Abstract
Background
Cervicitis is an inflammatory condition of the cervix associated with upper genital tract infection and reproductive complications. Although cervicitis can be caused by several known pathogens, the etiology frequently remains obscure. Here we investigate vaginal bacteria associated with bacterial vaginosis as potential causes of cervicitis.
Methods
Associations between vaginal bacteria and cervicitis were assessed in a retrospective case control study of women attending a Seattle STD clinic. Individual bacterial species were detected using two molecular methods: quantitative PCR (qPCR) and broad range 16S rRNA gene PCR with pyrosequencing. The primary finding from this initial study was evaluated using qPCR in a second cohort of Kenyan women.
Results
The presence of Mageeibacillus indolicus, formerly BVAB3, in the cervix was associated with cervicitis, while the presence of Lactobacillus jensenii was inversely associated. Quantities of these bacteria did not differ between cervicitis cases and controls, though in a model inclusive of presence and abundance, M. indolicus remained significantly associated with cervicitis after adjustment for other cervicitis-causing pathogens. M. indolicus was not associated with cervicitis in our study of Kenyan women, possibly due to differences in the clinical definition of cervicitis.
Conclusions
Colonization of the endocervix with M. indolicus may contribute to the clinical manifestations of cervicitis, but further study is needed to determine whether this finding is repeatable and applicable to diverse groups of women. Colonization of the cervix with L. jensenii could be a marker of health, perhaps reducing inflammation or inhibiting pathogenic infection.
Keywords: cervicitis, bacterial vaginosis, BVAB
INTRODUCTION
Cervicitis, presenting as inflammation of the uterine cervix, is a syndrome usually caused by infection. Cervicitis has classically been associated with Chlamydia trachomatis or Neisseria gonorrhoeae infection1, but can also accompany Trichomonas vaginalis, Mycoplasma genitalium, and herpes simplex virus infection2, 3. However, more than half of cervicitis cases lack known etiology4. Clinical signs of cervicitis include purulent endocervical exudate, increased numbers of polymorphonuclear leukocytes (PMNs) in the cervix, and easily induced cervical bleeding (friability)2. Cervicitis is a predictor of upper genital tract inflammation5 and has been associated with poor pregnancy outcomes6 and increased HIV-1 shedding7.
Intriguing evidence points to a link between cervicitis and bacterial vaginosis (BV)8, 9. BV is a complex polymicrobial infection10 associated with early pregnancy loss and preterm birth11, as well as pelvic inflammatory disease12. In the largest study of BV and cervicitis to date, 15% of 423 women with BV had coincident cervicitis and the majority of these women (87%) had no conventional pathogen detected13. In a separate study of women with both BV and cervicitis, adding treatment for BV in the form of metronidazole vaginal gel to a standard regimen of doxycycline and ofloxacin improved cure rates for cervicitis14. These data suggest that some BV-associated bacteria may contribute to cervicitis.
Detection of fastidious urogenital bacterial species with quantitative PCR (qPCR) has been associated with preterm birth15, persistent BV16, and urethritis in men17. Here we applied this methodology to investigate the relationship of eight BV-associated bacterial species/genera and three of the most common vaginal lactobacilli with cervicitis in a retrospective case control study of women attending a Seattle STD clinic. We also examined the cervical microbiotas of these women by deeply sequencing broad-range 16S rRNA gene amplicons. Our findings suggest the cervical microbiota is not markedly altered in women with cervicitis, but the presence of certain species in the cervix may be associated with clinical manifestations of cervicitis.
MATERIALS AND METHODS
Clinical Procedures
Our overarching approach was to explore associations of bacterial species with cervicitis in one cohort and subsequently confirm or refute our findings using a separate cohort. The Fred Hutchinson Cancer Research Center institutional review board approved this protocol and written informed consent was obtained from all participants. For the initial study we recruited women age 18 years and older seeking health care for a new problem at the Seattle-King County STD clinic between September 2006 and June 2010. Women were interviewed regarding their medical and sexual history prior to undergoing a standardized pelvic exam with speculum. After collecting a vaginal swab specimen from the lateral wall, visible adherent discharge was removed from the ectocervix with a large cotton swab prior to inserting a second swab into the endocervical canal for sample collection. The endocervical swab was rolled onto a sterile glass slide for Gram staining and both swabs were stored at −80° C until DNA extraction. Assessment of sexually transmitted infections (STI) is described in the Supplemental Methods provided as Supplemental Digital Content (SDC_Text).
Cohort Selection
Our Seattle study enrolled 238 women, 210 of whom had complete clinical data and a cervical exam. From these 210 women, 14 cervicitis cases (6.7%) were identified according to the following criteria: 1) purulent exudate visible in the endocervical canal or on an endocervical swab specimen; or 2) presence of cervical friability with sustained bleeding of the endocervix upon gentle passage of a swab through the cervical os; or 3) an average of 30 or more polymorphonuclear leukocytes (PMNs) per high power field on endocervical Gram stain. Of the remaining 196 women, 8 were excluded due to missing STI infection data. Eligible controls were further limited to those who enrolled within 140 days of a given case and matched on DNA extraction method prior to random selection of 3 controls for each case.
Molecular Analyses
DNA extraction is described in the Supplemental Methods (SDC_Text). Taxon-directed quantitative PCR assays targeting common vaginal bacteria and those with high specificity for BV were employed along with a PCR inhibition control as previously described10, 18. The lower limit of detection for qPCR runs was 2.5 to 10 gene copies per reaction, with the exception of three runs with a detection limit of 100 gene copies per reaction. Excluding these lower sensitivity runs from the analysis had no effect on our conclusions. No-template PCR and sham DNA extraction controls were run with each PCR assay to monitor for contamination.
To detect other cervical bacteria, cervical DNA samples from each case and 2 of its 3 controls were subjected to broad-range 16S rRNA gene PCR and the resulting amplicons sequenced with 454 Life Sciences Titanium pyrosequencing technology (Roche, Branford, CT)19, 20. Samples were multiplexed using 6 bp barcodes (SDC_Table_S1). We generated 337,303 reads with an average of 8,031 reads/sample (SRP058048). However, owing to low DNA concentration, only 456 reads were generated for one cervicitis case, (p7z1tr80). Read sequences were classified using the pplacer 21 phylogenetic placement tool with a curated reference set of vaginal bacteria19; 97.8% of the reads were classified to the species level.
Kenyan Study
A nested case control cohort was drawn from a longitudinal open cohort study of high-risk women in Mombasa, Kenya. Women 16 years or older who presented to the clinic and reported engaging in transactional sex were eligible to enroll. Women provided written informed consent for a protocol approved by the institutional review boards at the Kenyatta National Hospital (Nairobi, Kenya), the University of Washington, and the Fred Hutchinson Cancer Research Center. Visits consisted of a face-to-face interview to collect information on medical and sexual history, followed by a speculum-assisted pelvic examination. Cervicitis was diagnosed by observation of mucopurulent discharge at the cervical os or ≥30 PMN counts per high power field on endocervical Gram stain, as described above; data on friability were not collected. Of 608 women considered for the study, 603 had complete data on mucopus and cervical PMN counts; 21 (3.5%) of these women had cervicitis. Three controls were randomly chosen for each case from the remaining 582 eligible participants. STI detection and DNA extraction information is provided in the Supplemental Methods (SDC_Text).
Statistical Analyses
Bivariate associations were assessed using Fisher's exact tests and Wilcoxon rank sum tests. Due to small sample size, regression analyses were performed using exact logistic regression with p-values and confidence intervals calculated using the sufficient statistic. Statistical analyses were completed using Stata SE 13.1 (Stata, College Station, Texas) and all p-values reported at the 95% confidence level. The Venn diagram was constructed using eulerAPE22. To visualize the pyrosequencing data, Euclidean distances between asinh-transformed (‘inverse hyperbolic sin’ transforms data with near-zero values) sequence read abundances were hierarchically clustered using average linkage. Lasso regression with binomial response was used to model the relationship between asinh-transformed species abundance and cervicitis status in R's glmnet package23. This statistical technique forces some parameter estimates to zero, identifying a subset of taxa influencing cervicitis.
RESULTS
Seattle STD Clinic Study
Seattle study participants were predominately young Caucasian women with a low prevalence of genital infections; there were no significant differences between women with and without cervicitis with regards to race, ethnicity, sexual behaviors, or the presence of co-infections (p >0.1, Table 1). Women with cervicitis were more likely to have Mageeibacillus indolicus, formerly BVAB3, detected in the cervix (42.9% vs. 11.9%, p=0.020) and in the vagina (42.9% vs. 16.7%, p=0.067) than women without cervicitis (Table 2). In addition, there was a trend towards an association between detection of Megasphaera species type 2 in the cervix and cervicitis (28.6% vs. 7.1%, p=0.058). Lactobacillus jensenii was the only lactobacillus species inversely associated with cervicitis, detected in the cervix of 52.4% of women without cervicitis and only 14.3% of women with cervicitis (p=0.015). No statistically significant associations between cervicitis and the concentration of bacterial species were observed among women with detectable levels of the species (Figures S1–S2 in SDC_Text); 16S and 18S rRNA gene copy numbers (indicators of sampling error) also showed no association with cervicitis (data not shown).
Table 1.
Clinical and Behavioral Characteristics of Seattle Participants With and Without Cervicitis*
| Characteristic | Cervicitis Present N=14 | Cervicitis Absent N=42 | All Participants N=56 |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 26.5 (21–32) | 27 (24–33) | 27 (23–32) |
| Range | 19–46 | 17–57 | 17–57 |
| Race | |||
| Black/African American | 5 (35.7%) | 8 (19.1%) | 13 (23.2%) |
| Caucasian | 5 (35.7%) | 28 (66.7%) | 33 (58.9%) |
| Other | 3 (21.4%) | 3 (7.1%) | 6 (10.7%) |
| Not known/provided | 1 (7.1%) | 3 (7.1%) | 4 (7.1%) |
| Ethnicity | |||
| Hispanic | 2 (14.3%) | 4 (9.5%) | 6 (10.7%) |
| Not hispanic | 12 (85.7%) | 36 (85.7%) | 48 (85.7%) |
| Not known/provided | 0 | 2 (4.8%) | 2(3.6%) |
| Male sex partners | |||
| New partner, past 60 days | 2 (14.3%) | 11 (26.2%) | 13 (23.2%) |
| ≥ 1sex partner, past 90 days | 12 (85.7%) | 33 (78.6%) | 43 (23.2%) |
| Median # (IQR), past 90 days | 1 (1 – 1) | 1 (1 – 2) | 1 (1–1.5) |
| Female sex partners | |||
| New partner, past 60 days | 0 | 1 (2.4%) | 1 (1.8%) |
| Any, past 90 days | 2 (14.3%) | 4 (9.5%) | 6 (10.7%) |
| Used condom at last vaginal sex | 4 (28.6%) | 15 (35.7%) | 19 (33.9%) |
| Ever douched | 8 (57.1%) | 20 (47.6%) | 28 (50.0%) |
| Took oral antibiotics, past month | 0 | 4 (9.5%) | 4 (7.1%) |
| Co-infections | |||
| Chlamydia trachomatis | 1 (7.1%) | 1 (2.4%) | 2 (3.6%) |
| Neisseria gonorrhoeae | 1 (7.1%) | 0 | 1 (1.8%) |
| Trichomonas vaginalis | 2 (14.3%) | 4 (9.5%) | 6 (10.7%) |
| Vaginal candidiasis | 0 | 8 (19.1%) | 8 (14.3%) |
| Bacterial vaginosis | |||
| Amsel criteria | 8 (57.1%) | 15 (35.7%) | 23 (41.1%) |
| Nugent score | |||
| 0 – 3 – Negative | 3 (21.4%) | 21 (50.0%) | 24 (42.9%) |
| 4 – 6 – Intermediate | 1 (7.1%) | 2 (4.8%) | 3 (5.4%) |
| 7 – 10 – Positive | 10 (71.4%) | 19 (45.2%) | 29 (51.79%) |
p > 0.1 for all tests (Wilcoxon rank sum test for age and Fisher exact tests for categorical variables) comparing cases and controls.
Table 2.
Presence of BV-Associated Bacteria and Lactobacilli in Seattle Participants With and Without Cervicitis
| Detection in the Cervix n (%) | Detection in the Vagina n (%) | |||||
|---|---|---|---|---|---|---|
| BV-associated Bacteria | Cervicitis Present N=14 | Cervicitis Absent N=42 | p-value* | Cervicitis Present N=14 | Cervicitis Absent N=42 | p-value* |
| Gardnerella vaginalis | 12 (85.7) | 34 (81.0) | 1.000 | 13 (92.9) | 36 (85.7) | 0.666 |
| Atopobium vaginae | 12 (85.7) | 25 (59.5) | 0.106 | 12 (85.7) | 29 (69.1) | 0.307 |
| Leptotrichia/Sneathia | 9 (64.3) | 21 (50.0) | 0.537 | 8 (57.1) | 22 (52.4) | 1.000 |
| Megasphaera type 1 | 8 (57.1) | 17 (40.5) | 0.357 | 9 (64.3) | 19 (45.2) | 0.355 |
| Megasphaera type 2 | 4 (28.6) | 3 (7.1) | 0.058 | 4 (28.6) | 5 (11.9) | 0.206 |
| BVAB1 | 3 (21.4) | 6(14.3) | 0.676 | 3 (21.4) | 9 (21.4) | 1.000 |
| BVAB2 | 8 (57.1) | 15 (35.7) | 0.213 | 9 (64.3) | 16 (38.1) | 0.123 |
| Mageeibacillus indolicus | 6 (42.9) | 5 (11.9) | 0.020 | 6 (42.9) | 7 (16.7) | 0.067 |
| Lactobacilli | ||||||
| Lactobacillus crispatus | 4 (28.6) | 20 (47.6) | 0.350 | 8 (57.1) | 22 (52.4) | 1.000 |
| Lactobacillus jensenii | 2 (14.3) | 22 (52.4) | 0.015 | 3 (21.4) | 19 (45.2) | 0.205 |
| Lactobacillus iners | 12 (85.7) | 39 (92.9) | 0.590 | 14 (100.0) | 39 (92.9) | 0.565 |
Fisher exact tests. p-values were not adjusted for multiple comparisons.
BV = bacterial vaginosis.
Although our small study size precluded extensive multivariable analysis, we performed exact logistic regression to assess associations between the presence and quantity of specific bacteria and cervicitis, adjusting for the presence of Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC), and Trichomonas vaginalis (TV) (Table 3). We modeled the risk of cervicitis with 10-fold increments of 16S rRNA gene copies in the cervix, assigning samples in which the bacterium was not detected a value of half the lower limit of detection; in this way both the presence and load of each bacterial species contributed to the model. After adjusting for STIs, each 10-fold increase of M. indolicus bacterial load in the cervix and vagina was associated with a 53% (p=0.064) and 63% (p=0.019) increased risk of cervicitis, respectively.
Table 3.
Multivariate Analyses of the Relationship Between the Presence and Load of Specific Bacterial Species in Seattle Participants and Cervicitis
| Presence Alone | Detection in the Cervix | Detection in the Vagina | ||
|---|---|---|---|---|
| aOR (95% CI)† | p-value* | aOR (95% CI)† | p-value* | |
| Mageeibacillus indolicus | 4.38 (0.84–23.68) | 0.086 | 2.93 (0.57–14.78) | 0.232 |
| Lactobacillus jensenii | 0.17 (0.02–0.89) | 0.032 | 0.41 (0.06–2.17) | 0.401 |
| Presence and Load‡ | Detection in the Cervix | Detection in the Vagina | ||
|---|---|---|---|---|
| aOR (95% CI)† | p-value* | aOR (95% CI)† | p-value* | |
| Mageeibacillus indolicus | 1.53 (0.98–2.44) | 0.064 | 1.63 (1.08–2.56) | 0.019 |
| Lactobacillus jensenii | 0.61 (0.29–1.02) | 0.061 | 0.68 (0.40–1.02) | 0.068 |
Each bacterial species was modeled independently as they do not always co-occur. Each model was adjusted for the presence of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis, but not multiple comparisons.
Calculated using exact logistic regression with p-values reported by the sufficient statistic.
Odds ratio interpreted as increase in odds of cervicitis for each 10-fold increase in bacterial load (16S copies) for each individual species. Models include all 56 subjects; those in which the species in question was not detected were assigned a value of half the lower limit of detection. As such, the differential prevalence and load of each species in women with and without cervicitis influences the odds ratios shown.
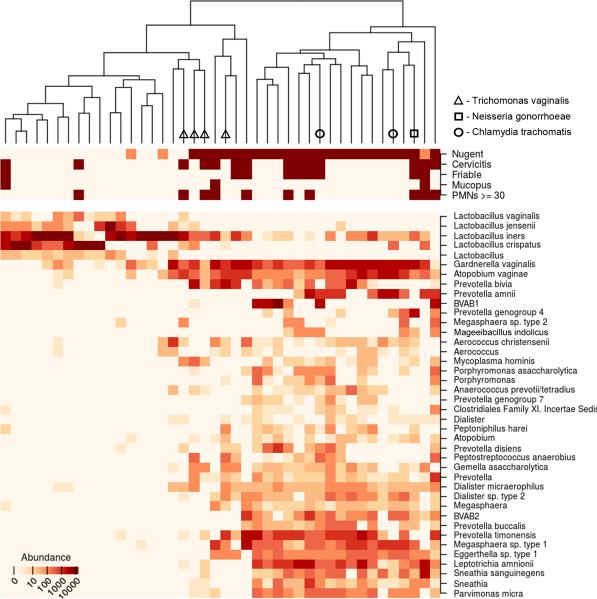
To further examine the role of the cervical microbiota in cervicitis, we deeply sequenced broad-range 16S rRNA gene amplicons from a subset of our Seattle cohort. Unsupervised hierarchical clustering separated cervical bacterial communities by BV status (Nugent score), but no cluster groups were associated with cervicitis case/control status (Figure 1). We also examined associations of bacteria with cervicitis using penalized linear regression models to determine linkages between cervicitis and specific bacterial species, including those not targeted by qPCR. A single bacterium, M. indolicus, had a positive association with cervicitis (regression coefficient 0.175). No bacterium had a negative association in the model. No reads were classified as the cervicitis-associated bacterium Mycoplasma genitalium, though we cannot completely rule out the possibility that a single nucleotide mismatch between the M. genitalium 16S rRNA gene sequence and one of our 16S broad range primers may have impacted our ability to detect this organism.
Figure 1.
Heat map of bacterial abundances in association with bacterial vaginosis and clinical indicators of cervicitis. Dendrogram shows association of meta data with vaginal bacterial communities. Nugent score for bacterial vaginosis and cervicitis-defining clinical criteria are expressed as negative (light) and positive (dark). The most abundant bacterial taxa (top 30%) are shown (see SDC_Table_S2 for complete data). PMNs = polymorphonuclear leukocytes.
Kenyan Study
We subsequently examined whether M. indolicus was associated with cervicitis in a separate case control cohort of Kenyan women that included 21 cervicitis cases. Like in the Seattle cohort, detection of cervicitis-causing pathogens was relatively uncommon, although 40.5% of the women in our Kenyan cohort were HIV positive (Table 4). The prevalence of BV was lower in the Kenyan cohort than in the Seattle cohort (Amsel 15.7%; Nugent 33.3%), but as shown in Table 4, these and other clinical characteristics did not differ between groups of women with and without cervicitis.
Table 4.
Characteristics of Women and Presence of Mageeibacillus indolicus in Kenyan Participants With and Without Cervicitis*
| Characteristic | Cervicitis Present N=21 | Cervicitis Absent N=63 | All Participants N=84 |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 29 (26-33) | 29 (25-34) | 29 (21-42) |
| Range | 22-41 | 19-46 | 19-46 |
| HIV Positive | 7 (33.3%) | 27 (42.9%) | 34 (40.5%) |
| Co-infections | |||
| Chlamydia trachomatis | 0 | 1 (1.6%) | 1 (1.2%) |
| Neisseria gonorrhoeae | 1 (4.8%) | 2 (3.2%) | 3 (3.6%) |
| Trichomonas vaginalis | 4 (19.1%) | 5 (7.9%) | 9 (10.7%) |
| Vaginal candidiasis | 5 (23.8%) | 10 (15.9%) | 15 (17.9%) |
| Bacterial vaginosis | |||
| Amsel criteria | 2 (9.5%) | 11 (17.7%) | 13 (15.7%) |
| Nugent score(≥7) | 4 (19.1%) | 24 (38.1%) | 28 (33.3%) |
| Bacteria | Cervicitis Present N=21 | Cervicitis Absent N=63 | p-value† |
|---|---|---|---|
| Mageeibacillus indolicus | |||
| Vaginal detection | 11 (52.4%) | 36 (57.1%) | 0.80 |
p > 0.1 for all tests (Wilcoxon rank sum test for age and Fisher exact tests for categorical variables) comparing cases and controls.
Fisher exact test.
By qPCR, M. indolicus was present in over 55% of vaginal samples obtained from Kenyan women, compared to 23% of those from Seattle women. However, there was no significant difference in the frequency with which M. indolicus was detected vaginally in women with and without cervicitis (52.4% vs. 57.1%; p=0.800; Table 4). There was also no association between the presence of M. indolicus and cervicitis in an exact logistic regression model adjusted for DNA extraction method and the presence of other cervicitis-causing infections (aOR = 0.74, 0.24–2.32, p=0.611). Finally, there was no difference in M. indolicus bacterial load in Kenyan women with and without cervicitis (Figure S3 in SDC_Text).
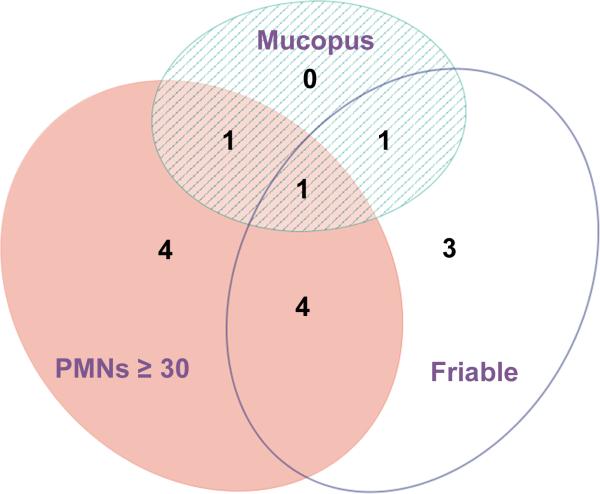
We hypothesized that one possible reason M. indolicus was not associated with cervicitis in the Kenyan study was that cervicitis was defined differently in the two studies. Because data on cervical friability were not available for the Kenyan women, we reevaluated the Seattle STD clinic data with this clinical marker of cervicitis factored separately (Figure 2). Upon refitting our multivariate model, we found M. indolicus was not significantly associated with mucopus or high cervical PMN counts in the Seattle STD clinic (aOR 2.53, 0.40–14.43, p=0.228; Table 5). M. indolicus was, however, significantly associated with cervical friability (aOR 8.24, 1.25– 63.89, p=0.013).
Figure 2.
Area-proportional Venn diagram depicting the individual clinical criteria used to define cervicitis in the Seattle cohort.
Table 5.
Relationship between the Presence of Cervicitis-Associated Species in the Cervix and Clinical Criteria Used to Define Cervicitis in the Seattle Study
| Friability | Mucopus or PMNs ≥ 30 | |||
|---|---|---|---|---|
| BV-associated Bacteria | aOR (95% CI)† | p-value* | aOR (95% CI)† | p-value* |
| Mageeibacillus indolicus | 8.24 (1.25-63.89) | 0.013 | 2.53 (0.40-14.43) | 0.228 |
| Lactobacilli | ||||
| Lactobacillus jensenii | 0.35 (0.03-2.06) | 0.279 | 0.11 (0.00-0.85) | 0.018 |
Each bacterial species was modeled independently as they do not always co-occur. Each model was adjusted for the presence of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Calculated using exact logistic regression with a conditional probability test.
BV = bacterial vaginosis; PMNs = polymorphonuclear leukocytes.
DISCUSSION
This study explored novel bacterial etiologies of cervicitis using two molecular approaches: qPCR to measure presence and concentration of key vaginal bacteria and broad-range 16S rRNA gene PCR with pyrosequencing for more comprehensive species-level profiling. Cervicitis was present in 7% of women attending a Seattle STD clinic. While BV was common among women diagnosed with cervicitis, only 4 out of 14 cervicitis cases were associated with other identifiable STIs (CT, GC, TV). Our qPCR studies linked detection and increasing load of the BV-associated bacterial species M. indolicus with cervicitis. Pyrosequencing of broad-range PCR amplicons from cervicitis cases and a subset of controls confirmed the association of M. indolicus with cervicitis using a model that accounted for multiple comparisons.
M. indolicus is a Clostridiales order bacterium24 that has been linked to treatment failure in women with BV25. It was recently detected in men with non-gonoccocal urethritis (NGU) along with Megasphaera spp., Leptotrichia/Sneathia spp., and Atopobium spp17. In that study, M. indolicus was associated with report of prior episodes of NGU and STI. Together these data support the hypothesis that this bacterium may be sexually transmitted, potentially playing a pathogenic role in both the female and male genitourinary tracts.
Association of M. indolicus with cervicitis was not observed in the cohort of Kenyan women we examined, suggesting this finding may not be generalizable to all women. One reason for the lack of consistency may be that women of African origin appear to have higher carriage rates of many BV-associated bacteria compared to Caucasian women19. In Seattle studies with relatively few (<25%) African American women, the prevalence of M. indolicus was 16–31%18, 25. In contrast, in our Kenyan cohort and in a New Orleans study with 95% of the participants identifying as African American, the prevalence of M. indolicus was 56–57% 26. Although M. indolicus is often detected in women with BV and BV is typically more common in African and African-American women than Caucasian women27, this does not explain the higher prevalence of M. indolicus in our Kenyan cohort since it had a lower prevalence of BV than our Seattle cohort. We speculate that M. indolicus might be a relatively common member of the vaginal microbiota of Kenyan women due to race-related genetic differences that allow for higher immunological tolerance of the organism and perhaps a diminished role in inflammatory conditions like cervicitis. Another possible explanation for the discrepancy in our results stems from the behavioral practices of the women in our Kenyan study. As these women are sex workers who are known to engage in frequent vaginal washing practices28, it is possible that some of the bacteria detected in cross-sectional analysis were recently acquired and/or transient; this would reduce our ability to detect disease associations in this cohort.
A third factor likely contributing to the different results obtained in the Seattle and Kenyan studies are differing clinical definitions of cervicitis. Inspection of the relationship of M. indolicus with cervicitis-defining clinical criteria in the Seattle cohort failed to identify significant association with neutrophilic infiltrate in the form of visible mucopus or elevated PMN counts. M. indolicus was significantly associated with friability, but this clinical marker was not recorded in the Kenyan study. There are several reasons the cervix may become prone to bleeding, including cervical edema, vascular swelling, and erosion. With regard to cervicitis, these changes are generally thought to occur as part of the inflammatory response to a cervical pathogen; thus friability is often causally linked to mucopus and high numbers of PMNs. M. indolicus could affect the tendency of cervical tissue to bleed in the absence of a neutrophilic immune response. The periodontis-associated microbe Porphyromonas gingivalis is thought to directly contribute to gum bleeding during infection of gingival tissue by degrading fibrinogen to prevent blood coagulation29. If our data can be confirmed, it would be of interest to determine whether M. indolicus similarly encodes virulence factors that alter coagulation or vascular permeability; an equally plausible and testable hypothesis is that cervical bleeding promotes the growth of M. indolicus.
L. jensenii is the only species we observed having a protective effect against cervicitis and mucopus/high PMN counts. Shimazu et al. demonstrated L. jensenii strain TL2937 attenuates the inflammatory response mediated by toll-like receptors (TLRs) by altering the expression of both upstream and downstream signaling molecules, including NF-κB, in a porcine intestinal cell culture model30. The present study provokes the question of whether vaginal strains of L. jensenii may also deactivate the innate immune response and reduce cervical inflammation, or simply provide colonization resistance against BV-associated bacteria.
This study is not the first to examine the role of the cervical and vaginal microbiotas in cervicitis, but it is the first to link particular BV-associated species to the condition. Ling et al. previously examined the role of G. vaginalis, A. vaginae, Eggerthella, Prevotella, Leptotrichia/Sneathia, and Megasphaera species type 1 in cervicitis using PCR-denaturing gradient gel electrophoresis (PCR-DGGE) and qPCR31. In their Chinese cohort of 30 healthy women and 70 women with BV and/or cervicitis, no significant associations of these microbes with cervicitis were identified. Ling et al. did observe Lactobacillus genus was inversely associated with cervicitis, but associations with individual Lactobacillus species (L. crispatus, L. jensenii, and L. iners) did not achieve significance.
Our study has several strengths, including our approach of coupling the sensitivity of qPCR data with the exploratory breadth of 16S rRNA gene PCR with pyrosequencing. In addition, our primary study surveyed both the endocervix and vagina; although we and others found the microbiota at these different anatomical sites to be similar31, the observed associations between bacterial species and cervicitis were often more significant in cervical specimens and might have been missed had we only analyzed vaginal swabs. Finally, we employed NAATs and cultivation to ensure we controlled for the presence of most other infectious causes of cervicitis.
Our study also has limitations. Foremost is the low prevalence of cervicitis in both cohorts, which limited our power to detect associations with cervicitis. Second, our ability to detect M. genitalium by 16S rRNA gene PCR may have been limited by primer mismatch and we did not survey for viral causes of cervicitis such as HSV and cytomegalovirus (CMV). Third, we did not consistently collect information on hormonal birth control and thus were not able to adjust for this potential confounder13. Although our findings suggest M. indolicus could be associated with idiopathic cervicitis, additional epidemiological studies are needed to confirm this finding and increase our understanding of how microbes may interact with the host to produce clinical indicators of cervicitis.
Supplementary Material
Short summary.
A case control study of women attending an STD clinic in Seattle identified associations between the BV-associated bacterium Mageeibacillus indolicus and clinical indicators of cervicitis.
ACKNOWLEDGEMENTS
The authors thank Noah G. Hoffman for consultation regarding our pyrosequencing analyses.
Sources of support: NIH T32 A107140 (training fellowship to LKS), R01 AI061628 (DNF), R01 AI052228 (JMM)
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Brunham RC, Paavonen J, Stevens CE, et al. Mucopurulent cervicitis--the ignored counterpart in women of urethritis in men. N Engl J Med. 1984;311(1):1–6. doi: 10.1056/NEJM198407053110101. [DOI] [PubMed] [Google Scholar]
- 2.Kiviat NB, Paavonen JA, Wolner-Hanssen P, et al. Histopathology of endocervical infection caused by Chlamydia trachomatis, herpes simplex virus, Trichomonas vaginalis, and Neisseria gonorrhoeae. Hum Pathol. 1990;21(8):831–7. doi: 10.1016/0046-8177(90)90052-7. [DOI] [PubMed] [Google Scholar]
- 3.Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187(4):650–7. doi: 10.1086/367992. [DOI] [PubMed] [Google Scholar]
- 4.Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(10):598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peipert JF, Ness RB, Soper DE, et al. Association of lower genital tract inflammation with objective evidence of endometritis. Infect Dis Obstet Gynecol. 2000;8(2):83–7. doi: 10.1002/(SICI)1098-0997(2000)8:2<83::AID-IDOG4>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugent RP, Hillier SL. Mucopurulent cervicitis as a predictor of chlamydial infection and adverse pregnancy outcome. The Investigators of the Johns Hopkins Study of Cervicitis and Adverse Pregnancy Outcome. Sex Transm Dis. 1992;19(4):198–202. doi: 10.1097/00007435-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gitau RW, Graham SM, Masese LN, et al. Effect of acquisition and treatment of cervical infections on HIV-1 shedding in women on antiretroviral therapy. AIDS. 2010;24(17):2733–7. doi: 10.1097/QAD.0b013e32833f9f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keshavarz H, Duffy SW, Sadeghi-Hassanabadi A, et al. Risk factors for and relationship between bacterial vaginosis and cervicitis in a high risk population for cervicitis in Southern Iran. Eur J Epidemiol. 2001;17(1):89–95. doi: 10.1023/a:1010935723248. [DOI] [PubMed] [Google Scholar]
- 9.Schwebke JR, Weiss HL. Interrelationships of bacterial vaginosis and cervical inflammation. Sex Transm Dis. 2002;29(1):59–64. doi: 10.1097/00007435-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 11.Guerra B, Ghi T, Quarta S, et al. Pregnancy outcome after early detection of bacterial vaginosis. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):40–5. doi: 10.1016/j.ejogrb.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100(3):456–63. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 13.Marrazzo JM, Wiesenfeld HC, Murray PJ, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617–24. doi: 10.1086/500149. [DOI] [PubMed] [Google Scholar]
- 14.Schwebke JR, Weiss HL. Interrelationships of bacterial vaginosis and cervical inflammation. Sexually Transmitted Diseases. 2002;29(1):59–64. doi: 10.1097/00007435-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Nelson DB, Hanlon A, Nachamkin I, et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol. 2014;28(2):88–96. doi: 10.1111/ppe.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrazzo JM, Thomas KK, Fiedler TL, et al. Relationship of Specific Vaginal Bacteria and Bacterial Vaginosis Treatment Failure in Women Who Have Sex with Women. Annals of Internal Medicine. 2008;149(1):20–28. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manhart LE, Khosropour CM, Liu C, et al. Bacterial vaginosis-associated bacteria in men: association of Leptotrichia/Sneathia spp. with nongonococcal urethritis. Sex Transm Dis. 2013;40(12):944–9. doi: 10.1097/OLQ.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredricks DN, Fiedler TL, Thomas KK, et al. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45(10):3270–6. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan S, Morgan MT, Liu C, et al. More than meets the eye: associations of vaginal bacteria with gram stain morphotypes using molecular phylogenetic analysis. PLoS One. 2013;8(10):e78633. doi: 10.1371/journal.pone.0078633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsen FA, Kodner RB, Armbrust EV. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11:538. doi: 10.1186/1471-2105-11-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9(7):e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Austin MN, Rabe LK, Srinivasan S, et al. Mageeibacillus indolicus gen. nov., sp. nov.: A novel bacterium isolated from the female genital tract. Anaerobe. 2014;32C:37–42. doi: 10.1016/j.anaerobe.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrazzo JM, Thomas KK, Fiedler TL, et al. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med. 2008;149(1):20–8. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zozaya-Hinchliffe M, Lillis R, Martin DH, et al. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J Clin Microbiol. 2010;48(5):1812–9. doi: 10.1128/JCM.00851-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peipert JF, Lapane KL, Allsworth JE, et al. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association? Sex Transm Dis. 2008;35(4):363–7. doi: 10.1097/OLQ.0b013e31815e4179. [DOI] [PubMed] [Google Scholar]
- 28.McClelland RS, Lavreys L, Hassan WM, et al. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10-year prospective study. AIDS. 2006;20(2):269–73. doi: 10.1097/01.aids.0000196165.48518.7b. [DOI] [PubMed] [Google Scholar]
- 29.Imamura T, Potempa J, Pike RN, et al. Effect of free and vesicle-bound cysteine proteinases of Porphyromonas gingivalis on plasma clot formation: implications for bleeding tendency at periodontitis sites. Infect Immun. 1995;63(12):4877–82. doi: 10.1128/iai.63.12.4877-4882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimazu T, Villena J, Tohno M, et al. Immunobiotic Lactobacillus jensenii elicits anti-inflammatory activity in porcine intestinal epithelial cells by modulating negative regulators of the Toll-like receptor signaling pathway. Infect Immun. 2012;80(1):276–88. doi: 10.1128/IAI.05729-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling Z, Liu X, Chen X, et al. Diversity of Cervicovaginal Microbiota Associated with Female Lower Genital Tract Infections. Microb Ecol. 2011;61(3):704–14. doi: 10.1007/s00248-011-9813-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.