Abstract
Importance
Widening socioeconomic disparities in mortality in the United States are largely explained by slower declines in tobacco use between low- and high-socioeconomic status (SES) groups, suggesting the need for targeted tobacco cessation interventions. Documentation of smoking status in electronic health records (EHRs), provides health systems with an opportunity to proactively offer tobacco treatment to disadvantaged smokers.
Objective
To evaluate a proactive strategy to provide tobacco treatment that addresses socio-contextual mediators of tobacco use for low-SES smokers.
Design, Setting, Participants
Prospective, randomized controlled trial for low-SES adult smokers who described their race/ethnicity as black, Hispanic or white and received primary care at one of 13 practices in greater-Boston (intervention n=399, control n=308).
Intervention
We used interactive voice response (IVR)-facilitated outreach to eligible individuals using EHR-coded smoking status. Consenting patients were randomized to a control group which received usual care from their health care team or to an intervention group that included a treatment program of: (1) telephone-based motivational counseling, (2) 6-weeks of free nicotine replacement therapy (NRT), (3) access to community-based referrals to address socio-contextual mediators of tobacco use, and (4) integration of this program with an individual’s care team through the EHR.
Main Outcome
Self-reported past 7-day tobacco abstinence 9-months after randomization (“quitting”), assessed by automated caller or blinded study staff.
Results
The intervention group had a higher quit rate than the usual care group (17.8% vs. 8.1%, odds ratio 2.5, 95% confidence interval 1.5-4.0, number-needed-to-treat=10). We examined whether use of intervention components was associated with quitting among individuals in the intervention group; individuals who participated in the telephone counseling were more likely to quit compared to those who did not (21.2% vs. 10.4%, p=0.0009). There was no difference in quitting by use of NRT. Quitting did not differ by a request for a community referral, but individuals who used their referral were more likely to quit than those who did not (43.6% vs. 15.3%, p<0.0001).
Conclusions and Relevance
Proactive, IVR-facilitated outreach enables engagement with low-SES smokers. Providing counseling, nicotine replacement therapy, and access to community-based resources to address socio-contextual mediators among smokers reached in this setting is effective.
Keywords: tobacco control, surveillance, health disparities
Introduction
Although tobacco use in the United States (US) has declined,1 substantial socioeconomic and racial/ ethnic disparities in smoking prevalence, risk of addiction, and tobacco-related disease remain.2,3 Despite relatively similar rates of tobacco use, blacks suffer from a higher burden of tobacco-related disease, particularly lung cancer, than whites.3 Importantly, low socioeconomic status (SES) and racial/ ethnic minority smokers also have a more difficulty quitting for several reasons, including more limited access to treatment,4-7 misinformation about risks and benefits of nicotine replacement therapy (NRT),8 lack of social support,9 neighborhood disadvantage,10 discrimination,11 and other life stressors.12,13 Widening socioeconomic disparities in mortality in the US are largely explained by slower declines in tobacco use between low- and high-SES groups.14,15
While the majority of smokers visit a primary care provider (PCP) each year, PCPs do not have adequate time or training to provide tobacco treatment;16 therefore it is important to offer systematic opportunities for tobacco treatment beyond the provider’s office. The broad dissemination of electronic health records (EHRs), with coded data about smoking status, offers greater opportunity to outreach to smokers.17,18 Interactive Voice Response (IVR) is a phone technology that allows a computer to detect voice responses during a call, which may provide an efficient way to proactively reach large populations, such as patients identified in the EHR as smokers. IVR scripts can be translated into other languages, facilitating outreach to diverse populations. IVR has been used as part of multi-component smoking cessation programs to provide reminders and facilitate or sustain treatment delivery.19-21 This technology can also be used to engage smokers by providing direct linkage to tobacco treatment specialists and other resources.
Despite growing disparities in tobacco use and tobacco-related disease, few trials have specifically examined smoking cessation interventions in low-SES populations.22 Because of the substantial burden of tobacco in these populations, the objective of this study was to develop and evaluate a proactive approach to tobacco treatment for low-SES smokers that addressed broader socio-contextual mediators of tobacco use. While conceptual models of smoking cessation stress the importance of addressing the broader context of smoking,23 we do not know of other empirical studies that have incorporated referrals to community resources as part of a cessation program. The intervention was designed so that it could be incorporated into health system through IVR outreach.
Methods
Overview
Project CLIQ (Community LInk to Quit) was a prospective, randomized controlled trial (RCT) for low-SES smokers that compared usual care from a patient’s health care team to a “proactive” treatment program that included: (1) a series of telephone-based motivational counselling calls with a tobacco treatment specialist (TTS) based in the health care system, (2) access to free NRT patches, (3) personalized, community based referrals to reduce socio-contextual mediators of tobacco use, and (4) integration of this program with an individual’s health care team through updated documentation in the EHR. The EHR identified low-SES smokers who described their race/ ethnicity as white, black or Hispanic and used IVR for recruitment. The protocol was reviewed and approved by the Institutional Review Board of Partners HealthCare and was registered at Clinicaltrials.gov (NCT01156610).
Setting
Smokers were recruited from 13 primary care practices affiliated with Partners HealthCare, a large health care delivery system in eastern Massachusetts. Participating primary care practices included 6 community health centers, 2 community-based practices, 4 hospital-based practices, and 1 medical home. These sites did not have on-site tobacco treatment programs during the study period. Providers can refer patients to the Massachusetts Smokers’ Helpline (http://makesmokinghistory.org). Practices share the use of a common, web-based, fully functional EHR that allows coded documentation of smoking status; this allows identification of smokers by querying a data repository, therefore providing opportunity for outreach. These tools and services provide the basis for “usual care” for tobacco treatment in these practices.
Eligibility
Eligibility included adults with a visit to a PCP at a participating clinic in the prior month, coded as a smoker in the EHR, with their race/ethnicity documented as white, black, or Hispanic, had English or Spanish language preference noted, and who lived in a “low” (<$45,000) or “moderate” ($45,000 - $67,050) median household income census tract. These income thresholds were chosen to reflect the income characteristics of Massachusetts. In 2008-2012, the median household income in Massachusetts was $66,658.24 These income thresholds also divided our population into thirds; the highest income group was not eligible. We used ArcMap 10.0 (ESRI, Redland, California) to geocode the patient’s mailing address to append median household income estimates based on 2010 census tract as a proxy for socioeconomic status, since income is not captured in the EHR.25 We targeted individuals following a visit to facilitate current documentation of smoking status and contact information. Because of the proactive, population-based design, we reached out to all individuals who met these eligibility criteria regardless of their interest in quitting. We excluded patients without a telephone number.
Recruitment and Randomization
Recruitment for this study occurred between November 7, 2011 and June 3, 2013. Every two weeks the EHR generated a list of individuals who met the eligibility criteria. Within 1 month of a PCP visit, EHR-identified smokers received an informational letter that described the study and included a toll-free phone number to call if they wished to “opt out.” Patients in both study groups received identical mailings. We contacted patients who did not opt-out within 2 weeks using an IVR script that called up to 15 times over a 2-week period on different days of the week and times of the day. Patients could choose to hear the IVR script in English or Spanish. The IVR platform did not leave messages. If all initial call attempts failed to make contact, patients remained eligible for an additional attempt at contact 4 months later. Recruitment was done solely using automated phone scripts.
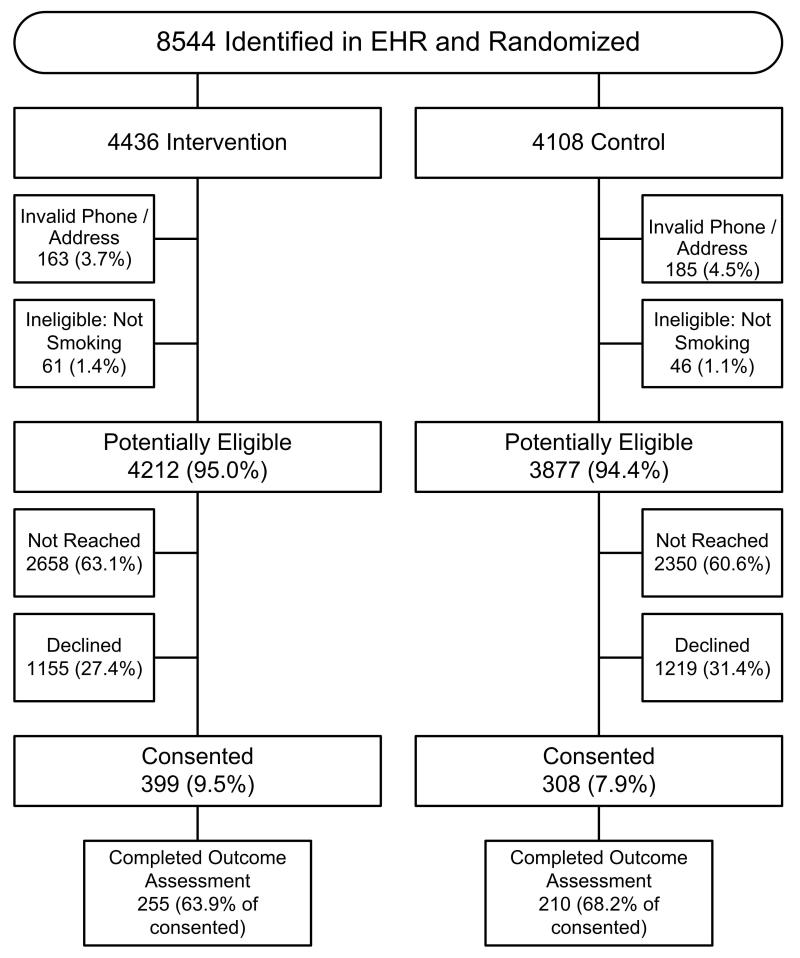
EHR-identified smokers were randomly allocated approximately 1:1 to intervention or control status. Randomization was done in batches based on the date of the clinic visit. The first patient randomized in each batch was randomized to intervention status; batches with an odd number of participants therefore resulted in an imbalance in the size of the intervention and control groups (Figure 1). Randomization occurred before consent for logistical reasons (IVR caller could not do randomization in real time).
Figure 1.
CONSORT Diagram.
FOOTNOTE: Smokers identified from the electronic health record (EHR) were randomly allocated 1:1 to intervention or control status. Random numbers for treatment assignment were pre-calculated in batches and assigned to potential participants sequentially by project staff using an Excel spreadsheet macro. These potential participants were sent an informational letter that described the study and included a toll-free phone number to call if they wished to opt out. Individuals in both study groups received identical mailings and were blinded to allocation status until the end of the baseline interactive voice response (IVR) call (i.e., they were blind to randomization status at the time of consent) and all baseline calls were made by automated calls. We contacted patients who did not opt-out within 2 weeks using the IVR platform. A small number of smokers identified in the EHR were excluded because they had an invalid phone or address and could not be reached (n = 348) or reported that they were not a smoker (n = 107). Potentially eligible participants (n= 8089) were not reached by the automated calls (n = 5008), declined participation (n = 2374), or consented to participate (n= 707). The individuals who consented were the denominator for our analyses.
Baseline Call
After patients who answered the IVR call confirmed their identity, they heard a standard informed consent script. All individuals who agreed to participate then completed a brief smoking history. We excluded patients who reported that they had not smoked even a puff of a cigarette in the prior week or who had not smoked at least 100 cigarettes in his/ her lifetime (i.e., they were considered to be a non-smoker). The baseline IVR call was identical for intervention and control patients until the final question. After completion of the smoking history, patients in the intervention group were asked if they wanted to speak with a TTS for advice about how to quit smoking and to receive a free 6-weeek supply of NRT patches. Patients in the control group were not offered treatment.
Intervention
We designed the intervention based on the Chronic Care Model,26,27 and the Social Contextual Model for Reducing Tobacco Use.23 The IVR system sent an automated email to the TTS when a patient requested contact. Patients in the intervention group were eligible for up to 4 counseling calls (total of approximately 75-100 minutes for all calls, over 8-10 weeks), with additional calls scheduled between the patient and TTS at the request of the patient. The TTS employed motivational interviewing (MI) techniques. MI helps a participant resolve ambivalence about behavior change regardless of readiness to quit.28 The counseling calls included standard content, as well as tailored content for the individual based on intent and confidence to quit, and patients could select optional modules based on their needs (e.g., stress, weight gain, menthol use). The TTS encouraged patients to receive a 6-week supply of free NRT patches (patients who smoked ≥10 cigarettes per day offered 2-weeks of 21 mg/day, 2-weeks of 14 mg/day, and 2-weeks of 7 mg/day patches; patients who smoked <10 cigarettes per day offered 4-weeks of 14 mg/day and 2-weeks of 7 mg/day patches); the TTS instructed patients on use of NRT, and addressed knowledge gaps around safety and efficacy.
To address the socio-contextual mediators of tobacco use, the TTS also encouraged participants to receive a personalized referral from a community resource database, HelpSteps.com. HelpSteps.com is a web-based referral system for the greater-Boston area designed to help users select referrals to over 1700 local health and human service agencies, categorized into 13 social domains (e.g. resources for food, education, employment) and has resources related to over 90 different types of services (e.g. education offerings include literacy classes, domestic violence hotline).29,30 At each call the TTS encouraged participants to pick two domains and then selected referrals near the participant’s address and mailed a personalized referral document to the participant. Costs for these referrals, if any, were determined by the particular agency or service and were paid for by the participants (i.e., not paid for by the study). The majority of these resources are free. Finally the tobacco treatment specialist could coordinate additional treatment needs (e.g., prescription medications) with the patient’s PCP. We tracked use of intervention components (speaking with the TTS, use of NRT, request for and use of a HelpSteps.com referral).
Outcome Assessment
Our primary outcome measure was self-reported 7-day tobacco abstinence at 9-months following randomization.31 We assessed outcomes by IVR call with live follow-up of non-respondents (52.9% of completed outcome calls were done by IVR with the remainder done by blinded study staff). Patients were considered to be quitters if they responded “no” to the question “Have you smoked a cigarette, even a puff, in the past 7 days?” The IVR platform automatically sent updated information about smoking status to the EHR for the provider’s review. We also obtained self-reported information about the use of tobacco treatment during the study period by asking: “Did you use any counseling over the phone or in person to help you to quit smoking?” and “Have you used any nicotine products to help you to quit smoking, like the patch, gum, or inhaler?” Participants in both the intervention and the control group who completed the outcome assessment were eligible for a monthly drawing for one of two $100 gift cards.
Data analysis
The primary analysis used an intent-to-treat approach. We compared participants’ characteristics by group using two-sample t-tests, Wilcoxon tests, and chi-square tests. For the primary analysis, we assumed that non-respondents at follow-up were smokers. We conducted a sensitivity analysis using multiple imputation of both baseline characteristics and the outcome variable using the FCS option in SAS PROC MI.32 We explored the effect of the intervention in subgroups based on sex, race/ ethnicity, SES, and baseline tobacco use. To assess whether there was a differential effect of the intervention for subgroups based on race/ ethnicity and SES, we constructed logistic regression models that included interaction terms between variables representing the subgroups and intervention status.
Results
Recruitment and retention
During the enrollment period, we attempted to contact 8,544 individuals identified by EHR data as smokers (Figure 1). Of these 455 were not eligible for the study (348 did not have valid phone number or address and 107 reported that they were not a smoker when reached). Of the 8,089 potentially eligible adults, 5,008 (61.9%) were never reached by the IVR system (no answer/ answering machine to all of the call attempts) and 2,374 (29.3%) declined participation. Overall, 707 (8.3% of eligible) agreed to participate; 66% of participants completed the outcome assessment call 9-months following enrollment.
Study Population
Overall, the median age of the participants was 50 years, 68% female, 20% self-reported Hispanic ethnicity, 28% black, 36% reported their highest level of educational attainment to be high school or less, and 35% had Medicaid (Table 1). Common comorbidities included hypertension (10%), depression or anxiety (8%), high cholesterol (5%) and diabetes (5%). At enrollment, participants were daily smokers with a median of 15 cigarettes per day, 88% reported smoking within 30 minutes of waking, and 77% said that they planned to quit in the next 30 days. The majority had made a quit attempt within the prior year. There were no significant differences between the enrolled intervention and control smokers in demographic, comorbidity, or baseline tobacco use. Demographic information from the EHR allowed us to compare the characteristics of participants to non-participants. Compared to participants, non-participants were younger (median age 47), less likely female (54.9%, more likely Hispanic (24.7%), and less likely black (17.1%, all p < 0.001).
Table 1.
Characteristics of the Participants
| Intervention | Control | p-value | |
|---|---|---|---|
| N | 399 | 308 | |
| Median age (range) | 49 (19-82) | 51 (21-77) | 0.98 |
| Female | 271 (67.9%) | 211 (68.5%) | 0.87 |
| Self-reported race/ ethnicity* | |||
| Hispanic ethnicity | 85 (21.3%) | 58 (18.8%) | 0.42 |
| White | 245 (61.4%) | 191 (62.0%) | 0.87 |
| Black | 107 (26.8%) | 89 (28.9%) | 0.54 |
| Other | 55 (13.8%) | 36 (11.7%) | 0.41 |
| Born in the United States (US)** | 265 (85.2%) | 214 (85.3%) | 0.99 |
| Education: | |||
| High school or less** | 113 (35.8%) | 89 (35.3%) | 0.91 |
| Health Insurance** | 0.08 | ||
| Medicare | 101 (26.2%) | 80 (26.6%) | |
| Medicaid | 139 (36.1%) | 101 (33.5%) | |
| Private | 135 (35.1%) | 119 (39.5%0 | |
| Self-pay | 10 (2.6%) | 1 (0.3%) | |
| Census Tract Median Household Income: | 0.26 | ||
| Low | 197 (49.4%) | 139 (45.1%) | |
| Moderate | 202 (50.6%) | 169 (54.9%) | |
| Married** | 104 (27.1%) | 78 (26.3%) | 0.81 |
| Comorbidity 1 | |||
| Hypertension | 44 (11.0%) | 30 (9.7%) | 0.58 |
| Depression or anxiety | 36 (9.0%) | 23 (7.5%) | 0.46 |
| High cholesterol | 21 (5.3%) | 14 (4.6%) | 0.66 |
| Diabetes | 23 (5.8%) | 12 (3.9%) | 0.26 |
| Chronic lung disease2 | 16 (4.0%) | 14 (4.6%) | 0.73 |
| Cardiovascular disease | 13 (3.3%) | 10 (3.3%) | 0.99 |
| Non-smoking-related cancer4 | 6 (1.5%) | 4 (1.3%) | 0.82 |
| Smoking-related cancer3 | 1 (0.25%) | 0 | 0.99 |
| Baseline smoking characteristics | |||
| Median number of days per week** | 7 | 7 | 0.35 |
| Median number of cigarettes per day** | 15 | 15 | 0.38 |
| Quit attempt in the last 12 months** | 206 (60.6%) | 171 (64.0%) | 0.38 |
|
Used nicotine replacement therapy (NRT) last
12 months** |
133 (40.8%) | 109 (40.8%) | 0.99 |
| Smoke within 30 minutes of waking** | 299 (89.3%) | 228 (86.4%) | 0.28 |
| Allows smoking in car or home** | 219 (65.8%) | 166 (63.1%) | 0.50 |
| Lives with someone who smokes** | 117 (35.5%) | 95 (36.7%) | 0.76 |
| Plan to quit in next 30 days** | 265 (78.6%) | 201 (74.7%) | 0.26 |
EHR race reported where self-reported race missing.
Missing: Education 139; Born in the US 145; Married 26; Health insurance 21; Median number of days per week 56; median number cigarettes per day 96; Quit attempt in the last 12 months 100; Used NRT last 12 months 114; Smoke within 30 minutes of waking 108; Allows smoking in car or home 111; Lives with someone who smokes 118; Plan to quit in the next 30 days 101.
Medical problems were obtained from the electronic health record problem list.
Chronic lung disease included: chronic obstructive pulmonary disease, chronic bronchitis, asthma and reactive airways disease.
Smoking-related cancers included: lung, throat, esophagus, head, neck, larynx, kidney and bladder.
Non-smoking related cancers included all other cancers excluding skin cancers.
Effect of the Intervention on Quit Rates and Use of Tobacco Treatment
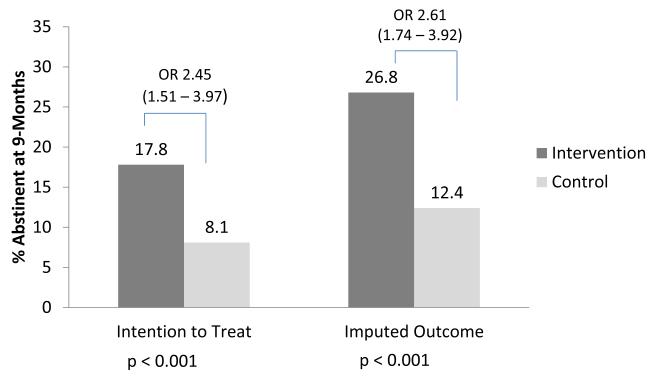
Individuals in the intervention group were significantly more likely to report quitting at the time of outcome assessment than individuals in the control group (Figure 2). In the primary intention-to-treat analysis 17.8% of intervention patients quit compared with 8.1% in the control group (p<0.001, odds ratio 2.5, 95% confidence interval 1.5–4.0). The number-needed-to-treat (NNT) was 10. The sensitivity analysis, using multiple imputation, confirmed these findings with quit rates of 26.8% and 12.4% respectively (p < 0.001, odds ratio 2.6, 95% confidence interval 1.7 – 3.9; NNT 7). Intervention patients were more likely than control patients to respond affirmatively that they had used any counseling for tobacco treatment during the follow-up period (49.6% vs. 8.4%, p < 0.0001) or had used NRT (63.6% vs. 41.8%, p < 0.0001).
Figure 2.
Self-reported 7-day Tobacco Abstinence 9-months Following Randomization. Primary outcome was intention-to-treat. Sensitivity analysis used multiple imputation of both baseline characteristics and the outcome variable.
OR = odds ratio with (95% confidence interval in parens).
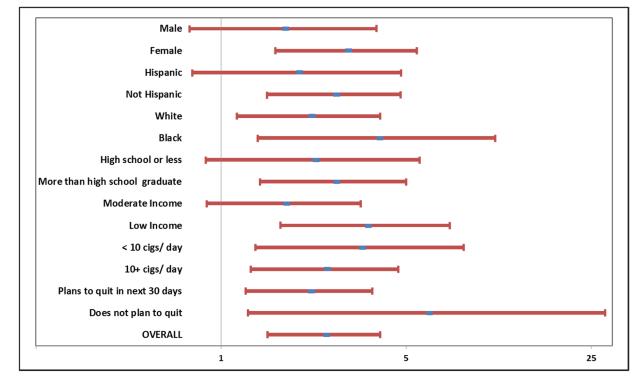
Quit Rates for Sub-groups
We examined the odds of quitting for intervention vs. control patients for sub-groups specified by demographic characteristics and baseline tobacco use (Figure 3). Women, blacks and whites, individuals with more than a high school education and those who lived in a low income census tract were all significantly more likely to quit in the intervention vs. control group. The odds of quitting for Hispanics was not significant. The intervention was effective in all baseline tobacco use subgroups including those who planned/ did not plan to quit in the next 30 days. There were no significant interactions between intervention status and any of the demographic or baseline smoking characteristics.
Figure 3.
Quit Rates by Sub-groups
Odds ratio and 95% confidence intervals are displayed for selected sub-groups defined by sex, race/ ethnicity, level of education, income (low or moderate median household income of the census tract as defined in text), baseline use of cigarettes (less than 10 cigarettes per day vs. 10 or more), and intention to quit during the next 30 days. There were no significant interactions between subgroups and intervention status.
Effect of Intervention Components
Among individuals in the intervention group, we examined use of each intervention component (i.e., speaking to the TTS, receiving NRT, request or use of a community referral) and whether use of a specific component of the intervention was associated with quitting (Table 2). Of those in the intervention arm (n = 399), 274 (68.7%) spoke with the TTS at least once; of these 274 individuals, 79.6% received NRT, 46.7% requested a Healthsteps.com referral, and 20.1% reported using this referral. The domains most commonly requested for community resources included physical activity (n = 379), educational opportunities (n = 235), and job counseling (n = 69).
Table 2.
Use of the intervention components among individuals in the intervention group and quitting by component (n = 399)
|
Quitting Among Individuals in the
Intervention Group Who: |
|||
|---|---|---|---|
|
Used intervention
component |
Did not use intervention
component |
p-value | |
| N (%) | N (%) | ||
| Spoke to the tobacco treatment specialist (TTS): |
58/ 274 (21.2%) | 13/ 125 (10.4%) | .009 |
| Received nicotine replacement patches* | 50/ 218 (22.9%) | 8/ 56 (14.3%) | 0.158 |
| Received a HelpSteps.com referral* | 30/ 128 (23.4%) | 28/ 146 (19.2%) | 0.389 |
| Reported using a HelpSteps.com referral** | 24/ 55 (43.6%) | 34/ 219 (15.3%) | <.0001 |
Among intervention subjects who spoke to the tobacco treatment specialist.
Among intervention subjects who spoke to the tobacco treatment specialist and received a HelpSteps.com referral.
Among individuals in the intervention arm, individuals who spoke to the TTS were more likely to quit compared to those who did not (21.2% vs. 10.4%, p=0.009). There was no difference in quitting by use of NRT. Quitting status did not differ by a request for a community referral, but individuals who reported using their referral for the community resource were much more likely to quit than those who did not (43.6% vs. 15.3%, p< 0.0001). In a multivariate model that included the intervention components, the only intervention component associated with quitting was use of a community referral (odds ratio 5.43; 95% confidence interval 2.45 – 12.04).
Discussion
Project CLIQ, one of the first RCTs that specifically targets low-SES smokers, demonstrates that a proactive, systematic, phone-based intervention including counselling, NRT, and referrals to community resources to address socio-contextual mediators of tobacco use, doubles smoking cessation rates compared to “usual care” for this population with access to primary care. Among participants, use of counselling and the community referrals were important components of this intervention; referral to community resources was one of the novel aspects of this treatment program.
We designed our intervention to take advantage of effective strategies for smoking cessation and address individual socio-contextual mediators that promote tobacco use. First, we recruited individuals from primary care. While 70% of smokers see a primary care provider each year, minority and low-SES smokers are less likely to report tobacco counselling.4,5 The use of a common EHR allowed us to identify smokers outside of the context of a visit, independent of their interest in quitting. Second, the intervention used automated calls to provide a systematic, proactive outreach to link interested smokers to treatment. Third, the phone-based program allowed for the delivery of established effective treatments including personalized counselling and subsidized NRT.33 Finally, our intervention was designed to consider the socio-contextual challenges to tobacco cessation faced by minority and low-SES smokers.34 Among participants, community resources for physical activity, educational opportunities, and job counselling were the most common referrals requested and use of these referrals was associated with quitting among participants suggesting that attempts to address the broader lived experience of smokers was an important component of our intervention. Our findings suggest that the use of these community resources was one of the important mediators of the effectiveness of our program. For the 8,544 registry identified smokers in our clinics, the design and implementation of this intervention was $283,023 ($33.13 per registry-identified smoker). The rates of tobacco abstinence were similar to other proactive treatment programs not specifically targeting low SES smokers,35 and higher than visit-based physician advice for smoking cessation.36 The Veterans Victory over Tobacco Study (VVTS), a pragmatic RCT, used a registry of current smokers at four VA medical centers to test a proactive outreach with either phone or in-person cessation services compared to usual care.37 At 12-months following randomization, the 6-month abstinence rate was 13.5% in the outreach group vs. 10.9% in the usual care group. The VVTS had a similar proportion of blacks to Project CLIQ, but few Hispanics and was largely men. Project CLIQ extends the result of a previous study in which smokers from one health center were proactively contacted by mail to offer telephone counseling and free NRT; that study found increased self-reported quit rates at 3 month follow-up compared to usual care (5.3% vs. 1.1%).38
EHRs allow for identification and management of a population of smokers in a health system.17,39 Coupled with IVR or other technology for systematic outreach (e.g., texting), this infrastructure allows for the possibility of large scale, linkage to care teams for tobacco treatment.40 Systematic intervention facilitated by EHRs may be particularly important for low-SES smokers who experience substantial barriers to treatment.19,41 While only 8.3% of the potentially eligible population in Project CLIQ responded to the proactive outreach, this is not surprising as only a minority of smokers may be interested in hearing about cessation at any given time and the difficulties of reaching people by phone (screening of calls, intermittent service particularly among low-SES groups). Our participation rate is higher than that of quitlines, which get at best 1-2% reach into populations.42 Population-based approaches may reach a relatively small proportion of smokers, but settings with a large number of smokers will still realize a large number of quits. Future implementation of this type of program could consider an ongoing schedule of outreach, informed by qualitative work to understand how to better engage men, younger participants and Hispanics, the groups less likely to participate in this study. This model could be generalized to other health systems with an EHR, which are increasingly promoted to improve the safety and quality of health care.
This study has several limitations. This was a pragmatic trial, conducted within a primary care network. This is also a strength, as our findings speak more directly to clinical effectiveness. Our randomization protocol led to an imbalance of the group sizes; despite this limitation the groups were well balanced on measured characteristics. Our outcome was measured by self-report and was not biochemically verified, an approach similar to other population-based interventions.31 Project CLIQ was designed to be generalizable to clinical care where providers use self-reported smoking status to guide treatment and risk assessment. While our outcome survey completion rate was good (66%), there is the potential for non-response bias. Because we had a higher outcome assessment rate in the control group the assumption that non-respondents were smokers would bias against finding an effect. Our sensitivity analysis also suggests that our findings are robust. Our intervention was designed to address barriers to cessation experienced by low-SES patients but we needed to use a census tract based proxy for income. EHRs should include measures of individual SES to enable interventions to reduce health disparities. Widening socioeconomic disparities in tobacco use and mortality support the importance of the broad collection of individual socioeconomic measures in EHRs to support interventions designed to promote health equity.14
Project CLIQ demonstrates that proactive, systematic, phone-based treatment to provide counselling, pharmacotherapy, and access to community-based resources to address the social context of smoking can promote tobacco cessation in disadvantaged populations. Interventions to reduce tobacco use for these populations may reduce disparities in preventable deaths in the US, an important public health goal.
Acknowledgements
This work was conducted with support from The Lung Cancer Disparities Center at the Harvard School of Public Health (National Cancer Institute Award # P50 CA148596) and the Harvard Catalyst ∣ The Harvard Clinical and Translational Science Center (NIH Grant #1 UL1 RR 025758-01 and financial contributions from participating institutions). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers or the NIH. The funders did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and did not approve the manuscript; or participate in the decision to submit the manuscript for publication. Dr. Haas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ms. Brawarsky conducted the data analysis.
Footnotes
The authors report the following conflict of interest disclosures:
Dr. Haas: Dr. Haas reports grants from National Institutes of Health (NIH) during the conduct of the study.
Dr. Rigotti reports grants from NIH during the conduct of the study; royalties from UpToDate, and serving as an unpaid consultant to Pfizer, outside the submitted work.
Dr. Fleegler reports grants that funded the initial development of HealthSteps.com from the Home for Little Wanderers Service Grant, the Aerosmith Endowment Fund for Prevention and Treatment of AIDS and HIV Infections, the Verizon Foundation, Thrive in 5/Boston Mayor’s Office Ready Families program, the Highland Street Foundation, and the Boston Foundation. Dr. Fleegler receives ongoing support from Boston Children’s Hospital and the Boston Public Health Commission, during the conduct of the study. HelpSteps.com is freely available on the web for general use. There are no intended patents.
Ms. Klinger reports grants from NIH, during the conduct of the study.
Ms. St. Hubert reports grants from NIH, during the conduct of the study.
Dr. Linder, Park, Gonzalez, Kontos, Zaslavsky, Williams and Ms. Brawarsky and Mr. Marinacci have nothing to disclose.
References
- 1.Schroeder SA, Koh HK. Tobacco control 50 years after the 1964 surgeon general’s report. JAMA : the journal of the American Medical Association. 2014;311:141–3. doi: 10.1001/jama.2013.285243. [DOI] [PubMed] [Google Scholar]
- 2.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults - United States, 2005-2012. MMWR Morbidity and mortality weekly report. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 3.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, Le Marchand L. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 4.Browning KK, Ferketich AK, Salsberry PJ, Wewers ME. Socioeconomic disparity in provider-delivered assistance to quit smoking. Nicotine Tob Res. 2008;10:55–61. doi: 10.1080/14622200701704905. [DOI] [PubMed] [Google Scholar]
- 5.Cokkinides VE, Halpern MT, Barbeau EM, Ward E, Thun MJ. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34:404–12. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Jamal A, Dube SR, Malarcher AM, Shaw L, Engstrom MC. Tobacco use screening and counseling during physician office visits among adults--National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005-2009. MMWR - Morbidity & Mortality Weekly Report. 2012;61(Suppl):38–45. [PubMed] [Google Scholar]
- 7.Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. American journal of public health. 2011;101:699–706. doi: 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen B, Reeder K, Hill M, Baker TB, Fiore MC. Barriers to effective tobacco-dependence treatment for the very poor. Journal of studies on alcohol and drugs. 2012;73:874–84. doi: 10.15288/jsad.2012.73.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slopen N, Dutra LM, Williams DR, Mujahid MS, Lewis TT, Bennett GG, Ryff CD, Albert MA. Psychosocial stressors and cigarette smoking among African American adults in midlife. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2012;14:1161–9. doi: 10.1093/ntr/nts011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, Mazas CA, Vidrine JI, Cinciripini PM, Greisinger AJ, Wetter DW. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2010;29:262–73. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendzor DE, Businelle MS, Reitzel LR, Rios DM, Scheuermann TS, Pulvers K, Ahluwalia JS. Everyday Discrimination is Associated With Nicotine Dependence in African American, Latino, and White Smokers. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2013 doi: 10.1093/ntr/ntt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffer CEP, Stitzer MP, Landes RP, Brackman SLMPH, Munn TBA, Moore PP. Socioeconomic Disparities in Community-Based Treatment of Tobacco Dependence. American Journal of Public Health. 2012;102:e8–e16. doi: 10.2105/AJPH.2011.300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Cofta-Woerpel LM, Li Y, Mazas CA, Vidrine JI, Cinciripini PM, Greisinger AJ, Wetter DW. Financial strain and smoking cessation among racially/ethnically diverse smokers. American journal of public health. 2010;100:702–6. doi: 10.2105/AJPH.2009.172676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meara ER, Richards S, Cutler DM. The gap gets bigger: changes in mortality and life expectancy, by education, 1981-2000. Health Aff (Millwood) 2008;27:350–60. doi: 10.1377/hlthaff.27.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A Report of the Surgeon General. U.S. Department of Health and Human Services; Atlanta, GA: 2014. The Health Consequences of Smoking—50 Years of Progress. [Google Scholar]
- 16.Thorndike AN, Regan S, Rigotti NA. The treatment of smoking by US physicians during ambulatory visits: 1994-2003. Am J Public Health. 2007;97:1878–83. doi: 10.2105/AJPH.2006.092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle RG, Solberg LI, Fiore MC. Electronic medical records to increase the clinical treatment of tobacco dependence: a systematic review. American journal of preventive medicine. 2010;39:S77–82. doi: 10.1016/j.amepre.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Linder JA, Rigotti NA, Brawarsky P, Kontos EZ, Park ER, Klinger EV, Marinacci L, Li W, Haas JS. Use of practice-based research network data to measure neighborhood smoking prevalence. Preventing chronic disease. 2013;10:E84. doi: 10.5888/pcd10.120132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlini BH, McDaniel AM, Weaver MT, Kauffman RM, Cerutti B, Stratton RM, Zbikowski SM. Reaching out, inviting back: using Interactive voice response (IVR) technology to recycle relapsed smokers back to Quitline treatment--a randomized controlled trial. BMC public health. 2012;12:507. doi: 10.1186/1471-2458-12-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan S, Reyen M, Lockhart AC, Richards AE, Rigotti NA. An interactive voice response system to continue a hospital-based smoking cessation intervention after discharge. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2011;13:255–60. doi: 10.1093/ntr/ntq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient education and counseling. 2007;66:319–26. doi: 10.1016/j.pec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Cox LS, Okuyemi K, Choi WS, Ahluwalia JS. A review of tobacco use treatments in U.S. ethnic minority populations. American journal of health promotion : AJHP. 2011;25:S11–30. doi: 10.4278/ajhp.100610-LIT-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen G, Barbeau E, Hunt MK, Emmons K. Reducing social disparities in tobacco use: a social-contextual model for reducing tobacco use among blue-collar workers. Am J Public Health. 2004;94:230–9. doi: 10.2105/ajph.94.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.State & County QuickFacts . Massachusetts: [Accessed May 19, 2014]. 2014. at http://quickfacts.census.gov/qfd/states/25000.html. [Google Scholar]
- 25.Krieger N, Gordon D. Re: “Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples”. Am J Epidemiol. 1999;150:892–6. doi: 10.1093/oxfordjournals.aje.a010095. [DOI] [PubMed] [Google Scholar]
- 26.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4:12–25. [PubMed] [Google Scholar]
- 27.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–44. [PubMed] [Google Scholar]
- 28.Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147–55. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 29.Fleegler EW, Lieu TA, Wise PH, Muret-Wagstaff S. Families’ health-related social problems and missed referral opportunities. Pediatrics. 2007;119:e1332–41. doi: 10.1542/peds.2006-1505. [DOI] [PubMed] [Google Scholar]
- 30.Hassan A, Blood EA, Pikcilingis A, Krull EG, McNickles L, Marmon G, Wylie S, Woods ER, Fleegler EW. Youths’ health-related social problems: concerns often overlooked during the medical visit. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53:265–71. doi: 10.1016/j.jadohealth.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Biochemical verification of tobacco use and cessation. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 32.Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction (Abingdon, England) 2007;102:1564–73. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 33.Fiore MC, Jaen CR, Baker TB. Treating Tobacco Use and Dependence: 2008 Update. US Department of Health and Human Services. Public Health Service; Rockville, MD: 2008. al. e. [Google Scholar]
- 34.Webb MS, Carey MP. Tobacco smoking among low-income Black women: demographic and psychosocial correlates in a community sample. Nicotine Tob Res. 2008;10:219–29. doi: 10.1080/14622200701767845. [DOI] [PubMed] [Google Scholar]
- 35.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. The Cochrane database of systematic reviews. 2013;8:CD002850. doi: 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. The Cochrane database of systematic reviews. 2013;5:CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu SS, van Ryn M, Sherman SE, Burgess DJ, Noorbaloochi S, Clothier B, Taylor BC, Schlede CM, Burke RS, Joseph AM. Proactive Tobacco Treatment and Population-Level Cessation: A Pragmatic Randomized Clinical Trial. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.177. [DOI] [PubMed] [Google Scholar]
- 38.Rigotti NA, Bitton A, Kelley JK, Hoeppner BB, Levy DE, Mort E. Offering population-based tobacco treatment in a healthcare setting: a randomized controlled trial. American journal of preventive medicine. 2011;41:498–503. doi: 10.1016/j.amepre.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser D, Christiansen BA, Adsit R, Baker TB, Fiore MC. Electronic health records as a tool for recruitment of participants’ clinical effectiveness research: lessons learned from tobacco cessation. Transl Behav Med. 2013;3:244–52. doi: 10.1007/s13142-012-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitton A, Flier LA, Jha AK. Health information technology in the era of care delivery reform: to what end? JAMA : the journal of the American Medical Association. 2012;307:2593–4. doi: 10.1001/jama.2012.6663. [DOI] [PubMed] [Google Scholar]
- 41.Carlini BH, Zbikowski SM, Javitz HS, Deprey TM, Cummins SE, Zhu SH. Telephone-based tobacco-cessation treatment: re-enrollment among diverse groups. American journal of preventive medicine. 2008;35:73–6. doi: 10.1016/j.amepre.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barry MB, Saul J, Bailey LA. U.S. quitlines at a crossroads: Utilization, budget, and service trends 2005–2010. Phoenix, AZ: 2010. [Google Scholar]