Abstract
Compared with autosomes, the X chromosome shows different patterns of evolution as a result of its hemizygosity in males. Additionally, inactivation of the X during spermatogenesis can make the X chromosome an unfavorable location for male-specific genes. These factors can help to explain why in many species gene content of the X chromosome differs from that of autosomes. Indeed, the X chromosome in mouse is enriched for male-specific genes while they are depleted on the X in Drosophila but show neither of these trends in mosquito. Here, we will discuss recent findings on the ancestral and neo-X chromosomes in Drosophila that support sexual antagonism as a force shaping gene content evolution of sex chromosomes and suggest that selection could be driving male-biased genes off the X.
Introduction
In many animal species males and females differ genetically by only one chromosome. There are several chromosomal sex-determination systems found in nature, with XY chromosomes being the most familiar one. The XY sex-determination system is present in most mammals, including humans, and in Drosophila. Here, males are the heterogametic sex, XY, and females are homogametic, XX. In the ZW sex-determination system, which is found in birds, butterflies, and many reptiles, females are the heterogametic sex, ZW, while males are homogametic, ZZ.
Sex chromosomes originated from ordinary autosomes, and their evolutionary differentiation is driven by the progressive gene loss on the chromosome that is present only in the heterogametic sex (Y/W chromosome). The difference in gene content evolution between the degrading Y/W chromosome and the autosomes is immediately apparent and has been studied extensively [1]. By contrast, X/Z chromosomes have traditionally been viewed as similar to autosomes, with little change occurring that would distinguish the X from the autosome it was derived from. However, multiple recent studies have found that the gene content of X/Z chromosomes can be quite different from that of autosomes (reviewed in reference [2]).
What might be driving unusual patterns of gene content evolution on the X? The fact that one sex possesses two copies of the X/Z chromosome while the other has only one copy can result in different selective pressures in males and females, which could be particularly apparent in the distribution of genes with different fitness consequences in the two sexes. Indeed, X chromosome gene content is not stable across animal taxa and can show opposite tendencies in different species (reviewed in references [2–4]). For example, genes that benefit males are depleted from the X chromosome of Drosophila [5], while in mammals such genes are overrepresented on the X [6]. Understanding these differences between taxonomic groups and identifying the underlying selective forces that shape the gene content of the X chromosome can not only shed light on how and why differences between genders are achieved, but also leads to a better understanding of general features driving genome evolution.
Several theories can explain different trends in gene content evolution of the X chromosome, and empirical progress has been made to uncover the factors that affect the genetic makeup of the X. In this review, we will briefly describe these theories and discuss the distribution of sex-biased genes on the X chromosome of several well-studied species, with a particular focus on recent findings in the genus Drosophila.
Sexual antagonism, hemizygosity, sex-biased transmission, and X inactivation
Not all mutations will have the same selective effects in both sexes; instead, some alleles may benefit only one sex and either have no fitness consequences in the other sex (sex-specific mutations), or actually be deleterious to the other sex (sexually antagonistic mutations). Theory predicts that X chromosomes might be a hot spot for sexually antagonistic fitness variation since the X shows female-biased transmission and is hemizygous in males [2,7]. Hemizygosity of the X allows recessive or partially recessive mutations to fix more efficiently on the X chromosome relative to autosomes. Since the X is hemizygous in males, this effect is particularly pronounced for mutations that are beneficial to males [7,8]. Thus, the occurrence of a significant number of recessive male-beneficial mutations in the genome can lead to an accumulation of male-specific genes on the X chromosomes (i.e. masculinization of the X chromosome). On the contrary, the X chromosome is transmitted 2/3 of the time through females and only 1/3 of the time through males. As a consequence, selection acts more often on mutations that are beneficial for females, which could result in the accumulation of female-beneficial mutations on the X chromosome (i.e. feminization of the X chromosome). Thus, depending on the nature of the underlying genetic variation, the X chromosome could be either a preferred or unpreferred location for male-beneficial and female-beneficial genes [7].
Differential expression of genes present in both sexes (sex-biased genes) is the main cause of phenotypic differences between the two sexes [9,10]. Some of these genes have higher expression in one sex relative to the other, while others are exclusively expressed in one sex. Genes with sex-biased expression constitute a significant fraction of the transcriptome in many species. In Drosophila, for example, ~4–16% of genes show female-biased expression and ~8–16% of genes are male-biased [11••]. Owing to differential expression in the two sexes, sex-biased genes may be subject to different selective pressures relative to genes that are expressed similarly in the two sexes (i.e. they might more often be under sexually antagonistic selection). This may be particularly apparent for sex-biased genes located on sex chromosomes, owing to the sexual antagonism scenarios described above.
Another property of the X chromosome that can affect its gene content is X-inactivation during spermatogenesis [12]. This process is part of a temporary inactivation of both the X and Y chromosomes in primary spermatogenesis in heterogametic males of many animal species [13–16]. As a result of inactivation, transcription of genes located on sex chromosomes is temporarily silenced [17]. Therefore, genes with male-specific function late in spermatogenesis are expected to be selectively favored on autosomes, where they can avoid inactivation [12].
Gene content evolution of the X chromosome in Drosophila
Multiple studies have found an underrepresentation of male-biased genes on the X chromosome in Drosophila [5,3,18,19••]. In Drosophila melanogaster, only 10% of X-linked genes were found to exhibit male-biased expression while among autosomal genes 14–17% are male-biased [5]. These early studies also detected an excess of female-biased genes on the X chromosome in Drosophila [5,20]. A recent, more extensive gene expression study confirmed underrepresentation of X-linked male-biased genes in all seven Drosophila species that were investigated [19••]. Female-biased genes, however, were found to be distributed randomly across the chromosomes in Drosophila [19••].
The mechanisms to achieve an underrepresentation of male-biased genes on the X and its causes have been central to many studies [reviewed in references [2,18]. There are several events that can lead to X chromosome demasculinization. Male-biased genes can switch their expression bias and become female-biased or unbiased; they can be lost from the X; fail to arise on the X chromosome; or move from the X chromosome onto autosomes. The past mechanism has been observed in several species and shown to be an important contributor to the demasculinization of the X chromosome in Drosophila [21–23]).
Genes can change their genomic location by means of retroposition, which is an RNA-based gene duplication mechanism. A gene duplication occurs when mRNA is reverse transcribed and inserted into the genome. The resulting copy is intronless, contains a polyA tract, and can be located anywhere in the genome [24]. After the duplication, the original copy can become degraded if the new location is more beneficial. Several studies in Drosophila have demonstrated a large flow of genes from the X chromosome onto the autosomes due to retroposition, while cases of genes moving onto the X are rare [21,25,26,22,23]). Most of the genes that are retroposed off the X chromosome have been shown to be male-specific. For example, of 24 young retroposed genes found in Drosophila melanogaster, 12 were duplicated from the X chromosome onto an autosome and only three moved in the opposite direction [21]. Most of these X-derived genes were found to have a testis-specific expression pattern. A recent study taking advantage of the genome sequence of nine Drosophila species found a similar excess of genes duplicated off the X onto autosomes in all species examined (Meisel et al., unpublished).
The ancestral X chromosome in the genus Drosophila is homologous across all species studied. Thus, the observed patterns of gene content evolution could be a property of this specific chromosome rather than a result of selection. However, this possibility is excluded by recent findings on the neo-X chromosomes of Drosophila pseudoobscura and Drosophila willistoni [[19••], Meisel et al., unpublished]. In both species, a neo-X chromosome evolved independently when an autosome fused to the X chromosome, about ~8–12 million years ago for the neo-X of D. pseudoobscura and ~20 million years ago for the neo-X of D. willistoni [27,28] (Figure 1). After such a fusion, the former autosomes start to segregate and behave like true sex chromosomes. Interestingly, the neo-X chromosome of Drosophila pseudoobscura is not only similar to the ancestral X in sequence composition [29] but also shows a similar depletion of male-biased genes as the ancestral X chromosome of other Drosophila species [[19••], Meisel et al., unpublished]. Specifically, 11% of genes with male-specific function are lost from the neo-X chromosome in D. pseudoobscura and 3% of ancestral male-specific genes are lost from the neo-X owing to relocation onto the autosomes, where they continue to maintain their original function [19••]. Moreover, another recent study has shown that there was a burst of duplication of genes off the neo-X to autosomes following the creation of the neo-X chromosome [Meisel et al., unpublished]. In contrast with male-biased genes relocated off the D. melanogaster X, Meisel et al. did not find a significant excess of testis-expressed genes duplicated off the X or neo-X chromosome in D. pseudoobscura relative to relocations between autosomes. However, they did find such a trend on the neo-X in D. willistoni. The fact that the same trend is present on both the ancestral X chromosome of several Drosophila lineages and on the neo-X provides evidence that relocation of male-biased genes is caused by selective forces rather than a property of the ancestral X chromosome.
Figure 1.
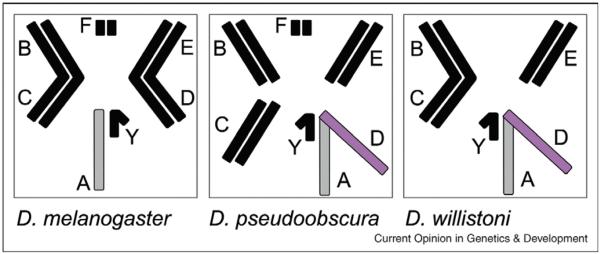
Karyotypes for D. melanogaster, D. pseudoobscura, and D. willistoni (males are shown). Chromosomes from which movement of male-biased genes has been observed are in gray (ancestral X chromosome or Muller element A) and in purple (the neo-X chromosome in D. pseudoobscura and D. willistoni, which evolved when Muller element D fused to the ancestral X chromosome).
Less frequent emergence of novel genes with male-biased function is likely to be another reason for the observed depletion of male-biased genes on the neo-X chromosomes of Drosophila. Genes with male-biased expression in the genus Drosophila show higher turnover rates than female-biased or unbiased genes (i.e. higher rates of gene formation and extinction [11••]). In Drosophila pseudoobscura, novel male-biased genes become established significantly more often on autosomes than on the neo-X chromosome, and male-biased genes are more likely to be lost from the genome if they are located on the neo-X chromosome compared with autosomes [19••]. These findings suggest that there is an evolutionary advantage for male-specific genes to be located on autosomes rather than on the X chromosome.
Several recent studies have found that novel genes derived from non-coding DNA often show male-biased expression in Drosophila [30–32]. Interestingly, they are overrepresented on the X chromosome in D. melanogaster and the D. yakuba/D. erecta clade [30,31], but no such enrichment of de novo evolved male-biased genes was found on the X chromosome of D. pseudoobscura [32]. A possible explanation for this pattern is that novel male-biased genes arise and/or fix more readily on the X chromosome but then move to an autosome, resulting in a deficiency of male-biased genes on the X [5]. In the three studies mentioned above, D. pseudoobscura novel genes may be older than the novel genes identified in D. melanogaster [30,32]. It is thus possible that they have already moved off the X in D. pseudoobscura, while the novel genes in the other two lineages have not had enough time yet to retropose. Alternatively, this difference may also be attributed to sampling effects due to a very small number of genes considered in these studies, and more research is needed to resolve this question.
In sum, these recent studies point to two explanations for the observed gene content patterns of X chromosomes in Drosophila. First, X inactivation during spermatogenesis clearly plays an important role for genes functioning late in spermatogenesis to avoid X-linkage [14]. However, recent findings show that not only genes that are expressed in male germ cells are depleted from the X but also genes with male-biased expression in somatic cells [19••]. Since the X is only inactivated in germ cells but remains fully functional in somatic cells, X inactivation cannot be solely responsible for the observed demasculinization of the X chromosome [12] and suggests that sexual antagonism also plays an important role in shaping the gene content of the X in Drosophila. The observed underrepresentation of male-biased genes and possible excess of female-biased genes both suggest that mutations on the X chromosome are not recessive on average, resulting in the observed demasculinization of the X chromosome.
X chromosome gene content in other species
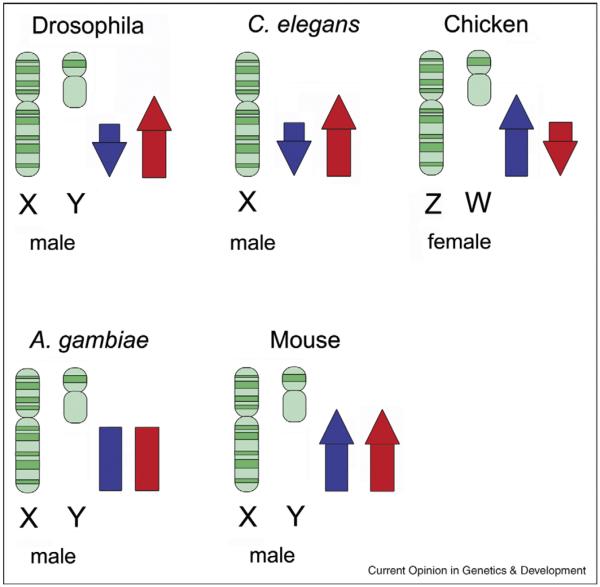
Other than Drosophila, underrepresentation of male-biased genes on the X chromosome has also been shown in Caenorhabditis elegans, where only 3% of the expected number of spermatogenic genes is located on the X [33] (Figure 2). However, unlike Drosophila, in C. elegans only genes expressed in the male germline are underrepresented on the X, but not genes with male-biased expression in somatic cells [33]. This is consistent with X inactivation playing a major role in the demasculinization of the X chromosome in C. elegans. Hermaphroditespecific genes expressed in somatic cells are enriched on the X [33], suggesting that sexually antagonistic selection might play a role for the genomic distribution of these types of genes. In Anopheles gambiae male-biased genes are evenly distributed across the genome [34]. These examples accentuate that the patterns of X chromosome gene content are far from being universal, and more research is needed to shed light on these differences among taxa.
Figure 2.
Trends in sex-biased gene content on the X chromosome in different species. Karyotypes for the heterogametic sex are shown for each species. Blue arrows indicate the overall trend for male-specific genes; red arrows indicate the overall trend for female-specific genes. In the genus Drosophila, male-biased genes are depleted on the X chromosome while female-biased genes are enriched. C. elegans shows the same overall trend as Drosophila. Chicken is a species with ZW sex-determination system where the Z chromosome is enriched for male-biased genes and depleted for female-biased genes. A. gambiae has an even distribution of male-biased genes among the chromosomes. In the mouse, both male-biased and female-biased genes are enriched on the X.
Studies in mammals found that genes that are preferentially expressed in the male somatic tissues are generally enriched on the X chromosome [6]. In humans, brain-expressed genes as well as genes related to muscle function, sex, and reproduction are enriched on the X [35–38]. This implies that sexual antagonism plays a role in gene content evolution of X chromosomes in mammals. Interestingly, in mouse a clear relationship between the stages of spermatogenesis (and associated stages of X inactivation) and the presence of male-specific genes on the X chromosome was observed [39]. Mouse genes expressed in mitotic stages of spermatogenesis (before X inactivation takes place) are overrepresented on the X, while genes expressed later in spermatogenesis (when the X chromosome becomes transcriptionally inactivated) are depleted, and genes expressed in meiotic stages of spermatogenesis are completely absent [39]. These findings led to the conclusion that in mouse there is a selective advantage of having male-specific genes on the X – as expected for recessive male-beneficial mutations – but that inactivation of the X chromosome later in spermatogenesis causes a deficiency of late-acting spermatogenesis genes on the X. A recent study, however, found that the X chromosome in mouse is also enriched for multicopy testis genes that have postmeiotic expression [40••]. Additionally, this study found that many genes that are expressed earlier in spermatogonia undergo a low level of postmeiotic reactivation. These findings not only confirm that the X chromosome is a preferential location for male-biased genes in mouse, but also suggest that increased copy number of an X-linked gene allows it to compensate for repressive chromatin environment in postmeiotic stages [40••]. Like in Drosophila, genes on the X chromosome of mammals undergo increased rates of retroposition off the X chromosome, with the majority of X-derived retrogenes showing testis-specific expression [41–45,46•]. X chromosome inactivation is thought to be the driving force underlying this out-of-X gene movement [42].
In chicken, a species with a ZW sex-determination system, ovary-specific genes are significantly underrepresented on the Z chromosome but no enrichment for testis-expressed genes was found [47•,48]. In addition, for genes that are expressed in the brain an enrichment of male-biased genes and an underrepresentation of female-biased genes were found on the Z chromosome [47•]. This broadly mimics patterns of gene content evolution of species with XY sex determination systems. Sex-chromosome inactivation has not been observed in heterogametic female individuals, implying that Z-inactivation is not a likely factor affecting gene distribution in chicken. While these findings are compatible with sexual antagonism driving gene content evolution on the Z, a lack of dosage compensation in chicken can also account for the observed excess of male-biased and deficiency of female-biased genes on the Z chromosome [49]. More research is needed to better understand patterns of gene content evolution in species where females are the heterogametic sex.
Conclusions
The X chromosome evolves differently from autosomes as a result of its sex-biased transmission, hemizygosity in males, and X chromosome inactivation during spermatogenesis. How exactly these different forces influence patterns of gene content evolution of the X chromosome has been extensively studied in the past few years with particular focus on genes showing sex-biased expression. In the genus Drosophila, ancestral and recently formed neo-X chromosomes show an underrepresentation of male-biased genes on the X. Comparative genomics studies have shown that this depletion of male-biased genes is caused by higher rates of gene loss and lower rates of establishment of novel male-biased genes, and some existing genes with male-biased expression escape from the X to autosomes through retroposition. While X inactivation certainly plays an important role explaining the observed deficiency of male-biased genes on the X, recent research shows that selection on a gene-level and sexual antagonism can also be driving male-biased genes off the X chromosome. To further address this question, it will be useful to look at patterns of gene content evolution in even younger neo-sex chromosomes in Drosophila. Recently established neo-sex chromosomes will make it possible to study the initial stages of sex chromosome evolution and observe the population genetic forces that shape gene content evolution of the X. For example, Drosophila miranda has a neo-X chromosome that was formed only about 1 million years ago. Interestingly, rates of adaptive evolution on this neo-X chromosome have been found to be increased about 10-fold relative to background levels of adaptation in the genome [Bachtrog et al., submitted for publication], indicating that genes on this newly formed neo-X chromosome are undergoing rampant evolutionary change to adjust to their altered genomic environment. D. miranda may thus be an ideal model for comparison of selective forces acting upon recently established X-linked genes and their autosomal homologs.
Acknowledgements
This work was funded by the National Institute of Health Grant (GM076007) and an AP Sloan Fellowship in Molecular and Computational Biology to DB.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Bachtrog D. A dynamic view of sex chromosome evolution. Curr Opin Genet Dev. 2006;16:578–585. doi: 10.1016/j.gde.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Vicoso B, Charlesworth B. Evolution on the X chromosome: unusual patterns and processes. Nat Rev Genet. 2006;7:645–653. doi: 10.1038/nrg1914. [DOI] [PubMed] [Google Scholar]
- 3.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007;8:689–698. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- 4.Graves JAM. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–914. doi: 10.1016/j.cell.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Parisi M, Nuttall R, Naiman D, Bouffard G, Malley J, Andrews J, Eastman S, Oliver B. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science. 2003;299:697–700. doi: 10.1126/science.1079190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang PJ, Mccarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- 7.Rice WR. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–742. doi: 10.1111/j.1558-5646.1984.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 8.Hurst LD. Evolutionary genomics. Sex and the X. Nature. 2001;411:149–150. doi: 10.1038/35075697. [DOI] [PubMed] [Google Scholar]
- 9.Connallon T, Knowles LL. Intergenomic conflict revealed by patterns of sex-biased gene expression. Trends Genet. 2005;21:495–499. doi: 10.1016/j.tig.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 11 ••.Zhang Y, Sturgill D, Parisi M, Kumar S, Oliver B. Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature. 2007;450:233–237. doi: 10.1038/nature06323. [DOI] [PMC free article] [PubMed] [Google Scholar]; A survey of sex-biased expression in several Drosophila species that shows that male-biased genes have higher rates of turnover as well as increased DNA sequence and expression divergence relative to female-biased genes.
- 12.Wu C-I, Xu EY. Sexual antagonism and X inactivation—the SAXI hypothesis. Trends Genet. 2003;19:243–247. doi: 10.1016/s0168-9525(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 13.Kelly WG, Schaner CE, Dernburg AF, Lee M-H, Kim SK, Villeneuve AM, Reinke V. X-chromosome silencing in the germline of C. elegans. Development. 2002;129:479–492. doi: 10.1242/dev.129.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hense W, Baines JF, Parsch J. X chromosome inactivation during Drosophila spermatogenesis. PLoS Biol. 2007;5:2288–2295. doi: 10.1371/journal.pbio.0050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner JMA. Meiotic sex chromosome inactivation. Development. 2007;134:1823–1831. doi: 10.1242/dev.000018. [DOI] [PubMed] [Google Scholar]
- 16.Kelly WG, Aramayo R. Meiotic silencing and the epigenetics of sex. Chromosome Res. 2007;15:633–651. doi: 10.1007/s10577-007-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lifschytz E, Lindsley DL. The role of X-chromosome inactivation during spermatogenesis. Proc Nat Acad Sci U S A. 1972;69:182–186. doi: 10.1073/pnas.69.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver B, Parisi M. Battle of the Xs. Bioessays. 2004;26:543–548. doi: 10.1002/bies.20034. [DOI] [PubMed] [Google Scholar]
- 19 ••.Sturgill D, Zhang Y, Parisi M, Oliver B. Demasculinization of X chromosomes in the Drosophila genus. Nature. 2007;450:238–241. doi: 10.1038/nature06330. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using species-specific microarrays, the authors find a paucity of male-biased genes on the ancestral X chromosome in multiple Drosophila species and on the newly established neo-X chromosome in Drosophila pseudoobscura. Additionally, they show that underrepresentation of male-specific genes is not only limited to male germ cells but is also observed in somatic cells, a finding that does not fit a simple X inactivation model.
- 20.Ranz JM, Castillo-Davis CI, Meiklejohn CD, Hartl DL. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science. 2003;300:1742–1745. doi: 10.1126/science.1085881. [DOI] [PubMed] [Google Scholar]
- 21.Betrán E, Thornton K, Long M. Retroposed new genes out of the X in Drosophila. Genome Res. 2002;12:1854–1859. doi: 10.1101/gr.604902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H, Yoshimatsu TF, Long M. Retrogene movement within-and between- chromosomes in the evolution of Drosophila genomes. Gene. 2006;385:96–102. doi: 10.1016/j.gene.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Bai Y, Casola C, Feschotte C, Betrán E. Comparative genomics reveals a constant rate of origination and convergent acquisition of functional regrogenes in Drosophila. Genome Biol. 2007;8:R11. doi: 10.1186/gb-2007-8-1-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W-H. Molecular evolution. Sinauer Associates Sunderland; MA: 1997. [Google Scholar]
- 25.Betrán E, Long M. Dntf-2r, a young Drosophila retroposed gene with specific Male expression under positive darwinian selection. Genetics. 2003;164:977–988. doi: 10.1093/genetics/164.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betrán E, Emerson JJ, Kaessmann H, Long M. Sex chromosomes and male functions: where do new genes go? Cell Cycle. 2004;3:873–875. [PubMed] [Google Scholar]
- 27.Tamura K, Subramanian S, Kumar S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol. 2004;21:36–44. doi: 10.1093/molbev/msg236. [DOI] [PubMed] [Google Scholar]
- 28.Richards S, Liu Y, Bettencourt BR, Hradecky P, Letovsky S, Nielsen R, Thornton K, Hubisz MJ, Chen R, Meisel RP, et al. Comparative genome sequencing of Drosophila pseudoobscura: chromosomal, gene, and cis-element evolution. Genome Res. 2005;15:1–18. doi: 10.1101/gr.3059305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallach M, Arnau V, Marín E. Global patterns of sequence evolution in Drosophila. BMC Genomics. 2007;8:408. doi: 10.1186/1471-2164-8-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine MT, Jones CD, Kern AD, Lindfors HA, Begun DJ. Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. Proc Nat Acad Sci U S A. 2006;103:9935–9939. doi: 10.1073/pnas.0509809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begun BJ, Lindfors HA, Kern AD, Jones CD. Evidence for de novo evolution of testis-expressed genes in the Drosophila yakuba/Drosophila erecta clade. Genetics. 2007;176:1131–1137. doi: 10.1534/genetics.106.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metta M, Schlötterer C. Male-biased genes are overrepresented among novel Drosophila pseudoobscura sex-biased genes. BMC Evol Biol. 2008;8:182. doi: 10.1186/1471-2148-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 34.Hahn MW, Lanzaro GC. Female-biased gene expression in the malaria mosquito Anopheles gambiae. Curr Biol. 2005;15:R192. doi: 10.1016/j.cub.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Bortoluzzi S, Rampoldi L, Simionati B, Zimbello R, Barbon A, d'Alessi F, Tiso N, Pallavicini A, Toppo S, Cannata N, et al. A comprehensive, high-resolution genomic transcript map of human skeletal muscle. Genome Res. 1998;8:817–825. doi: 10.1101/gr.8.8.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurst LD, Randerson JP. An eXceptional chromosome. Trends Genet. 1999;15:383–385. doi: 10.1016/s0168-9525(99)01809-0. [DOI] [PubMed] [Google Scholar]
- 37.Lercher MJ, Urrutia AO, Hurst LD. Evidence that the human X chromosome is enriched for male-specific but not female-specific genes. Mol Biol Evol. 2003;20:1113–1116. doi: 10.1093/molbev/msg131. [DOI] [PubMed] [Google Scholar]
- 38.Saifi GM, Chandra HS. An apparent excess of sex- and reproduction-related genes on the human X chromosome. Proc Biol Sci. 1999;266:203–209. doi: 10.1098/rspb.1999.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khil PP, Smirnova NA, Romanienko PJ, Camerini-Otero RD. The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation. Nat Genet. 2004;36:642–646. doi: 10.1038/ng1368. [DOI] [PubMed] [Google Scholar]
- 40 ••.Mueller JL, Mahadevaiah SK, Park PJ, Wartburton PE, Page DC, Turner JMA. The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression. Nat Genet. 2008;40:794–799. doi: 10.1038/ng.126. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates an enrichment of X-linked multicopy testis genes that show postmeiotic expression, opposite to the pattern found for single-copy genes. The authors also show that many genes expressed earlier in spermatogonia undergo a low level of postmeiotic reactivation.
- 41.Khil PP, Oliver B, Camerini-Otero RD. X for intersection: retrotransposition both on and off the X chromosome is more frequent. Trends Genet. 2005;21:3–7. doi: 10.1016/j.tig.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Emerson JJ, Kaessmann H, Betran E, Long M. Extensive gene traffic on the mammalian X chromosome. Science. 2004;303:537–540. doi: 10.1126/science.1090042. [DOI] [PubMed] [Google Scholar]
- 43.Vinckenbosch N, Dupanloup I, Kaessmann H. Evolutionary fate of retroposed gene copies in the human genome. Proc Natl Acad Sci U S A. 2006;103:3220–3225. doi: 10.1073/pnas.0511307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marques A, Dupanloup I, Vinckenbosch N, Reymond A, Kaessmann H. Emergence of young human genes after a burst of retroposition in primates. PLoS Biol. 2005;4:e357. doi: 10.1371/journal.pbio.0030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang PJ. X chromosomes, retrogenes and their role in male reproduction. Trends Endocrinol Metab. 2004;15:79–83. doi: 10.1016/j.tem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 46 ••.Potrzebowski L, Vinckenbosch N, Marques AC, Chalmel F, Jégou B, Kaessmann H. Chromosomal gene movements reflect the recent origin and biology of therian sex chromosomes. PLoS Biol. 2008;6:e80. doi: 10.1371/journal.pbio.0060080. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors found that in eutherians and marsupials genes preferentially retropose off the X chromosome onto autosomes, with the majority of X-derived retrogenes showing testis-specific expression. Additionally, the study supports the notion that therian sex chromosomes emerged in the therian ancestor and not in mammalian common ancestor, more recently than usually thought.
- 47 ••.Storchová R, Divina P. Nonrandom representation of sex-biased genes on chicken Z chromosome. J Mol Evol. 2006;63:676–681. doi: 10.1007/s00239-006-0022-1. [DOI] [PubMed] [Google Scholar]; The study shows that sex-biased genes are distributed non-randomly on the Z chromosome in chicken, with brain-expressed male-specific genes overrepresented and female-specific genes underrepresented on the Z. This is the first study to show non-random distribution of genes on a homogametic sex chromosome in a species with heterogametic females.
- 48.Mank JE, Hultin-Rosenberg L, Webster MT, Ellegren H. The unique genomic properties of sex-biased genes: insights from avian microarray data. BMC Genomics. 2008;9:148. doi: 10.1186/1471-2164-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellegren H, Hultin-Rosenberg L, Brunström B, Dencker L, Kultima K, Scholz B. Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. doi: 10.1186/1741-7007-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]