Abstract
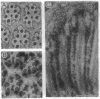
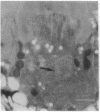
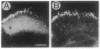
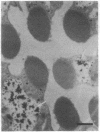
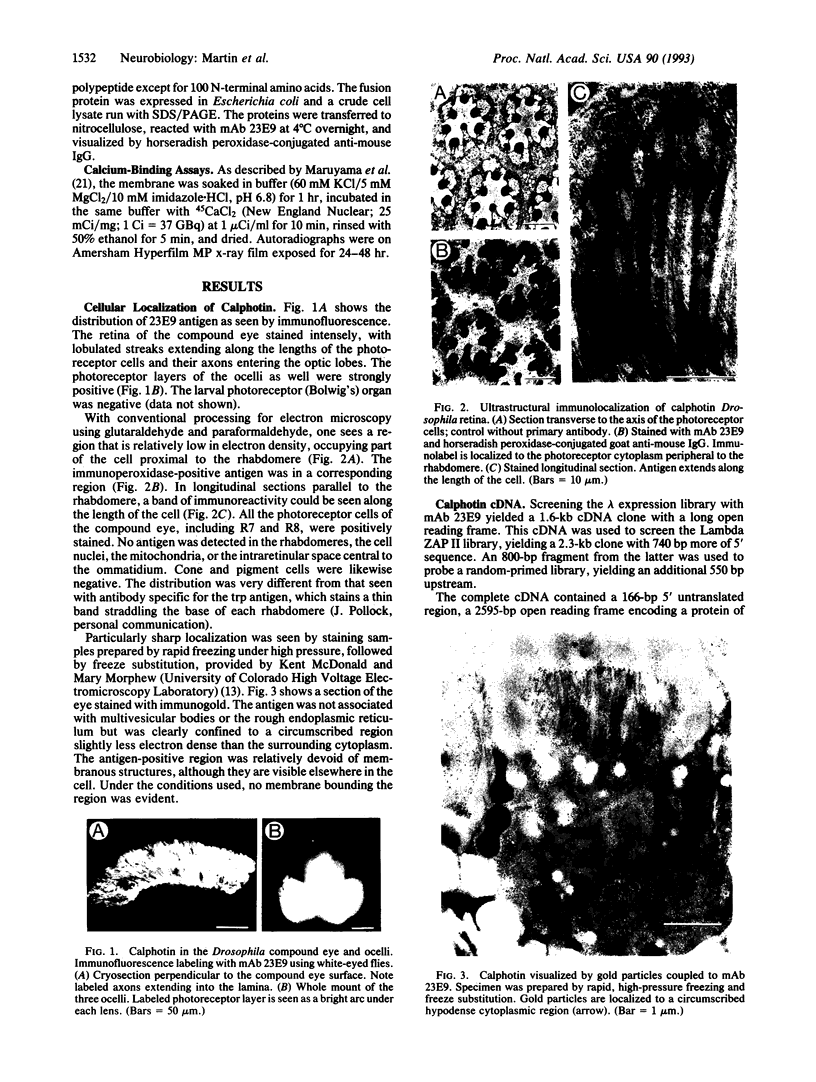
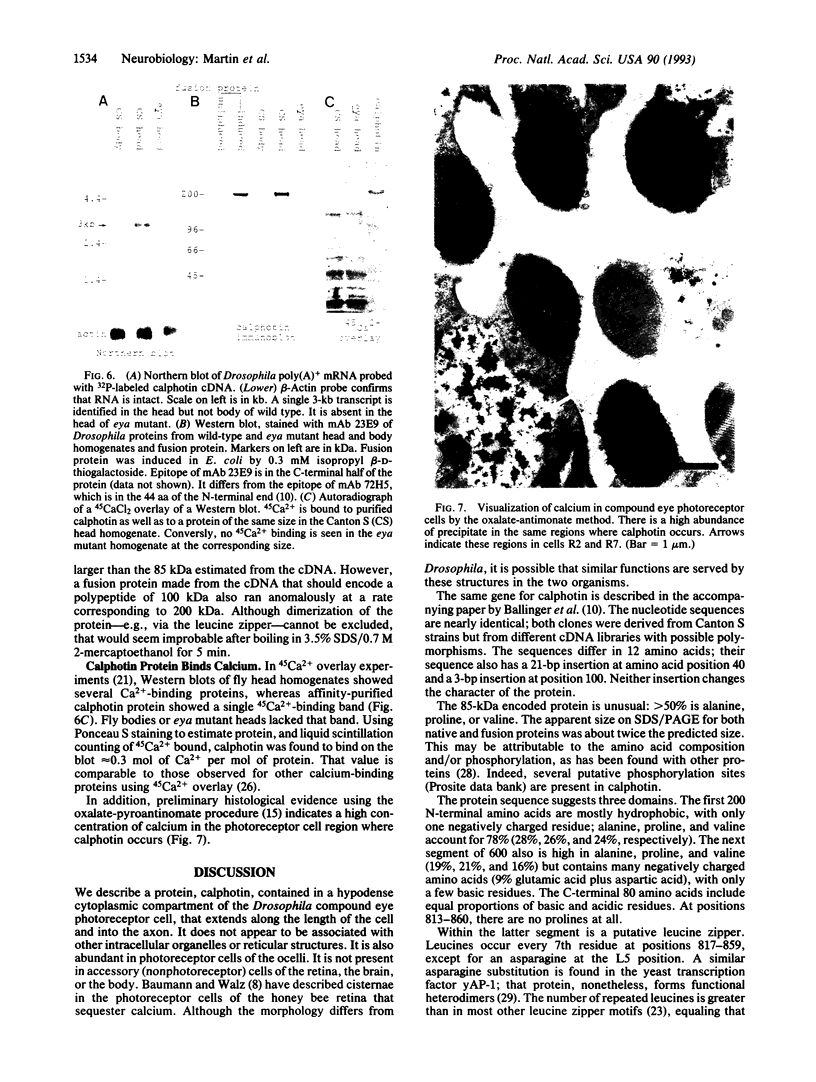
Monoclonal antibody 23E9 identifies a calcium-binding protein, calphotin, in photoreceptor cells of the Drosophila melanogaster compound eyes and ocelli. The antigen is restricted to a defined cytoplasmic region; it is not present in the rhabdomeres, nuclei, mitochondria, or rough endoplasmic reticulum. A corresponding cDNA recognizes a 3-kb mRNA with retinal specificity similar to the antigen and maps to band 86E/F-87A/B on chromosome 3. An open reading frame of 2595 bp encodes an estimated 85-kDa protein of unusual amino acid composition, with > 50% proline, alanine, and valine and very few basic residues. The C-terminal segment contains a leucine zipper motif uninterrupted by prolines. We found no significant similarities with the GenBank or National Biomedical Resource Foundation data bases. The location of the protein within a distinct cytoplasmic region suggests that it might function as a calcium-sequestering "sponge" to regulate the amount of free cytoplasmic calcium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baimbridge K. G., Celio M. R., Rogers J. H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992 Aug;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Benzer S. Targeted gene mutations in Drosophila. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9402–9406. doi: 10.1073/pnas.86.23.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
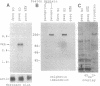
- Ballinger D. G., Xue N., Harshman K. D. A Drosophila photoreceptor cell-specific protein, calphotin, binds calcium and contains a leucine zipper. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1536–1540. doi: 10.1073/pnas.90.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Salivary proline-rich proteins. Mol Cell Biochem. 1982 Jun 11;45(2):83–99. doi: 10.1007/BF00223503. [DOI] [PubMed] [Google Scholar]
- Busch S. J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990 Feb;6(2):36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L., Burns K., MacLennan D. H., Reithmeier R. A., Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989 Dec 25;264(36):21522–21528. [PubMed] [Google Scholar]
- Fliegel L., Ohnishi M., Carpenter M. R., Khanna V. K., Reithmeier R. A., MacLennan D. H. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S. C., Zipursky S. L., Benzer S., Ferrús A., Shotwell S. L. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7929–7933. doi: 10.1073/pnas.79.24.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigos M., Deschamps S., Viel A., Lund S., Champeil P., Møller J. V., le Maire M. Detection of Ca(2+)-binding proteins by electrophoretic migration in the presence of Ca2+ combined with 45Ca2+ overlay of protein blots. Anal Biochem. 1991 Apr;194(1):82–88. doi: 10.1016/0003-2697(91)90154-l. [DOI] [PubMed] [Google Scholar]
- Gmachl M., Berger H., Thalhammer J., Kreil G. Dermal glands of Xenopus laevis contain a polypeptide with a highly repetitive amino acid sequence. FEBS Lett. 1990 Jan 15;260(1):145–148. doi: 10.1016/0014-5793(90)80088-z. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Perin M. S., Reynolds G. A., Wasserman S. A., Südhof T. C. Two novel annexins from Drosophila melanogaster. Cloning, characterization, and differential expression in development. J Biol Chem. 1990 Jul 5;265(19):11382–11388. [PubMed] [Google Scholar]
- Kaufmann E., Geisler N., Weber K. SDS-PAGE strongly overestimates the molecular masses of the neurofilament proteins. FEBS Lett. 1984 May 7;170(1):81–84. doi: 10.1016/0014-5793(84)81373-3. [DOI] [PubMed] [Google Scholar]
- Kelly L. E. Purification and properties of a 23 kDa Ca2(+)-binding protein from Drosophila melanogaster. Biochem J. 1990 Nov 1;271(3):661–666. doi: 10.1042/bj2710661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Mitchell R. D., Simmerman H. K., Jones L. R. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988 Jan 25;263(3):1376–1381. [PubMed] [Google Scholar]
- Montell C., Rubin G. M. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989 Apr;2(4):1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Moye-Rowley W. S., Harshman K. D., Parker C. S. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989 Mar;3(3):283–292. doi: 10.1101/gad.3.3.283. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peute J., van Linder A. T., Zandbergen M. A., de Bruijn W. C. Ultrastructural localization of calcium and Ca(2+)-ATPase activity in gonadotropes and stellate cells of the catfish pituitary. Histochemistry. 1990;94(6):601–607. doi: 10.1007/BF00271987. [DOI] [PubMed] [Google Scholar]
- Ranganathan R., Harris W. A., Zuker C. S. The molecular genetics of invertebrate phototransduction. Trends Neurosci. 1991 Nov;14(11):486–493. doi: 10.1016/0166-2236(91)90060-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinós J., Maroto M., Garesse R., Marco R., Cervera M. Drosophila melanogaster paramyosin: developmental pattern, mapping and properties deduced from its complete coding sequence. Mol Gen Genet. 1992 Feb;231(3):385–394. doi: 10.1007/BF00292707. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz B. Calcium-sequestering smooth endoplasmic reticulum in retinula cells of the blowfly. J Ultrastruct Res. 1982 Nov;81(2):240–248. doi: 10.1016/s0022-5320(82)90079-x. [DOI] [PubMed] [Google Scholar]