SUMMARY
Aims:
This retrospective study determined the survival of glioblastoma patients with or without pseudoprogression.
Methods:
A total of 68 patients were included. Overall survival was compared between patients showing pseudoprogression (in most cases diagnosed using perfusion MRI with ferumoxytol) and in patients without pseudoprogession. MGMT methylation status was also analyzed in the pseudoprogression cases.
Results:
Median survival in 24 (35.3%) patients with pseudoprogression was 34.7 months (95% CI: 20.3–54.1), and 13.4 months (95% CI: 11.1–19.5) in 44 (64.7%) patients without pseudoprogression (p < 0.0001). The longest survival was a median of 54.1 months in patients with combination of pseudoprogression and (MGMT) promoter methylation.
Conclusion:
Pseudoprogression is associated with better outcome, especially if concurring with MGMT promoter methylation. Patients never diagnosed with pseudoprogression had poor survival. This study emphasizes the importance of differentiating tumor progression and pseudoprogression using perfusion MRI.
KEYWORDS : ferumoxytol, glioblastoma, perfusion MRI, pseudoprogression, temozolomide
Summary points.
Pseudoprogression
The prevalence of pseudoprogression is between 30 and 40+% in glioblastoma multiforme (GBM) patients treated with chemoradiation.
Patients showing pseudoprogression have increased survival of 34.7 months (95% CI: 20.3–54.1) compared with patients without pseudoprogression of 13.4 months (95% CI: 11.1–19.5).
The mechanism of pseudoprogression and its beneficial effect on survival needs further testing.
MGMT status
Hypermethylated MGMT status may predict further improved survival in patients with pseudoprogression.
Perfusion MRI
Increased contrast enhancement with low relative cerebral blood volume (<1.75) on perfusion MRI indicate pseudoprogression (‘inflammation’), whereas high relative cerebral blood volume (>1.75) indicate tumor progression (‘vascular’).
Ferumoxytol as a blood pool agent can be used to differentiate between progression and pseudoprogression.
Current standard of care in patients with newly diagnosed glioblastoma multiforme (GBM) consists of postoperative radiation therapy with concomitant and adjuvant temozolomide (TMZ). This paradigm is based on a randomized Phase III trial that showed addition of TMZ significantly improved median survival in comparison to radiotherapy alone by approximately 10 weeks, as well as increased 2-year survival (26.5 vs 10.4%) [1].
The rate of reactive inflammatory response to treatment, so-called pseudoprogression, has increased with the addition of TMZ to radiation therapy relative to radiation alone (31 vs 9%, respectively) [2–4]. Differentiation of pseudoprogression from tumor progression is crucial since the former represents treatment response requiring continuation of TMZ, and the latter represents treatment failure requiring treatment change. Based on clinical presentation, pseudoprogression cannot reliably be differentiated from tumor progression. However, clinical deterioration can be seen in 67% of tumor progression cases while only in 33% of pseudoprogression [5]. Both entities have similar appearance on conventional MRI, providing little value in differential diagnosis [6]. The importance and difficulties in the differentiation of pseudoprogression versus tumor progression resulted in a review and update of response evaluation criteria by the Response Assessment in Neuro-Oncology Working Group (RANO 2010) [7]. Perfusion MRI and assessment of cerebral blood volume (CBV) may better resolve this diagnostic dilemma, especially if an intravascular contrast agent, such as ferumoxytol (a superparamagnetic iron oxide nanoparticle) is used [8,9].
TMZ acts by methylating cancer cell DNA; therefore, its efficacy is decreased by the enzyme MGMT, which acts to demethylate DNA [10]. Hypermethylation of the MGMT promoter, blocking expression of the enzyme, is a commonly used prognostic marker in GBM [2,10]. In fact, it has been suggested that only patients with hypermethylated MGMT may benefit from TMZ.
The goal of this study was to use perfusion MRI to determine and compare the overall survival (OS) of GBM patients with or without pseudoprogression. We also tested whether MGMT hypermethylation status correlated with pseudoprogression.
Methods
We conducted a retrospective study of all patients with newly diagnosed GBM (WHO grade IV) between January 2006 and June 2012 who received neuro-oncology care at Oregon Health and Science University (OR, USA). The study was approved by the institutional review board (IRB# 8365). All patients underwent surgical intervention followed by standard upfront chemoradiotherapy (CRT) including fractionated external beam radiation therapy (60 Gy) with concurrent TMZ 75 mg/m2 daily for 6 weeks. After a 4-week break, patients received adjuvant TMZ 150–200 mg/m2 for 5 days every 28 days. Patients stayed on adjuvant TMZ until tumor progression, intolerable side effects or patient preference to discontinue treatment. During adjuvant TMZ therapy no patients received prophylaxis against Pneumocystis jiroveci pneumonia (PJP) [11]. Blood counts were obtained on days 21 and 28 after initiation of TMZ. Nonstandard upfront therapy such as antiangiogenic treatment, immunotherapy and local chemotherapy, as well as insufficient records for analysis were exclusion criteria.
Patients with radiographic worsening on MRI, such as a new lesion or a 25% increased area of a previously seen enhancing lesion within the radiation field, with or without clinical deterioration, regardless of time after CRT, were evaluated for pseudoprogression versus tumor progression. A total of 50 out of 68 patients underwent perfusion MRI using ferumoxytol (AMAG Pharmaceuticals, Inc., MA, USA) as a contrast agent to assess CBV. Details and utility of CBV measurement by perfusion MRI with ferumoxytol for differentiation of pseudoprogression from tumor progression were reported by our group elsewhere [8,9]. In brief, using the dynamic susceptibility contrast technique serial magnetic resonance (MR) images were obtained during 1 mg/kg ferumoxytol bolus followed by 20 ml saline injected intravenously at a flow rate of 3 ml/s. CBV parametric maps were created using Nordic Ice perfusion software (Nordic Neurolab, Bergen, Norway). Regions of interests were placed in tumor hotspots within the gadolinium enhancing area. Larger blood vessels were avoided during hotspot selection. Relative CBV (rCBV) was calculated by normalizing to the noninvolved white matter, and rCBV values were analyzed. rCBV below 1.75 was considered an indicator of pseudoprogression, while higher than 1.75 was considered tumor progression. The updated RANO criteria were used in patients (18 out of 68 patients) who did not undergo ferumoxytol perfusion imaging. In these patients the diagnosis of pseudoprogression was determined on follow-up imaging [7]. Adjuvant TMZ was continued in all pseudoprogression cases. In order to decrease mass effect (based on clinical decision) patients may have received bevacizumab (Avastin®, Genentech/Roche, CA, USA) according to Levin et al. [12].
After progression on adjuvant TMZ, patients were switched to second-line therapy which included bevacizumab alone or in combination with chemotherapy (carboplatin, irinotecan, melphalan, cyclophosphamide, etoposide and procarbazine), or underwent repeat craniotomy for tumor resection. If patients had distant tumor recurrence, radiation therapy could be applied again to the new lesion.
In order to test if hypermethylation of MGMT was associated with survival in pseudoprogression cases, MGMT status was assessed retrospectively by pyrosequencing if tumor tissue was available.
Patient and treatment characteristics were described using summary statistics for the whole patient population, and separately in patients with or without pseudoprogression. Summary statistics were used to analyze treatment related toxicity by duration of TMZ chemotherapy. Progression-free survival (PFS), OS and probability of OS at 6, 12, 24, 36 and 48 months from the date of diagnosis were calculated using the Kaplan–Meier method. Difference in OS by potential prognostic variables including age, Karnofsky performance status (KPS), extent of resection, presence of pseudoprogression and MGMT status were analyzed using both univariate and multivariate Cox proportional hazard regression models. p-values less than 0.05 were considered significant. All analyses were performed using Statistical Application System (Version 9.2; SAS Institute, Inc., NC, USA).
Results
• Patient characteristics
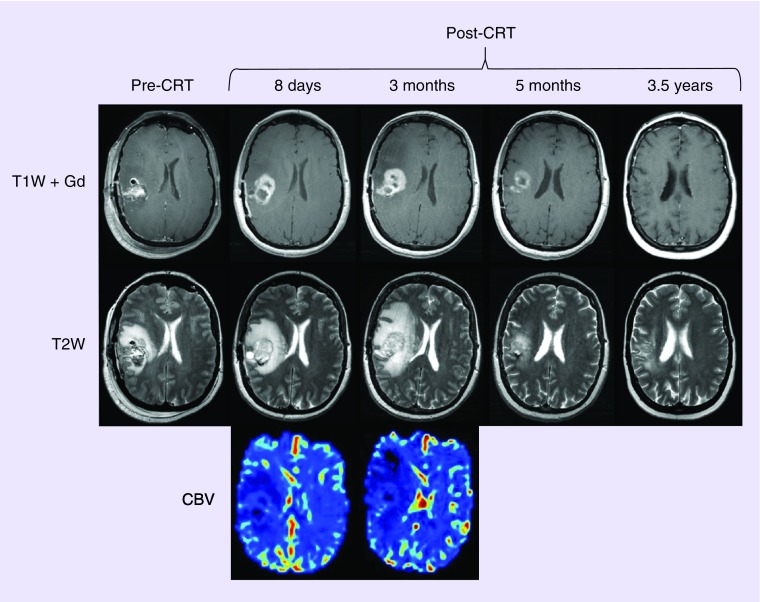
A total of 68 patients were included in the study. The median follow-up was 39 months (range: 15–87 months). A total of 24 (35.3%) patients were diagnosed with pseudoprogression, 21 by rCBV assessment using ferumoxytol perfusion MRI and three using updated RANO. Figure 1 represents a case of pseudoprogression diagnosed using perfusion MRI with ferumoxytol. The median interval from end of CRT to diagnosis of pseudoprogression was 6 weeks (range: 0–46 weeks). In this subset 18 (75%) patients were diagnosed within 12 weeks and six (25%) patients beyond 12 weeks (including two cases over 6 months). The 44 patients (64.7% of the entire study group) who had never been diagnosed with pseudoprogression had either partial/complete response (n = 3), stable disease (n = 1) or tumor progression (n = 28) (Figure 2) or nonevaluable progression status. In particular, ten patients received bevacizumab or increased doses of steroids prior to the MRI scan due to radiographic and/or clinical worsening after CRT/adjuvant TMZ therapy. In these cases, assessment of pseudoprogression from tumor progression was not possible. MR follow-up was not available in two cases.
Figure 1. . Pseudoprogression diagnosed using perfusion MRI with ferumoxytol.
Axial images of 47-year-old woman with glioblastoma multiforme pseudoprogression. T1W + Gd and T2W postoperative images prior to CRT (pre-CRT) and different time points after CRT (post-CRT) show radiographic worsening 8 days after CRT completion followed by further deterioration on follow-up MRI while the patient continued adjuvant temozolomide chemotherapy, indicative of tumor progression by updated Response Assessment in Neuro-Oncology Working Group (RANO) criteria. Blood volume of the lesion was low on CBV maps, indicative of pseudoprogression. The patient received only three courses of bevacizumab and continued adjuvant temozolomide. Substantial improvement is seen on 5-month follow-up MRI after completion of bevacizumab therapy. 3.5 years after completion of CRT, the image shows patient is stable without evidence of recurrence/progression (the patient is still on adjuvant temozolomide chemotherapy). The patient is still being followed 5 years after CRT and there is still no sign of disease recurrence/progression.
CBV: Cerebral blood volume; CRT: Chemoradiotherapy; T1W + Gd: Gadolinium-based contrast-enhanced T1-weighted; T2W: T2-weighted.
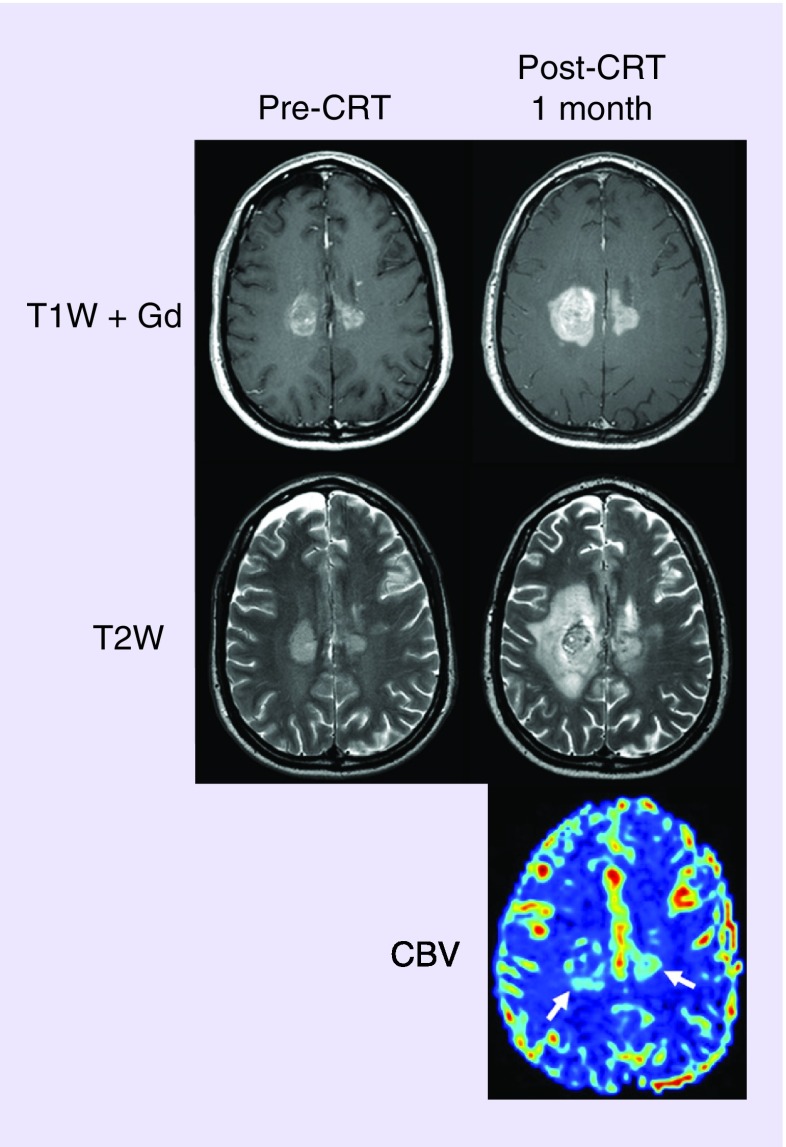
Figure 2. . Tumor progression diagnosed using perfusion MRI with ferumoxytol.
Axial images of 43-year-old man with GBM tumor progression. T1W + Gd and T2W images after surgery but prior to CRT (pre-CRT) and after CRT (post-CRT) show radiographic worsening 1 month after CRT completion. Blood volume of the lesions was high on the CBV map, indicative of tumor progression. Bevacizumab and carboplatin chemotherapy were started. The patient died 4 months later.
CBV: Cerebral blood volume; CRT: Chemoradiotherapy; T1W + Gd: Gadolinium-based contrast-enhanced T1-weighted; T2W: T2-weighted.
Minor differences were noted in the patient characteristics between patients with and without pseudoprogression (Table 1). The median age was 58 years in the whole group. However, patients with pseudoprogression were slightly younger than those without pseudoprogression, 51 versus 60 years, respectively. KPS at the time of pseudoprogression diagnosis was 90%, similar to baseline status, while at the time of tumor progression the KPS score dropped to 60–70%. Surgical debulking was performed in 56 (82%) patients, with 92% in the pseudoprogression group compared with 77% among patients without pseudoprogression. MGMT status was available in 22 of 24 patients with pseudoprogression, and was hypermethylated in 12 (55%) patients and nonhypermethylated in ten (45%).
Table 1. . Demographic characteristics of the patients.
| Patient characteristics | All patients | Patients with pseudoprogression | Patients without pseudoprogression |
|---|---|---|---|
| Patients (n) | 68 | 24 | 44 |
| Age (years): | |||
| – Median | 58 | 51 | 60 |
| – Range | 22–79 | 22–77 | 27–79 |
| Age, n (%): | |||
| – <50 years | 20 (29) | 12 (50) | 8 (18) |
| – ≥50 years | 48 (71) | 12 (50) | 36 (82) |
| Sex, number (%): | |||
| – Male | 39 (57) | 15 (62.5) | 24 (55) |
| – Female | 29 (43) | 9 (37.5) | 20 (45) |
| KPS at the time of GBM diagnosis: | |||
| – Median | 80 | 90 | 80 |
| – Range | 50–100 | 60–100 | 50–100 |
| KPS at the time of progression: | |||
| – Median | 60 | 70 | 60 |
| – Range | 50–100 | 50–100 | 50–90 |
| KPS at the time of pseudoprogression: | |||
| – Median | – | 90 | – |
| – Range | – | 60–100 | – |
| Extent of surgery, n (%): | |||
| – Biopsy | 12 (18) | 2 (8) | 10 (23) |
| – Debulking | 56 (82) | 22 (92) | 34 (77) |
| – Partial resection | 15 (22) | 7 (29) | 8 (18) |
| – Gross total resection | 41 (60) | 15 (63) | 26 (59) |
| Time from diagnosis to chemoradiotherapy (weeks): | |||
| – Median | 5 | 5 | 5 |
| – Range | 2–9 | 2–9 | 2–8 |
| Time from end of chemoradiotherapy to diagnosis of pseudoprogression: | |||
| – Median (weeks) | – | 6 | – |
| – Range (weeks) | – | 0–46 | – |
| – ≤12 weeks, n (%) | – | 18 (75) | – |
| – >12 weeks, n (%) | – | 6 (25) | – |
| Tumor location, n (%): | |||
| – Supratentorial | 66 (97) | 23 (96) | 43 (98) |
| – Right hemisphere | 31 (46) | 15 (63) | 16 (36) |
| – Left hemisphere | 31 (46) | 8 (33) | 23 (52) |
| – Bihemispheric | 4 (6) | 0 (0) | 4 (9) |
| – Frontal lobe | 25 (37) | 6 (25) | 19 (43) |
| – Temporal lobe | 16 (24) | 6 (25) | 10 (23) |
| – Parietal lobe | 15 (22) | 8 (33) | 7 (16) |
| – Occipital lobe | 7 (10) | 2 (8) | 5 (11) |
| – Basal ganglia/intraventricular | 3 (4) | 1 (4) | 2 (5) |
| – Infratentorial | 2 (3) | 1 (4) | 1 (2) |
| MGMT status (available in 22 patients), n (%): | |||
| – Hypermethylated | – | 12 (55) | – |
| – Nonhypermethylated | – | 10 (45) | – |
GBM: Glioblastoma multiforme; KPS: Karnofsky performance status.
• Treatment
All patients completed CRT except one who discontinued treatment because of intracerebral hemorrhage. Treatment details are presented in Table 2. Two patients completed CRT in 8 weeks and one in 11 weeks due to toxicity. In total, 60 patients (88%) started adjuvant TMZ and received a median of six cycles (range: 1–68); 55% received no more than six cycles and 45% more than six cycles. All patients with pseudoprogression started adjuvant TMZ and completed a median of 13 cycles (range: 3–68) and 83% received over six cycles. A total of 36 (82%) out of 44 patients who had never had pseudoprogression started adjuvant TMZ and completed a median of four cycles (range: 1–38) with only 19% receiving more than six cycles. Eight patients (12%) were unable to start adjuvant TMZ after completion of CRT because of either disease progression/poor clinical status (n = 7) or an allergic reaction to TMZ (n = 1). Discontinuation of adjuvant TMZ was due to disease progression (83%), treatment-related toxicity (6%) or patient decision to stop treatment (11%). Continuous steroid therapy was required in three (12.5%) patients with pseudoprogression and in 25 (57%) patients without pseudoprogression. Bevacizumab was started in 53 patients (78%) at the time of symptomatic clinical worsening or tumor progression as determined by perfusion MRI. After tumor progression, salvage chemotherapy was administered in 34 patients (50%), CRT was repeated in seven (10%) patients. Repeat surgery for tumor debulking was performed in six patients with pseudoprogression and in five patients without pseudoprogression. In these 11 cases pathology confirmed GBM at recurrence.
Table 2. . Intensity of treatment.
| Clinical characteristics | All patients | Patients with pseudoprogression | Patients without pseudoprogression |
|---|---|---|---|
| Chemoradiotherapy n (%): | |||
| – Started | 68 (100) | 24 (100) | 44 (100) |
| – Early discontinuation | 1 (1.5) | 0 | 1 (1.5) |
| Radiation dose (Gy): | |||
| – Median | 59.45 | 59.4 | 59.4 |
| – Range | 4–60 | 59.4–60 | 54–60 |
| Radiation duration (weeks): | |||
| – Median | 6 | 6 | 6 |
| – Range | 5–11 | 6–7 | 5–11 |
| Started adjuvant temozolomide, n (%) | 60 (88) | 24 (100) | 36 (82) |
| Cycles: | |||
| – Median (n) | 6 | 13 | 4 |
| – Range (n) | 1–68 | 3–68 | 1–38 |
| – Completed ≤six cycles, n (%) | 33 (55) | 4 (17) | 29 (81) |
| – Completed >six cycles, n (%) | 27 (45) | 20 (83) | 7 (19) |
| – Completed >12 cycles, n (%) | 15 (25) | 12 (50) | 3 (8) |
| – Completed >24 cycles, n (%) | 7 (12) | 5 (21) | 2 (6) |
| Temozolomide discontinued, n (%) | 54 (90) | 18 (75) | 36 (100) |
| Reason for discontinuation, n (%): | |||
| – Disease progression | 45 (83) | 15 (83) | 30 (83) |
| – Toxicity | 3 (6) | 1 (6) | 2 (6) |
| – Decision by patient | 6 (11) | 2 (11) | 4 (11) |
| Corticosteroid therapy, n (%): | |||
| – Yes | 28 (41) | 3 (12.5) | 25 (57) |
| – No | 40 (59) | 21 (87.5) | 19 (43) |
| Started bevacizumab, n (%) | 53 (78) | 18 (75) | 35 (80) |
| Doses: | |||
| – Median (n) | 9 | 10 | 9 |
| – Range (n) | 1–55 | 1–55 | 1–50 |
| – ≤three doses, n (%) | 15 (28) | 5 (28) | 10 (29) |
| – >three doses, n (%) | 38 (72) | 13 (72) | 25 (71) |
| Salvage chemotherapy, n (%) | 34 (50) | 12 (50) | 22 (50) |
| Repeat irradiation, n (%) | 7 (10) | 2 (8) | 5 (11) |
| Repeat debulking surgery, n (%) | 11 (16) | 6 (25) | 5 (11) |
• Treatment-related toxicity
We analyzed adverse events during CRT and adjuvant TMZ. Grade 3 and 4 hematologic toxicity most commonly occurred during CRT and within 6 months of adjuvant TMZ (Table 3). Lymphocyte counts were most affected by radiation and chemotherapy. Hematologic toxicity was rarely seen in patients who stayed on adjuvant TMZ for more than 6 months and none over 24 months. The lack of excess toxicity in patients who received more than 6 months of TMZ demonstrates that longer treatment duration is safe and feasible.
Table 3. . Grade 3 and 4 hematologic toxicity.
| Hematologic parameters | CRT (n = 68) | Adjuvant temozolomide (n = 60) | |||
|---|---|---|---|---|---|
| <6 months | 6–12 months | 12–24 months | >24 months | ||
| Leukopenia, n (%) | 4 (5.9) | 4 (6.7) | 0 (0) | 1 (1.7) | 0 (0) |
| Neutropenia, n (%) | 3 (4.4) | 3 (5) | 1 (1.7) | 1 (1.7) | 0 (0) |
| Lymphocytopenia, n (%) | 13 (19.1) | 13 (21.7) | 0 (0) | 0 (0) | 0 (0) |
| Thrombocytopenia, n (%) | 1 (1.5) | 2 (3.3) | 0 (0) | 1 (1.7) | 0 (0) |
| Anemia, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
CRT: Chemoradiotherapy.
One patient was diagnosed with PJP after completion of CRT but before adjuvant TMZ. This patient also developed shingles and Aspergillus pneumonia. No other patient developed PJP despite no prophylaxis at our institution. Other nonhematologic adverse effects included peripheral neuropathy (n = 1), surgical wound infection (n = 1), and allergic reaction (n = 1).
• Survival
At the end of follow-up, 13 (54%) patients in the pseudoprogression group and 43 (98%) without pseudoprogression had died. The median follow-up in the pseudoprogression group was 45.5 months, higher than the 38.5 months in the group without pseudoprogression. The 44 patients (64.7% of the entire study group) who had never been diagnosed with pseudoprogression had either partial/complete response (n = 3), stable disease (n = 1) or tumor progression (n = 28), as determined by standard MRI criteria, or had nonevaluable progression status.
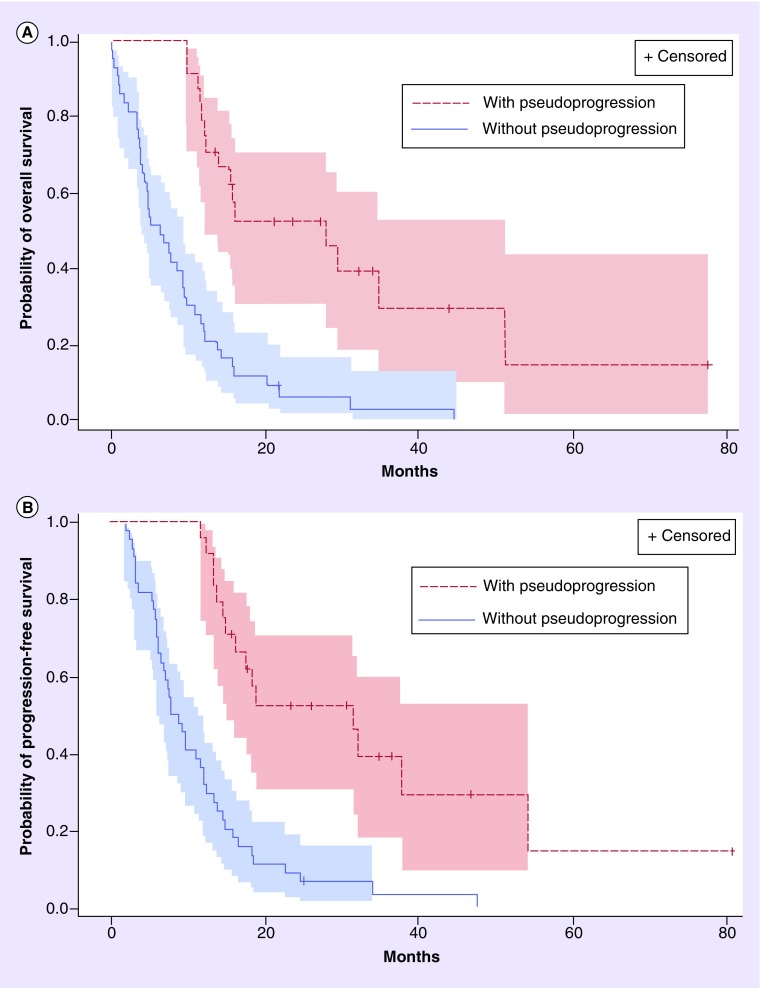
The median PFS was 13.4 (95% CI: 9.8–14.9) months for all patients. Median PFS in patients with pseudoprogression and without pseudoprogression was 31.4 (95% CI: 14.9–53.8) months and 8.4 (95% CI: 6.2–12.2) months (p < 0.0001), respectively (Figure 3). Median PFS after diagnosis of pseudoprogression was 17.8 months (95% CI: 10.8–48.8). At analysis, 15 patients with pseudoprogression subsequently progressed; 12 of these patients were diagnosed with pseudoprogression before 12 weeks after CRT and progressed in a median interval of 11 months (range: 9–49), the three patients with pseudoprogression diagnosis beyond 12 weeks after CRT, progressed in a median interval of 18 months (range: 10–24).
Figure 3. . Kaplan–Meier estimates.
Kaplan–Meier estimates of (A) overall and (B) progression-free survival by the presence or absence of pseudoprogression.
Median OS in the study population was 19.9 months (95% CI: 15.1–22.5) (Table 4). Survival was significantly greater in patients with pseudoprogression than in patients without pseudoprogression, 34.7 months (95% CI: 20.3–54.1) versus 13.4 months (95% CI: 11.1–19.5), respectively (p < 0.0001) (Figure 3). The hazard ratio for death comparing the pseudoprogression versus without pseudoprogression group was 0.24 (95% CI: 0.13–0.47; p < 0.0001) indicating a 76% relative reduction in death risk for patients with pseudoprogression in comparison to patients without pseudoprogression. Survival rates were 100% (95% CI: 100–100) at 1 year, 54.7% (95% CI: 32.2–72.5) at 2 years, 47.8% (95% CI: 25.2–67.4) at 3 years in patients with pseudoprogression, and 61.4% (95% CI: 45.4–73.9) at 1 year, 20.5% (95% CI: 10.1–33.3) at 2 years, 7.8% (95% CI: 2.1–18.6) at 3 years without pseudoprogression. The subgroup of patients with pseudoprogression and hypermethylated MGMT had longer median survival than patients with pseudoprogression and nonhypermethylated MGMT (54.1 vs 28.2 months; p = 0.23). However, this did not reach statistical significance, probably due to the small number of patients.
Table 4. . Kaplan–Meier overall survival analysis.
| Death/patients (n) | Hazard ratio (95% CI) | Median survival (95% CI); months | 6 months (%) | 12 months (%) | 24 months (%) | 36 months (%) | 48 months (%) | |
|---|---|---|---|---|---|---|---|---|
| Overall survival | 56/68 | – | 19.9 (15.1–22.5) | 94.1 | 75.0 | 32.5 | 21.4 | 15.8 |
| Patients without pseudoprogression | 43/44 | Referent | 13.4 (11.1–19.5) | 90.9 | 61.4 | 20.5 | 7.8 | 2.6 |
| Patients with pseudoprogression | 13/24 | 0.24 (0.13–0.47) | 34.7 (20.3–54.1) | 100.0 | 100.0 | 54.7 | 47.8 | 47.8 |
| MGMT status: | ||||||||
| – Nonhypermethylated | 6/10 | Referent | 28.2 (19.0–49.1) | 100.0 | 100.0 | 50.0 | 33.3 | – |
| – Hypermethylated | 6/12 | 0.49 (0.15–1.60) | 54.1 (19.9–79.9) | 100.0 | 100.0 | 58.3 | 58.3 | – |
| Extent of surgery: | ||||||||
| – Biopsy | 11/12 | Referent | 11.9 (5.4–19.8) | 83.3 | 50.0 | 8.3 | – | – |
| – Gross total resection | 35/41 | 0.36 (0.17–0.73) | 22.0 (17.3–25.3) | 97.6 | 82.9 | 37.9 | 24.5 | 17.9 |
| – Partial resection | 10/15 | 0.41 (0.17–0.97) | 19.9 (7.8–24.2) | 93.3 | 66.7 | 28.1 | – | – |
| Age: | ||||||||
| – <50 years | 13/20 | Referent | 20.1 (11.1–22.8) | 95.0 | 75.0 | 32.3 | – | – |
| – ≥50 years | 43/48 | 1.42 (0.76–2.65) | 19.8 (13.0–23.1) | 93.8 | 75.0 | 30.3 | 17.8 | 11.9 |
| Sex: | ||||||||
| – Male | 32/39 | Referent | 19.9 (13.8–23.2) | 92.3 | 74.4 | 32.1 | 26.2 | 22.5 |
| – Female | 24/29 | 1.26 (0.73–2.18) | 20.1 (12.4–22.8) | 96.6 | 72.4 | 29.4 | 13.1 | 0.0 |
| KPS: | ||||||||
| – <80 | 18/21 | Referent | 12.2 (7.8–18.9) | 85.7 | 47.6 | 14.3 | – | – |
| – ≥80 | 38/47 | 0.44 (0.25–0.79) | 21.3 (19.5–25.3) | 97.9 | 85.1 | 38.4 | 25.7 | 19.1 |
KPS: Karnofsky performance status.
After adjusting for age at diagnosis, gender, baseline KPS, and type of surgery, patients with pseudoprogression lived significantly longer than the patients without pseudoprogression (HR: 0.23; 95% CI: 0.11–0.50; p = 0.0002). No other variables were significant in the model.
Discussion
Median OS in our study was higher compared with the Stupp study (19.9 vs 14.6 months). The rate of survival at 2 and 3 years was also higher, 31.4 and 20% versus 27.2 and 16%, respectively, although the differences may not be statistically significant. The improved survival in our series might be explained by the extension of adjuvant TMZ. There are several studies suggestive for OS benefit with extension of adjuvant TMZ. The Brandes et al. study, in which patients with GBM received adjuvant TMZ until complete response (a minimum of 12 months) or tumor progression, reported OS of 20.7 months [2]. Another retrospective study showed improved OS in GBM patients treated with adjuvant TMZ for more than 6 months compared with 6 months (due to institutional policy and not tumor progression), 24.6 versus 16.5 months (p = 0.031), respectively [13].
A second possibility for the improved survival in our series compared with the Stupp results is the application of bevacizumab therapy [1]. However, two recent randomized Phase III trials (AVAglio and RTOG 0825) failed to demonstrate OS benefit with addition of upfront bevacizumab to standard therapy [14–16]. The median OS in patients receiving bevacizumab at the time of progression in the AVAglio and RTOG 0825 trials (16.7 and 16.1 months, respectively) was shorter than in our and the above mentioned studies. Therefore, the improvement in survival in our study is unlikely related to the presence of bevacizumab.
Median survival was significantly longer in patients with pseudoprogression compared with those without pseudoprogression. The rate of pseudoprogression diagnosed at any time point after CRT in our study was higher than in the Brandes et al. study and in the AVAglio trial, in which pseudoprogression was diagnosed within 1 month after CRT (35, 31 and 9.3%, respectively) [2]. Usage of upfront bevacizumab in the AVAglio trial decreased the rate of pseudoprogression to 2.2% [17]. This could reflect solely suppression of contrast enhancement, thus suppressing the imaging findings of radiation effects or tumor progression. However, this may also suggest that upfront bevacizumab decreases blood–brain barrier permeability, which is substantially disrupted in patients who developed pseudoprogression, and subsequently led to a decline of TMZ delivery to tumor cells. If this is true, use of VEGF antibody in newly diagnosed GBM should be used with caution. The correct timing of bevacizumab introduction in the treatment regimen can be crucial.
According to the updated RANO criteria, pseudoprogression is considered only within 12 weeks after completion of CRT and increase of enhancement beyond 12 weeks on stable/increased steroids is indicative of progressive disease [7]. However, 25% of all pseudoprogression cases in our study were diagnosed beyond the 12-week cutoff including two cases of pseudoprogression beyond the 6-month interval. Pseudoprogression beyond 12 weeks after CRT has been reported only in a few cases with onset between 2 and 6 months post-treatment, and there are only two reported cases of pseudoprogression 6 months after therapy [18–20]. Clinicians should be aware of the possibility of pseudoprogression even 6 months after CRT, and consider this while making treatment decision and designing investigational trials. It is likely that the incidence of early and late pseudoprogression will increase in the future with increased usage of combination of several chemotherapeutic medications or regimens with immunostimulative effects. Therefore, accurate diagnosis of pseudoprogression is essential.
We previously reported perfusion MRI with ferumoxytol (a blood pool agent at early time points) as a simple, accurate and a reliable tool to differentiate pseudoprogression from tumor progression [8,9]. While our protocols may differ in terms of timing, combination and dose of contrast agents, they use the same methods to assess perfusion. The patients were not on a therapeutic protocol, just an imaging protocol. This study showed again the utility and feasibility of advanced imaging with ferumoxytol in the setting of a clinical diagnostic dilemma. In the clinical practice the current diagnosis of pseudoprogression is retrospective and based on updated RANO guidance according to which diagnosis of pseudoprogression can be made on follow-up MRI if the patient is on stable or decreased steroids, and no further radiographic worsening is seen compared with prior MRIs acquired within 12 weeks after CRT. Figure 1 represents the obvious failure of the guidance by RANO indicating the necessity of better diagnostic tools. Moreover, clinicians may need to increase steroids or use short-term bevacizumab therapy, as suggested by some authors, due to clinical deterioration associating with radiographic worsening after CRT, which makes diagnosis of pseudoprogression even harder to determine [12].
The predictive value of MGMT methylation status in light of survival outcome and response to therapy is well established [2,10,21]. We report the longest survival in patients with combination of MGMT promoter methylation and pseudoprogression, a median of 54.1 months. Although 45% of pseudoprogression cases had nonhypermethylated MGMT, they lived longer than patients without pseudoprogression (28.2 vs 13.4 months; p = 0.01). This emphasizes that outcome of patients with GBM is significantly influenced not only by MGMT status, but also by presence of pseudoprogression.
The limitations of the study include the retrospective observational design so causal relationship could not be made. The baseline prognostic factors may be imbalanced between patients with and without pseudoprogression. While multivariable regression models were fitted to control for the potential confounding of some prognostic factors, there may be residual confounding in the multivariable analyses. Furthermore, since the treatment regimen in this study was to use adjuvant TMZ until progression, all patients who lived longer would likely get more TMZ and it is neither statistically or clinically sound to adjust for extended TMZ use in the multivariable model that assesses the association between pseudoprogression and survival. Patients may live longer due to the extended TMZ, or get extended TMZ because they lived longer. The two situations could not be separated and independent control group without extended TMZ use is needed to address confounding due to extended TMZ use.
Other limitations include the low number of patients, the use of bevacizumab (based on clinical indications) and various second-line treatment regimens. Altogether, ten patients received bevacizumab or increased dose of steroids prior to MRI scan, which can affect the diagnosis of pseudoprogression. Therefore, these patients were not considered for the pseudoprogression group. However, these patients were included in the group ‘never diagnosed with pseudoprogresion’. While this may introduce bias, it will likely bias the results of comparing OS between patients with versus without pseudoprogression towards the null without increasing type I error, since the latter group may include patients of pseudoprogression with longer OS. Such inclusion also allowed for a broader analysis to produce an estimated difference that more accurately reflected the clinical situation in practice. Additionally, MGMT status was not available in patients without pseudoprogression, and this study was only able to assess the association between MGMT status and survival in patients with pseudoprogression. Therefore, we could not examine whether or not the association between pseudoprogression and survival benefit was independent of MGMT methylation status in a multivariable model. However, given the retrospective study design and tissues were not available for many patients, even an expanded analysis would have been very incomplete. The results of this study warrant a prospective trial to establish diagnostic and predictive value of perfusion MRI with ferumoxytol, since our results with ferumoxytol call the RANO criteria into question [22]. Ferumoxytol also permits steady state rCBV, which increases greatly spatial resolution in GBM and may thereby increase the diagnostic accuracy of pseudoprogression. Steady state rCBV cannot be done with gadolinium-based contrast due to vascular leak, and is a major advancement in CBV assessment using MRI [23].
Conclusion
In conclusion, pseudoprogression is associated with better outcome, and survival benefits may be best for patients with concurrent pseudoprogression and MGMT promoter methylation. However, larger studies are needed to provide more reliable estimates. Clinicians should recognize the importance of differentiating tumor progression and pseudoprogression for treatment decisions and survival prediction by taking advantage of the currently widely accessible MR perfusion imaging.
The way in which tumor progression is assessed today will change very soon. Even the currently used ‘updated’ response criteria is outdated. Imaging will focus on physiologic and metabolic changes rather than changes in tumor ‘size’. In other words ‘quality’ will gain more attention over ‘quantity’. Perfusion MRI is no longer a luxury diagnostic tool. Optimization and standardization of perfusion MRI will warrant more reliable assessment of tumors. High-resolution steady-state blood volume mapping using blood pool contrast agents such as ferumoxytol, will gain some indications, especially in cases in which the detection of small and highly active tumor components is crucial.
Future perspective
In 5–10 years from now, we would expect that this clinically relevant phenomenon currently referred to as ‘pseudoprogression’, versus ‘vascular progression’, will receive a more specific term reflecting its currently not well understood pathomechanism.
More studies are needed to have a better understanding about the association between survival, pseudoprogression and MGMT status. Patients could not be randomized to either pseudoprogression or MGMT status, and new evidence has to come from large, well-designed prospective observational studies, which carefully use design and statistical strategies to minimize the confounding and bias of a nonrandomized study.
Acknowledgements
The authors thank Aliana Culp for her editorial assistance.
Footnotes
Financial & competing interests disclosure
This work was supported by a Veterans Administration merit review grant and by NIH grants NS53468, NS44687 and CA137488, in part with Federal funds from the National Cancer Institute, NIH, under Contract No. HHSN261200800001E, and by the Walter S and Lucienne Driskill Foundation to EA Neuwelt. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 3.De Wit MC, De Bruin HG, Eijkenboom W, Sillevis Smitt PA, Van Den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 4.Gerstner ER, Mcnamara MB, Norden AD, Lafrankie D, Wen PY. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J. Neurooncol. 2009;94(1):97–101. doi: 10.1007/s11060-009-9809-4. [DOI] [PubMed] [Google Scholar]
- 5.Taal W, Brandsma D, De Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 6.Young RJ, Gupta A, Shah AD, et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76(22):1918–1924. doi: 10.1212/WNL.0b013e31821d74e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 8.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(2):514–523. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266(3):842–852. doi: 10.1148/radiol.12111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 11.Neuwelt AJ, Nguyen TM, Fu R, et al. Incidence of Pneumocystis jirovecii penumonia after temozolomide for CNS malignancies without prophylaxis. CNS Oncol. 2014 doi: 10.2217/cns.14.24. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(5):1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roldan Urgoiti GB, Singh AD, Easaw JC. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J. Neurooncol. 2012;108(1):173–177. doi: 10.1007/s11060-012-0826-3. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy – temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 16.Fine HA. Bevacizumab in glioblastoma – still much to learn. N. Engl. J. Med. 2014;370(8):764–765. doi: 10.1056/NEJMe1313309. [DOI] [PubMed] [Google Scholar]
- 17.Wolfgang W, Cloughesy TF, Nishikawa R, et al. Tumor response based on adapted Macdonald criteria and assessment of pseudoprogression (PsPD) in the Phase III AVAglio trial of bevacizumab (Bv) plus temozolomide (T) plus radiotherapy (RT) in newly diagnosed glioblastoma (GBM) J. Clin. Oncol. 2002;2013;31(Suppl. 15) [Google Scholar]
- 18.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J. Neurooncol. 2007;82(1):81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 19.Chaskis C, Neyns B, Michotte A, De Ridder M, Everaert H. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg. Neurol. 2009;72(4):423–428. doi: 10.1016/j.surneu.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Stuplich M, Hadizadeh DR, Kuchelmeister K, et al. Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J. Clin. Oncol. 2012;30(21):e180–e183. doi: 10.1200/JCO.2011.40.9565. [DOI] [PubMed] [Google Scholar]
- 21.Dunn J, Baborie A, Alam F, et al. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br. J. Cancer. 2009;101(1):124–131. doi: 10.1038/sj.bjc.6605127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasseri M, Gahramanov S, Netto JP, et al. Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro. Oncol. 2014;16(8):1146–1154. doi: 10.1093/neuonc/not328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varallyay CG, Nesbit E, Fu R, et al. High-resolution steady-state cerebral blood volume maps in patients with central nervous system neoplasms using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J. Cereb. Blood Flow Metab. 2013;33(5):780–786. doi: 10.1038/jcbfm.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]