Abstract
Certain tastants inhibit oral irritation by capsaicin, whereas anesthesia of the chorda tympani (CT) enhances oral capsaicin burn. We tested the hypothesis that tastants activate the CT to suppress responses of trigeminal subnucleus caudalis (Vc) neurons to noxious oral stimuli. In anesthetized rats, we recorded Vc unit responses to noxious electrical, chemical (pentanoic acid, 200 μm) and thermal (55 °C) stimulation of the tongue. Electrically evoked responses were significantly reduced by a tastant mix and individually applied NaCl, monosodium glutamate (MSG), and monopotassium glutamate. Sucrose, citric acid, quinine and water (control) had no effect. Pentanoic acid-evoked responses were similarly attenuated by NaCl and MSG, but not by other tastants. Responses to noxious heat were not affected by any tastant. Transection and/or anesthesia of the CT bilaterally affected neither Vc neuronal responses to electrical or pentanoic acid stimulation, nor the depressant effect of NaCl and MSG on electrically evoked responses. Calcium imaging showed that neither NaCl nor MSG directly excited any trigeminal ganglion cells or affected their responses to pentanoic acid. GABA also had no effect, arguing against peripheral effects of GABA, NaCl or MSG on lingual nocicepive nerve endings. The data also rule out a central mechanism, as the effects of NaCl and MSG were intact following CT transection. We speculate that the effect is mediated peripherally by the release from taste receptor cells (type III) of some mediator(s) other than GABA to indirectly inhibit trigeminal nociceptors. The results also indicate that the CT does not exert a tonic inhibitory effect on nociceptive Vc neurons.
Keywords: calcium imaging, chorda tympani, tastant, trigeminal ganglion cell
Introduction
The gustatory and trigeminal somatosensory systems of mammals are intimately associated in the task of processing respective taste and tactile signals arising from the mouth during feeding. On the one hand, there is substantial evidence for modulation of taste processing by the trigeminal system (Wang et al. 1995; Boucher et al. 2003; Simons et al. 2003; Felizardo et al., 2009). On the other hand, available data suggest that the gustatory system can also modulate trigeminal inputs. For example, a mixture of NaCl, a gustatory stimulus, and capsaicin, a trigeminal stimulus, evoked a stronger burning sensation than capsaicin alone (Prescott et al., 1993). Conversely, oral burn elicited by capsaicin was suppressed in the presence of a mixture of menthol, acid, and sucrose (Lugaz, 2004). Stevens & Lawless (1986) reported decreased oral burn caused by capsaicin and piperine when various tastants were delivered to the mouth. The potential modulation of trigeminal pain pathways by the gustatory system has important clinical implications. It has been hypothesized that trigeminal inputs are inhibited by the chorda tympani (CT) and facial (VII) nerves (Bartoshuk, 2000), similarly to the inhibitory interactions between the glossopharyngeal (IX) and facial nerves (Catalanotto et al., 1993; Yanagisawa et al., 1998). In favor of this hypothesis, anesthesia of the CT increased the burning sensation elicited by capsaicin applied to the surface of the tongue (Tie et al., 1999). Also, patients suffering from burning mouth syndrome show gustatory deficits (Eliav et al., 2007). Further support comes from preclinical studies showing that transection of the CT acutely increased the expression of neuronal nitric oxide synthase in the trigeminal subnucleus caudalis (Vc; Takemura et al., 1994), and that CT stimulation elicited expression of Fos, a marker of nociceptive neurons, in the Vc (Harrison, 2001).
The aims of the present electrophysiological study were threefold. First, we wished to test whether tastants affect the responses of nociceptive Vc neurons to noxious electrical, chemical or thermal stimulation of the tongue. We found that NaCl and glutamate had inhibitory effects on Vc neuronal responses. The second aim was to investigate the role of the CT in modulating trigeminal nociception, by determining whether disruption of the CT affected baseline nociceptive responses of Vc neurons and/or tastant modulation of Vc neuronal responses to noxious stimuli. We observed that disruption of the CT input had no effect, suggesting a peripheral site for gustatory–trigeminal modulation. Our third aim was to investigate this possibility, using calcium imaging of trigeminal ganglion (TG) cells. We tested whether tastants directly activated TG cells, and whether they affected responses of TG cells elicited by pentanoic acid. Portions of this study have been reported in abstract form (Boucher et al., 2012).
Materials and methods
Single-unit recording from the Vc
Surgery and CT anesthesia/transection
The experiments were conducted under a protocol approved by the University of California, Davis Institutional Animal Care and Use Committee, and followed the ethical guidelines of the International Association for the Study of Pain and the European Community Council directive of 24 November 1986 (86/609/EEC). Forty-seven adult male Sprague–Dawley rats (Simonsen, Gilroy, CA, USA), weighing 400–500 g, were anesthetized with pentobarbital (65 mg/kg, intraperitoneal). After induction, a catheter was placed in the external jugular vein, and anesthesia was maintained by constant intravenous infusion of pentobarbital at a rate sufficient to maintain areflexia, as described previously (Carstens et al., 1998; Dessirier et al., 2000). The electrocardiogram was recorded continually with a Powerlab interface and chart software (AD Instruments, Colorado Springs, CO, USA), and core body temperature was monitored and maintained with an electrically thermostatically controlled blanket coupled to a rectal probe. The head was fixed in a stereotaxic system with atraumatic hollow ear bars. The eyes were covered with petroleum jelly to avoid drying of the cornea. A laminectomy exposed the upper cervical spinal cord and lower brainstem to allow access to the Vc (for details, see Carstens et al., 1998; Dessirier et al., 2000). The mouth of the rat was kept open to allow access to the oral cavity, and regularly irrigated with distilled water to prevent desiccation before chemical or electrical stimulation of the tongue.
Anesthesia and transection of the CT were performed in separate experiments with different approaches. Anesthesia of the CT was accomplished according to the procedure described by Dinkins & Travers (1998). The tympanic bulla was exposed under a surgical microscope, and the tympanic membrane was carefully perforated to allow the diffusion of lidocaine to the CT. Lidocaine (2%) was injected onto the tympanic membrane in a volume of 0.02 mL with a Hamilton microsyringe connected to a polyethylene tube that was passed through the hollow ear bar such that its tip was located at the tympanic membrane. This procedure was reported to result in anesthesia of the CT in < 1 min (Dinkins & Travers, 1998). Then, physiological saline was injected through the tubing, which has been reported to lead to recovery of CT responses in < 10 min (Dinkins & Travers, 1998). At the end of the experiment, an equal volume of Evans blue was applied in the same manner, and confirmed to flood the tympanic bulla to access the CT.
For transection of the CT, the mandibular condyle was exposed by dissection of the masseter and temporal muscles, and resected. The lateral and medial pterygoid muscles were carefully dissected to avoid bleeding of the adjacent venous sinuses, and, under a surgical microscope, the CT was isolated and separated from the lingual nerve central to its entry into the tympanic bulla. A cotton thread was placed around the CT and loosely knotted, and saline-soaked cotton was placed over the CT to prevent desiccation. The CT was transected by pulling the thread, and was verified visually at the end of the experiment.
Recording
Single-unit (n = 29 units) or multiple-unit (17 dual-unit recordings = 34 units; five triple-unit recordings = 15 units) recordings were made with a tungsten recording microelectrode (10–14 MΩ; Frederick Haer, Brunswick, ME, USA) that was advanced into the superficial laminae (< 300 μm from the brainstem surface) of the dorsomedial Vc at a position 0–2 mm caudal to the obex and 1.5–1.7 mm lateral to the midline. Extracellular action potentials were amplified, digitized by conventional means (CED 1401, CED 1901; Cambridge Instruments, Cambridge, UK), and sent to a computer for later analysis with spike 2 software (CED; Cambridge Instruments). In cases in which 2 or 3 units were recorded simultaneously, they were distinguished on the basis of spike amplitude and waveform. Vc single units responsive to pinching of the tongue were isolated, and additionally tested for responses to noxious stimuli, as described below.
Stimulus paradigm
Vc units isolated by pinching the tongue were then tested for responses to noxious heat (55 °C water) and application of pentanoic acid (250 μm). Pentanoic acid was selected because it elicits responses in Vc neurons that are reproducible across trials, showing neither sensitization nor desensitization (Dessirier et al., 2000; Fig. 3C), in contrast to capsaicin, which excites Vc neurons in a manner showing strong desensitization (Dessirier et al., 2000). Responsive units were also tested with electrical stimulation of the tongue (or the lip). Electrical stimuli were delivered through an isolation transformer with a Grass S88 stimulator (Astro-Med, W. Warwick, RI, USA). The parameters for electrical stimulation (brief trains of 1–5 square-wave pulses of duration 0.2–10 ms, 1–5 Hz, 5–15 V) were adjusted such that the electrical stimulus train elicited a response that approximately matched the peak firing rate elicited by heat and pentanoic acid stimuli (Fig. 2). Electrical stimulus trains were delivered at 120-s intervals, which was sufficient for evoked responses of all units to return to the prestimulus baseline firing level.
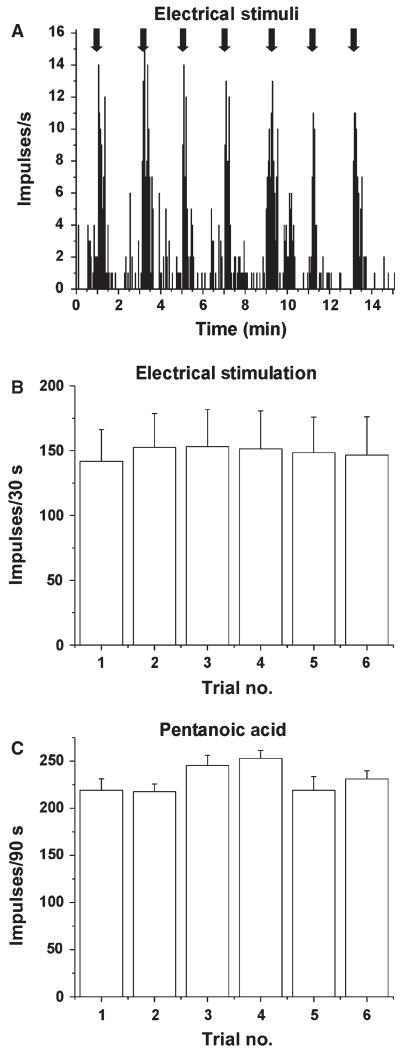
Fig. 3.
Reproducibility of responses of Vc units to electrical and chemical stimuli. (A) Peristimulus–time histogram showing an individual example of a Vc unit’s responses to repeated electrical tongue stimulation. (B) Mean responses of 19 Vc units to repetitive electrical stimuli; trials separated by 2 min. (C) Mean responses of 5 Vc units to repeated application of 250 μm pentanoic acid to the tongue; trials separated by 15 min.
Fig. 2.
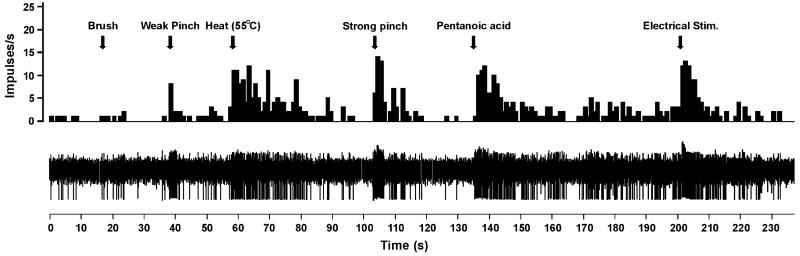
Example of Vc unit responses. Upper row – peristimulus–time histogram (bins – 1 s) of a unit’s responses to, from left to right, brush, weak pinch, noxious heat, strong pinch, pentanoic acid, and electrical stimulation (at arrows) delivered to the tongue. Lower row – raw spike traces.
The effect of gustatory stimulation on electrically evoked responses of Vc neurons was tested as follows. The gustatory stimuli were sucrose (300 mm), NaCl (100 mm), citric acid (30 mm), quinine (1 mm), monosodium glutamate (MSG; 200 mm), and monopotassium glutamate (MKG; 50 mm), or a mixture of sucrose, MSG, citric acid, NaCl, and quinine. Either an individual tastant or the tastant mixture was superfused at a constant flow rate at room temperature (23 °C) for 30 s over the anterior dorsal lingual surface with a hand-held syringe, prior to delivery of the electrical stimulus. Water delivered in the same manner served as a control. In a subset of experiments, the lower lip was electrically stimulated, and the effects of tastants were studied in the same way.
To test the effect of gustatory stimulation on chemonociceptive responses of Vc units, we used pentanoic acid. Repeated delivery of pentanoic acid (250 μm) to the tongue elicits consistent responses in Vc units (Dessirier et al., 2000). Pentanoic acid was delivered at a constant flow rate to the dorsal anterior tongue over a 30-s period, and this was followed by rinsing with saline, twice with a 15-min interstimulus interval. The first pentanoic acid stimulus was preceded by a 30-s period of application of water as control, and the second was preceded by application of the tastant.
The effects of gustatory stimulation on thermally evoked responses of Vc units were tested in two ways. In the first experiment, either hot water (55 °C) or a hot tastant mixture at 55 °C was applied to the anterior dorsal tongue for a period of 3 s in a blinded manner. In the second experiment, a filter paper (diameter, 0.5 mm) that had previously been immersed in either hot (55 °C) water or hot tastant mixture was placed onto the anterior dorsal tongue ipsilateral to the recorded Vc unit. In either case, the hot water and hot taste mixture stimuli were repeated three times, and responses were averaged.
Data analysis
Unit responses were quantified as number of action potentials per 30 s for electrical stimulation and number of action potentials per 60 s for pentanoic acid stimulation. For each experiment, unit responses to the electrical, chemical or thermal stimulus under control (water pretreatment) or tastant conditions were compared by use of either a Friedman test for comparison of three groups or a Wilcoxon signed rank test for comparison of paired data. Statistical tests were performed with spss version 9.0 (IBM, Chicago, IL, USA) or graphpad prism (GraphPad Software, La Jolla, CA, USA), with P < 0.05 considered to be significant.
Histology
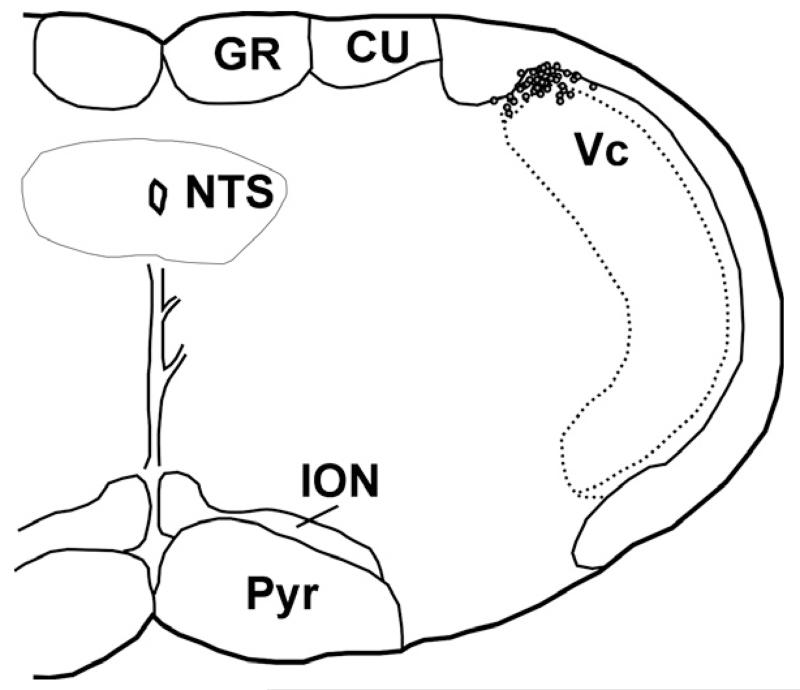
At the end of each recording session, an electrolytic lesion was made at the recording site by passing direct current (6 V) through the electrode for 45 s. The rats were killed with an overdose of anesthetic delivered through the jugular catheter, and the brains were harvested and fixed in 10% formalin for a minimum of 10 days, after which time they were frozen, cut into 50-μm sections, collected onto glass slides, and counterstained with neutral red. Lesion sites were identified under a light microscope and plotted onto a representative brainstem section (Fig. 1).
Fig. 1.
Vc recording sites in superficial laminae of the dorsomedial Vc. CU, cuneate nucleus; GR, gracile nucleus; ION, inferior olivary nucleus; NTS, nucleus of the solitary tract; Pyr, pyramid.
Calcium imaging of TG cells
Under a protocol approved by the UC Davis Institutional Animal Care and Use Committee, TGs were extracted and prepared as described in our recent publications (Klein et al., 2011a,b,c,d). Briefly, under isoflurane anesthesia, 12 juvenile (~3 weeks, ~100 g) male Sprague–Dawley rats (Simonsen) were killed, and TGs were extracted, minced, and incubated in 40 μL of papain (no. 3126; Worthington Biochemical Company, Lakewood, NJ, USA) with 1 mg of l-cysteine (Sigma); this was followed by centrifugation at 200 g for 2 min, and incubation in 2 mg/mL collagenase type II (CLS2; Worthington) at 37 °C, followed by centrifugation at 200 g for 1 min. Cells were then triturated and plated onto glass coverslips (Bellco, Vineland, NJ, USA) coated with 1 mg/mL poly(d-lysine) (Sigma). On the next day, cells were loaded 1 mm Fura 2AM (F1221; Invitrogen Life Sciences, Carlsbad, CA, USA) and 0.1% Pluronic (F127; Invitrogen). The coverslip was placed in a perfusion block and viewed through an inverted fluorescence microscope (Nikon Eclipse TS100). Fluorescence images at 340/380-nm wave-lengths were obtained at 3-s intervals with Nikon elements software. Solutions were delivered with a solenoid-operated perfusion system (ValveLink 8.2; AutoMate Scientific, Berkeley, CA, USA). The chemicals applied were sucrose (300 mm), NaCl (100 mm), citric acid (3 mm), quinine (0.5 mm), MSG (200 mm), pentanoic acid (20 μm), and GABA (100 μm), all dissolved in Ringer’s solution. All cells reported responded to Ringer’s solution containing 144 mm K+. A positive response to a chemical was defined as at least a 20% change in corrected ratio response (response post-application – baseline prior to application).
The effects of NaCl, glutamate and GABA on TG cell responses to pentanoic acid were tested as follows. In control experiments, pentanoic acid was delivered for 30 s, three times in succession at either 3- or 5-min interstimulus intervals. Baseline-corrected responses were normalized to the mean response to the first pentanoic acid stimulus. To test the effects of tastants, the same stimulus paradigm was followed, except that either NaCl, MSG or GABA was delivered for a 30-s period, 30 s prior to the second application of pentanoic acid. Normalized responses to each application of pentanoic acid were compared by use of repeated measures anova with Tukey post hoc tests for between-group comparisons. Statistical tests were performed with spss version 9.0 or graphpad prism, with P < 0.05 considered to be significant.
Results
Single-unit recordings from the Vc
Unit sample
Of the 78 Vc units recorded, 61 were classified as wide dynamic range type, in that they responded to noxious pinch, heat and/or pentanoic acid as well as innocuous pressure on the tongue; 17 responded to noxious stimuli but not to innocuous mechanical stimulation, and were classified as nociceptive-specific. All units had mechanosensitive receptive fields on the ipsilateral anterior tongue, and 22 of these also had mechanical receptive fields on the ipsilateral lower lip. Histologically localized recording sites were in the superficial laminae of the dorsomedial aspect of the Vc (Fig. 1), as previously reported (Carstens et al., 1998; Dessirier et al., 2000).
All units selected responded to noxious heat, lingual application of pentanoic acid, and electrical stimulation of the tongue. Figure 2 shows an individual example of a Vc unit’s responses to various stimuli.
Effects of tastants on electrically evoked Vc responses
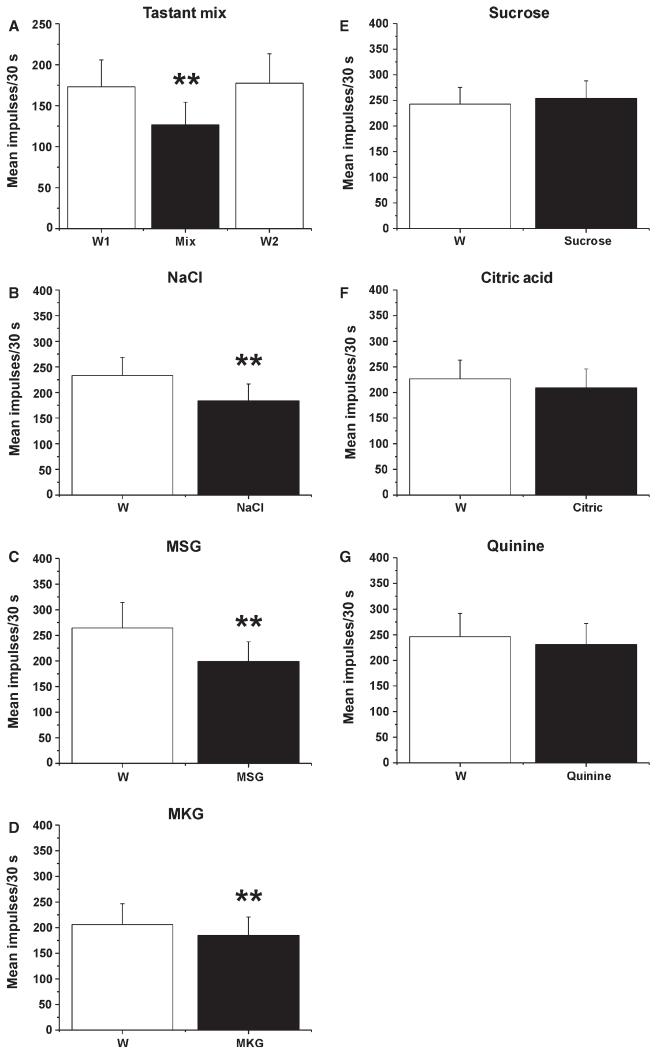
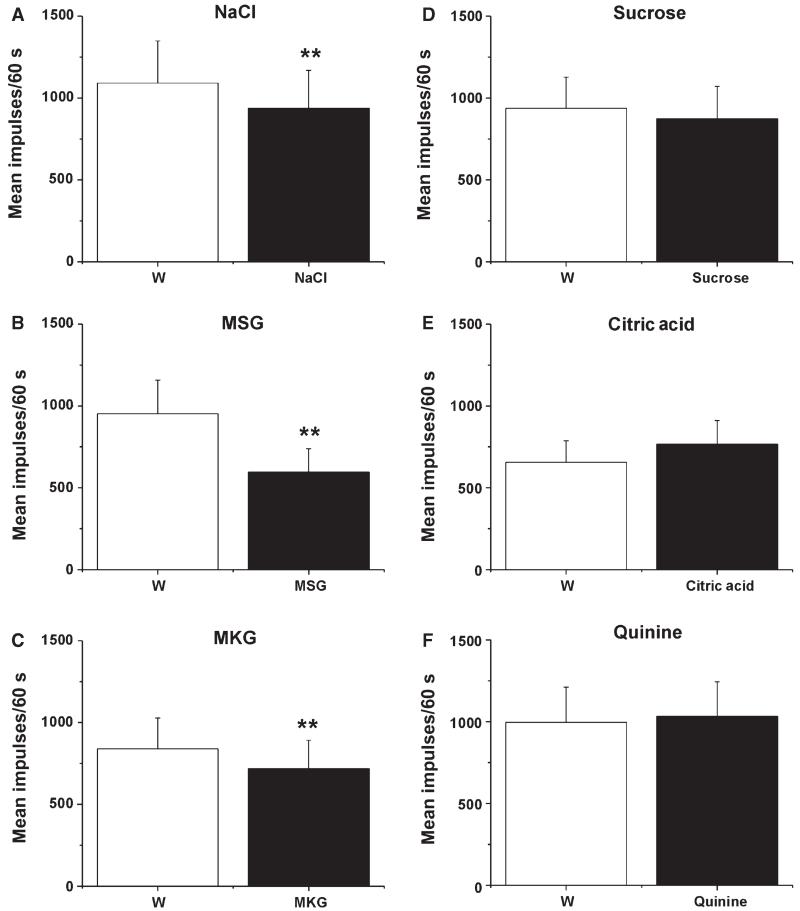
Electrical stimulation of the tongue elicited consistent responses. Figure 3A shows an individual example, and Fig. 3B shows that the mean responses of Vc units to repeated electrical stimuli were stable across trials, providing a stable baseline for us to test the conditioning effects of gustatory stimulation. Moreover, the mean responses of 19 Vc units to electrical stimulation of the tongue were not significantly different when this was preceded by superfusion with water or not (data not shown). When it was preceded by tongue superfusion with the tastant mixture, there was a significant reduction in the magnitude of the mean electrically evoked response (Fig. 4A; mix, Friedman, P = 0.0015, χ2 = 13, n = 14) as compared with when it was preceded by water (Fig. 4A, W1). This effect was transient; the electrically evoked response returned to baseline on the subsequent trial with water superfusion (Fig. 4A, W2). On testing of the individual tastants, there were significant reductions in the mean electrically evoked responses when the tongue was superfused with NaCl (Fig. 4B; Wilcoxon, P < 0.0005, Z = −4.39, n = 30), MSG (Fig. 4C; Wilcoxon, P < 0.0005, Z = −3.58, n = 19) or MKG (Fig. 4D; Wilcoxon, P = 0.003, Z = −2.98, n = 12). Superfusion with sucrose (n = 18), citric acid (n = 17) or quinine (n = 16) had no effect (Fig. 4E–G). Because of the electrical conductivity of the salt solutions, we performed additional experiments in which the tongue was dried with absorbent paper at the end of the superfusion and just prior to electrical stimulation. Under this condition, NaCl superfusion also resulted in a statistically significant decrease in the mean electrically evoked response (Wilcoxon, P < 0.001, Z = −3.62, n = 17).
Fig. 4.
Tastant modulation of electrically evoked responses of Vc units. (A) Mean responses of 14 Vc units to electrical tongue stimulation, when preceded by tongue superfusion with water (W1), the tastant mixture (Mix), and again with water (W2). **Significant difference between Mix and W1 (P < 0.001, anova). (B–F) Mean responses of 14 Vc units to electrical tongue stimulation, when preceded by tongue superfusion with water (W), NaCl, MSG, MKG, sucrose, citric acid, or quinine. (B) NaCl. **Significant difference between W and NaCl treatments (n = 30). (C) MSG. **Significant difference between W and MSG treatments (n = 19). (D) MKG. **Significant difference between W and MKG treatments (n = 12). (E) Sucrose (n = 18). (F) Citric acid (n = 17). (G) Quinine (n = 16).
To test whether the suppressant effect of sodium and glutamate was restricted to the tongue or generalized to other orofacial areas, we repeated the experiment with electrical stimulation of the ipsilateral lower lip. Neither superfusion of the tongue with NaCl or MSG, nor superfusion of the lower lip with NaCl, had any significant effect on the mean response of Vc units to electrical lip stimulation (Wilcoxon, P > 0.05, n = 13, n = 12, and n = 9 Vc units, respectively).
Effects of tastants on Vc responses to pentanoic acid
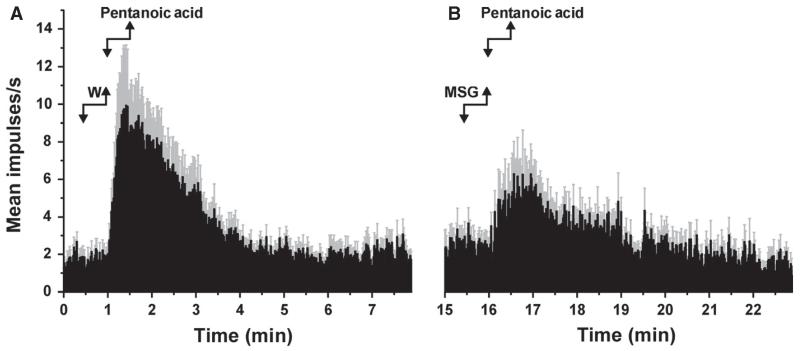
Pentanoic acid activated the present Vc units (Fig. 2), and the mean responses to repeated application of pentanoic acid of 5 Vc units tested were consistent across trials (Fig. 3C). Similarly to the results obtained with electrical stimulation, superfusion of the tongue with NaCl (Fig. 5A; Wilcoxon, P = 0.007, Z = −2.67, n = 16), MSG (Fig. 5B; Wilcoxon, P = 0.001, Z = −3.24, n = 15) or MKG (Fig. 5C; Wilcoxon, P = 0.008, Z = −2.67, n = 9) resulted in significant reductions in the mean responses of Vc units to pentanoic acid. Figure 6 illustrates the mean responses of 15 units to pentanoic acid, which were significantly depressed when pentanoic acid was preceded by MSG (Fig. 6B) as compared with water (Fig. 6A). In contrast, sucrose (n = 20), citric acid (n = 16) and quinine (n = 15) had no effect on pentanoic acid-evoked responses (Fig. 5D–F).
Fig. 5.
Tastant modulation of Vc unit responses to pentanoic acid. Mean responses of 16 Vc units to lingual application of pentanoic acid, when preceded by tongue superfusion with water (W), NaCl, MSG, MKG, sucrose, citric acid, or quinine. (A) NaCl. **Significant difference between W and NaCl treatments (P < 0.001, anova). (B) MSG. **Significant difference between W and MSG treatments (n = 15). (C) MKG. **Significant difference between W and MKG treatments (n = 9). (D) Sucrose (n = 20). (E) Citric acid (n = 16). (G) Quinine (n = 15).
Fig. 6.
MSG suppression of Vc unit responses to pentanoic acid. (A) Averaged peristimulus–time histogram (PSTH; bin width – 1 s) of responses of 15 Vc units to pentanoic acid when preceded by water. Gray error bars – SEM. (B) Averaged PSTH of responses of the same units shown in A to a second application of pentanoic acid when preceded by MSG.
Effects of tastants on Vc responses to noxious heat
There were no significant differences between the mean Vc responses elicited by hot water and those elicited by the heated tastant mixture (water, 286.8 ± 28.7 impulses/30 s; tastant mix, 268.7 ± 23.6/30 s). Similarly, there were no differences between the mean Vc unit responses elicited by filter papers dipped in hot water and those elicited by any of the individual heated tastants [water vs. sucrose, 101.8 ± 36.4 (standard error of the mean (SEM)) vs. 102.6 ± 45.1 impulses/30 s; water vs. NaCl, 78.0 ± 28.5 vs. 83.0 ± 30.3 impulses/30 s; water vs. citric acid, 71.8 ± 25.6 vs. 77 ± 23.9 impulses/30 s; water vs. MSG, 78.0 ± 23.9 vs. ± 81.6 ± 30.0 impulses/30 s; n = 5 for all].
Lack of effect of anesthesia or transection of the CT on Vc responses or tastant modulation
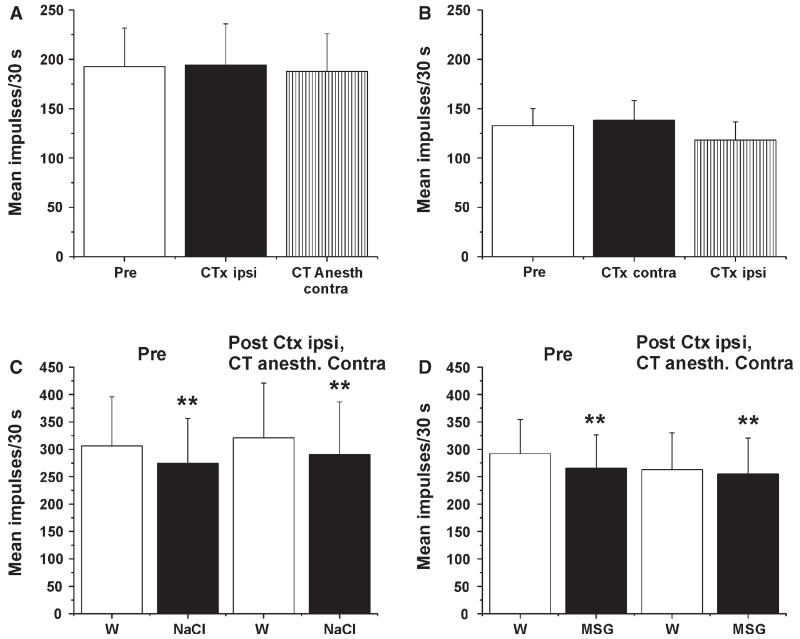
Electrically evoked responses of Vc units were recorded before and after sequential transection of the ipsilateral CT, followed by anesthesia of the contralateral CT. Neither procedure had any significant effect on the magnitude of electrically evoked responses (Fig. 7A; n = 17) or on basal activity [before, 2.25 ± 0.54 (SEM) impulses/30 s; ipsilateral CT transection, 2.34 ± 0.57 impulses/30 s; contralateral CT anesthesia, 2.38 ± 0.52 impulses/30 s]. We also altered the sequence by first transecting the contralateral CT and then transecting the ipsilateral CT. Neither procedure affected Vc neuronal responses (Fig. 7B; n = 11) or basal activity [before, 1.17 ± 0.29 (SEM) impulses/30 s; contralateral CT transection, 1.31 ± 0.39 impulses/30 s; ipsilateral CT transection, 1.12 ± 0.37 impulses/30 s].
Fig. 7.
Lack of effect of CT transection and anesthesia on electrically evoked responses of Vc neurons or modulation by NaCl and MSG. (A) Mean electrically evoked response of 17 Vc units before (Pre; open bar) and following (Ctx; filled bar) transection of the ipsilateral CT, and again following anesthesia of the contralateral CT (striped bar). (B) Mean electrically evoked response of 11 Vc units before (Pre) and following (Ctx) transection of the contralateral CT, and again following transection of the ipsilateral CT. (C) Electrically evoked response of Vc units when preceded by water (W) or NaCl, before (Pre) and following transection of the ipsilateral CT and anesthesia of the contralateral CT. **Significant difference between W and NaCl (n = 10). (D) As in C for MSG. **Significant difference between W and MSG (n = 18).
We additionally tested whether interfering with the CT affected tastant modulation of responses of Vc neurons to electrical tongue stimulation. Figure 7C shows that both before and after transection of the ipsilateral CT and anesthesia of the contralateral CT, tongue superfusion with NaCl significantly reduced the electrically evoked response (n = 10). Similar data were obtained with MSG superfusion (Fig. 7D; n = 18).
Calcium imaging of TG cells
Response to tastants
None of the TG cells tested responded to NaCl (n = 135), sucrose (n = 160), or MSG (n = 160). Consistent with a previous study (Liu & Simon, 1998), 80 of 148 (54%) TG cells responded to quinine. In addition, 43 of 145 (30%) responded to citric acid.
Tastant modulation of TG cell responses to pentanoic acid
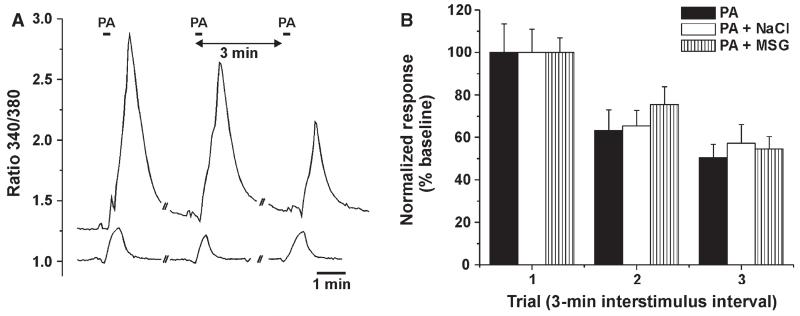
Of a total of 391 TG cells, 297 (76%) responded to the initial application of pentanoic acid. Figure 8A shows examples of the responses of two TG cells to three repeated applications of pentanoic acid. We reasoned that NaCl and MSG might diffuse through the lingual epithelium to reach trigeminal nerve endings and affect their nociceptive responses. To test this, we applied each tastant 30 s prior to a second application of pentanoic acid, with the first application of pentanoic acid serving as baseline control. The rationale was that, if either tastant exerted an inhibitory effect, the response to the second application of pentanoic acid would be significantly lower than the reduction caused by tachyphylaxis. There was a significant decrease (F2,180 = 53.8, P < 0.0005) in the normalized response magnitude over time (Fig. 8B, filled bars), indicative of tachyphylaxis, but no significant difference between groups over time (F4,180 = 0.5, P > 0.05, repeated measures anova). Thus, neither NaCl nor MSG directly affected the responses of TG cells to pentanoic acid.
Fig. 8.
Lack of effect of NaCl or MSG on pentanoic acid (PA)-evoked responses of TG cells. (A) Example of responses of two TG cells to repeated application of PA with 3-min interstimulus intervals. (B) Mean normalized responses of TG cells to three repeated PA applications, with either nothing (PA; n = 24), NaCl (PA + NaCl; n = 44) or MSG (PA + MSG; n = 25) applied 30 s prior to the second PA application. There was a significant decrease in responses over time (F2,180 = 53.8, P < 0.0005) but no significant difference between groups over time (F4,180 = 0.5, P > 0.05, repeated measures anova).
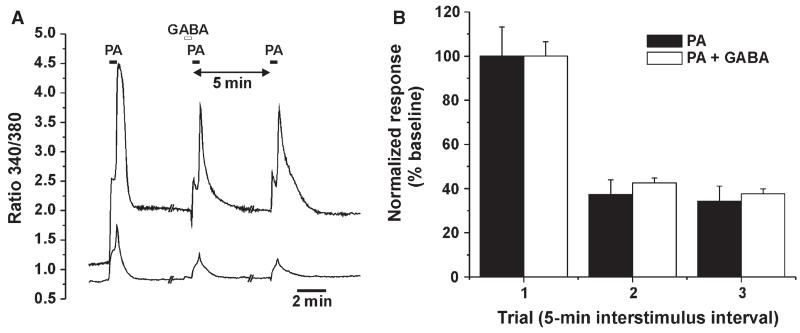
Conceivably, NaCl and MSG might indirectly affect TG cells via the release of inhibitory mediators such as GABA from taste receptor cells (Huang et al., 2011). We used the same paradigm to determine whether GABA affected pentanoic acid-evoked responses of TG cells. Figure 9A shows an example of TG cell responses to repeated applications of pentanoic acid, with GABA preceding the second application, and Fig. 9B shows the averaged normalized responses for TG cells. There was a significant decrease in responses over time (F2,332 = 57.6, P < 0.0005) but no significant difference between groups over time (F2,332 = 0.075, P > 0.05, repeated measures anova), indicating that GABA had no effect on TG neuronal responses to pentanoic acid. Four of 195 (2%) TG cells responded to application of GABA.
Fig. 9.
Lack of effect of GABA on pentanoic acid (PA)-evoked responses of TG cells. (A) Example of responses of two TG cells to repeated application of PA with 5-min interstimulus intervals, with the second PA application preceded by GABA. (B) Mean normalized responses of TG cell to three repeated PA applications, without GABA (PA) and with GABA (PA + GABA) applied 30 s prior to the second PA application. There was a significant decrease in responses over time (F2,332 = 57.6, P < 0.0005) but no significant difference between groups over time (F2,332 = 0.075, P > 0.05, repeated measures anova).
Discussion
The present results are, to our knowledge, the first to demonstrate gustatory modulation of responses of central nociceptive trigeminal neurons. Delivery of a mixture of tastants, or individual application of NaCl or MSG, to the tongue resulted in a significant reduction in responses of Vc neurons to both electrical and noxious chemical (pentanoic acid) stimuli. This effect was restricted to the tongue, as responses of Vc neurons to noxious stimulation of the lower lip were not affected by tastants applied onto the tongue. Another main finding of this study is the lack of acute modulatory effects of the CT nerve on nociceptive processing at the level of the Vc. Bilateral anesthesia and/or transection of the CT had no effect on the nociceptive responses of Vc neurons, nor did it affect the depressant actions of NaCl and MSG on electrically evoked Vc neuronal responses. Moreover, neither NaCl nor MSG directly excited TG neurons, and nor did GABA inhibit their nociceptive responses. These results imply that NaCl and MSG act peripherally to modulate the sensitivity of trigeminal nociceptive afferents innervating the tongue, possibly via the release of some mediator(s) other than GABA from taste receptor cells.
Methodological considerations
Electrical and pentanoic acid stimulation of the tongue elicited responses in Vc neurons that were stable across repeated trials (Fig. 3), with no evidence of sensitization or habituation. In contrast, TG neurons showed significant tachyphylaxis in response to the second application of pentanoic acid (Figs 8 and 9). Conceivably, the decreased response of trigeminal nociceptors was compensated for by an increased response, such as wind-up, of Vc neurons to the first two stimulus applications. In this regard, it is interesting that, in human microneurography experiments, noxious mechanical stimulation of the skin elicited responses in mechanical nociceptors that markedly adapted over time while the concomitant pain sensation progressively increased, a ‘paradox’ explained by wind-up of central pain-transmitting neurons (Adriaensen et al., 1984).
The reductions in electrically and chemically evoked responses were attributed to NaCl and glutamate. Superfusion of the tongue with water (or sucrose, citric acid, or quinine) had no effect on electrically or chemically evoked Vc neuronal responses, ruling out non-specific thermal or mechanical effects. The effect of glutamate was the same, regardless of the cation (Na+ or K+). We cannot rule out the possibility that K+ may have contributed to the effect observed with MKG. KCl depolarizes taste cells (Tomchik et al., 2007) and, at high concentrations, can excite trigeminal primary afferents (Wang et al., 1993). In addition, changes in osmolarity can sensitize trigeminal nociceptive afferents to capsaicin (Liu et al., 2007). However, such excitatory effects are opposite to the observed depressant effects of MSG and MKG on Vc neuronal responses, implying that any direct excitatory effect of cations on peripheral trigeminal afferents was overcome by the inhibitory effect of glutamate. We were additionally concerned that the salt solutions may have facilitated electrical current spread, although, again, this would have increased, rather than decreased, electrically evoked responses of Vc neurons. In any event, the depressant effects of NaCl and MSG were observed when the tongue was dried just prior to the electrical stimulus. Finally, the depressant effects of NaCl and glutamate were shown to be site-specific, as application of these tastants to the tongue, but not to the lower lip, depressed Vc responses evoked by electrical tongue stimulation.
Curiously, none of the tastants affected responses of Vc neurons to noxious heating of the tongue. This was tested in two paradigms: first by comparing responses of Vc neurons to superfusion of the tongue with hot water vs. a heated tastant; and second, by applying filter papers soaked with heated water or tastant. One difference between this and the electrical and chemical stimulus paradigms is that the noxious thermal stimulus was delivered simultaneously with the tastant, rather than 30 s later. This may have left insufficient time for the tastant to act at its target site.
Mechanisms and functional significance of tastant modulation of trigeminal nociception
The observation that transection and/or anesthesia of the CT did not affect NaCl or glutamate modulation of Vc neuronal responses points to a peripheral site of action. The trigeminal afferents activated by electrical and chemical stimulation of the tongue are presumably transient receptor potential cation channel subfamily V member 1-expressing nociceptors. Lingual trigeminal afferents form a dense plexus around the basal end of taste buds (Nagy et al., 1982; Yamasaki et al., 1984; Silverman & Kruger, 1990; Kinnman & Aldskogius, 1991), but with numerous fibers extending into the taste bud and some coming close to the taste pore (Nishimoto et al., 1982; Montavon et al., 1996; Ishida et al., 2009). This affords an opportunity for NaCl and glutamate to directly contact trigeminal afferents. Additionally, NaCl and KCl can diffuse through tight junctions to potentially access trigeminal afferents surrounding the basolateral end of taste buds (Wang et al., 1993). An increased Na+ concentration at trigeminal nerve endings would presumably exert a depolarizing effect (Onizuka et al., 2011). Similarly, glutamate can depolarize and excite some nociceptive afferent fibers (Agrawal & Evans, 1986; Ault & Hildebrand, 1993; Hwang et al., 2011). However, our calcium imaging data showed that neither NaCl nor MSG directly excited any TG cells. In contrast, quinine and citric acid excited 54 and 30% of TG cells, respectively. The effect of quinine is consistent with a previous study (Liu & Simon, 1998), and the effect of citric acid may be attributable to activation of transient receptor potential cation channel subfamily V member 1 and/or acid-sensing ion channels expressed in TG cells. The concentrations of NaCl and MSG used in the present study (100 and 200 mm, respectively) were lower than the threshold NaCl concentrations (> 500 mm) needed to excite lingual nerve fibers (Wang et al., 1993). Thus, the inability of NaCl or MSG to excite nociceptive TG neurons argues against the possibility of a trigeminally mediated central inhibitory mechanism for the depressant effects of NaCl and MSG on nociceptive Vc responses.
Another possibility is that NaCl and glutamate indirectly affect peripheral trigeminal nerve endings, e.g. by depolarizing taste receptor cells (Avenet & Lindemann, 1991; Bigiani et al., 1997) that release mediators such as ATP (Huang et al., 2009), glutamate (Vandenbeuch et al., 2010), noradrenaline, serotonin (Huang et al., 2008), or GABA (Huang et al., 2011). Taste receptor cells responsive to NaCl are thought to be mainly presynaptic (type III) cells (Tomchik et al., 2007), which can release GABA or serotonin under certain conditions (Huang et al., 2011). GABA is a well-known inhibitory neurotransmitter, and GABAA receptors have been detected in nociceptive afferents, where they mediate antinociceptive effects (Carlton et al., 1999). Thus, GABA released from salt-sensitive taste receptor cells might reduce the excitability of trigeminal nociceptors, accounting for the present observations. Glutamate binds to the T1R1–T1R3 heterodimer as well as metabotropic glutamate receptor 4 expressed in taste receptor cells, and excites a subset of amiloride-sensitive cells that presumably express epithelial sodium channels (Lin & Kinnamon, 1999). Thus, glutamate may also induce the release from taste receptor cells of various mediators, including GABA, to indirectly inhibit trigeminal nociceptors. However, in the present study we showed that GABA had no effect on pentanoic acid-evoked responses of TG cells. It is possible that some other mediator, not presently tested, may be released from taste receptor cells to exert an inhibitory effect on trigeminal nociceptive nerve endings in the tongue.
The functional significance of suppression of nociceptive Vc neurons by NaCl and MSG remains speculative. As our data largely rule out a central inhibitory mechanism, we infer that the depressant effects of NaCl and MSG on trigeminal nociception occur locally, and would not be expected to extend beyond the oral cavity. The only relevant human study that we know of found that NaCl enhanced capsaicin-evoked oral burn (Prescott et al., 1993), in contrast to the inhibitory effect of NaCl on oral pain that would be predicted by the present data. There is evidence for a more global antinociceptive effect of certain tastants. Sucrose has been reported to elicit antinociception in juveniles (Blass et al., 1987; Miller et al., 1994; Bucher et al., 2000; Anseloni et al., 2005) and in adults (Stevens & Lawless, 1986; Kakeda, 2010; Schöbel et al., 2012). Water, sucrose and self-initiated ingestion of chocolate produced analgesia in rats in a manner requiring intact central antinociceptive pathways (Foo & Mason, 2009). Oral ingestion of NaCl elicited antinociception in salt-deprived (but not undeprived) rats (Foo & Mason, 2011). It has been proposed that centrally mediated antinociception maintains feeding behavior and other physiologically essential functions (Mason, 2011). Given the importance of dietary sodium, glutamate and sugars for electrolyte balance and protein and carbohydrate intake, it is reasonable to speculate that salt, umami and sweet taste qualities might trigger central mechanisms to maintain the ingestion of these important foods. What remains unclear is how these tastants would activate central antinociceptive pathways, given the present results showing that only NaCl and MSG inhibited Vc neurons via a CT-independent mechanism, and that sucrose did not affect TG neurons. It is noteworthy that the antinociceptive effect induced by ingestion of sweets was attenuated by conditioned taste aversion to sucrose, indicating that the hedonic valence of the food plays a key role in triggering antinociception (Foo & Mason, 2009). It may be that NaCl and MSG ‘mask’ oral pain via a local effect restricted to the tongue, whereas sweeteners suppress pain via a centrally mediated mechanism that depends on the rewarding properties of the food.
In the present study, we observed that bilateral transection or anesthesia of the CT did not affect nociceptive responses of Vc neurons. This argues against the idea that the gustatory system tonically inhibits the trigeminal system, and that damage to the taste pathway disinhibits trigeminal nociceptive mechanisms, resulting in chronic orofacial pain such as burning mouth syndrome (Bartoshuk, 2000; Grushka et al., 2003; Femiano, 2004; Nasri-Heir et al., 2011). It is important to note that our study assessed the effect of acute CT blockade on responses of Vc neurons to acute noxious stimuli in healthy adult male rats, and may not ideally model complex pain disorders.
Acknowledgements
This work was supported by grants from the NIH (DE013685, AR057194) and Institut Francais pour la la Recherche en Odontologie (IFRO).
Abbreviations
- CT
chorda tympani
- MKG
monopotassium glutamate
- MSG
monosodium glutamate
- SEM
standard error of the mean
- TG
trigeminal ganglion
- Vc
trigeminal subnucleus caudalis
Footnotes
None of the authors declares any conflict of interest.
References
- Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Nociceptor discharges and sensations due to prolonged noxious mechanical stimulation – a paradox. Hum. Neurobiol. 1984;3:53–58. [PubMed] [Google Scholar]
- Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Brit. J. Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anseloni VC, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–243. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Ault B, Hildebrand LM. l-glutamate activates peripheral nociceptors. Agents Actions. 1993;39:C142–C144. doi: 10.1007/BF01972747. [DOI] [PubMed] [Google Scholar]
- Avenet P, Lindemann B. Noninvasive recording of receptor cell action potentials and sustained currents from single taste buds maintained in the tongue: the response to mucosal NaCl and amiloride. J. Membrane Biol. 1991;124:33–41. doi: 10.1007/BF01871362. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Comparing sensory experiences across individuals: recent psychophysical advances illuminate genetic variation in taste perception. Chem. Senses. 2000;25:447–460. doi: 10.1093/chemse/25.4.447. [DOI] [PubMed] [Google Scholar]
- Bigiani A, Delay RJ, Chaudhari N, Kinnamon SC, Roper SD. Responses to glutamate in rat taste cells. J. Neurophysiol. 1997;77:3048–3059. doi: 10.1152/jn.1997.77.6.3048. [DOI] [PubMed] [Google Scholar]
- Blass E, Fitzgerald E, Kehoe P. Interactions between sucrose, pain and isolation distress. Pharmacol. Biochem. Be. 1987;26:483–489. doi: 10.1016/0091-3057(87)90153-5. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Simons CT, Faurion A, Azérad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res. 2003;973:265–274. doi: 10.1016/s0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Iodi Carstens M, Carstens E. Gustatory modulation of the responses of trigeminal subnucleus caudalis (Vc) neurons to noxious stimulation of the tongue in rats. Chem. Senses. 2012;38:274. doi: 10.1111/ejn.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher HU, Baumgartner R, Bucher N, Seiler M, Fauchère JC. Artificial sweetener reduces nociceptive reaction in term newborn infants. Early Hum. Dev. 2000;59:51–60. doi: 10.1016/s0378-3782(00)00085-2. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93:713–722. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J. Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- Catalanotto FA, Bartoshuk LM, Östrum KM, Gent JF, Fast K. Effects of anesthesia of the facial nerve on taste. Chem. Senses. 1993;18:461–470. [Google Scholar]
- Dessirier JM, Simons CT, Sudo M, Sudo S, Carstens E. Sensitization, desensitization and stimulus-induced recovery of trigeminal neuronal responses to oral capsaicin and nicotine. J. Neurophysiol. 2000;84:1851–1862. doi: 10.1152/jn.2000.84.4.1851. [DOI] [PubMed] [Google Scholar]
- Dinkins ME, Travers SP. Effects of chorda tympani nerve anesthesia on taste responses in the NST. Chem. Senses. 1998;23:661–673. doi: 10.1093/chemse/23.6.661. [DOI] [PubMed] [Google Scholar]
- Eliav E, Kamran B, Schaham R, Czerninski R, Gracely RH, Benoliel R. Evidence of chorda tympani dysfunction in patients with burning mouth syndrome. J. Am. Dent. Assoc. 2007;138:628–633. doi: 10.14219/jada.archive.2007.0234. [DOI] [PubMed] [Google Scholar]
- Felizardo R, Boucher Y, Braud A, Carstens E, Dauvergne C, Zerari-Mailly F. Trigeminal projections on gustatory neurons of the nucleus of the solitary tract: a double-label strategy using electrical stimulation of the chorda tympani and tracer injection in the lingual nerve. Brain Res. 2009;1288:60–68. doi: 10.1016/j.brainres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Femiano F. Damage to taste system and oral pain: burning mouth syndrome. Minerva Stomatol. 2004;53:471–478. [PubMed] [Google Scholar]
- Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J. Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo H, Mason P. Ingestion analgesia occurs when a bad taste turns good. Behav. Neurosci. 2011;126:956–961. doi: 10.1037/a0025542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushka M, Epstein JB, Gorsky M. Burning mouth syndrome and other oral sensory disorders: a unifying hypothesis. Pain Res. Manag. 2003;8:133–135. doi: 10.1155/2003/654735. [DOI] [PubMed] [Google Scholar]
- Harrison TA. Chorda tympani nerve stimulation evokes Fos expression in regionally limited neuron populations within the gustatory nucleus of the solitary tract. Brain Res. 2001;904:54–66. doi: 10.1016/s0006-8993(01)02449-0. [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD. Norepinephrine is core-leased with serotonin in mouse taste buds. J. Neurosci. 2008;28:13088–13093. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J. Neurosci. 2009;29:13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS ONE. 2011;6:e25471. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SM, Koo NY, Jin M, Davies AJ, Chun GS, Choi SY, Kim JS, Park K. Intracellular acidification is associated with changes in free cytosolic calcium and inhibition of action potentials in rat trigeminal ganglion. J. Biol. Chem. 2011;286:1719–1729. doi: 10.1074/jbc.M109.090951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Ugawa S, Ueda T, Yamada T, Shibata Y, Hondoh A, Inoue K, Yu Y, Shimada S. P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J. Comp. Neurol. 2009;514:131–144. doi: 10.1002/cne.22000. [DOI] [PubMed] [Google Scholar]
- Kakeda T. Potential of sucrose-induced analgesia to relieve pain in male adults: a preliminary study. Jpn. J. Nurs. Sci. 2010;7:169–173. doi: 10.1111/j.1742-7924.2010.00150.x. [DOI] [PubMed] [Google Scholar]
- Kinnman E, Aldskogius H. The role of substance P and calcitonin gene-related peptide containing nerve fibers in maintaining fungiform taste buds in the rat after a chorda tympani nerve injury. Exp. Neurol. 1991;113:85–91. doi: 10.1016/0014-4886(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Klein AH, Iodi Carstens M, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011a;106:1078–1088. doi: 10.1152/jn.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Iodi Carstens M, McCluskey TS, Blancher G, Simons CT, Slack JP, Furrer S, Carstens E. Novel menthol-derived cooling compounds activate primary and second-order trigeminal sensory neurons and modulate lingual thermosensitivity. Chem. Senses. 2011b;36:649–658. doi: 10.1093/chemse/bjr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Iodi Carstens I, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, Carstens E. Self- and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem. Senses. 2011c;36:199–208. doi: 10.1093/chemse/bjq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Sawyer CM, Zanotto KL, Ivanov MA, Cheung S, Iodi Carstens M, Furrer S, Simons CT, Slack JP, Carstens E. A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats. J. Neurophysiol. 2011d;105:1701–1710. doi: 10.1152/jn.00922.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kinnamon SC. Co-localization of epithelial sodium channels and glutamate receptors in single taste cells. Biol. Signal. Recept. 1999;8:360–365. doi: 10.1159/000014609. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Responses of cultured rat trigeminal ganglion neurons to bitter tastants. Chem. Senses. 1998;23:125–130. doi: 10.1093/chemse/23.2.125. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Liedtke W, Simon SA. Changes in osmolality sensitize the response to capsaicin in trigeminal sensory neurons. J. Neurophysiol. 2007;97:2001–2015. doi: 10.1152/jn.00887.2006. [DOI] [PubMed] [Google Scholar]
- Lugaz O. Convergence des sensations gustatives et somesthésiques: cas particulier des acides. Université Paris-Sud XI; 2004. Thèse de Doctorat. [Google Scholar]
- Mason P. From descending pain modulation to obesity via the medullary raphe. Pain. 2011;152(3 Suppl.):S20–S24. doi: 10.1016/j.pain.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Barr RG, Young SN. The cold pressor test in children: methodological aspects and the analgesic effect of intraoral sucrose. Pain. 1994;56:175–183. doi: 10.1016/0304-3959(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Montavon P, Hellekant G, Farbman A. Immunohistochemical, electrophysiological, and electron microscopical study of rat fungiform taste buds after regeneration of chorda tympani through the non-gustatory lingual nerve. J. Comp. Neurol. 1996;367:491–502. doi: 10.1002/(SICI)1096-9861(19960415)367:4<491::AID-CNE2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Goedert M, Hunt SP, Bond A. The nature of the substance P-containing nerve fibres in taste papillae of the rat tongue. Neuroscience. 1982;7:3137–3151. doi: 10.1016/0306-4522(82)90236-6. [DOI] [PubMed] [Google Scholar]
- Nasri-Heir C, Gomes J, Heir GM, Ananthan S, Benoliel R, Teich S, Eliav E. The role of sensory input of the chorda tympani nerve and the number of fungiform papillae in burning mouth syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;112:65–72. doi: 10.1016/j.tripleo.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Akai M, Inagaki S, Shiosaka S, Shimizu Y, Yamamoto K, Senba E, Sakanaka M, Takatsuki K, Hara Y, Takagi H, Matsuzaki T, Kawai Y, Tohyama M. On the distribution and origins of substance P in the papillae of the rat tongue: an experimental and immunohistochemical study. J. Comp. Neurol. 1982;207:85–92. doi: 10.1002/cne.902070108. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Yonaha T, Tamura R, Hosokawa N, Kawasaki Y, Kashiwada M, Shirasaka T, Tsuneyoshi I. Capsaicin indirectly suppresses voltage-gated Na+ currents through TRPV1 in rat dorsal root ganglion neurons. Anesth. Analg. 2011;112:703–709. doi: 10.1213/ANE.0b013e318204ea5b. [DOI] [PubMed] [Google Scholar]
- Prescott J, Allen S, Stephens L. Interactions between oral chemical irritation, taste and temperature. Chem. Senses. 1993;18:389–404. [Google Scholar]
- Schöbel N, Kyereme J, Minovi A, Dazert S, Bartoshuk L, Hatt H. Sweet taste and chorda tympani transection alter capsaicin-induced lingual pain perception in adult human subjects. Physiol. Behav. 2012;107:368–373. doi: 10.1016/j.physbeh.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Analysis of taste bud innervation based on glycoconjugate and peptide neuronal markers. J. Comp. Neurol. 1990;292:575–584. doi: 10.1002/cne.902920407. [DOI] [PubMed] [Google Scholar]
- Simons CT, Boucher Y, Carstens E. Suppression of central taste transmission by oral capsaicin. J. Neurosci. 2003;23:978–985. doi: 10.1523/JNEUROSCI.23-03-00978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DA, Lawless HT. Putting out the fire: effects of tastants on oral chemical irritation. Percept. Psychophys. 1986;39:346–350. doi: 10.3758/bf03203002. [DOI] [PubMed] [Google Scholar]
- Takemura M, Wakisaka S, Yoshida A, Nagase Y, Bae YC, Shigenaga Y. Nadph-diaphorase in the spinal trigeminal nucleus oralis and rostral solitary tract nucleus of rats. Neuroscience. 1994;61:587–595. doi: 10.1016/0306-4522(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Tie K, Fast K, Kveton J, Cohen Z, Duffy V, Green B, Prutkin J, Bartoshuk L. Anesthesia of chorda tympani nerve and effect on oral pain (abstract) Chem. Senses. 1999;24:609. [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. J. Neurosci. 2007;27:10840–10848. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Tizzano M, Anderson CB, Stone LM, Goldberg D, Kinnamon SC. Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neurosci. 2010;11:77. doi: 10.1186/1471-2202-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. Selectivity of lingual nerve fibers to chemical stimuli. J. Gen. Physiol. 1993;101:843–866. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. Modulation of rat chorda tympani nerve activity by lingual nerve stimulation. J. Neurophysiol. 1995;73:1468–1483. doi: 10.1152/jn.1995.73.4.1468. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Kubota Y, Tagaki H, Tohyama M. Immunoelectron-microscopic study on the fine structure of substance-P-containing fibers in the taste buds of the rat. J. Comp. Neurol. 1984;227:380–392. doi: 10.1002/cne.902270308. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Bartoshuk LM, Catalanotto FA, Karrer TA, Kveton JF. Anesthesia of the chorda tympani nerve and taste phantoms. Physiol. Behav. 1998;63:329–335. doi: 10.1016/s0031-9384(97)00423-x. [DOI] [PubMed] [Google Scholar]