Abstract
The objective of this study was to evaluate the difference in the pharmacokinetics of zolpidem tatrate in subjects from five Chinese ethnicities (Han, Mongolian, Uigur, Korean and Hui). Healthy subjects (10 Hans, 10 Mongolians, 10 Uigurs, 10 Koreans and 9 Huis) were recruited and each received a 10 mg tablet-dose of zolpidem tatrate. A total of 12 plasma samples were collected over a 12 h period after administration. The concentrations of zolpidem in plasma were determined by an HPLC-FLU method, after which the pharmacokinetic parameters were determined using DAS 2.0 software and analyzed by SPSS 16.0 software. After normalization by weight, no differences were noted in the pharmacokinetic parameters of zolpidem tatrate among the five ethnic groups (P>0.05). However, there were statistically significant differences between males and females for the pharmacokinetic parameters (P<0.05). The metabolism of zolpidem tatrate in males was faster than in females. Results indicate that ethnicity has no significant impact on the pharmacokinetics of zolpidem tatrate after a single oral dose in healthy Chinese subjects. However, an effect of gender on the pharmacokinetics of zolpidem tatrate can be noted.
KEY WORDS: Zolpidem tartrate, Pharmacokinetics, Chinese population, Gender, Ethnicity
Graphical abstract
The objective of this study was to evaluate the difference in the pharmacokinetics of zolpidem tatrate in subjects from five Chinese ethnicities (Han, Mongolian, Uigur, Korean and Hui).
1. Introduction
Zolpidem (N,N,6-trimethyl-2-(4-methylphenyl)imidazo[1,2-a]pyridine-3-acetamide hemitartrate) is a non-benzodiazepine imidazopyridine hypnotic which has been use for the treatment of insomnia and the induction of anesthesia1–3. It was reported that zolpidem can be rapidly and completely absorbed from the gastrointestinal tract with a limited first-pass effect with an absolute bioavailability of approximately 70%. In vitro studies using human liver microsomes and heterologously expressed human cytochromes P450 indicated that zolpidem undergoes extensive hepatic metabolism with up to 96% eliminated in the bile, urine and feces as inactive hydroxylated metabolites by a series of CYP enzymes including CYP3A4, CYP2C9, CYP1A2, CYP2D6 and CYP2C194,5. Differences in the expression or activity of cytochromes P450 (CYP), an important family of drug-metabolising enzymes, can lead to inter-individual differences in the response to drugs and thus result in adverse effects as a result of genetic variations6. Ethnic differences in CYP3A4 substrate pharmacokinetics have been observed. After being given oral triazolam, Asians achieved higher maximum plasma concentrations (Cmax) and had a shorter time to reach Cmax as compared to Caucasians7.
China is a multi-ethnic country which has a Han group and 55 ethnic minorities that includes the main groups of Mongolian, Korean, Hui, Uygur and Tibetan. Each ethnic group has developed its own population genetics and characteristics as a result of geographical and cultural separation, as well as differences in origin. Importantly, genetic differences in various ethnic Chinese populations may contribute to differences in drug responses8–11. For example, the pharmacokinetic parameters of tinidazole clearance were different among Han, Mongolian, Korean, Hui and Uigur healthy subjects after a single oral dose. The t1/2, AUC(0–∞), CL and ke values were significantly different between Uigur and other Chinese ethnic groups, and the clearance of tinidazole in Uigur subjects was increased by 20% compared to that of other ethnicities12. This ethnic difference was also observed in a comparative pharmacokinetic study on midazolam clearance in healthy volunteers of the Han, Mongolian, Uygur, Hui and Korean13. For this reason using the same dosage regimen of zolpidem for every ethnic group may be inappropriate. Therefore, we characterized the pharmacokinetics of oral zolpidem among different Chinese ethnic groups, including Han, Mongolian, Uygur, Hui and Korean. This is the first evaluation of the effect of ethnicity on zolpidem pharmacokinetics in healthy Chinese volunteers.
2. Materials and methods
This was a controlled study which was conducted in Shenyang Northern Hospital (Shenyang, China) in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice, and the protocol was approved by the Ethical Committee of Shenyang Northern Hospital (Shenyang, China). Written informed consent was obtained from all participants before enrollment in the study. The volunteers were free to withdraw from the study at any time without any reason.
2.1. Inclusion and exclusion criteria
The ethnic origin of each subject׳s parents, maternal and paternal grandparents was required to ensure homogeneity. Principal exclusion criteria included medical or psychiatric conditions that could have affected the performance of the pharmacokinetics study, positive screening test for alcohol, smoking or drugs of abuse within 14 days before the study and use of any prescription or over-the-counter medication within 7 days before the study. All females were tested during the luteal phase of their menstrual cycle. There was no significant difference in average height or weight between the five ethnic groups.
2.2. Study design
The current study was designed as a single-dose, parallel-group protocol performed in young healthy male and female adults across five different ethnicities in China. Following an overnight fast, an oral dose of 10 mg zolpidem tablets (Hangzhou Sanofi-Aventis Minsheng Pharmaceutical Co., Ltd., Hangzhou, China) was given to each subject. All subjects were not allowed to consume food or beverage until the 2 h blood sample had been obtained. The lunch and dinner provided during experiment was low fat and given at 4 and 9 h after administration, respectively. Participants were required to abstain from alcohol, grapefruit, caffeine-containing products and strenuous exercise during the course of the study. During the test period, all participants remained under close medical supervision.
2.3. Blood sampling and analysis
Blood samples (4 mL) were collected into heparinized tubes prior to zolpidem administration (0 h) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 9 and 12 h following oral dosage. Plasma samples were immediately separated by centrifugation and the plasma was stored at –25 °C in polypropylene tubes until analysis.
Concentrations of zolpidem in plasma were analyzed by means of validated high-performance liquid chromatography14. Briefly, 0.5 mL plasma sample was pipetted into a 15 mL centrifuge tube to which was added 100 μL methanol, 50 μL internal standard solution (50 μg/L alfuzosin hydrochloride) and 50 μL sodium hydrate solution (4.0 mol/L). The mixture was vortex-mixed for 1 min, then after addition of 5 mL ether, vortex-mixed for 3 min and centrifuged at 3000 rpm for 10 min. The organic layer was transferred to a clean tube and evaporated under a steady stream of nitrogen to dryness in a water bath at 37 °C. The residue was reconstituted in 100 μL mobile phase and vortex-mixed for 30 s, and a 20 μL aliquot of supernatant was injected into the HPLC system.
2.4. Pharmacokinetic and statistical analysis
Pharmacokinetic parameters for zolpidem were estimated by non-compartmental methods using the Drug and Statistics software (DAS, version 2.0, Mathematical Pharmacology Professional Committee of China). For each subject, the Cmax and the time reach to Cmax (Tmax) were determined by visual inspection of plasma concentration-time profiles. The terminal elimination rate constant (λz) was determined by linear regression of the terminal portion of the log concentration-time profile. The terminal half-life (t1/2Z) was calculated as 0.693/λz. The area under the curve to the last measured point (AUC0–t) was calculated using the trapezoidal rule. The area under the plasma concentration versus time curve from 0 h to infinite time (AUC0–∞) was calculated as the sum of AUC0–t and Clast/λz, while Clast was the last quantifiable concentration. Total body weight-normalized plasma oral clearance (CLz/F) was calculated from the ratio of D/(AUC0–∞·W), where D represents dosage and W represents total body weight of participant. The total body weight-normalized apparent volume of distribution (Vz/F) was calculated as CLz/(λz·W).
Statistical analyses were performed by using SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). Normal distribution and equality of variances of pharmacokinetic parameter values for each ethnic group were determined by Kolmogoroff-Smirnov test or Levene׳s test, prior to the statistic analyses of the selected parameter. Differences among the various groups were determined using either Analysis of variance (ANOVA) or Kruskal–Wallis rank test. P<0.05 was considered statistically significant.
3. Results
3.1. Demographic data
Fifty healthy young male and female volunteers were recruited from 2007 to 2009 from Shenyang in Liaoning, Chifeng in Inner Mongolia, Yanji in Jilin, Urumqi in Xinjiang, and Yinchuan in Ningxia, thus comprising the five ethnic groups (n=10/group). After collection of the plasma samples, the same researcher determined the plasma concentration of zolpidem as described in Materials and Methods. One Hui female subject withdrew from the study after the oral administration at her own request. There was no significant difference in height and weight among five ethnic groups (Table 1).
Table 1.
Participant demographic data for subjects of five ethnic groups.
| Character | Han | Mongolian | Uigur | Korean | Hui |
|---|---|---|---|---|---|
| Gender | 5M, 5F | 5M, 5F | 5M, 5F | 5M, 5F | 5M, 4F |
| Height (cm) | 164±5 | 168±6 | 171±6 | 165±8 | 166±6 |
| Weight (kg) | 55.0±5.2 | 57.8±5.1 | 58.5±6.9 | 55.0±7.6 | 57.8±6.0 |
| Age (years) | 22±0.9 | 20.8±1.3 | 20.3±1.5 | 22.4±0.5 | 21.3±1.9 |
M: male; F: female.
3.2. Method validation
The regression equation for zolpidem concentration was described as: y=–0.022+0.041x (r=0.9994), where y means the plasma concentration of zolpidem, and x mean the ratio of peak area of zolpidem to that of internal standard. The calibration curve was in good linearity within the range of 2.0–250.0 ng/mL with inter- and inter-day relative standard deviations of less than 8.8%. The retention times of zolpidem and internal standard were 9.3 and 5.3 min, respectively (Fig. 1).
Figure 1.
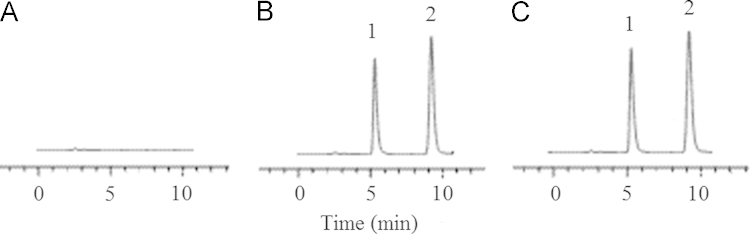
HPLC chromatograms of zolpidem tatrate in plasma. A: a blank plasma sample; B: a blank plasma sample spiked with IS and zolpidem tatrate and; C: a volunteer plasma sample. 1: alfuzosin hydrochloride (internal standard); 2: zolpidem tatrate.
3.3. Pharmacokinetics
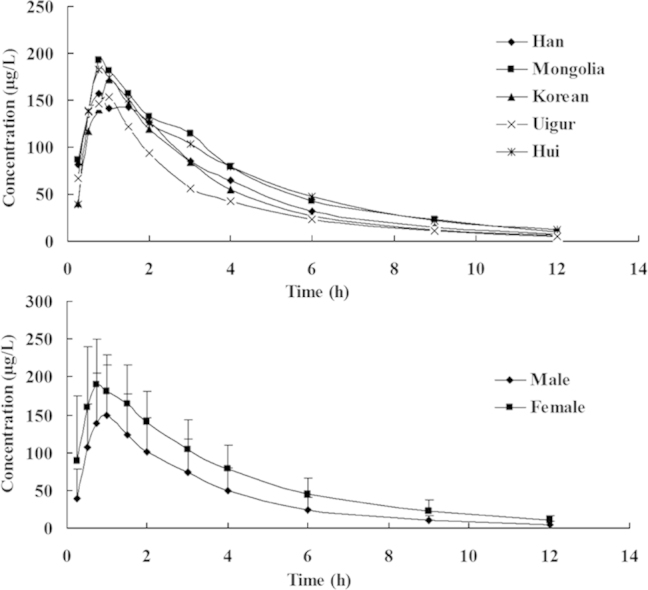
The mean plasma concentration vs. time curves for zolpidem clearance for different ethnic subjects and genders are depicted in Figs. 2 and 3. Pharmacokinetic parameters of the different ethnicities and genders are presented in Tables 2 and 3. Comparative analysis of the pharmacokinetic parameters indicated that there were no significant differences in all pharmacokinetic parameters among groups of Han, Mongolian, Uygur, Hui and Korean (P>0.05). The average t1/2 values of 2.04±0.47 h (Korean), 2.1±0.75 h (Uigur) were 1.4 times shorter than that of the Hui (2.84±0.81 h), and the CL value of 31.87±35.96 L/h (Uigur) was higher than that of the other four groups; however, there were large individual variations within each group, and differences were not statistically significant.
Figure 2.
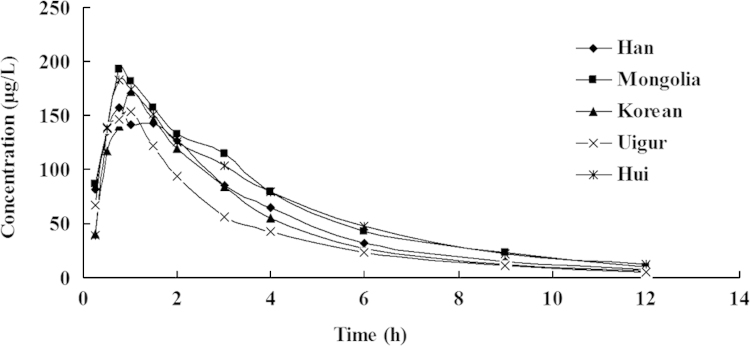
Mean plasma concentration-time profiles of zolpidem tartrate tablet in healthy volunteers of five ethnic groups (Han, Mongolian, Uygur and Korean: n=10; Hui, n=9).
Figure 3.
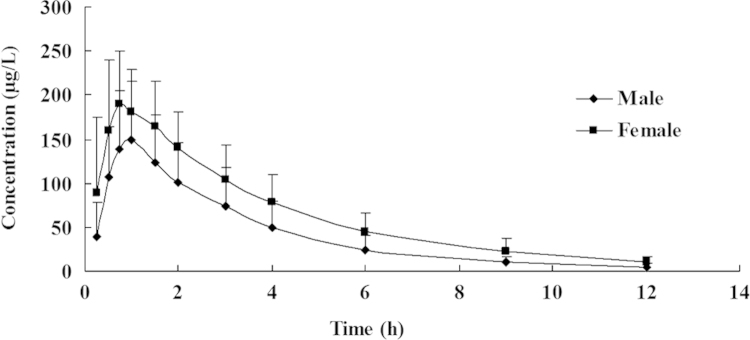
Mean plasma concentration-time profiles of zolpidem tartrate tablet in male and female healthy volunteers (nmale=25, nfemale=24).
Table 2.
Pharmacokinetic parameters for 49 healthy subjects after a single oral dose of 10 mg zolpidem tartrate tablet (Means±SD).
| Parameter | Han | Mongolian | Korean | Hui | Uigur | P |
|---|---|---|---|---|---|---|
| Cmax (μg/L) | 190.81±70.59 | 221.85±109.97 | 178.13±43.07 | 199.64±55.48 | 175.12±78.19 | 0.671 |
| Tmax (h) | 0.93±0.47 | 0.9±0.37 | 0.98±0.22 | 0.75±0.18 | 0.78±0.25 | 0.368 |
| t1/2 (h) | 2.22±0.77 | 2.48±0.6 | 2.04±0.47 | 2.84±0.81 | 2.1±0.75 | 0.088 |
| Vd (L) | 49.52±7.99 | 58.4±36.43 | 53.07±12.89 | 58.98±34.76 | 68.74±27.34 | 0.239 |
| CL (L/h) | 17.95±10.06 | 18.76±15.1 | 19.56±8.32 | 15.82±12.32 | 31.87±35.96 | 0.160 |
| AUC0–12 h (μg·h/L) | 624.46±192.15 | 770±405.64 | 578.89±216.25 | 742.11±234.34 | 493.03±240.37 | 0.166 |
| AUC0–∞ (μg·h/L) | 649.58±210.17 | 808.85±434.10 | 597.48±227.97 | 803.94±272.08 | 510.99±254.54 | 0.160 |
Table 3.
The comparison of pharmacokinetic parameters between male and female subjects after a single oral dose of 10 mg zolpidem tartrate tablet (Means±SD).
| Parameter | Malea | Femaleb | P1 | P2 |
|---|---|---|---|---|
| Cmax (μg/L) | 169.11±70.20 | 217.83±71.27 | 0.020 | 0.354 |
| Tmax (h) | 0.94±0.34 | 0.79±0.28 | 0.104 | |
| t1/2 (h) | 2.02±0.60 | 2.63±0.70 | 0.002 | |
| Vd (L) | 66.59±31.64 | 47.50±10.30 | 0.008 | 0.098 |
| CL (L/h) | 27.51±24.69 | 13.58±5.78 | 0.011 | 0.029 |
| AUC0–12 h (μg·h/L) | 507.86±246.34 | 784.20±240.36 | 0.001 | 0.010 |
| AUC0–∞ (μg·h/L) | 524.78±260.03 | 831.63±266.67 | 0.000 | 0.008 |
n=25.
n=24.
P1 with weight-normalization; P2, without weight-normalization.
There were significant differences between males and females (P<0.05). The Cmax value and Vd value of females were significant different from those of males, but no significant differences were found after weight-normalization. The t1/2 value of 2.63±0.70 h (female) was significantly longer than that of males (P<0.002). The CL value 27.51±24.69 L/h was significantly higher in males than in females (P<0.01), and AUC value 507.86±246.34 μg.h/L (male) was significantly lower than in females (P<0.001). The differences in CL and AUC retained significance after weight normalization.
3.4. Safety assessments
One Mongolian participant suffered from vomiting and 2–3 suffered from nausea in each ethnic group. There were no serious adverse experiences in the other subjects throughout the study.
4. Discussion
The objective of this investigation was to characterize the ethnic and gender-related pharmacokinetics of zolpidem in Chinese Han, Mongolian, Uygur, Hui and Koreans. Ten healthy volunteers of each ethnicity were selected according to the “Guidance for Clinical Pharmacokinetic Study of Chemicals in China” which determined that the sample size in each group in a Phase I pharmacokinetic study might be 8–12 healthy volunteers with the sex ratio at 1:1. In order to ensure the homogeneity of ethnicity, all volunteers were students recruited from the local universities whose parents and grandparents had the same descent.
The results indicate that there were no significant differences in any pharmacokinetic parameter among five groups, indicating that ethnicity has no impact on the pharmacokinetics of zolpidem for Han, Mongolian, Uygur, Hui and Koreans. However, individual gender factors should be considered; for example, the half-life of zolpidem in females was statistically significant longer than in males. The CL value in males was about 2 times higher than that of females, and the AUC value of females was about 1.5 times larger than males. These differences in CL and AUC values were significant, with and without normalization for body weight. These observations are consistent with previous reports1,15. The sex difference in the pharmacokinetics of zolpidem is similar to that seen for estrogen16.
5. Conclusions
The results of the current study indicate that no dose adjustment is required when zolpidem is given to Han, Mongolian, Uygur, Hui or Korean subjects. However, gender has a significant impact on the oral clearance and half-life of zolpidem. Whether the sex difference might lead to adverse effects should be considered when the same dosage is given to male or female patients.
Acknowledgment
This research work was supported financially by the Entire Armed Forces “11th 5-year plan” Medicine Health Science and Technology Attach Topic (06G023).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Olubodun J.O., Ochs H.R., von Moltke L.L., Roubenoff R., Hesse L.M., Harmatz J.S. Pharmacokinetic properties of zolpidem in elderly and young adults: possible modulation by testosterone in men. Br J Clin Pharmacol. 2003;56:297–304. doi: 10.1046/j.0306-5251.2003.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenblatt D.J., Harmatz J.S., Singh N.N., Roth T., Harris S.C., Kapil R.P. Influence of food on pharmacokinetics of zolpidem from fast dissolving sublingual zolpidem tartrate tablets. J Clin Pharmacol. 2013;53:1194–1198. doi: 10.1002/jcph.159. [DOI] [PubMed] [Google Scholar]
- 3.Kolla B.P., Lovely J.K., Mansukhani M.P., Morgenthaler T.I. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6. doi: 10.1002/jhm.1985. [DOI] [PubMed] [Google Scholar]
- 4.von Moltke L.L., Greenblatt D.J., Granda B.W., Duan S.X., Grassi J.M., Venkatakrishnan K. Zolpidem metabolism in vivo: responsible cytochromes, chemical inhibitors, and in vitro correlations. Br J Clin Pharmacol. 1999;48:89–97. doi: 10.1046/j.1365-2125.1999.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pichard L., Gillet G., Bonfils C., Domergue J., Thénot J.P., Maurel P. Oxidative metabolism of zolpidem by human liver cytochrome P450s. Drug Metab Dispos. 1995;23:1253–1262. [PubMed] [Google Scholar]
- 6.Johnson J.A. Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther. 2000;38:53–60. doi: 10.5414/cpp38053. [DOI] [PubMed] [Google Scholar]
- 7.Kinirons M.T., Lang C.C., He H.B., Ghebreselasie K., Sha S., Robin D.W. Triazolam pharmacokinetics and pharmacodynamics in Caucasians and Southern Asians: ethnicity and CYP3A activity. Br J Clin Pharmacol. 1996;41:69–72. doi: 10.1111/j.1365-2125.1996.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 8.Catherino W.H., Eltoukhi H.M., Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31:370–379. doi: 10.1055/s-0033-1348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzer T., Fujimoto V.Y. Ethnic differences in assisted reproductive technologies outcomes. Semin Reprod Med. 2013;31:360–364. doi: 10.1055/s-0033-1348894. [DOI] [PubMed] [Google Scholar]
- 10.Hilmi A., Pasternak Y., Friger M., Loewenthal N., Haim A., Hershkovitz E. Ethnic differences in glycemic control and diabetic ketoacidosis rate among children with diabetes mellitus type 1 in the Negev area. Isr Med Assoc J. 2013;15:267–270. [PubMed] [Google Scholar]
- 11.Balant L.P., Balant-Gorgia E.A. Cultural differences: implications on drug therapy and global drug development. Int J Clin Pharmacol Ther. 2000;38:47–52. doi: 10.5414/cpp38047. [DOI] [PubMed] [Google Scholar]
- 12.Chang X.Y., Guo T., Xia D.Y. Pharmacokinetics of tinidazole in Chinese subjects: comparison of Mongolian, Korean, Hui, Uighur and Han nationalities. J Pharm Pharm Sci. 2009;12:175–180. doi: 10.18433/j3kk50. [DOI] [PubMed] [Google Scholar]
- 13.Guo T., Mao G.F., Xia D.Y., Su X.Y., Zhao L.S. Pharmacokinetics of midazolam tablet in different Chinese ethnic groups. J Clin Pharm Ther. 2011;36:406–411. doi: 10.1111/j.1365-2710.2010.01178.x. [DOI] [PubMed] [Google Scholar]
- 14.Xia D.Y., Yang L., Guo T. Pharmacokinetics study on zolpidem tartrate tablet in Han healthy volunteers. China Pharm. 2010;21:254–256. [Google Scholar]
- 15.Greenblatt D.J., Harmatz J.S., von Moltke L.L., Wright C.E., Durol A.L., Harrel-Joseph L.M. Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex-dependent differences. J Pharmacol Exp Ther. 2000;293:435–443. [PubMed] [Google Scholar]
- 16.Zhang S.B., Zhou H.H. Gender difference of drug metabolism and its mechanism. Pharmacol Bull. 2007;23:292–294. [Google Scholar]



