Abstract
Purpose of review
Mitochondria are cellular organelles that are required for energy production. Emerging evidence demonstrates their role in oocyte development and reproduction. In this review, we examine recent animal and clinical studies on the role of mitochondria in fertility. We also analyse the impact of assisted reproductive techniques (ARTs) on mitochondrial function and discuss the future clinical implications of mitochondrial nutrients and mitochondrial replacement.
Recent findings
Mitochondria affect all aspects of mammalian reproduction. They are essential for optimal oocyte maturation, fertilization and embryonic development. Mitochondrial dysfunction causes a decrease in oocyte quality and interferes with embryonic development. ART procedures affect mitochondrial function, while mitochondrial nutrients may increase mitochondrial performance in oocytes. New mitochondrial replacement procedures using mitochondria obtained from polar bodies or from the patient’s own oogonial stem cells are promising and may address concerns related to the induction of high-levels of heteroplasmy, which could potentially result in negative long-term health effects.
Summary
Optimal energy production is required for oocyte and embryo development, and mitochondrial abnormalities have devastating reproductive consequences. Improvement of oocyte mitochondrial function via intake of compounds that boost mitochondrial activity may have clinical benefits, and mitochondrial replacement could potentially be used for the prevention of mitochondrial diseases.
Keywords: fertility, mitochondria, oocyte, reproduction
INTRODUCTION
Mitochondria are energy factories of the cell separated from the cytoplasm by a double membrane. They synthesize ATP, which is the essential energy currency for many cellular processes. Moreover, mitochondria have important roles in metabolism, calcium homeostasis, fatty acid oxidation and apoptosis.
Evolutionarily, mitochondria are the relics of bacteria that were in symbiosis with eukaryotic cells. Over millions of years of evolution, most of the bacterial genes have translocated to the nucleus, while mitochondria became a cellular organelle. Importantly, although mitochondrion is the only animal organelle containing DNA outside of the nucleus, most of the proteins essential for mitochondrial function are encoded by the nuclear genome [1–3].
Tissues have varying numbers of mitochondria and mitochondrial DNA (mtDNA) copy number, determined by their metabolic activity [1]. Human mtDNA is circular, 16.6 kB long and contains 37 genes. It encodes two ribosomal RNAs, 22 transport RNAs and 13 proteins. All proteins encoded by the mitochondrial genome are part of the electron transport chain (ETC) [4,5] (Table 1). Mitochondrial DNA is prone to mutations because it lacks protective histones and lays in close proximity of ETC, which is notorious for the generation of reactive oxygen species (ROS). Therefore, mtDNA accumulates mutations and deletions over time, which is believed to reduce the fitness of the organism [6]. In fact, mitochondrial dysfunction is associated with and possibly contributes to the pathogenesis of atherosclerosis, type 2 diabetes and neurodegenerative disorders [7▪].
Table 1.
The list of human mtDNA genes
| Protein encoding genes | Complex I | MT-ND1, MT-ND2, MT-ND3, MT-ND4. MT-ND4L, MT-ND5, MT-ND6 |
| Complex III | MT-CYB | |
| Complex IV | MT-COl, MT-C02, M1T-C03 | |
| Complex V | MT-ATP6, MT-ATP8 | |
| rRNA-encoding genes | MT-RNR1, MT-RNR2 | |
| tRNA-encoding genes | MT-TA, MT-TN, MT-TR, MT-TC, MT-TD, MT-TE, MT-TQ. MT-TG, MT-TH, MT-TI, MT- TLI, MT-TL2, MT-TK, MT-TM MT-TF, MT-TP, MT-TSI, MT-TS2, MT-TT, MT-TW, MT-TY, MT-TV | |
In this review, we discuss the role of mitochondria in oocyte biology and reproduction. We also provide a summary of the recent studies which examine the impact of assisted reproductive techniques (ARTs) on mitochondrial function and discuss the future clinical implications of mitochondrial nutrients and mitochondrial replacement.
THE ROLE OF MITOCHONDRIA IN OOCYTE BIOLOGY
Mitochondria are inherited uniparentally, as sperm mitochondrial proteins are ubiquitinated and degraded via proteasomes and autophagy following their entry into the oocyte [8–10]. The reason for this phenomenon is unknown. However, it is thought that the sperm mitochondria are destroyed to protect the embryo from deleterious mutations that they carry as a result of exposure to high ROS levels during spermatogenesis [7▪,11]. It is also possible that the degradation of sperm mitochondria protects the offspring from the high levels of heteroplasmy that would have been observed if sperm mitochondria were retained in the oocyte [10].
There are about 100 000 mitochondria per fully-grown human oocyte. Mitochondria in mammalian oocytes are spherical with little cristae [2]. These mitochondria are transcriptionally and bioenergetically silent and this functional state seems to be evolutionarily conserved [12,13], especially in immature eggs, which have slow ATP production [14]. This quiescent state is believed to be important to keep the number of mitochondrial DNA mutations to minimum, as these mutations will then be passed down to the embryo [13]. After fertilization, oocyte mitochondria undergo structural changes and resemble their somatic counterparts by the time of blastocyst formation [7▪].
Thousands of mitochondria in oocytes are formed from a few hundred present in primordial germ cells (PGCs). Although mitochondrial replication starts in PGCs [15] and continues during early oogenesis, a major increase in mitochondria numbers is observed during later stages of folliculogenesis [16]. Oocyte mitochondria are required to support early embryonic development for two reasons. First, glycolysis is limited during oocyte maturation and early preimplantation embryo development, until the blastocyst stage [17,18]. In addition, mitochondria replication is suppressed and mtDNA count does not change from metaphase II oocytes till hatched blastocyst stage [16,19,20]. Therefore, mitochondria derived from the oocyte are the major source of ATP during preimplantation embryonic development.
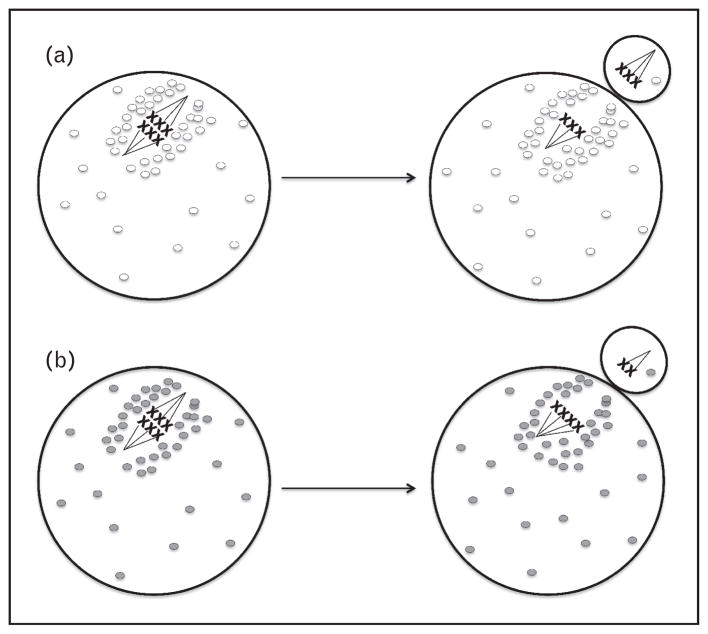
Mitochondria are dynamic structures: they move inside the cell and undergo fusion (two mitochondria join to form a single mitochondrion) and fission (a single mitochondrion divides into two mitochondria) [21,22]. They aggregate around the spindle during the first meiotic division, migrate to the oocyte cortex in contact with the spindle and then distribute asymmetrically during the division wherein almost all mitochondria remain in the oocyte [23] (Fig. 1). This is important because polar body will degenerate and mitochondria in the oocyte are essential energy suppliers to the developing embryo. During the second meiotic division, mitochondria aggregate around the spindle and then disperse to the cytoplasm [23]. ATP levels also peak during polar body extrusion, further emphasizing the role of mitochondria in meiosis [18]. The regulation of mitochondrial dynamics is important for oocyte maturation. Overexpression of mitochondrial fusion proteins leads to the formation of mitochondrial aggregates in oocyte cytoplasm and interferes with spindle organization and spatiotemporal endoplasmic reticulum (ER) distribution [24]. Moreover, oocyte-specific knockout of mitochondrial fission factor Drp1 impairs calcium signalling and intercellular communication, and leads to abnormalities in follicle development and ovulation [25].
FIGURE 1.
Schematic representations of mitochondrial behaviour and mitochondrial function in oocytes during meiosis I. Mitochondria accumulate around meiotic spindle during meiosis I and remain in the oocyte following cell division. (a) Chromosomes distribute evenly when mitochondria function is optimal (white circles). (b) In cases of mitochondrial dysfunction (grey circles), meiotic nondisjunction occurs. This mechanism is thought to be responsible for the observed increase in aneuploidy rate in aged oocytes.
Heteroplasmy is the presence of more than one mitochondrial DNA variant in the cell. Each individual carries one heteroplasmy on average [26▪▪]. It is impossible to predict the heteroplasmy level in the oocyte on the basis of the heteroplasmy level in a woman’s somatic tissue. Individual oocytes have various levels of heteroplasmy. As a result, allele frequency of different mtDNA variants changes in subsequent generations [27]. A number of mechanisms were proposed as a cause of this genetic drift, such as the drastic reduction of mtDNA number from oocytes to PGCs (mitochondrial bottleneck), assembly of homoplasmic mtDNA variants into segregation units and preferential amplification of a subgroup of mtDNAs during oogenesis or post-natal folliculogenesis [15,28,29]. Regardless of the mechanism of this phenomenon, it seems to be in place to protect the offspring from deleterious mutations, because in the opposite scenario, the accumulation of mitochondrial mutations can lead to devastating consequences, as oocyte mitochondria populate new embryos in each successive generation [7▪,29,30]. It was demonstrated that one in eight individuals carry disease-associated heteroplasmy [26▪▪]. The frequency of disease-associated allele determines the phenotype. The nature of the mutation is also important, as mutations in the mtDNA region encoding tRNAs seem to be tolerated better than others [4].
MITOCHONDRIA AND REPRODUCTION
Mitochondria play important roles in reproduction, and transgenic mice with induced mtDNA mutations experience severe decline in fertility [31]. Subplasmalemmal mitochondria affect fertilization ability of human oocytes [32]. Mitochondria also regulate calcium oscillations during fertilization, which are crucial for embryonic development [33]. The pattern of calcium oscillations changes when mitochondria function is inhibited [34]. Moreover, mitochondrial function and DNA content determines fertilization outcome of porcine oocytes [35,36]. Mitochondrial DNA content also correlates with the ability of the human oocytes to be fertilized. Unfertilized oocytes have lower mtDNA copy number and degenerated oocytes demonstrate even lower levels [37,38]. Fertilization failure observed in oocytes with low mtDNA number may indicate defective cytoplasmic maturation.
In pig oocytes, mitochondrial copy number and activity are important for parthenogenetic activation; however, mitochondrial copy number does not seem to be crucial for porcine oocyte maturation. On the contrary, pig oocytes with depleted mitochondrial membrane potential experience problems with maturation [39]. The same is true for mouse oocytes. In mice, mitochondrial activity is important for oocyte maturation and subsequent embryo development. However, alterations in mtDNA copy number do not seem to affect maturation, although they interfere with embryo development to the blastocyst stage following in-vitro fertilization (IVF) [40]. In another study, inhibition of oxidative phosphorylation in mouse oocytes severely reduces their development to hatched blastocyst stage [41]. It was also demonstrated that, although low mtDNA copy number in mouse oocytes could be enough to support fertilization and preimplantation development, these embryos do not do well after implantation. Threshold of mtDNA copy number in oocytes required for mouse embryonic development following implantation was determined to be 40 000–50 000 by genetically manipulating mtDNA copy number at different stages of germline development through deletion of Tfam, an essential component of mitochondrial nucleoid [42]. In humans, higher ATP levels in oocytes correlate with better embryo development and implantation rates [41]. On the basis of these studies, it is possible to conclude that oocyte has much more mitochondria than is required for oocyte maturation. However, high numbers of mitochondria observed in oocytes are essential to support early embryonic development, especially considering that the mitochondrial replication is inhibited in preimplantation embryos.
Mitochondrial dysfunction in the oocytes of diabetic mothers may be the mechanism responsible for the occurrence of developmental defects and high rate of miscarriages. Oocytes from diabetic mice have altered mitochondria ultrastructure, function and mtDNA copy number, and demonstrate high frequency of spindle abnormalities [43,44]. Cumulus cells of type 1 diabetic mice also display increased apoptosis, abnormal mitochondria distribution and function [45]. Moreover, oocytes and cumulus cells from mice on high-fat diet demonstrate mitochondrial structural defects and increased spindle abnormalities [46,47]. Altered mitochondria number and function are also associated with male subfertility [1].
Age-related decline in fertility is largely due to decrease in oocyte quality. Embryos obtained using donor oocytes from young women demonstrate high implantation rates when transferred to older women [48]. It was shown that human euploid embryos are much more likely to develop to blastocysts and euploid oocytes almost always form euploid embryos [49,50]. However, oocyte aneuploidy rate increases with age and increased aneuploidy rate is believed to be the main mechanism responsible for the decrease in oocyte quality [51,52]. It was demonstrated that mouse oocyte ATP production decreases with age [53]. Ageing mice and hamster oocytes have less ATP, and mtDNA copy number, and demonstrate ultrastructural mitochondrial abnormalities [54]. Aged mare oocytes also have lower mtDNA copy number [55]. Moreover, mitochondrial mutations increase with age [19]. In one recent study, authors examined the frequency of various mitochondrial alleles in 39 healthy mother–child pairs. Interestingly, the number of heteroplasmies in children positively correlated with maternal age at conception. This points to the accumulation of mtDNA mutations in maternal oocytes with age [26▪▪]. Furthermore, mtDNA copy number is lower in unfertilized oocytes and uncleaved embryos of women more than 40 years old than younger women [56]. Interestingly, Wells et al. [57▪▪] recently reported that elevated mitochondrial DNA in human preimplantation embryos is associated with aneuploidy. Although definitive evidence is lacking, the aforementioned studies and others suggest that the mitochondrial abnormalities affect energy production, lead to spindle abnormalities, result in aneuploidy and affect developmental competence of the oocytes [58–60] (Fig. 1). The relationship between mitochondrial DNA content in human oocytes and embryos and how they affect developmental potential is a complex process and will require further investigation.
Emerging evidence demonstrates the effects of ARTs on mitochondria function and the role of mitochondria in determining IVF outcomes. Human oocytes with dark zona pellucida have lower fertilization, implantation and clinical pregnancy rates, and demonstrate mitochondrial abnormalities [61]. Vitrification decreases mouse oocyte mitochondrial membrane potential [62] and germinal vesicle stage oocytes obtained from vitrified mouse ovarian tissue have lower fertilization rates and poor developmental potential than fresh samples. This could be due to mitochondrial dysfunction observed in vitrified oocytes [63]. Prolonged in-vitro maturation (IVM) reduces developmental rate of bovine oocytes to blastocysts, despite increasing their mitochondrial activity [64]. Epidermal growth factor-like peptides improve IVM outcomes in mouse cumulus oocyte complexes by inducing mitochondrial activity [65]. Mitochondrial copy number, membrane potential and ATP content are decreased in murine oocytes following controlled ovarian hyperstimulation (COH) [66]. It is possible that mitochondria of superovulated mouse oocytes have some defects, which impairs their embryonic development [67]. COH causes mitochondrial abnormalities in granulosa cells of Rhesus monkeys [68]. Growth hormone improves oocyte quality and increases the number of functional mitochondria when applied as part of a COH protocol in aged women with poor ovarian response [69]. The widespread use of ART necessitates extensive studies on the effects of these techniques on oocyte quality and mitochondrial function.
IMPROVEMENTS IN MITOCHONDRIAL PERFORMANCE AND CLINICAL IMPLICATIONS
As mitochondrial dysfunction negatively affects reproduction, an increase in mitochondrial number and/or improvement of mitochondrial function could potentially lead to improved fertility [7▪]. Mitochondrial nutrients are biological or chemical compounds that boost energy-producing capacity of mitochondria [14]. α Lipoic acid (ALA), a coenzyme important in mitochondrial metabolism, is required for embryonic survival in mice [70]. Resveratrol has gained much attention recently as an antiageing compound that increases mitochondrial numbers and improves mitochondrial function [14,71,72]. It was demonstrated to improve mitochondrial performance and developmental capacity of porcine oocytes [73]. Supplementation of mouse oocyte media with L-carnitine, a small antioxidant molecule important in fatty acid β oxidation, improves meiotic spindle configuration and mitochondrial distribution following IVM of vitrified oocytes [74]. Coenzyme Q10 (CoQ10) is a lipid-soluble electron transporter in ETC. Tissue levels of CoQ10 decrease with age [6,75]. Dietary supplementation of CoQ10 could possibly decrease aneuploidy rate in human oocytes [76]. Although the use of mitochondrial nutrients to improve fertility is appealing, well designed clinical studies are required to test the clinical efficacy and potential benefits of these compounds.
Efforts were also made to transfer ‘healthy’ mitochondria from young oocytes to defective oocytes in women with repeated IVF failures. Small amounts of cytoplasm obtained from young oocytes were used for this purpose. However, concerns over the impact of mitochondrial heteroplasmy on the long-term health of the offspring led to the discontinuation of the procedure, despite the initial success [7▪,77,78]. There is an intricate communication between the nucleus and mitochondria. Therefore, the presence of the third parent genetic material could lead to devastating consequences. These concerns were corroborated with mice studies in which heteroplasmy was associated with cognitive abnormalities [79] and a phenotype resembling early-onset metabolic syndrome [80].
Mitochondrial mutations may result in numerous devastating diseases [4,81]. Although preimplantation genetic diagnosis can reduce mutant mtDNA transmission to the next generation, it cannot eliminate disease occurrence [27]. Therefore, mitochondrial replacement has gained a lot of attention in recent years as a method to prevent these diseases. Experimental studies attempted to use spindle chromosome transfer or pronuclear transfer (PNT) of oocytes harbouring mitochondrial mutations to healthy enucleated oocytes. However, these procedures still result in transfer of some donor mitochondria and theoretical concern of significant negative health effects of heteroplasmy still exists. Despite this, the Human Fertilization and Embryology Authority (HFEA) in the UK concluded that preclinical evidence is sufficient to proceed with experimental studies in humans and recently, the UK Parliament approved the use of this technique in humans, but a further vote is required in the House of Lords (http://www.parliament.uk/business/news/2015/february/commons-debate-statutory-instrument-on-mitochondrial-donation/). However, in the USA, the Food and Drug Administration (FDA) decided to hold until further preclinical evidence is available [81].
In one recent study, authors proposed the use of polar bodies to eliminate the possibility of transfer of defective mitochondria. Polar bodies contain the same genetic material as their sister oocytes and have significantly less mitochondria. The experiments performed in mice are promising and either polar body 1 or polar body 2 transfer resulted in healthy offspring and, importantly, minimal mtDNA carryover [82▪▪] (Fig. 1). More studies are needed to explore the possibility of use of polar bodies in this regard, either alone or in combination with other techniques that may decrease the number of donor oocytes needed for mitochondrial replacement procedures [82▪▪]. Another possibility is the use of oogonial stem cell (OSC) mitochondria for replacement. Termed AUGMENT (autologous germline mitochondrial energy transfer), this would have certain advantages: first, these would be tissue-matched mitochondria derived from germ cells; second, these mitochondria have very high bioenergetic potential and low number of mutations; and third, as these mitochondria will be isolated from the patient’s own cells, the possibility of the presence of the third-party genetic material will be eliminated [7▪,83].
CONCLUSION
Available evidence points to the important role of mitochondria in reproduction. Optimal mitochondrial function is required for the oocyte maturation, fertilization and embryonic development. Improvement of the mitochondrial function either through the use of small molecules or procedures involving mitochondrial transfers could lead to better fertility outcomes. Moreover, mitochondrial replacement procedures could open a new page in the treatment of mitochondrial diseases. We are entering a new era wherein boosting mitochondrial performance with exciting procedures could improve oocyte quality and reproductive performance in humans.
KEY POINTS.
Mitochondria affect all aspects of mammalian reproduction, including oocyte maturation, fertilization and embryonic development.
The use of ARTs may alter mitochondria function in mammalian oocytes and granulosa cells.
The idea of the use of mitochondrial nutrients to boost mitochondrial performance and to increase oocyte quality is appealing, but well designed clinical studies are needed to test their efficacy.
New mitochondrial replacement procedures using mitochondria obtained from polar bodies or from the patient’s own oogonial stem cells are promising and may address concerns related to the induction of high levels of heteroplasmy.
Acknowledgments
Financial support and sponsorship
E.S. is supported by award no. R01HD059909 from the National Institutes of Health (NIH).
Footnotes
The authors have nothing to disclose.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Benkhalifa M, Ferreira YJ, Chahine H, et al. Mitochondria: participation to infertility as source of energy and cause of senescence. Int J Biochem Cell Biol. 2014;55C:60–64. doi: 10.1016/j.biocel.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Cummins JM. Mitochondria: potential roles in embryogenesis and nucleocytoplasmic transfer. Hum Reprod Update. 2001;7:217–228. doi: 10.1093/humupd/7.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheibye-Knudsen M, Fang EF, Croteau DL, et al. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2014 doi: 10.1016/j.tcb.2014.11.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentov Y, Casper RF. The aging oocyte – can mitochondrial function be improved? Fertil Steril. 2013;99:18–22. doi: 10.1016/j.fertnstert.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 7▪.Tilly JL, Sinclair DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17:838–850. doi: 10.1016/j.cmet.2013.05.007. This comprehensive literature review analyses available evidence on ageing, metabolism and female fertility. The effects of diet on oocyte quality and the role of mitochondria in ageing and in determination of oocyte competence are also discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummins JM, Wakayama T, Yanagimachi R. Fate of microinjected spermatid mitochondria in the mouse oocyte and embryo. Zygote. 1998;6:213–222. doi: 10.1017/s0967199498000148. [DOI] [PubMed] [Google Scholar]
- 9.Hajjar C, Sampuda KM, Boyd L. Dual roles for ubiquitination in the processing of sperm organelles after fertilization. BMC Dev Biol. 2014;14:6. doi: 10.1186/1471-213X-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song WH, Ballard JW, Yi YJ, Sutovsky P. Regulation of mitochondrial genome inheritance by autophagy and ubiquitin-proteasome system: implications for health, fitness, and fertility. Biomed Res Int. 2014;2014:981867. doi: 10.1155/2014/981867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- 12.Allen JF, de Paula WB. Mitochondrial genome function and maternal inheritance. Biochem Soc Trans. 2013;41:1298–1304. doi: 10.1042/BST20130106. [DOI] [PubMed] [Google Scholar]
- 13.de Paula WB, Agip AN, Missirlis F, et al. Female and male gamete mitochondria are distinct and complementary in transcription, structure, and genome function. Genome Biol Evol. 2013;5:1969–1977. doi: 10.1093/gbe/evt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentov Y, Yavorska T, Esfandiari N, et al. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet. 2008;40:1484–1488. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- 16.St John J. The control of mtDNA replication during differentiation and development. Biochim Biophys Acta. 2014;1840:1345–1354. doi: 10.1016/j.bbagen.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Barbehenn EK, Wales RG, Lowry OH. The explanation for the blockade of glycolysis in early mouse embryos. Proc Natl Acad Sci U S A. 1974;71:1056–1060. doi: 10.1073/pnas.71.4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229:353–361. doi: 10.1002/jcp.24457. [DOI] [PubMed] [Google Scholar]
- 19.Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:183024. doi: 10.1155/2013/183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thundathil J, Filion F, Smith LC. Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev. 2005;71:405–413. doi: 10.1002/mrd.20260. [DOI] [PubMed] [Google Scholar]
- 21.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalton CM, Carroll J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J Cell Sci. 2013;126(Pt 13):2955–2964. doi: 10.1242/jcs.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakai T, Harada Y, Miyado K, Kono T, et al. Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Mol Hum Reprod. 2014;20:1090–1100. doi: 10.1093/molehr/gau064. [DOI] [PubMed] [Google Scholar]
- 25.Udagawa O, Ishihara T, Maeda M, et al. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr Biol. 2014;24:2451–2458. doi: 10.1016/j.cub.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Rebolledo-Jaramillo B, Su MS, Stoler M, et al. Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 2014;111:15474–15479. doi: 10.1073/pnas.1409328111. This study demonstrates the heteroplasmy transmission in 39 healthy mother–child pairs. Importantly, authors estimate the size of mtDNA bottleneck, germ-line mutation rate and demonstrate a positive association between the number of heteroplasmies in a child and maternal age at fertilization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson J, Irving L, Hyslop LA, et al. Assisted reproductive technologies to prevent transmission of mitochondrial DNA disease. Stem Cells. 2014 doi: 10.1002/stem.1887. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao L, Shitara H, Horii T, et al. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat Genet. 2007;39:386–390. doi: 10.1038/ng1970. [DOI] [PubMed] [Google Scholar]
- 29.Cree LM, Samuels DC, de Sousa Lopes SC, et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet. 2008;40:249–254. doi: 10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- 30.Freyer C, Cree LM, Mourier A, et al. Variation in germline mtDNA heteroplasmy is determined prenatally but modified during subsequent transmission. Nat Genet. 2012;44:1282–1285. doi: 10.1038/ng.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 32.Van Blerkom J, Caltrider K. Sperm attachment and penetration competence in the human oocyte: a possible aetiology of fertilization failure involving the organization of oolemmal lipid raft microdomains influenced by the DeltaPsim of subplasmalemmal mitochondria. Reprod Biomed Online. 2013;27:690–701. doi: 10.1016/j.rbmo.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Ajduk A, llozue T, Windsor S, et al. Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability. Nat Commun. 2011;2:417. doi: 10.1038/ncomms1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakai T, Zhang N, Vangheluwe V, Fissore RA. Regulation of endoplasmic reticulum Ca(2+) oscillations in mammalian eggs. J Cell Sci. 2013;126(Pt 24):5714–5724. doi: 10.1242/jcs.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Shourbagy SH, Spikings EC, Freitas M, St John JC, et al. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- 36.Viet Linh N, Kikuchi K, Nakai M, et al. Fertilization ability of porcine oocytes reconstructed from ooplasmic fragments produced and characterized after serial centrifugations. J Reprod Dev. 2013;59:549–556. doi: 10.1262/jrd.2013-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Reynier P, May-Panloup P, Chretien MF, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 39.Lee SK, Zhao MH, Kwon JW, et al. The association of mitochondrial potential and copy number with pig oocyte maturation and developmental potential. J Reprod Dev. 2014;60:128–135. doi: 10.1262/jrd.2013-098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge H, Tollner TL, Hu Z, et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev. 2012;79:392–401. doi: 10.1002/mrd.22042. [DOI] [PubMed] [Google Scholar]
- 41.Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–424. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 42.Wai T, Ao A, Zhang X, et al. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Ratchford AM, Chi MM, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–1612. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion. 2010;10:403–410. doi: 10.1016/j.mito.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Frolova AI, Purcell S, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010;5:e15901. doi: 10.1371/journal.pone.0015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19:486–494. doi: 10.1093/molehr/gat026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luzzo KM, Wang Q, Purcell SH, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance – United States, 2005. MMWR Surveill Summ. 2008;57:1–23. [PubMed] [Google Scholar]
- 49.Sher G, Keskintepe L, Keskintepe M, et al. Oocyte karyotyping by comparative genomic hybridization [correction of hybrydization] provides a highly reliable method for selecting ‘competent’ embryos, markedly improving in vitro fertilization outcome: a multiphase study. Fertil Steril. 2007;87:1033–1040. doi: 10.1016/j.fertnstert.2006.08.108. [DOI] [PubMed] [Google Scholar]
- 50.Navot D, Bergh PA, Williams MA, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- 51.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 52.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simsek-Duran F, Li F, Ford W, et al. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8:e64955. doi: 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rambags BP, van Boxtel DC, Tharasanit T, et al. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology. 2014;81:959–965. doi: 10.1016/j.theriogenology.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Murakoshi Y, Sueoka K, Takahashi K, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–1375. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57▪▪.Wells D, Kaur K, Grifo J, et al. Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet. 2014;51:553–562. doi: 10.1136/jmedgenet-2014-102497. In this study, authors propose the use of next-generation sequencing for the diagnosis of aneuploidy in human embryos. Interestingly, elevated mtDNA content was associated with aneuploidy in embryos, either supporting the ‘quiet embryo’ hypothesis in which abnormally high metabolism decreases embryonic viability or demonstrating a compensatory increase in mtDNA to overcome the primary defect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- 59.Schon EA, Kim SH, Ferreira JC, et al. Chromosomal nondisjunction in human oocytes: is there a mitochondrial connection? Hum Reprod. 2000;15(Suppl 2):160–172. doi: 10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Wu XQ, Lu S, et al. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16:841–850. doi: 10.1038/sj.cr.7310095. [DOI] [PubMed] [Google Scholar]
- 61.Shi W, Xu B, Wu LM, et al. Oocytes with a dark zona pellucida demonstrate lower fertilization, implantation and clinical pregnancy rates in IVF/ICSI cycles. PLoS One. 2014;9:e89409. doi: 10.1371/journal.pone.0089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei T, Guo N, Tan MH, Li YF. Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. J Huazhong Univ Sci Technolog Med Sci. 2014;34:99–102. doi: 10.1007/s11596-014-1238-8. [DOI] [PubMed] [Google Scholar]
- 63.Salehnia M, Tohonen V, Zavareh S, Inzunza J. Does cryopreservation of ovarian tissue affect the distribution and function of germinal vesicle oocytes mitochondria? Biomed Res Int. 2013;2013:489032. doi: 10.1155/2013/489032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koyama K, Kang SS, Huang W. Aging-related changes in in vitro-matured bovine oocytes: oxidative stress, mitochondrial activity and ATP content after nuclear maturation. J Reprod Dev. 2014;60:136–142. doi: 10.1262/jrd.2013-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richani D, Sutton-McDowall ML, Frank LA, et al. Effect of epidermal growth factor-like peptides on the metabolism of in vitro- matured mouse oocytes and cumulus cells. Biol Reprod. 2014;90:49. doi: 10.1095/biolreprod.113.115311. [DOI] [PubMed] [Google Scholar]
- 66.Ge HS, Zhang F, Li XH, et al. Effects of controlled ovarian hyperstimulation on mitochondria copy number and functions in murine oocytes. Zhonghua Fu Chan Ke Za Zhi. 2013;48:858–861. [PubMed] [Google Scholar]
- 67.Komatsu K, Iwase A, Mawatari M, et al. Mitochondrial membrane potential in 2-cell stage embryos correlates with the success of preimplantation development. Reproduction. 2014;147:627–638. doi: 10.1530/REP-13-0288. [DOI] [PubMed] [Google Scholar]
- 68.Dong G, Guo Y, Cao H, et al. Long-term effects of repeated superovulation on ovarian structure and function in rhesus monkeys. Fertil Steril. 2014;102:1452–1457. doi: 10.1016/j.fertnstert.2014.07.739. [DOI] [PubMed] [Google Scholar]
- 69.Weall BM, Al-Samerria S, Conceicao J, et al. A direct action for growth hormone in improving oocyte quality in poor responder patients. Reproduction. 2015;149:147–154. doi: 10.1530/REP-14-0494. [DOI] [PubMed] [Google Scholar]
- 70.Yi X, Maeda N. Endogenous production of lipoic acid is essential for mouse development. Mol Cell Biol. 2005;25:8387–8392. doi: 10.1128/MCB.25.18.8387-8392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato D, Itami N, Tasaki H, et al. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One. 2014;9:e94488. doi: 10.1371/journal.pone.0094488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moawad AR, Xu B, Tan SL, Taketo T. l-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytes. Hum Reprod. 2014;29:2256–2268. doi: 10.1093/humrep/deu201. [DOI] [PubMed] [Google Scholar]
- 75.Pignatti C, Cocchi M, Weiss H. Coenzyme Q10 levels in rat heart of different age. Biochem Exp Biol. 1980;16:39–42. [PubMed] [Google Scholar]
- 76.Bentov Y, Hannam T, Jurisicova A, et al. Coenzyme Q10 supplementation and oocyte aneuploidy in women undergoing IVF-ICSI treatment. Clin Med Insights Reprod Health. 2014;8:31–36. doi: 10.4137/CMRH.S14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barritt J, Willadsen S, Brenner C, Cohen J. Cytoplasmic transfer in assisted reproduction. Hum Reprod Update. 2001;7:428–435. doi: 10.1093/humupd/7.4.428. [DOI] [PubMed] [Google Scholar]
- 78.Cohen J, Scott R, Alikani M, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–280. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- 79.Sharpley MS, Marciniak C, Eckel-Mahan K, et al. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–343. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acton BM, Lai I, Shang X, et al. Neutral mitochondrial heteroplasmy alters physiological function in mice. Biol Reprod. 2007;77:569–576. doi: 10.1095/biolreprod.107.060806. [DOI] [PubMed] [Google Scholar]
- 81.Mitochondrial manipulations. Nat Med. 2014;20:451–452. doi: 10.1038/nm.3570. [DOI] [PubMed] [Google Scholar]
- 82▪▪.Wang T, Sha H, Ji D, et al. Polar body genome transfer for preventing the transmission of inherited mitochondrial diseases. Cell. 2014;157:1591–1604. doi: 10.1016/j.cell.2014.04.042. In this study, authors propose the use of polar body genome transfer to prevent the transmission of inherited mitochondrial diseases. They demonstrate that polar body transfer results in minimal mtDNA carryover in mice and mtDNA genotype remains stable in F2 generation. [DOI] [PubMed] [Google Scholar]
- 83.Woods DC, White YA, Tilly JL. Purification of oogonial stem cells from adult mouse and human ovaries: an assessment of the literature and a view toward the future. Reprod Sci. 2013;20:7–15. doi: 10.1177/1933719112462632. [DOI] [PMC free article] [PubMed] [Google Scholar]