Abstract
Of cancers affecting both men and women, colorectal cancer (CRC) is the second leading cancer killer among African Americans in the U.S. Compared to White men, African American men have incidence and mortality rates 25% and 50% higher from CRC. Despite the benefits of early detection and the availability of effective screening, most adults over age 50 have not undergone testing, and disparities in colorectal cancer screening (CRCS) persist. Owing to CRC’s high incidence and younger age at presentation among African American men, CRCS is warranted at age 45 rather than 50. However, the factors influencing young adult (i.e., age < 50) African American men’s intention to screen and/or their CRCS behaviors has not been systematically assessed. To assess whether the factors influencing young adult African American men’s screening intentions and behaviors are changeable through structured health education interventions, we conducted a systematic review, with the two-fold purpose of: (1) synthesizing studies examining African American men's knowledge, beliefs, and behaviors regarding CRCS; and (2) assessing these studies’ methodological quality. Utilizing Garrard’s Matrix Method, a total of 28 manuscripts met our inclusion/exclusion criteria: 20 studies followed a non-experimental research design, 4 comprised a quasi-experimental design, and 4, an experimental design. Studies were published between 2002 and 2012; the majority, between 2007 and 2011. The factors most frequently assessed were behaviors (79%), beliefs (68%), and knowledge (61%) of CRC and CRCS. Six factors associated with CRC and CRCS emerged: previous CRCS, CRC test preference, perceived benefits, perceived barriers, CRC/CRCS knowledge, and physician support/recommendation. Studies were assigned a methodological quality score (MQS – ranging from 0 to 21). The mean MQS of 10.9 indicated these studies were, overall, of medium quality and suffered from specific flaws. Alongside a call for more rigorous research, this review provides important suggestions for practice and culturally relevant interventions.
Keywords: African Americans, Colorectal Neoplasms, Early Detection of Cancer, Men, Review
Introduction
Of cancers affecting both men and women, colorectal cancer (CRC) is the second leading cancer to kill African Americans in the U.S. (American Cancer Society [ACS], 2014). Of the nearly 42 million African Americans comprising about 13% of the total population, the American Cancer Society estimates 18,110 African American (AA) men and women will be diagnosed with CRC in 2013—and 6,850 (38%) will die of the disease (ACS, 2013). Compared with Whites, AA men and women have poorer survival once a CRC diagnosis is made (Jemal et al., 2007). Compared to White men, AA men have incidence and mortality rates 25% and 50% higher from CRC (ACS, 2014).
Factors known to contribute to this disproportionate burden of CRC incidence and mortality among AA men vary, yet include differences in timely screening, diagnosis, and treatment (Jemal et al., 2007). In 2010, Holden and colleagues reviewed the barriers and facilitators associated with screening for CRC. Among the patient-level barriers were: having low income, less education, being uninsured, being of Hispanic or Asian descent, and having reduced access to care. Conversely, higher screening rates correlated with being non-Hispanic White, having higher income/education, being insured, participating in other cancer screenings, having a family history of CRC or personal history of another cancer, and receiving a physician recommendation. Intervention-related factors effectively increasing CRC screening (CRCS facilitators) included eliminating structural barriers, enacting system-level changes, adding patient reminders, and implementing one-on-one interactions.
The qualitative systematic review conducted by Guessous and colleagues (2010) provided an inventory of the facilitators and barriers to CRCS for older persons (ages ≥ 65), and documented the changes in barriers and facilitators since Medicare began covering the costs of screening colonoscopy in 2001. Guessous et al. (2010) recommended researchers and intervention planners pay particular attention to modifiable factors, and called for further research to address whether the facilitators/barriers to CRCS among older persons differ for younger persons.
Although these reviews make important contributions, neither specifically examined CRCS uptake among AA men, or the barriers and facilitators of CRCS uptake among adults younger than 50 (studies reviewed by Holden et al., 2010, included respondents 50–89 years old; most studies in the Guessous et al., 2010 review addressed an asymptomatic average-risk older population (defined as ≥ 65 years).
Since routine screening detects CRC at an earlier, more treatable stage, the U.S. Preventive Services Task Force (USPSTF) currently recommends routine screening at age 50 for all men at average risk (USPSTF, 2008). Nonetheless, because African Americans have the highest CRC incidence of any ethnic or racial group in the U.S, and because many cases among them occur at a younger age, beginning CRCS at 45 rather than 50 is a practice supported by many providers (Agrawal et al., 2005; Rex et al., 2009).
Despite the absence of official recommendations to begin screening before age 50, it may be beneficial to initiate education about CRC and screening practices, earlier (Powe et al., 2006; Rex et al., 2009). Moreover, if age guidelines are modified in the future, practitioners and health educators may lack knowledge of the complex factors shaping decisions to screen for CRC and screening behaviors among AA men who are younger than those traditionally assessed by researchers and clinicians. Thus, the importance of understanding factors influencing screening behaviors among AA men younger than age 50, and the contribution this review makes.
Purpose
To our knowledge, a systematic review of the factors influencing young adult AA men’s intention to screen and/or their CRCS behaviors have not been reported in the literature. Thus, in order to provide insight into which factors influencing young adult AA men’s screening intentions and behaviors are changeable through structured health education interventions, we conducted a systematic review of the extant literature. The two-fold purpose of the review was to (1) synthesize the evidence from published studies examining younger (< 50 years old) AA men's knowledge, beliefs, and behaviors regarding CRCS; and (2) assess the methodological quality of this evidence. This review contributes (a) a foundation for further analyses of specific factors influencing CRCS among AA men younger than 50, which, in turn, represent (b) points of intervention for this population.
Systematic literature reviews (SLRs) represent an efficient method for identifying these specific intervention points, and are useful for reducing unnecessary duplication, for helping ensure enquiry is informed by evidence (Bambra, 2011), and for supporting evidence-based clinical decisions (Cook et al., 1997). SLRs help counteract the generalizability deficiency often evident in studies conducted among one particular population (Egger et al., 2001; Light et al., 1984), and require transparency in its methods/procedures (Rosenthal, 1990). Furthermore, SLRs offer critical appraisals of primary studies’ methodological quality, through careful assessment of their reliability, relevance, and value (Belsey, 2009; Higgins & Green, 2008; Oxman et al., 1988).
Materials and Methods
Eligibility Criteria
For inclusion in this review, articles had to (a) be primary empirical studies with human subjects, reporting research findings, (b) be published in English-language peer-reviewed journals, (c) be published between January 2000 (two years before the USPSTF’s CRCS recommendations for screenings starting at age 50 or older were published) and February 2013, (d) be conducted in the United States, (e) have explored factors associated with CRCS, (f) have included AA men, (g) have assessed AA men's knowledge, beliefs, and behaviors regarding CRCS, and (h) have samples including AA men younger than 50.
Information Sources
Following procedures outlined in the Matrix Method (Garrard, 2014), we conducted the core search in four widely used bibliographic databases: Cinahl, Embase, Medline, and PsycInfo. MeSH and key terms included colorectal neoplasms, colonoscopy, sigmoidoscopy, occult blood; mass screening, and AA or Black. Using the Scopus database, we also assessed the cited references from each of the studies included in the review. The final sample comprised 28 studies.
Data Abstraction
To systematically organize and structure the information collected from each study, we employed a review matrix. This matrix captured information regarding the purpose/research question(s), keywords, sample characteristics, study design, study findings (in reference to knowledge, beliefs, behaviors) and other major factors/findings, limitations, and generalizability.
Methodological Quality Score (MQS)
To assess the conceptual and methodological characteristics of this body of literature, each reviewed study received an overall methodological quality score (MQS) (Lee et al., 2002). The highest possible MQS was 21 (Table 1). The criteria for the MQS included assessments of each study’s use of theory, its design, sample design and size, utilization of complex analytical techniques, reporting of the validity and reliability of the study’s data, and the inference of appropriate conclusions. Better methodological quality is reflected in a higher MQS. Seven studies (25%) were randomly selected and assigned to another reviewer to establish the reliability of the data abstraction and methodological quality scoring processes.
Table 1.
Criteria for assessment of reviewed studies’ methodological quality characteristics and frequency distributions for each characteristic.
| Methodological Quality Characteristic | Scoring Options (Maximum total score = 21 points) | Distribution of characteristics among (28) reviewed studies |
|
|---|---|---|---|
| Frequency (n) | Percent (%) | ||
| Conceptual | |||
| Does a theoretical framework drive the study? | Explicit use of theory = 2 points Implicit use of theory = 1 point Not reported = 0 points |
17 1 10 |
60.7 3.6 35.7 |
| Research Design | |||
| What is the research paradigm? | Experimental = 3 points [e.g., RCT] Quasi-experimental = 2 points [e.g., observational, comparison pre-test/post-test] Non-experimental = 1 point [e.g., exploratory and/or qualitative] |
4 4 20 |
14.3 14.3 71.4 |
| What is the study’s design? |
Longitudinal = 2 points Cross-sectional = 1 point |
5 23 |
17.9 82.1 |
| Does the study exclusively focus on African American men? | Yes = 1 point No = 0 points |
0 28 |
0 100 |
| Sampling | |||
| What is the sample design? | Random/Nationally Representative = 3 points Random/Not Nationally Representative = 2 points Convenience/Nonprobability = 1 point |
1 9 18 |
3.6 32.1 64.3 |
| What is the sample size? | Large (n >300) = 2 points Medium (100 ≥ n ≥ 300) = 1 points Small (n < 100) = 0 points |
3 10 3 |
10.7 35.7 10.7 |
| Data Analyses | |||
| What were the most advanced statistical techniques utilized? | Multivariate statistics = 4 points (e.g., Structural Equation Modeling) Multiple/Logistic Regression = 3 points ANOVA/Bivariate statistics = 2 points Descriptive/Univariate statistics = 1 point Qualitative analyses = 0 points (e.g., Grounded Theory, Content Analysis) |
9 8 4 5 2 |
32.1 28.6 14.3 17.9 7.1 |
| Was any validity reported? | Yes = 1 point No = 0 points |
8 20 |
28.6 71.4 |
| Was any reliability reported? | Yes = 1 point No = 0 points |
8 20 |
28.6 71.4 |
| Were appropriate conclusions inferred? | Yes = 1 point No = 0 points |
28 0 |
100 0 |
Results
Sample
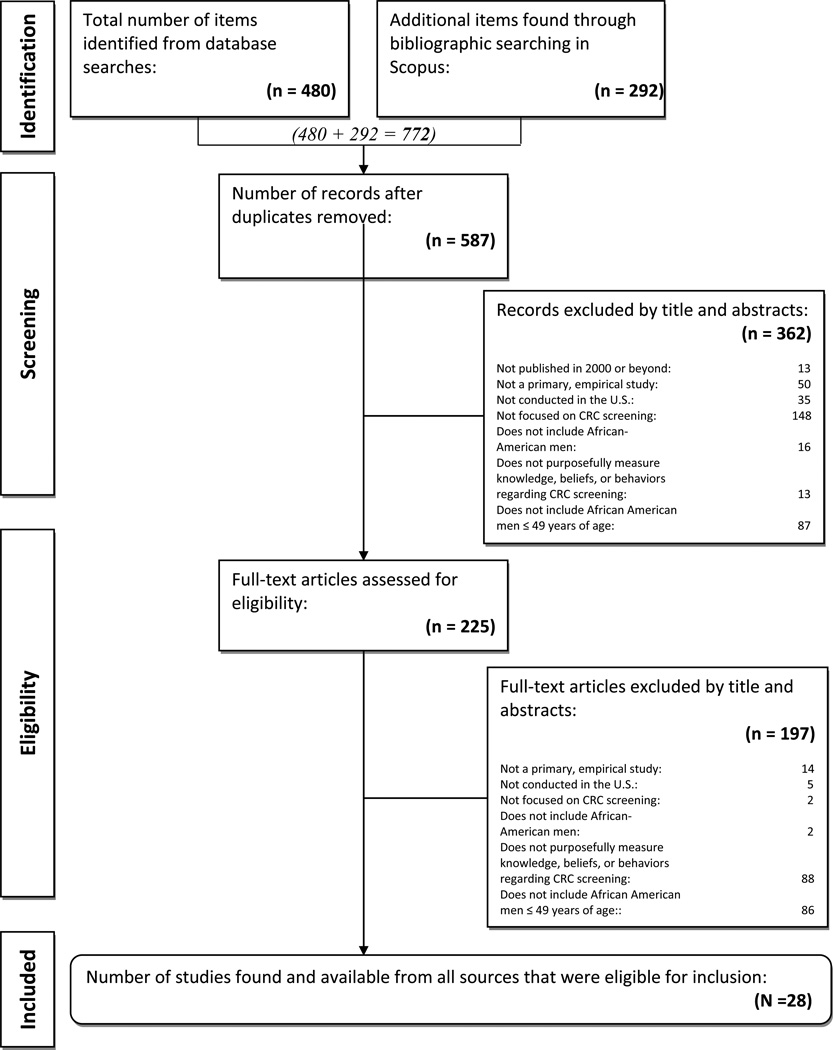
A total of 772 articles were initially identified. Among the total, 225 (29%) met the eligibility criteria for the first round of screening (titles and abstracts) and 28 (12%) of the 225 studies, met the criteria for the second round of screening (4% of the original sample – see Figure 1).
Figure 1.
PRISMA Flow Diagram
Studies’ Characteristics
A total of 28 manuscripts met the inclusion/exclusion criteria. These included 20 studies with a non-experimental research design, 4 with a quasi-experimental design, and 4 with an experimental design. Forty-three percent were published in the following journals: Health Psychology (n = 3), Preventive Medicine (n = 3), Gastroenterology Nursing (n = 2), Journal of Community Health (n = 2), and the Journal of General Internal Medicine (n = 2). The remaining 47% were featured in journals devoted to health promotion (e.g., Health Promotion Practice, Journal of Health Communication) and medical journals (e.g., Surgical Endoscopy, Journal of Occupational and Environmental Medicine). Studies were published between 2002 and 2012 with the largest number (n = 17) appearing between 2007 and 2011. A few authors published more than one study on the topic (36%), namely James (n = 3), Manne (n = 3), Greiner (n = 2), and Griffith (n = 2). Five studies (18%) evaluated an intervention. The factors most frequently assessed in the reviewed studies were behaviors (79%), beliefs (68%), and knowledge (61%) of CRC and CRCS. These three factors and additional key factors associated with CRC and CRCS among younger African American men are presented in Table 2.
Table 2.
Key factors associated with CRC and CRCS among younger African American men in a sample of (28) reviewed studies.
Findings: Behaviors, Beliefs, and Knowledge regarding CRC and CRCS
Behaviors
These were the most frequently examined factors associated with CRCS, reported by 22 reviewed studies (79%). Ten studies (45%) used the Health Belief Model and four (18%) used Social Cognitive Theory as a theoretical framework – two of the most widely used models in health promotion, for understanding behavior change.
Among these 22 studies, previous CRCS (screening history) emerged as a strong behavioral factor associated with being screened among 43% of the studies. For example, Fisher and colleagues (2007) assessed the proportion of 500 patients (ages 39–89) from a Veterans Affairs (VA) facility who completed an ordered fecal occult blood test (FOBT). Of this predominantly male sample (97%) which was 30% AA, current FOBT adherence was strongly associated with prior FOBT completion. According to Fisher et al. (2007), “this could reflect many factors, such as better understanding of instructions, increased interest in FOBT screening, higher level of compliance with medical recommendations in general, and increased understanding of the importance of CRCS” (p. 95). Other behaviors documented included cancer information seeking, screening intention, and avoidance of the health care system, but none were reported in more than two studies each.
Beliefs
Assessment of beliefs was reported in 19 reviewed studies (68%). Among these, CRCS test preference, perceived benefits, and perceived barriers emerged as factors influencing participants’ views of, and behaviors related to CRC and CRCS.
DeBourcy and colleagues (2007) determined the screening test preferences of 323 colonoscopy-naive participants ages 40–79 in Denver, CO who were predominately non-Latino whites (64%). When given time to consider comprehensive, written information about 2 CRCS tests, more than half of the sample preferred FOBT over colonoscopy. At least 40% preferred FOBT over colonoscopy in almost every demographic subgroup based on race/ethnicity, type of health insurance, employment, marital status, educational attainment, and age. Conversely, Greiner and colleagues (2005) assessed CRCS preferences among 55 African Americans over 40 years of age in their qualitative-focused study. Following an education lecture session at the end of each focus group, 33% of the participants reported a preference for colonoscopy followed by FOBT (26%).
Perceived benefits were a key factor in the study by Palmer and colleagues (2007). The researchers examined the relationship between health beliefs and attitudes toward CRCS, as well as the relationship between health beliefs, being appropriately screened for CRC, and the strength of family history. The sample comprised 511 patients between the ages of 35 and 55, with only 5% identifying as AA. Based on family history, participants’ perceived cancer risk and the potential influence from family and close friends to screen for CRC (subjective norms) increased.
In terms of perceived barriers to CRCS, James and colleagues (2008) conducted a prospective intervention trial of a predominantly African American sample (69%) to assess whether certain perceived barriers to CRCS were more common among 291 patients 40 years and older from a lower SES. Among their sample, the researchers determined the two most common barriers to undergoing a FOBT were fear that the results would show something bad (37%) and disgust (34%). Similarly, perceived barriers to CRCS were found by Holt and colleagues (2011) who evaluated the efficacy of a spiritually-based CRC educational intervention delivered by trained community health advisors to 122 individuals from one predominantly White and two predominantly AA churches in Alabama. The sample was predominately African American (84%) and the average age was 57 (SD = 7.41). The important role of perceived barriers and benefits of screening was inferred from the finding that CRC knowledge and perceived benefits of screening, colonoscopy specifically, increased from baseline to follow-up.
Knowledge
Findings related to CRC and CRCS knowledge were reported by 17 reviewed studies (61%). Powe, Finnie, and Ko (2006) compared knowledge and awareness of CRC among 345 participants (93% AA) in three age groups (20–29, 30–49, 50–75 years) who attended federally funded primary care centers. There were no significant differences in the CRC knowledge among the three age groups, and participants’ CRC knowledge was limited. Thirty-one percent of the sample recognized the increased risk associated with age and 51% knew a history of CRC among first-degree relatives increased their risk of CRC. Furthermore, the 20–29-year old group was not only less likely to know the relationship between CRC and diet, but less likely to acknowledge the relationship between increased CRC risk and family history.
Healthcare Provider Recommendation
Findings related to physician support/recommendation for CRCS were reported by 18% of the reviewed studies. Ford, Coups, and Hay (2006), for instance, examined CRCS knowledge and potential covariates (e.g., cancer information seeking, health care) among 3,131 adults of at least 45 years of age from the 2003 Health Information National Trends Survey. For this sample which was only 10% AA, participants were "less likely to have CRCS knowledge if they were not advised to have FOBT in the past year, had never been advised to receive sigmoidoscopy or colonoscopy, or had never had an FOBT, sigmoidoscopy, or colonoscopy" (Ford et al., 2006, p. 28). Furthermore, the researchers found those who were ages 45–49 or over 70 were less likely to have adequate screening knowledge. This difference by age not only places attention on the significant increase in CRCS knowledge at age 50, but may indicate providers are recommending CRCS at this age, exclusively (Ford et al., 2006).
Geiger and colleagues (2008) documented among 6,349 participants ages 18–64 in the Health Information National Trends Survey (HINTS 1), of those without a primary healthcare provider, only 9% had undergone a colonoscopy. For this nationally representative sample, the major difference between the group who had undergone a colonoscopy and the group that had not, was the behavior of their health care provider. A number of the participants (24%) indicated they had never had a colonoscopy or sigmoidoscopy because their primary care provider “did not order it or did not say they needed it” (Geiger et al., 2008, p. 529).
In the qualitative study conducted by Griffith et al. (2012), 14 AA men and women -- aged 40 or older with at least one first-degree family member affected by CRC -- participated in four focus groups to explore barriers and facilitators to screening for CRC and suggestions for improving screening among African Americans with affected first degree relatives. For some of these participants, strong physician recommendation was deemed instrumental in their decision to be screened. One participant stated,
“[M]y doctor determined that my brother had cancer, [and] he made me get my test. And [I] took the colonoscopy, first time I took that they found three polyps so they removed them and it hasn’t any more polyps showed up since then” (Griffith et al., 2012, p. 303).
Other Factors
Fear of pain or discomfort associated with the CRCS procedures and fear of illness or diagnosis emerged as determining factors for being screened for CRC in 14% of the reviewed studies. For example, Geiger and colleagues (2008) identified barriers to colonoscopy screening among 6,349 participants, 18–64 years of age, in the HINTS 1. Among their nationally representative sample, fear that CRCS results would show something bad, fear of injury to the colon from CRCS, and fear of embarrassment with CRCS were identified as perceived barriers, affective in nature.
Similarly, in the qualitative study conducted by Winterich et al. (2011), 30 White and 35 AA men, aged 40–64, with diverse education backgrounds were interviewed to compare how education, race, and screening status affected their knowledge about CRC and their views of 3 early detection screening practices (i.e., FOBT, sigmoidoscopy, colonoscopy). Specifically, men in each education group (e.g., low, medium, and high educational attainment) refused to comply with the FOBT as a result of their negative views of the test. Although attitudes varied with education, as education increased so did the men’s negative views (Winterich et al., 2011).
Perceived CRC severity was reported as a key factor, but only by 3 (11%) of the reviewed studies. Manne et al. (2003) tested a mediational model predicting CRCS intention among 534 siblings of patients from the northeastern U.S who were diagnosed with CRC prior to age 56. For these siblings who were greater than or equal to 35 years of age and predominately white (93%), the researchers found a significant positive association between perceived severity and colonoscopy intentions.
Methodological Quality Assessment
Many scholars recommend assigning an overall methodological quality score (MQS) to reviewed studies to assess their conceptual and methodological characteristics (Lee et al., 2002; Miller & Wilbourne, 2002; Wortman, 1994). Accordingly, each study in this review’s final sample was assessed and scored, to determine which ones met specific methodological standards (see Table 1). Seven studies (25%) were assessed by two reviewers, to check for inter-rater reliability and validity of the abstraction and methodological quality scoring processes. Raters achieved an agreement rate of 86% for all ten questions on the MQS form. On 5 of the questions (study type, the exclusive study of AA men, sample size, validity, and appropriate inference of conclusions), raters agreed 100%. Raters discussed their disagreements and achieved consensus prior to assigning the final MQS.
As expected, the reviewed studies varied in terms of their methodological quality (Table 1). The average MQS was 10.9 (SD = 3.44) with a median score of 10.5, within a range of 4 to 17 points (actual range, 0 to 21 total possible points). While none of the studies scored the maximum score, fourteen (50%) scored below average in terms of methodological quality.
In terms of conceptual quality, seventeen studies (60.7%) explicitly used one or more of the following theories: Health Belief Model (n = 12), Social Cognitive Theory (n = 4), Theory of Planned Behavior (n = 4), Dual Process Theory (n = 3), Social Support models (n = 3), Stages of Change/Transtheoretical Model (n = 3), Powe Fatalism Model (n = 2), Patient/Provider/System Theoretical Model (n = 1), Kleinman’s Explanatory Models of Illness (n = 1), Mediational Model (n = 1), Precaution Adoption Process Model (n = 1), PRECEDE-PROCEED Model (n = 1), Risk Reappraisal Hypothesis (n = 1), and the Social-Ecological Model (n = 1). Ten studies (35.7%) did not report a theoretical framework.
Regarding the research design, most reviewed studies (82.1%) comprised cross-sectional designs and more than a third (35.7%) examined medium (100 ≤ n participants ≤ 300) samples. Although all studies included AA men in their sample, none had samples comprising AA men, exclusively.
The majority of the studies utilized a non-experimental research paradigm (71.4%), a phenomenon that may have affected the overall methodological quality of the study. Of the 9 studies (32.1%) utilizing more robust statistical techniques, all but one were non-experimental in design.
Convenience/nonprobability sample designs (64.3%) were utilized the most, but the majority of researchers failed to report their data’s validity and reliability: only 28.6% reported any data validity and 28.6% reported any data reliability. It is important to note that we considered non-reporting of data validity and reliability as a function of overall methodological precision and care, not a function of the measures being used or the design itself (albeit only quantitative studies would require tests of data validity/reliability). Accordingly, we awarded the study a score if any reporting was available, including – although not ideal -- validity/reliability information from other samples, in previously conducted studies.
Two longitudinal intervention studies, Campbell et al. (2004) and Leone and colleagues (2010), obtained the highest MQS of 17 total points as they explicitly used theory, had large random but not nationally representative samples (> 300 participants), and utilized a 2 × 2 factorial research design. The WATCH (Wellness for African Americans through Churches) Project examined by the two teams of researchers was primarily guided by Social Cognitive Theory, the Stages of Change Transtheoretical framework, the Health Belief Model, and Social Support models (Campbell et al, 2004; Leone et al., 2010). Both of these studies also reported validity and reliability of their own data, and utilized multiple/logistic regression for analyses. Table 3 presents the theoretical, design, and methodological features of the 28 reviewed studies in detail.
Table 3.
Matrix of 28 reviewed studies, according to theoretical, design, and methodological features.
| Study | Theoretical Framework |
Research Design | Sample Design | Most Advanced Statistical Analysis |
Validity & Reliability Reported |
|---|---|---|---|---|---|
| Campbell et al., 2004 | Explicit use | Experimental | Random/Not Nationally Representative | Multiple/Logistic Regression |
Validity: Yes Reliability: Yes |
| DeBourcy et al., 2007 | Not reported | Non-experimental | Convenience/Nonprobability | Multivariate statistics |
Validity: No Reliability: No |
| Fisher et al., 2007 | Not reported | Non-experimental | Convenience/Nonprobability | Multiple/Logistic Regression |
Validity: No Reliability: No |
| Ford et al., 2006 | Not reported | Non-experimental | Random/Not Nationally Representative | Multiple/Logistic Regression |
Validity: No Reliability: No |
| Geiger et al., 2007 | Not reported | Non-experimental | Random/Nationally Representative | ANOVA/Bivariate statistics |
Validity: No Reliability: No |
| Glenn et al., 2011 | Explicit use | Experimental | Random/Not Nationally Representative | Multiple/Logistic Regression |
Validity: No Reliability: No |
| Good et al., 2010 | Not reported | Non-experimental | Convenience/Nonprobability | Descriptive/Univariate statistics |
Validity: No Reliability: No |
| Greiner et al., 2005 | Explicit use | Non-experimental | Convenience/Nonprobability | Qualitative Analyses |
Validity: No Reliability: No |
| Greiner et al., 2005 | Implicit use | Quasi-experimental | Convenience/Nonprobability | Multivariate statistics |
Validity: Yes Reliability: Yes |
| Griffith et al., 2008 | Not reported | Non-experimental | Random/Not Nationally Representative | Multivariate statistics |
Validity: No Reliability: No |
| Griffith et al., 2012 | Not reported | Non-experimental | Convenience/Nonprobability | Qualitative Analyses |
Validity: No Reliability: No |
| Holt et al., 2011 | Implicit use | Quasi-experimental | Convenience/Nonprobability | ANOVA/Bivariate statistics |
Validity: No Reliability: No |
| James et al., 2011 | Implicit use | Non-experimental | Convenience/Nonprobability | Descriptive/Univariate statistics |
Validity: No Reliability: No |
| James et al., 2008 | Not reported | Non-experimental | Convenience/Nonprobability | Multiple/Logistic Regression |
Validity: Yes Reliability: No |
| James et al., 2008 | Explicit use | Non-experimental | Convenience/Nonprobability | Multiple/Logistic Regression |
Validity: No Reliability: No |
| Leone et al., 2010 | Explicit use | Experimental | Random/Not Nationally Representative | Multiple/Logistic Regression |
Validity: Yes Reliability: Yes |
| Manne et al., 2009 | Implicit use | Non-experimental | Convenience/Nonprobability | Generalized estimating equations (GEE) |
Validity: Yes Reliability: Yes |
| Manne et al., 2003 | Implicit use | Non-experimental | Convenience/Nonprobability | Structural equation modeling (SEM) |
Validity: Yes Reliability: No |
| Manne et al., 2002 | Explicit use | Non-experimental | Convenience/Nonprobability | Generalized estimating equations (GEE); Hierarchical stepwise logistic regression |
Validity: Yes Reliability: No |
| McNeill et al., 2009 | Explicit use | Experimental | Random/Not Nationally Representative | Descriptive/Univariate statistics |
Validity: Yes Reliability: No |
| Menon et al., 2003 | Explicit use | Quasi-experimental | Random/Not Nationally Representative | Binomial logistic regression |
Validity: No Reliability: Yes |
| Palmer et al., 2007 | Explicit use | Quasi-experimental | Random/Not Nationally Representative | Multivariate logistic regression |
Validity: No Reliability: Yes |
| Powe et al., 2006 | Explicit use | Non-experimental | Convenience/Nonprobability | ANOVA/Bivariate statistics |
Validity: No Reliability: Yes |
| Purnell et al., 2010 | Explicit use | Non-experimental | Convenience/Nonprobability | Multiple/Logistic Regression |
Validity: No Reliability: No |
| Sheikh et al., 2004 | Not reported | Non-experimental | Convenience/Nonprobability | ANOVA/Bivariate statistics |
Validity: No Reliability: No |
| Tseng et al., 2009 | Implicit use | Non-experimental | Convenience/Nonprobability | Multiple logistic regression |
Validity: No Reliability: Yes |
| Winterich et al., 2011 | Explicit use | Non-experimental | Convenience/Nonprobability | Qualitative Analyses |
Validity: No Reliability: No |
| Yim et al., 2012 | Not reported | Non-experimental | Random/Not Nationally Representative | Descriptive/Univariate statistics |
Validity: No Reliability: No |
Discussion
In fulfilling its first purpose—to synthesize the evidence from published studies examining AA men's knowledge, beliefs, and behaviors regarding CRCS—this review identified 6 key factors associated with CRC and CRCS. These 6 factors included: previous CRCS (screening history), CRC test preference, perceived benefits, perceived barriers, CRC and CRCS knowledge, and physician support/recommendation.
Also supporting the findings in this review, previous screening (screening history) and test preference were significant factors associated with early detection screening for other diseases, besides CRC. For instance, Lerman and colleagues (1990) conducted a study of 910 women ages 50 years and over. The researchers learned “women who had a mammogram in the past 12 months…[believed] mammograms were effective in detecting early breast cancer” (Lerman et al., 1990, p. 238). Since AA women have a 41% higher rate of breast cancer death than their White counterparts, screening history is a factor that should not be taken lightly in the troubling, yet similar, racial divide for AA men who have CRC mortality rates 50% higher than White men (ACS, 2014; ACS, 2013).
Perceived benefits, perceived barriers, and lack of knowledge also have been reported as factors influencing decisions regarding adherence to, or underutilization of colonoscopy screening alone. For instance, in a study by Harewood and colleagues (2002), researchers studied the perceptions of patients who never had a CRCS procedure and previously screened patients to identify the colonoscopy screening barriers that were most critical in deterring participation. A substantial knowledge deficit of the curability of early stage CRC was reported as a factor that affected never-screened patients’ lack of participation in colonoscopy screening. Similarly, never-screened respondents in this study were more likely to overestimate the risk of complications from a colonoscopy (Harewood et al., 2002).
This review’s finding that physician support/recommendation is a critical factor is consistent with the literature. In the study conducted by Post and colleagues (2008), a questionnaire assessing patients’ knowledge, beliefs, and barriers regarding CRC and CRCS screening was completed by 104 participants who were at least 51 years of age. Physician recommendation for a CRCS test was significantly associated with CRCS. With a physician’s recommendation, participants showed odds of completing a CRCS test of 11.24 times those of other participants. Other research has confirmed the importance of physician involvement and communication (Bass et al., 2011; Epstein & Street, 2007).
Fear of any pain or discomfort associated with the CRCS procedures, fear of illness or diagnosis, and perceived CRC severity were other factors reported, yet not as frequently. For instance, fear/anxiety was a key theme in the qualitative study with sixteen patients (> age 50 with no previous colonoscopy or medical comorbidities) who received patient navigation services but did not complete a colonoscopy (Sly et al., 2013). “When asked specifically why they had not completed the scheduled colonoscopy, half of the participants said they were fearful or anxious about the colonoscopy and indicated this was the primary reason they did not keep their scheduled appointment” (Sly et al., 2013, p. 453).
The review we reported here has been useful in synthesizing the salient factors shaping young AA men’s view of CRC and CRCS behaviors. Armed with this knowledge, how should health promoters (and, in particular, health educators) proceed? Teutsch (2003) argues the ability to effectively communicate is critical and represents a potential solution to many health disparities issues. Communication between health promoters and the lay public, between health care providers and their patients, between scientists and practitioners – all forms of communication, if taking the factors synthesized in this review into account, may represent a strategy for changing the health disparities status-quo. Specifically supported in our findings is the suggestion medical providers capitalize on their influence and join policy makers in efforts to eliminate CRCS disparities among AA men.
A second purpose of this review was to assess the methodological quality of the reviewed studies. The mean methodological quality score (MQS) of 10.9 indicates these studies are, overall, of medium quality (relative to a perfect score totaling 21), and an array of significant flaws transpire from this analysis.
The first weakness of this body of literature involves the extensive use of non-experimental research designs. Only 4 of the 28 reviewed studies (14.3%) utilized the gold standard for research, experimental designs (specifically, Randomized Control Trials). The majority (n = 20; 71%) employed non-experimental research designs (e.g., exploratory and/or qualitative studies). Future research should strive to either be driven by methodologically rigorous designs that are also theory-based, or be guided by naturalistic inquiry approaches, in order to elicit the complexity of, and relationships among, the multi-level factors affecting screening behaviors. Granted, examination of factors influencing behaviors does not easily lend itself to neat, experimental designs, and most researchers must rely on convenience or clinical samples available to them. Furthermore, quantitative researchers often struggle with negative perceptions of qualitative inquiry and shy away from naturalistic approaches. Anderson and Taylor (2009) suggest such negative perceptions include weakened reliability since the process relies on the abilities and insights of the observer; small, selective samples that not only influence generalizability, but limit statistical descriptions of large populations; and unavoidable researcher bias and idiosyncrasies. Nonetheless, it is important researchers remain aware of the need for rigor, and strive to achieve the highest methodological standards in their studies, along with the most meaningful and useful data, possible.
A second weakness in this group of studies is the absence of samples comprised exclusively of AA men. A little more than a third of the studies (36%) involved a medium sample size sample (100 ≤ n ≤ 300) and 64% employed convenience/nonprobability sample designs. Although the sample sizes are respectable, the fact none of the studies exclusively examined AA men does not allow for generalizable results that can assist in developing effective interventions to decrease CRC and CRCS disparities among this population.
A third and final weakness involves data analyses. The most advanced statistical techniques (e.g., structural equation modeling) were only utilized by 32% of the studies. It appears some studies attempted to compensate for weak research and sample designs with more rigorous statistical analyses. Yet, when 71% of the reviewed studies did not report any tests of validity or reliability of their own data, it becomes difficult to determine the quality of the evidence being reported, thus undermining the confidence readers/consumers can have regarding the data analyses. Without testing for the data’s validity and reliability, there is no way to determine how much measurement error comes into play and may be weakening the evidence. The quality of the data, therefore, is being taken for granted and assumed to be high; policies, practices and interventions may be based on data for which there is, in fact, no evidence of quality. Future researchers, therefore, should strive to report evidence of the quality of their data, and tests of validity and reliability are among the most common types of evidence can be easily provided. Given validity and reliability are sample-specific, they should be documented in each research report (Thompson, 2002).
Alongside the weaknesses in the reviewed body of literature, the review itself suffers from specific limitations. One limitation is a weakness inherent in nearly all systematic literature reviews and meta-analyses: the possibility of having missed one or more relevant studies/reports. We made every effort, however, to ensure our search yielded all relevant data. For instance, to be as inclusive as possible throughout the search process, we not only searched electronic databases, we also added a manual search of cited references (i.e., reference lists of electronically-identified reports). This technique retrieved additional references which were not indexed appropriately in the databases originally searched.
Another limitation is the lack of validation of the MQS criteria we chose to use in this study, and its bias towards quantitative studies. Nonetheless, the criteria we developed were based on previously published reports (e.g., Goodson et al., 2006), and found to adequately capture most of the salient methodological characteristics of empirical studies.
Despite these limitations, this review contributes to the body of knowledge on younger AA men's knowledge, beliefs, and behaviors regarding CRCS, by organizing and assessing the quality of the available evidence. We hope that findings from this review can guide future research in terms of its focus and rigor, and foster the development of appropriate educational interventions promoting the health of African American men in the U.S.
Supplementary Material
Acknowledgements
This research was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA163184 (Drs. Jean Forster and Kola Okuyemi, Co-PIs). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Charles R. Rogers, University of Minnesota Medical School, Dept. of Family Medicine & Community Health, 717 Delaware St. SE, Suite 166, Minneapolis, MN 55414, Tel. 612-626-3894, Fax. 612-626-6782, crrogers@umn.edu.
Patricia Goodson, Texas A&M University, Dept. of Health & Kinesiology, TAMU 4243, College Station, TX 77843.
Margaret J. Foster, Texas A&M University, Medical Sciences Library, TAMU 4462, College Station, TX 77843.
References
- Agrawal S, Bhupinderjit A, Bhutani MS, Romero Y, Srinivasan R, Figueroa-Moseley C. Colorectal cancer in African Americans. The American Journal of Gastroenterology. 2005;100(3):515–523. doi: 10.1111/j.1572-0241.2005.41829.x. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Colorectal cancer facts & figures 2014–2016. 2014 Retrieved from http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures for African Americans Cancer Facts & Figures for African Americans 2013–2014. 2013 Retrieved from http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036921.pdf. [Google Scholar]
- Andersen ML, Taylor HF. Sociology: The essentials. Sixth Ed. Belmond, CA: Thomson Wadsworth; 2009. [Google Scholar]
- Bambra C. Real world reviews: A beginner’s guide to undertaking systematic reviews of public health policy interventions. Journal of Epidemiology & Community Health. 2011;65(1):14–19. doi: 10.1136/jech.2009.088740. [DOI] [PubMed] [Google Scholar]
- Bass SB, Gordon TF, Ruzek SB, Wolak C, Ward S, Paranjape A, Ruggieri D. Perceptions of colorectal cancer screening in urban African American clinic patients: Differences by gender and screening status. Journal of Cancer Education. 2011;26(1):121–128. doi: 10.1007/s13187-010-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsey J. What is evidence-based medicine? London, UK: Hayward Medical Communication; 2009. [Google Scholar]
- Calvocoressi L, Claus EB, Jones BA, Kasi SV, Stolar M. Applying recursive partitioning to a prospective study of factors associated with adherence to mammography screening guidelines. American Journal of Epidemiology. 2005;162(12):1215–1224. doi: 10.1093/aje/kwi337. [DOI] [PubMed] [Google Scholar]
- Campbell MK, James A, Hudson MA, Carr C, Jackson E, Oakes V, Tessaro I. Improving multiple behaviors for colorectal cancer prevention among African American church members. Health Psychology. 2004;23(5):492–502. doi: 10.1037/0278-6133.23.5.492. [DOI] [PubMed] [Google Scholar]
- Champion V, Russell K, Skinner C. Psychosocial factors related to repeat mammography screening over five years in African American women. Cancer Nursing. 2006;29(3):236–243. doi: 10.1097/00002820-200605000-00012. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: Synthesis of best evidence for clinical decisions. Annals of Internal Medicine. 1997;127(5):376–380. doi: 10.7326/0003-4819-126-5-199703010-00006. [DOI] [PubMed] [Google Scholar]
- DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. Journal of General Internal Medicine. 2007;23(2):169–174. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, O'Rourke K. Systematic reviews in health care: Meta-analysis in context, second edition. New York, NY: BMJ Publishing Group; 2001. Chapter 1. introduction: Rationale, potentials, and promise of systematic reviews; pp. 3–19. [Google Scholar]
- Epstein RM, Street RL. Patient-centered communication in cancer care: Promoting healing and reducing suffering. Bethesda, MD: National Cancer Institute; 2007. (NIH Publication No. 07-6225). [Google Scholar]
- Fisher DA, Johnson MR, Shaheen NJ. Fecal occult blood testing completion in a VA population: Low and strongly related to race. Journal of Clinical Outcomes Management. 2007;14(2):93–98. [Google Scholar]
- Ford JS, Coups EJ, Hay JL. Knowledge of colon cancer screening in a national probability sample in the United States. Journal of Health Communication. 2006;11(Suppl 1):19–35. doi: 10.1080/10810730600637533. [DOI] [PubMed] [Google Scholar]
- Garrard J. Health sciences literature review made easy: The matrix method. Sudbury, MA: Jones & Bartlett; 2014. [Google Scholar]
- Geiger TM, Miedema BW, Geana MV, Thaler K, Rangnekar NJ, Cameron GT. Improving rates for screening colonoscopy: Analysis of the health information national trends survey (HINTS I) data. Surgical Endoscopy. 2008;22(2):527–533. doi: 10.1007/s00464-007-9673-2. [DOI] [PubMed] [Google Scholar]
- Glenn BA, Herrmann AK, Crespi CM, Mojica CM, Chang LC, Maxwell AE, Bastani R. Changes in risk perceptions in relation to self-reported colorectal cancer screening among first-degree relatives of colorectal cancer cases enrolled in a randomized trial. Health Psychology. 2011;30(4):481–491. doi: 10.1037/a0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K, Niziolek J, Yoshida C, Rowlands A. Insights into barriers that prevent African Americans from seeking colorectal screenings: A qualitative study. Gastroenterology Nursing. 2010;33(3):204–208. doi: 10.1097/SGA.0b013e3181e379ed. [DOI] [PubMed] [Google Scholar]
- Goodson P, Buhi ER, Dunsmore SC. Self-esteem and adolescent sexual behaviors, attitudes, and intentions: A systematic review. Journal of Adolescent Health. 2006;38(3):310–319. doi: 10.1016/j.jadohealth.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Greiner KA, Born W, Nollen N, Ahluwalia JS. Knowledge and perceptions of colorectal cancer screening among urban African Americans. Journal of General Internal Medicine. 2005;20(11):977–983. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner KA, James AS, Born W, Hall S, Engelman KE, Okuyemi KS, Ahluwalia JS. Predictors of fecal occult blood test (FOBT) completion among low-income adults. Preventive Medicine. 2005;41:676–684. doi: 10.1016/j.ypmed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Griffith KA, McGuire DB, Royak-Schaler R, Plowden KO, Steinberger EK. Influence of family history and preventive health behaviors on colorectal cancer screening in African Americans. Cancer. 2008;113:276–285. doi: 10.1002/cncr.23550. [DOI] [PubMed] [Google Scholar]
- Griffith KA, Passmore SR, Smith D, Wenzel J. African Americans with a family history of colorectal cancer: Barriers and facilitators to screening. Oncology Nursing Forum. 2012;39(3):299–306. doi: 10.1188/12.ONF.299-306. [DOI] [PubMed] [Google Scholar]
- Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN. Colorectal cancer screening barriers and facilitators in older persons. Preventive Medicine. 2010;50(1–2):3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. London, UK: John Wiley & Sons, Inc.; 2008. [Google Scholar]
- Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: Enhancing the use and quality of colorectal cancer screening. Annals of Internal Medicine. 2010;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- Holt CL, Shipp M, Eloub M, Fouad MN, Britt K, Norena M. Your body is the temple: Impact of a spiritually based colorectal cancer educational intervention delivered through community health advisors. Health Promotion Practice. 2011;12(4):577–588. doi: 10.1177/1524839910370421. [DOI] [PubMed] [Google Scholar]
- James AS, Daley CM, Greiner KA. Knowledge and attitudes about colon cancer screening among African Americans. American Journal of Health Behavior. 2011;35(4):393–401. doi: 10.5993/ajhb.35.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AS, Hall S, Greiner KA, Buckles D, Born WK, Ahluwalia JS. The impact of socioeconomic status on perceived barriers to colorectal cancer testing. American Journal of Health Promotion. 2008;23(2):97–100. doi: 10.4278/ajhp.07041938. [DOI] [PubMed] [Google Scholar]
- James AS, Leone L, Katz ML, McNeill LH, Campbell MK. Multiple health behaviors among overweight, class i obese, and class ii obese persons. Ethnicity & Disease. 2008;18:157–162. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: A Cancer Journal for Clinicians. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Lee KP, Schotland M, Bacchetti P, Bero LA. Association of journal quality indicators with methodological quality of clinical research articles. Journal of the American Medical Association. 2002;287(21):2805–2808. doi: 10.1001/jama.287.21.2805. [DOI] [PubMed] [Google Scholar]
- Leone LA, James AS, Allicock M, Campbell MK. Obesity predicts differential response to cancer prevention interventions among African Americans. Health Education & Behavior. 2010;37(6):913–925. doi: 10.1177/1090198109353388. [DOI] [PubMed] [Google Scholar]
- Lerman C, Rimer B, Trock B, Balshem A, Engstrom PF. Factors associated with repeat adherence to breast cancer screening. Preventive Medicine. 1990;19(3):279–290. doi: 10.1016/0091-7435(90)90028-i. [DOI] [PubMed] [Google Scholar]
- Light RJ, Pillemer DB. Summing up: The science of reviewing research. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- Manne S, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, Winkel G. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Annals of Behavioral Medicine. 2009;37:207–217. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne S, Markowitz A, Winawer S, Guillem J, Meropol NJ, Duncan T. Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: A test of a mediational model. Preventive Medicine. 2003;36(1):71–84. doi: 10.1006/pmed.2002.1122. [DOI] [PubMed] [Google Scholar]
- Manne S, Markowitz A, Winawer S, Meropol NJ, Haller D, Rakowski W, Jandorf L. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychology. 2002;21(1):3–15. [PubMed] [Google Scholar]
- McNeill LH, Coeling M, Puleo E, Suarez EG, Bennett GG, Emmons KM. Colorectal cancer prevention for low-income, sociodemographically-diverse adults in public housing: Baseline findings of a randomized controlled trial. BMC Public Health. 2009;9(353) doi: 10.1186/1471-2458-9-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon U, Champion VL, Largin GN, Zollinger TW, Gerde PM, Vernon SW. Beliefs associated with fecal occult blood test and colonoscopy use at a worksite colon cancer screening program. Journal of Occupational and Environmental Medicine. 2003;45(8):891–898. doi: 10.1097/01.jom.0000083038.56116.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W, Wilbourne P. Mesa grande: A methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97(3):265–277. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Oxman AD, Guyatt GH. Guidelines for reading literature reviews. Canadian Medical Association Journal. 1988;138(8):697–703. [PMC free article] [PubMed] [Google Scholar]
- Palmer RC, Emmons KM, Fletcher RH, Lobb R, Miroshnik I, Kemp JA, Bauer M. Familial risk and colorectal cancer screening health beliefs and attitudes in an insured population. Preventive Medicine. 2007;45(5):336–341. doi: 10.1016/j.ypmed.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Post DM, Katz ML, Dickson SL, Lemeshow S, Paskett EE. Determinants of colorectal cancer screening in primary care. Journal of Cancer Education. 2008;23(4):241–247. doi: 10.1080/08858190802189089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe BD, Finnie R, Ko J. Enhancing knowledge of colorectal cancer among African Americans: Why are we waiting until age 50? Gastroenterology Nursing. 2006;29(1):42–49. doi: 10.1097/00001610-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Katz ML, Andersen BL, Palesh O, Figueroa-Moseley C, Jean-Pierre P, Bennett N. Social and cultural factors are related to perceived colorectal cancer screening benefits and intentions in African Americans. Journal of Behavioral Medicine. 2010;33(1):24–34. doi: 10.1007/s10865-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American college of gastroenterology guidelines for colorectal cancer screening 2008. American Journal of Gastroenterology. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. An evaluation of procedures and results. In: Wachter K, Straf M, editors. The future of meta-analysis. New York, NY: Russell Sage Foundation; 1990. pp. 123–134. [Google Scholar]
- Sheikh RA, Kapre S, Calof OM, Ward C, Raina A. Screening preferences for colorectal cancer: A patient demographic study. Southern Medical Journal. 2004;97(3):224–230. doi: 10.1097/01.SMJ.0000078619.39604.3D. [DOI] [PubMed] [Google Scholar]
- Sly JR, Edwards T, Shelton RC, Jandorf L. Identifying barriers to colonoscopy screening for nonadherent African American participants in a patient navigation intervention. Health Education & Behavior. 2013;40(4):449–457. doi: 10.1177/1090198112459514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutsch C. Patient-doctor communication. The Medical Clinics of North America. 2003;87(5):1115–1145. doi: 10.1016/s0025-7125(03)00066-x. [DOI] [PubMed] [Google Scholar]
- Thompson B. Score reliability: Contemporary thinking on reliability issues. Thousand Oaks, CA: SAGE Publications, Inc.; 2002. [Google Scholar]
- Tseng T, Holt CL, Shipp M, Eloubeidi M, Britt K, Norena M, Fouad MN. Predictors of colorectal cancer knowledge and screening among church-attending African Americans and Whites in the deep south. Journal of Community Health. 2009;34:90–97. doi: 10.1007/s10900-008-9128-2. [DOI] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group. United States cancer statistics: 1999–2009 incidence and mortality web-based report. 2013 Retrieved from http://apps.nccd.cdc.gov/uscs/cancersbyraceandethnicity.aspx.
- Winterich JA, Quandt SA, Grzywacz JG, Clark P, Dignan M, Stewart JH, Arcury TA. Men's knowledge and beliefs about colorectal cancer and 3 screenings: Education, race, and screening status. American Journal of Health Behavior. 2011;35(5):525–534. doi: 10.5993/ajhb.35.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortman PM. Judging research quality. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York, NY: Russell Sage Foundation; 1994. pp. 97–109. [Google Scholar]
- Yim M, Butterly LF, Goodrich ME, Weiss JE, Onega TL. Perception of colonoscopy benefits: A gap in patient knowledge? Journal of Community Health. 2012;37:719–724. doi: 10.1007/s10900-011-9506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.