Abstract
Emerging evidence suggests that synaptic dysfunction occurs prior to neuronal loss in neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS). Therefore, monitoring synaptic activity during early stages of neurodegeneration may provide valuable information for the development of diagnostic and/or therapeutic strategies. Here, we describe an electrophysiological method routinely applied in our laboratory for investigating synaptic activity of the neuromuscular junction (NMJ), the synaptic connection between motoneurons and skeletal muscles. Using conventional intracellular sharp electrodes, both spontaneous synaptic activity (miniature end-plate potentials) and evoked synaptic activity (end-plate potentials) can be readily recorded in acutely isolated nerve–muscle preparations. This method can also be adapted to various simulation protocols for studying short-term plasticity of neuromuscular synapses.
Keywords: Amyotrophic lateral sclerosis, Miniature end-plate potential, End-plate potential, Intracellular recording, Neuromuscular junction, Neurodegeneration
1. Introduction
Synaptic dysfunction/loss is among one of the earliest signs of neurodegeneration. Functional impairment of the neuromuscular junction (NMJ) occurs in amyotrophic lateral sclerosis (ALS) patients (1–3), and neuromuscular synapses degenerate prior to the loss of motoneurons (4–10). These observations lead to an emerging view that synaptic dysfunction/loss is likely the primary pathophysiological event, and therefore is an important target for developing diagnostic and/or therapeutic strategies (11–14).
Synaptic dysfunction of the NMJ can be conveniently assayed in acutely isolated nerve–muscle preparations. In this chapter, we describe methods routinely used in our laboratory for evaluating neuromuscular synaptic transmission. To illustrate synaptic dysfunction of the NMJ, we describe electrophysiological analyses of the NMJ in mutant mice deficient in ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) (15). These mutant mice develop progressive age-dependent paralysis reminiscent of ALS.
We apply conventional intracellular recordings to record spontaneous miniature end-plate potentials (mEPPs) and evoked end-plate potentials (EPPs). We use a number of nerve–muscle preparations, including diaphragm muscles, triangularis sterni muscles (16), levator auris longus muscles (17), flexor digitorum brevis (FDB) muscles (18), soleus and extensor digitorum longus (EDL) muscles (19–21). Each muscle preparation has some advantages and disadvantages. For example, the diaphragm muscles are easy to dissect, but they are relatively large, which require a large volume of perfusion and thus large amount of drug, such as Mu-conotoxin. The triangularis sterni muscles are difficult to dissect but they are best suited for visual identification of individual motor end-plates (22). The levator auris longus muscles are particular useful for chronic injection of drugs and toxins, as these muscles are conveniently located just underneath the skin covering the dorsal aspect of head and neck (17). We prefer the lumbrical muscle (23–26) because they are small. The muscles are located in the hindpaw and innervated by a distal branch of the sciatic nerve, the plantar nerve. Their small size is particularly advantageous for morphological analyses, especially at electron microscopy level.
2. Materials
2.1. Solutions and Reagents
All solutions are prepared with purified water (mili-Q, Millipore Co. Bedford MA, USA). The equipments listed below are currently used in our laboratory.
Normal mouse Ringer’s solution, based on Liley (27): 136.8 mM NaCl, 5 mM KCl, 12 mM NaHCO3, 1 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, and 11 mM d-glucose, pH 7.3, stored at 4°C.
Mu-conotoxin GIIIB (Peptides International, Louisville, KY): Working concentration 1–2 mM. Prepare 100 μM stock solution, make aliquots, and store at −20°C (see Note 1).
Intracellular solution for filling recording micropipettes: A mixture of 2 M potassium citrate and 10 mM potassium chloride (see Note 2). This solution is stable and can be stored at room temperature.
Isoflurane (Webster Veterinary Supply Inc., Devens, MA, USA).
2.2. Dissection
Sylgard-lined dishes. The Sylgard 184 Silicone Elastomer kit (Dow Corning Co, Midland, MI, USA) is composed of two parts: Sylgard base and curing agent. Combine in a ratio of ten parts of base to one part of curing agent, by weight. Mix gently by inverting the tubes (avoid air bubbles) and pour into Petri dishes. Use a 100-mm dish (in diameter) for dissection, and a 35-mm dish for recording chamber. Keep Sylgard-lined dishes in a dust-free and flat area overnight at room temperature (see Note 3). More detailed information is described in manufacture instructions (http://www3.dowcorning.com/DataFiles/090007c8801d3f9e.pdf).
Minutien pins: Item No.26002-10 or No.26002-20 (Fine Science Tools, USA).
Fine forceps and scissors.
Stereomicroscope (Stemi 2000, Zeiss).
Oxygenated (95% O2 and 5% CO2) Ringer’s solution.
2.3. Electrophysiology Equipments
Vibration isolation table and Farraday Cage.
Upright fixed stage microscope with water immersion objectives (Olympus BX51WI).
Micromanipulator: Model MP-225 (Sutter Instrument Co., CA, USA).
Stimulator: Grass-Telefactor SD9 (West Warwick, RI, USA).
Amplifier: AxoClamp-2B (Molecular Devices, Sunnyvale, CA, USA).
Computer and data acquisition software: Digidata 1332 (Molecular Devices), pClamp 9.0 (Molecular Devices) and Mini Analysis Program (Synaptosft, Inc., Decatur, GA, USA).
Micropipette puller: Model P-97 or model P-30 (Sutter Instrument Co., CA, USA).
Glass capillaries for recording micropipettes: Cat. No. TW100F-3 (1 mm OD with filament, World Precision Instruments Inc., Sarasota, FL, USA).
Glass capillaries for suction electrode: Cat. No. 628500 (A-M Systems Inc. Carlsborg, WA, USA).
Suction electrode Cat. No. 573000 (A-M Systems Inc. Carlsborg, WA, USA). This product includes tubing.
Audible baseline monitor: ABM (World Precision Instruments Inc. Sarasota, FL, USA).
3. Methods
3.1. Dissection of Plantar Nerve–Lumbrical Muscle
The lumbrical muscles are located between the digits of the hind-paw (see also ref. 24).
Anesthetize the mice with isoflurane and sacrifice the animal by cervical dislocation.
Remove one foot by cutting above the ankle.
Pin the foot in a Sylgard-lined dish with oxygenated Ringer’s solution, ventral side up.
Make a medial incision to the skin from the ankle toward the toes to expose hypodermal surface.
Remove the surface muscles, including the FDB to expose the flexor digitorium longus (FDL) tendon.
Grasp the cut end of the FDL tendon and gently lift it from the proximal to distal (from the ankle to the toes) while carefully cutting the underlying connective tissues. Lumbrical muscles are attached to the tendon.
Isolate plantar nerves attached to the lumbrical muscles.
Cut the distal side of FDL tendons. Now, four lumbrical muscles attached to the FDL tendon can be removed as a whole.
Cut tendon and separate single nerve–muscle preparation. No need to remove the tendon attached to the proximal part of the muscle.
Place the preparation in oxygenated Ringer’s.
3.2. Preparation of Suction Electrodes for Nerve Stimulation
We modify a commercially available suction electrode (from A-M Systems Inc.) by soldering a piece of silver wire (as a reference electrode) to the negative end of the BNC cable.
3.2.1. Preparation of Glass Pipettes for Suction Electrodes
Expose a glass capillary over the flame of a burner or alcohol lamp and pull manually (see Note 4).
Brake off the tip of the glass capillary.
Fire-polish the broken end of the pipette tips using an alcohol lamp (see Note 5).
Under a stereomicroscope, match the size of the pipette tip with the nerve so that a good seal can be achieved.
3.2.2. Assembly of Suction Electrodes
Cut a 30–40 cm length of tubing (see Subheading 2.3, item 10).
Connect one end of the tubing to the side port of the suction electrode holder.
Connect the other end of the tubing to a 3–5 ml syringe.
Connect a BNC connector of the suction electrode to a stimulator (SD9, see Subheading 2.3, item 4).
For a reference electrode, solder a thin silver wire to the outer conductor of the BNC cable, and then run it alongside of the suction electrode down to the length of the glass pipette.
Insert the glass pipette into the electrode holder. Be sure the silver wire of the holder is inside the glass pipette.
3.3. Preparation of Sharp Electrodes for Intracellular Recording
Adjust the heater and weights on the pipette puller till the tips of micropipettes are sharp and small, with resistances around 20–40 MΩ. Use thin-wall glass capillaries with filament to facilitate filling (see Subheading 2.3, item 8).
Fill the micropipette using a fine flexible plastic capillary connected to a 1-ml syringe (see Note 6).
Hold the micropipette vertically (tip down) and gently tap the pipette to remove air bubbles.
Connect the micropipette to a pipette holder of the amplifier (see Note 7).
3.4. Recording Procedures
Place the nerve–muscle preparation in a recording chamber with oxygenated Ringer’s solution. It is advisable to measure the volume of the Ringer’s solution, as the volume is used to determine the amount of Mu-conotoxin being added later (see Note 8).
Draw the nerve into the suction electrode by applying gentle suction. It is critical to obtain a good seal. Test if muscle responds to nerve stimulation by applying a single pulse of stimulation.
Add Mu-conotoxin to a final concentration 1 mM (see Note 9).
Locate the tip of the micropipette under the microscope by adjusting the X-Y-Z position using a micromanipulator. Start with a low-power objective (e.g., 4×, then 10×).
Use a high-power objective (e.g., 40×) to locate an end-plate area of the muscle.
Move the recording micropipette gently toward the end-plate area of the muscle. Use the audio baseline monitor (MBA) as a guide when approaching to the surface of the muscle membrane.
When muscle is impaled, the resting potential (RP) drops to around −70 to −80 mV.
If the micropipette is near or at synaptic sites, spontaneous mEPPs with rise time less than 1.5 ms can be detected.
Record mEPPs (see Note 10). Examples of spontaneous mEPP traces are shown in Fig. 1.
Record nerve-evoked EPPs. Examples of nerve-evoked EPP traces are shown in Fig. 2.
You may apply various stimulation protocols (see Note 11) to obtain a variety of synaptic responses. For example, paired-pulse stimulation (see Fig. 3a, b) or repetitive stimulation (see Fig. 3c) can be applied to examine short-term plasticity of the NMJ.
Fig. 1.
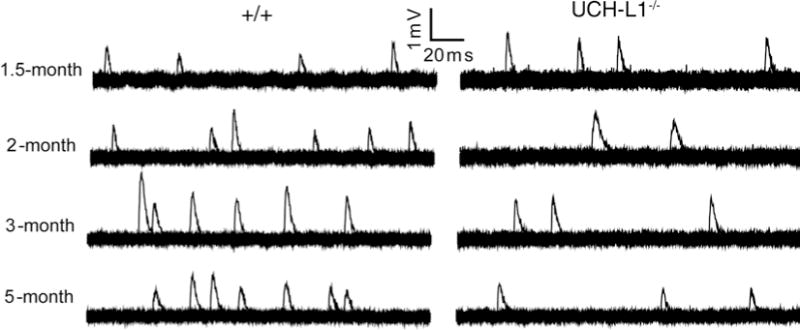
Reduced spontaneous neuromuscular synaptic activity in UCH-L1−/− mice. Sample traces of mEPPs from wild-type (WT, +/+) and UCH-L1−/− mice at 1.5-, 2-, 3-, and 5 months of age: each trace is a set of 20 superimposed 200 ms-sweeps representing a 4-s continuous recording from an individual muscle fiber. MEPP frequencies are reduced in UCH-L1−/− mice after 2 months of age, compared with age-matched WT mice (modified from ref. 15).
Fig. 2.
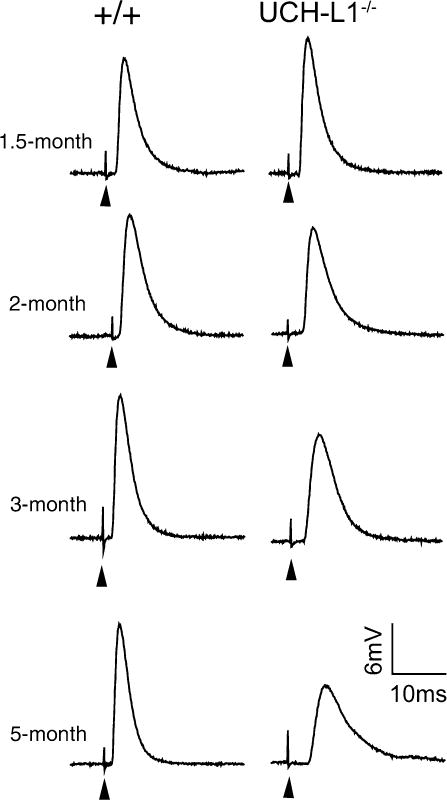
Progressive reduction in evoked neuromuscular synaptic activity in UCH-L1−/− mice. Sample EPP traces from wild-type (WT, +/+) and UCH-L1−/− mice at 1.5-, 2-, 3-, and 5 months of age: EPP amplitudes are markedly decreased in UCH-L1−/− mice at 3- and 5 months of age, compared with age-matched WT mice. Arrowheads point to stimulus artifacts (modified from ref. 15).
Fig. 3.
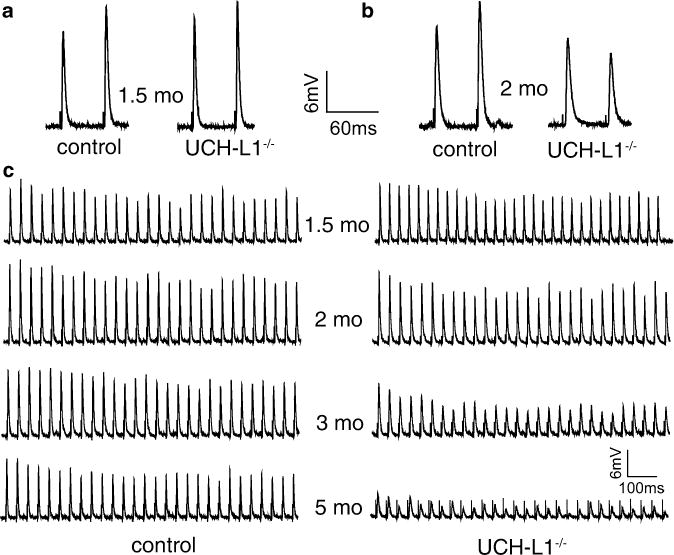
Impaired short-term neuromuscular synaptic plasticity in UCH-L1−/− mice. (a and b): Twin-pulse stimulation induces paired-pulse facilitation (PPF) in WT mice at both 1.5- and 2 months of age. In UCH-L1−/− mice, twin-pulse stimulation induces PPF at 1.5 months (a), but paired-pulse depression 2 months of age (b). (c) Sample EPP traces in WT and UCH-L1−/− mice during repetitive nerve stimulation (30 Hz for 1 s) at 1.5-, 2-, 3-, and 5 months of age. Increased synaptic depression in UCH-L1−/− mice is observed at 2-, 3-, and 5 months of age, compared with age-matched WT mice (modified from ref. 15).
4. Notes
To prevent muscle contraction, we use Mu-conotoxin GIIIB. It binds voltage-gated sodium channels in skeletal muscles and prevents the generation of muscle action potentials thus blocking muscle contraction (28, 29).
We prefer this solution (2 M potassium citrate and 10 mM potassium chloride) over 3 M potassium chloride because the latter often causes drifting of membrane potential, especially in small muscle fibers, such as the lumbrical muscles, due to the leakage of chloride channels (25).
The time required for the polymerization is dependent on the temperature (faster at higher temperature).
Any capillary glass with outer diameters from 1.2 to 1.5 mm fits the electrode.
Fire polishing sometimes makes the tip completely close. Expose to the flame shorter time and observe the tip with a stereoscope.
Fine flexible plastic capillaries are made from yellow pipette tips. Expose a tip to flames and pull. It is also commercially available (MicroFil, World Precision Instruments Inc., Sarasota, FL, USA).
Glass micropipette can be prepared a day before an experiment and kept in a container.
For example, use 2.5 ml Ringer’s: add a 25 ml of 100 mM stock solution to make a final concentration of 1 mM.
In adult mouse muscles, the final concentration 1 mM of Mu-conotoxin is usually effective to prevent muscle contraction. Occasionally, we double the dose to 2 mM if muscle contraction persists after 30 min of incubation.
During the recording, the RP should remain stable.
Various stimulation protocols can be programmed in pClamp.
References
- 1.Stalberg E, Schwartz MS, Trontelj JV. Single fibre electromyography in various processes affecting the anterior horn cell. J Neurol Sci. 1975;24:403–415. doi: 10.1016/0022-510x(75)90166-5. [DOI] [PubMed] [Google Scholar]
- 2.Killian JM, Wilfong AA, Burnett L, Appel SH, Boland D. Decremental motor responses to repetitive nerve stimulation in ALS. Muscle Nerve. 1994;17:747–754. doi: 10.1002/mus.880170708. [DOI] [PubMed] [Google Scholar]
- 3.Similowski T, Attali V, Bensimon G, Salachas F, Mehiri S, Arnulf I, Lacomblez L, Zelter M, Meininger V, Derenne JP. Diaphragmatic dysfunction and dyspnoea in amyotrophic lateral sclerosis. Eur Respir J. 2000;15:332–337. doi: 10.1034/j.1399-3003.2000.15b19.x. [DOI] [PubMed] [Google Scholar]
- 4.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 7.Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 8.Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7:1427–1431. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- 9.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 10.Wong F, Fan L, Wells S, Hartley R, Mackenzie FE, Oyebode O, Brown R, Thomson D, Coleman MP, Blanco G, Ribchester RR. Axonal and neuromuscular synaptic phenotypes in Wld(S), SOD1(G93A) and ostes mutant mice identified by fiber-optic confocal microendoscopy. Mol Cell Neurosci. 2009;42:296–307. doi: 10.1016/j.mcn.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer’s disease. Neurobiol Aging. 2003;24:1023–1027. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Nelson PG. Activity-dependent synapse modulation and the pathogenesis of Alzheimer disease. Curr Alzheimer Res. 2005;2:497–506. doi: 10.2174/156720505774932232. [DOI] [PubMed] [Google Scholar]
- 13.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 14.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Sugiura Y, Myers KG, Liu Y, Lin W. Ubiquitin carboxyl-terminal hydrolase L1 is required for maintaining the structure and function of the neuromuscular junction. Proc Natl Acad Sci USA. 2010;107:1636–1641. doi: 10.1073/pnas.0911516107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArdle JJ, Angaut-Petit D, Mallart A, Bournaud R, Faille L, Brigant JL. Advantages of the triangularis sterni muscle of the mouse for investigations of synaptic phenomena. J Neurosci Methods. 1981;4:109–115. doi: 10.1016/0165-0270(81)90044-3. [DOI] [PubMed] [Google Scholar]
- 17.Angaut-Petit D, Molgo J, Connold AL, Faille L. The levator auris longus muscle of the mouse: a convenient preparation for studies of short- and long-term presynaptic effects of drugs or toxins. Neurosci Lett. 1987;82:83–88. doi: 10.1016/0304-3940(87)90175-3. [DOI] [PubMed] [Google Scholar]
- 18.Carlsen RC, Larson DB, Walsh DA. A fast-twitch oxidative-glycolytic muscle with a robust inward calcium current. Can J Physiol Pharmacol. 1985;63:958–965. doi: 10.1139/y85-158. [DOI] [PubMed] [Google Scholar]
- 19.Lomo T, Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972;221:493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balice-Gordon RJ, Thompson WJ. The organization and development of compartmentalized innervation in rat extensor digitorum longus muscle. J Physiol. 1988;398:211–231. doi: 10.1113/jphysiol.1988.sp017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balice-Gordon RJ, Thompson WJ. Synaptic rearrangements and alterations in motor unit properties in neonatal rat extensor digitorum longus muscle. J Physiol. 1988;398:191–210. doi: 10.1113/jphysiol.1988.sp017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribchester RR. Mammalian neuromuscular junctions: modern tools to monitor synaptic form and function. Curr Opin Pharmacol. 2009;9:297–305. doi: 10.1016/j.coph.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Aickin CC, Betz WJ, Harris GL. Intracellular chloride and the mechanism for its accumulation in rat lumbrical muscle. J Physiol. 1989;411:437–455. doi: 10.1113/jphysiol.1989.sp017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark AW, Bandyopadhyay S, DasGupta BR. The plantar nerveslumbrical muscles: a useful nerve-muscle preparation for assaying the effects of botulinum neurotoxin. J Neurosci Methods. 1987;19:285–295. doi: 10.1016/0165-0270(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 25.Harris GL, Betz WJ. Evidence for active chloride accumulation in normal and denervated rat lumbrical muscle. J Gen Physiol. 1987;90:127–144. doi: 10.1085/jgp.90.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross JJ, Duxson MJ, Harris AJ. Neural determination of muscle fibre numbers in embryonic rat lumbrical muscles. Development. 1987;100:395–409. doi: 10.1242/dev.100.3.395. [DOI] [PubMed] [Google Scholar]
- 27.Liley AW. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956;132:650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- 29.Hong SJ, Chang CC. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol. 1989;97:934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


