, in addition, completely released the drug in the kidney as early as 3 min post-injection via disulfide bond reduction. These results indicate the potential of G3-C12 peptide as a novel kidney-targeted carrier.
2.2. Prodrugs
2.2.1. Sugar-modified prodrugs
In recent years, the significance of protein–sugar interactions in pharmacology and pathology has been increasingly realized. Sugar-recognition plays a key role in cell–cell, cell–matrix and cell–molecule interactions including receptor-mediated endocytosis. This led Suzuki and co-workers to propose glycoconjugates as potential renal targeting vectors32–34. They used arginine–vasopressin (AVP) as a model drug and introduced different glycosylated derivatives to form glycosylated conjugates via an octamethylene group. On the basis of in vivo and in vitro studies, they concluded that the alkylglucoside structure (Glc-S-C8-) was necessary for kidney-targeted, and the targeting efficiency depended on the type of sugar moieties (the length of the alkyl chain, structure of the peptide and type of linkage) and the size and charge of the molecule. Potential therapeutic agents were those with a lower molecular weight and neutral charge.
Lin et al.35,36 successfully synthesized a prednisolone succinate–glucosamine conjugate (PGC) and a 2-deoxy-2-aminodiglucose–prednisolone conjugate (DPC) as potential prodrugs of prednisolone. They conducted cytotoxicity and uptake studies on PGC in HK-2 and MDCK cell lines and, compared with prednisolone, PGC showed lower cytotoxicity combined with a 2.2-fold greater cellular uptake. In addition, tissue distribution studies showed that the concentration of DPC in kidney was 4.9-fold higher than that of prednisolone. The authors concluded that 2-glucosamine may be a potential carrier for renal drug targeting.
Liang et al.37 prepared a zidovudine–chitosan oligomer conjugate and evaluated the in vitro release of zidovudine in a pharmacokinetic study in mouse after i.v. administration. The results indicated the mean residence time of the conjugate was 2.5 times that of zidovudine and that it accumulated in kidney more than in any other organ.
2.2.2. Amino acid modified prodrugs
Some endogenous enzymes such as those involved in amino acid l-decarboxylation and γ-glutamyltranspeptidase have relatively high concentrations in kidneys. On this basis, some researchers used substrates of these enzymes to chemically modify drugs in the hope that drug would be released in proximal tubular cells via action of the relevant enzyme. Wilk et al.38 synthesized γ-glutamyl-dopamine (GGDA) and studied its tissue distribution after administration to mice. They found that the dopamine concentration in kidneys produced by GGDA was higher than that of an equivalent dose of dopamine suggesting GGDA was degraded by renal enzymes. The dopamine targeted to the kidneys significantly increased renal blood flow without any significant effect on the heart or on blood pressure. After oral administration of GGDA, the concentration of free dopamine in plasma was very low while that in urine was relatively high39,40. These results indicate that GGDA is a useful way to target dopamine to the kidney.
Su et al.41 produced an N-acetyl-glutamyl prodrug of prednisone and investigated its in vivo distribution and effect on bone density after administration to rats. The results showed that, compared with prednisone, the renal concentration of prodrug was enhanced and the incidence of prednisolone-induced osteoporosis was reduced.
2.2.3. Folate modified prodrugs
The kidney plays an important role in reducing loss of folate. Folate exists in the body as 5-methylenetetrahydrofolate which is filtered at the glomerulus and reabsorbed into the renal vascular circulation via a high-affinity folate binding protein (FBP) concentrated in the proximal tubular epithelium. In pioneering work by Wang et al.42, folic acid was attached to diethylene triamine pentaacetic acid (DTPA) via an ethylenediamine spacer to give a DTPA-folate conjugate which was rapidly excreted in urine after i.v. administration to rat. In a subsequent pharmacodynamic study in athymic tumor-bearing mice, DTPA–folate administered by i.v. injection was not only taken up by the tumor but also largely distributed to the kidneys. This observation, together with the rapid clearance of the DTPA–folate conjugate from FR-negative tissues, illustrates the crucial role played by folate receptors in the renal uptake of such conjugates42. To date, the use of folate binding for renal drug targeting has received limited attention and, since folate receptors are expressed in organs other than kidney, the physicochemical properties of the conjugate may be important determinants of the success of such targeting.
3. Other kidney-targeted drug delivery systems
3.1. Nanoparticles
Nanoparticles show great potential as carriers for drug delivery not least because they can reduce the accumulation of drugs in renal tubules resulting in decreased renal tubular toxicity. The physicochemical properties of nanoparticles such as size, surface charge, shape and density are all important in dictating their ability to overcome biological barriers and reach their designated cellular destination.
Manil et al.43 prepared actinomycin D (AD)-loaded isobutyl acrylate nanoparticles (ADNP) and showed in in vitro and in vivo experiments that they concentrated in glomerular mesangial cells. Thus, when 3H–ADNP or 3H–AD were injected into rats with experimental glomerulonephritis, the uptake ratios (3H–ADNP/3H–AD) were respectively 6.9-fold (30 min) and 4.0-fold (120 min) higher than in normal rats. In vitro experiments showed that the uptake by glomerular mesangial cells was 6-fold higher than by epithelial cells. Targeting the glomerular mesangium is particularly valuable for anti-inflammatory drugs such as cortisone to treat glomerular inflammation.
Choi et al.44 found that nanoparticles with diameter 75±25 nm could be targeted to the kidney mesangium. By doing so, they established design criteria for constructing nanoparticle-based therapeutics for targeting diseases that involve the kidney mesangium.
3.2. Liposomes
Liposomes are a well-studied drug delivery system and a component of several currently marketed products. Singh et al. studied small unilamellar vesicles (SUVs) loading with methotrexate [(MTX)SUVs)] linked to Dal K29, an IgG1 monoclonal antibody against human renal cancer, normal mouse IgG or a nonspecific mouse myeloma IgGl45. After incubation with human kidney CaKi-1 cancer cells for 2 h, the Dal K29-linked (MTX)SUVs showed respectively 6- and 8-fold more binding to CaKi-1 cells than nonspecific mouse myeloma IgGl-linked (MTX)SUV or unlinked (MTX)SUVs. A colony inhibition assay also showed that the Dal K29-linked (MTX)SUVs were respectively 5 and 40 times better than Dal K29-MTX and free MTX in inhibiting the growth of the target CaKi-1 cells. The results suggested that the Dal K29-[(MTX)SUV] system could be useful in the targeted treatment of renal cancer.
Tuffin et al.46 prepared OX7-coupled immunoliposomes (OX7-IL) by coupling liposomes with Fab fragments of OX7 mAb directed against Thy1.1 antigen. The mean diameters of the liposomes and immunoliposomes were 130 and 170 nm, respectively. Because the glomerular endothelium is fenestrated and no basement membrane separates glomerular capillaries from the mesangium, mesangial cells represent a particularly suitable target for drug delivery by OX7-IL. After i.v. administration to rats, OX7-IL was found to specifically target mesangial cells but its kidney-targeted was blocked when free OX7 F(ab)2 fragments were co-administered. Rats injected with low-dose doxorubicin encapsulated in OX7-IL showed extensive glomerular damage whereas other parts of the kidney and other organs were spared.
4. Conclusions and future perspectives
At present, kidney-targeted drug delivery of drugs is focused on improving targeting efficiency and seeking new carriers. Pharmacokinetic and pharmacodynamic studies in pathological conditions involving severe reductions in glomerular filtration and proteinuria need to be done to reveal the mechanism of renal uptake of carriers and prodrugs. A pressing need is to find ways to target drugs to the glomerulus since it plays an important role in the development of renal disease. With greater understanding of renal structure, further development of molecular pharmacology and more in-depth research into novel carriers, drug targeting to mesangial cells and media fibroblasts will greatly improve the clinical treatment of renal diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation (No. 81130060) of China and the National Science & Technology Major Project of China (No. 2011ZX09310-002).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Zhang L.X., Wang F., Wang L., Wang W.K., Liu B.C., Liu J. Prevalence of chronic kidney disease in China: a cross-sectional survey. The Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 2.Liu K.X., Kato Y., Kino I., Nakamura T., Sugiyama Y. Ligand-induced downregulation of receptor-mediated clearance of hepatocyte growth factor in rats. Am J Physiol. 1988;275:835–842. doi: 10.1152/ajpendo.1998.275.5.E835. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe Y., Suzuki H., Suzuki K., Ando T., Nakabayashi S., Sugiyama Y. Detection of the membrane protein recognized by the kidney-specific alkylglucoside vector. Pharm Res. 2000;17:49–54. doi: 10.1023/a:1007566408323. [DOI] [PubMed] [Google Scholar]
- 4.Kirk A.D. Location, location, location: regional immune mechanisms critically influence rejection. Nat Med. 2002;8:553–555. doi: 10.1038/nm0602-553. [DOI] [PubMed] [Google Scholar]
- 5.Haas M., Mooletmar F., Meijer D.K., de Zeeuw D. Specific drug delivery to the kidney. Cardiovasc Drug Ther. 2002;16:489–496. doi: 10.1023/a:1022913709849. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura M., Fine L.G. The concept of glomerular self-defense. Kidney Int. 1999;55:1639–1671. doi: 10.1046/j.1523-1755.1999.00425.x. [DOI] [PubMed] [Google Scholar]
- 7.Christensen E.I., Bim H., Verroust P., Moestrup S.K. Membrane receptors for endocytosis in the renal proximal tubule. Int Rev Cytol. 1998;180:237–284. doi: 10.1016/s0074-7696(08)61772-6. [DOI] [PubMed] [Google Scholar]
- 8.Leung S., Bendayan R. Role of P-glycoprotein in the renal transport of dideoxynucleoside analog drugs. Can J Physiol Pharmacol. 1999;77:625–630. [PubMed] [Google Scholar]
- 9.van Ginneken C.A., Russel F.G. Saturable pharmacokineticsin the renal excretion of drugs. Clin Pharmacokinet. 1989;16:38–54. doi: 10.2165/00003088-198916010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Maack T., Johnson V., Kau S.T., Figueiredo J., Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16:251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- 11.Haas M., de Zeeuw D., van Zanten A., Meijer D.K. Quantification of renal low-molecular-weight protein handling in the intact rat. Kidney Int. 1993;43:949–954. doi: 10.1038/ki.1993.133. [DOI] [PubMed] [Google Scholar]
- 12.Franssen E.J., Koiter J., Kuipers C.A., Bruins A.P., Moolenaar F., de Zeeuw D. Low molecular weight proteins as carriers for renal drug targeting: preparation of drug–protein conjugates and drug-spacer derivatives and their metabolism in renal cortex homogenates and lysosomal lysates. J Med Chem. 1992;35:1246–1259. doi: 10.1021/jm00085a012. [DOI] [PubMed] [Google Scholar]
- 13.Franssen E.J.F., van Amsterdam R.G.M., Visser J., Moolenaar F., de Zeeuw D., Meijer D.K. Low molecular weight proteins as carriers for renal drug targeting: naproxen–lysozyme. Pharm Res. 1991;8:1223–1230. doi: 10.1023/a:1015835325321. [DOI] [PubMed] [Google Scholar]
- 14.Hass M., Kluppel A.C., Wartna E.S., Moolenaar F., Meijer D.K., de Jong P.E. Drug targeting to the kidney: renal delivery and degradation of a naproxen–lysozyme conjugate in vivo. Kidney Int. 1997;52:1693–1699. doi: 10.1038/ki.1997.504. [DOI] [PubMed] [Google Scholar]
- 15.Franssen E.J.F., Moolenaar F., de Zeeuw D., Meijer D.K. Low molecular weight proteins as carriers for renal drug targeting: naproxen coupled to lysozyme via the spacer l-lactic acid. Pharmacol Res. 1993;10:963–969. doi: 10.1023/a:1018946219057. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z.R., Zheng Q., Han J., Gao G.P., Liu J., Gong T. The targeting of 14-succinate triptolide–lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterial. 2009;30:1372–1381. doi: 10.1016/j.biomaterials.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Q., Gong T., Sun X., Zhang Z.R. Synthesis, characterization and in vitro evaluation of triptolide–lysozyme conjugate for renal targeting delivery of triptolide. Arch Pharm Res. 2006;29:1164–1170. doi: 10.1007/BF02969309. [DOI] [PubMed] [Google Scholar]
- 18.Kok R.J., Grijpstra F., Walthuis R.B., Moolenaar F., de Zeeuw D., Meijer D.K. Specific delivery of captopril to the kidney with the prodrug captopril–lysozyme. J Pharmacol Exp Ther. 1999;288:281–285. [PubMed] [Google Scholar]
- 19.Haverdings R.F., Haas M., Navis G., van Loenen-Weemaes A.M., Meijer D.K., De Zeeuw D. Renal targeting of captopril selectively enhances the intrarenal over the systematic effects of ACE inhibitor in rats. Br J Pharmacol. 2002;136:1107–1116. doi: 10.1038/sj.bjp.0704814. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Dolman M.E.E.M., Harmsen S., Pieters E.H.E., Sparidans R.W., Lacombe M., Szokol B. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int J Nanomed. 2012;7:417–433. doi: 10.2147/IJN.S26485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolman M.E., Harmsen S., Storm G., Hennink W.E., Kok R.J. Drug targeting to the kidney: advances in the active targeting of therapeutics to proximal tubular cells. Adv Drug Deliv Rev. 2010;62:1344–1357. doi: 10.1016/j.addr.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Z.X., Sun X., Gong T., Ding H., Fu Y., Zhang Z.R. Randomly 50% N-acetylated low molecular weight chitosan as a novel renal targeting carrier. J Drug Target. 2007;15:269–278. doi: 10.1080/10611860701289875. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Z.X., Zhang Z.R., Zhu D., Sun X., Gong T., Liu J. Specific renal uptake of randomly 50% N-acetylated low molecular weight chitosan. Mol Pharm. 2009;6:305–314. doi: 10.1021/mp800078a. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Z.X., Li J., Zhu D., Sun X., Gong T., Zhang Z.R. Enhanced accumulation of low molecular weight chitosan in kidneys: a study on the influence of N-acetylation of chitosan on the renal targeting. J Drug Target. 2011;19:540–551. doi: 10.3109/1061186X.2010.521158. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto Y., Tsutsumi Y., Yoshioka Y., Kamada H., Sato-Kamada K., Okamoto T. Poly(vinylpyrrolidone-co-dimethylmaleicacid) as a novel renal targeting carrier. J Control Release. 2004;95:229–237. doi: 10.1016/j.jconrel.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Kodaira H., Tsutsumi Y., Yoshioka Y., Kamada H., Kaneda Y., Yamamoto Y. The targeting of anionized polyvinylpyrrolidone to the renal system. Biomaterials. 2004;25:4309–4315. doi: 10.1016/j.biomaterials.2003.10.097. [DOI] [PubMed] [Google Scholar]
- 27.Kishida A. A site-specific polymeric drug carrier for renal disease treatment. Trends Pharmacol Sci. 2003;24:611–613. doi: 10.1016/j.tips.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Zou J., Glinsky V.V., Landon L.A., Matthews L., Deutscher S.L. Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancercell adhesion. Carcinogenesis. 2005;26:309–318. doi: 10.1093/carcin/bgh329. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S.R., Deutscher S.L. 111In-labeled galectin-3−targeting peptide as a SPECT agent for imaging breasttumors. J Nucl Med. 2008;49:796–803. doi: 10.2967/jnumed.107.048751. [DOI] [PubMed] [Google Scholar]
- 30.Deutscher S.L., Figueroa S.D., Kumar S.R. Tumor targeting and SPECT imaging properties of an in-labeled galectin-3 binding peptide in prostate carcinoma. Nucl Med Biol. 2009;36:137–146. doi: 10.1016/j.nucmedbio.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Geng Q., Sun X., Gong T., Zhang Z.R. Peptide−drug conjugate linked via a disulfide bond for kidney targeted drug delivery. Bioconjug Chem. 2012;23:1200–1210. doi: 10.1021/bc300020f. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K., Susaki H., Okuno S., Sugiyama Y. Renal drug targeting using a vector alkylglycoside. J Pharmacol Exp Ther. 1999;288:57–64. [PubMed] [Google Scholar]
- 33.Suzuki K., Susaki H., Okuno S., Yamada H., Sugiyama Y. Specific renal delivery of sugar-modified low-molecular-weight peptides. J Pharmacol Exp Ther. 1999;288:888–897. [PubMed] [Google Scholar]
- 34.Shirota K., Kato Y., Suzuki K., Sugiyama Y. Characterization of novel kidney-specific delivery system using an alkylglucosidevector. J Pharmacol Exp Ther. 2001;299:459–467. [PubMed] [Google Scholar]
- 35.Lin Y., Sun X., Gong T., Zhang Z.R. Prednisolone succinate-glucosamine conjugate: synthesis, characterization and in vitro cellular uptake by kidney cell lines. Chin Chem Lett. 2012;23:25–28. [Google Scholar]
- 36.Lin Y., Sun X., Gong T., Zhang Z.R. Synthesis and in vivo distribution of 2-deoxy-2-aminodiglucose-prednisolone conjugate (DPC) Chin Chem Lett. 2012;23:557–560. [Google Scholar]
- 37.Liang Z., Gong T., Sun X., Tang J.Z., Zhang Z.R. Chitosan oligomers as drug carriers for renal delivery of zidovudine. Carbohydrate Polymers. 2012;87:2284–2290. [Google Scholar]
- 38.Wilk S., Mizoguchi H., Orlowski M. γ-Glutamyldopa:akidney-specific dopamineprecursor. J Pharmacol Exp Ther. 1978;206:227–232. [PubMed] [Google Scholar]
- 39.Pestana M., Soares-da-Silva P. The renal handling of dopamine originating from l-dopa and gamma-glutamyl-l-dopa. Br J Pharmacol. 1994;112:417–422. doi: 10.1111/j.1476-5381.1994.tb13088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizoguchi H., Odowski M., Wilk S., Green J.P. Gamma-glutamyl DOPA and gamma-glutamyl dopamine: effect on plasma glucose levels. Eur J Pharmacol. 1979;57:239–245. doi: 10.1016/0014-2999(79)90371-6. [DOI] [PubMed] [Google Scholar]
- 41.Su M., He Q., Zhang Z.R., Hu B., Liu S.W. Kidney-targeting characteristics of N-acetyl-l-glutamic prednisolone prodrug. Acta Pharm Sin. 2003;38:627–630. [PubMed] [Google Scholar]
- 42.Wang S., Luo J., Lantrip D.A., Waters D.J., Mathias C.J., Green M.A. Design and synthesis of [111In] DTPA-folate for use as a tumor-targeted radiopharmaeutical. Bioconjug Chem. 1997;8:673–679. doi: 10.1021/bc9701297. [DOI] [PubMed] [Google Scholar]
- 43.Manil L., Davin J.C., Duchenne C., Kubiak C., Foidart J., Couvreur P. Uptake of nanoparticles by rat glomerular mesangial cells in vivo and in vitro. Pharm Res. 1994;11:1160–1165. doi: 10.1023/a:1018993000633. [DOI] [PubMed] [Google Scholar]
- 44.Choi C.H., Zuckerman J.E., Webster P., Davis M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc Natl Acad Sci U S A. 2011;108:6656–6661. doi: 10.1073/pnas.1103573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh M., Ghose T., Faulkner G., Kralovec J., Mezei M. Targeting of methotrexate-containing liposomes with a monoclonal antibody against human renal cancer. Cancer Res. 1989;49:3976–3984. [PubMed] [Google Scholar]
- 46.Tuffin G., Waelti E., Huwyler J., Hammer C., Marti H.P. Immunoliposome targeting to mesangial cells: a promising strategy for specific drug delivery to the kidney. J Am Soc Nephrol. 2005;16:3295–3305. doi: 10.1681/ASN.2005050485. [DOI] [PubMed] [Google Scholar]