Abstract
Recent regulatory initiatives in the United States and Europe have transformed the pediatric clinical trials landscape by significantly increasing capital investment and pediatric trial volume. The purpose of this manuscript is to review the impact of these initiatives on the pediatric cardiovascular trials landscape when compared to other pediatric sub-specialties. We also evaluate factors that may have contributed to the success or failure of recent major pediatric cardiovascular trials so as to inform the optimal design and conduct of future trials in the field.
Introduction
Clinical trials represent the gold-standard for developing an evidence base in medicine, however children have historically been underrepresented in clinical trials. Consequently most drugs and devices are used “off-label” in children with their safety, efficacy and dosing extrapolated from trial data in adults.1–3 This practice is suboptimal as children have unique developmental differences that can affect drug metabolism and response, as well as device safety.
Recognizing the importance of conducting clinical trials in children, regulatory agencies in Europe and the United States have enacted several recent initiatives aimed at stimulating pediatric drug / device development and research.4–11 Collectively these initiatives have transformed the pediatric clinical trials landscape with an unprecedented injection of resources and financial capital. With congenital heart disease remaining the number one birth defect worldwide, the purpose of this article is to review the impact of these initiatives on the pediatric cardiovascular clinical trials landscape with a focus on ways that we can optimize future trials so as to maximize the return for children with heart disease.
Regulatory initiatives – brief historical overview
Although a comprehensive overview of pediatric drug / device development regulation is beyond the scope of this manuscript, a brief review of recent legislative initiatives is helpful to better understand the current clinical trials landscape. Figure 1 summarizes initiatives from the past two decades designed to encourage pediatric trials in the United States and Europe. Landmark initiatives include the 1998 “Pediatric Exclusivity” provision, the 2002 Best Pharmaceuticals for Children’s Act, the 2003 Pediatric Research Equity Act and the 2007 Paediatric Regulation. Collectively they have established regulatory mandates, incentives, and oversight mechanisms designed to advance the pediatric evidence base. These efforts have been tremendously successful. In the United States more than 480 pediatric trials enrolling > 175,000 study subjects have been conducted over the past decade with similar recent successes documented in Europe.12–14 From a financial standpoint, these trials have injected an enormous amount of capital into pediatric research. Li et al. estimated costs for a subset of trials conducted under the auspices of the Pediatric Exclusivity provision between 2002 and 2004.15 The median cost to the sponsor to conduct the required pediatric drug studies was $12.34 million (range: $5.13 to 43.80 million). Despite this up-front expense, the economic return from patent extension (the principal financial incentive for study sponsors) is typically well worth the investment with an estimated median net economic benefit of $134 million (range: −$8.9 to +$507 million) for the nine products studied.15 Not surprisingly, after decades of inaction, the pharmaceutical industry has now enthusiastically embraced pediatric drug study with an almost six-fold increase in the average annual number of trials conducted in children to evaluate drug safety.16–18
Figure 1.
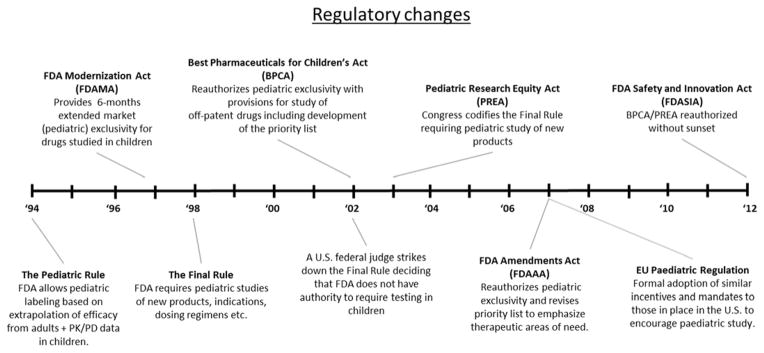
Time line depicting recent regulatory initiatives to encourage paediatric drug study in the United States and Europe
Measuring our successes and failures
With such significant changes in the pediatric clinical trials landscape, we sought to evaluate progress within the field of pediatric cardiology. How does our trial infrastructure and volume compare to our adult colleagues or to other pediatric sub-specialties? What types of trials are we conducting? What are the important drivers of trial design and conduct and most importantly, are we optimally advancing the evidence base in pediatric cardiology? To address these questions, we will focus on the U.S. clinical trials landscape as the U.S. Congress has established several mechanisms to evaluate progress. First, was the creation of a clinical trials registry; ClinicalTrials.gov is a searchable database that was mandated by Congress under the 1997 Food and Drug Administration Modernization Act and was made publically available in February of 2000.19,20 ClinicalTrials.gov includes information on trial design, study cohort, outcome measures, trial timeline, and more recently, trial results. In 2005 the International Committee of Medical Journal Editors began requiring trial registration as a condition for publication and in 2007 the U.S. Congress began requiring registration of all clinical trials conducted in the U.S.9,21 These actions have greatly increased trial registration; there are now more than 180,000 registered trials on ClinicalTrials.gov (Figure 2). A second mechanism to evaluate progress in pediatric cardiovascular clinical trials is the U.S. Food and Drug Administration “New Pediatric Labeling Information Database”.22 This public database was mandated by Congress in 2007 and includes a listing of all trials conducted under the auspices of recent legislative efforts, as well as the reviews of these trials and the associated labelling changes. Together, ClinicalTrials.gov and the Pediatric Labeling Information Database include a wealth of information that can provide important insight into pediatric cardiovascular trials and their outcomes.
Figure 2.
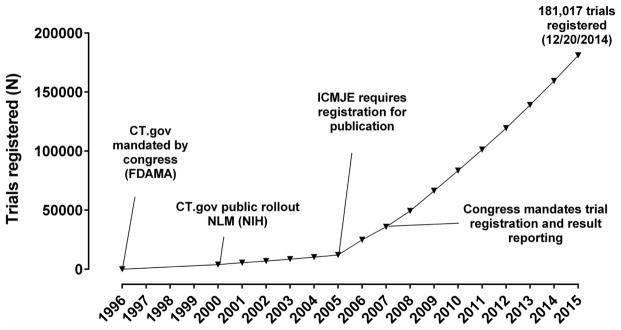
Clinical trials registered on ClinicalTrials.gov and associated milestones
ClinicalTrials.gov
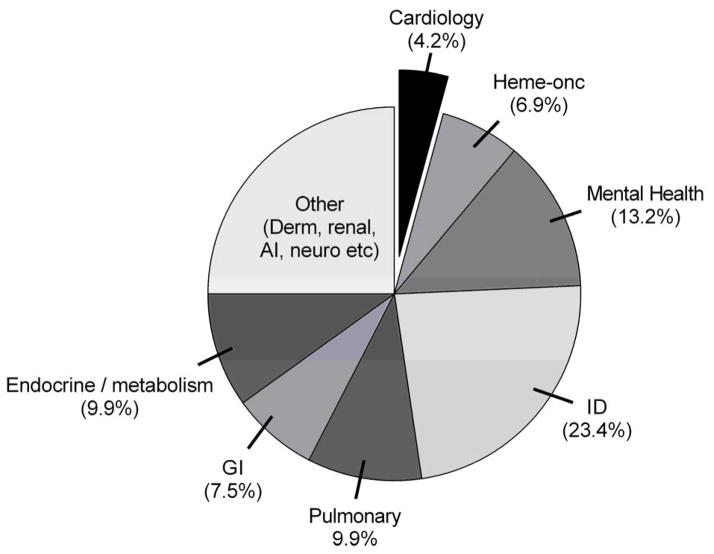
Numerous publications have used the ClinicalTrials.gov database to evaluate the clinical trials landscape. Califf et al. evaluated adult cardiovascular trials in comparison to oncology and mental health trials.23 Over a three year span from October 2007 to September 2010, 3437 adult cardiovascular trials were registered. Critically important, U.S. National Institutes of Health-sponsored trials performed substantially better across all trial quality metrics when compared to industry or other sponsor sources. In a follow up to this assessment, Pasquali et al. evaluated the pediatric clinical trials landscape.16 In this analysis which spanned from July 2005 until September 2010, 5035 trials restricted to children and adolescents (ages < 18 years) were registered. Pediatric trials were dominated by infectious disease trials which comprised ~ 23% of all registered trials (Figure 3). Other pediatric specialties with a relatively larger volume included: mental health/psychiatry (~13%), neurology/anesthesia/pain (~11%), pulmonary (~10%), endocrine/metabolic (~10%) and gastrointestinal/nutrition (~7.5%). In comparison, pediatric cardiology trials represented a relatively small subset of the overall trial landscape (~4.5%); the only sub-specialties with significantly fewer trials were hematology, dermatology and nephrology.
Figure 3.
Pediatric trials registered on ClinicalTrials.gov (09/2005–10/2010). AI = allergy/immunology, GI = gastroenterology, ID = infectious diseases
Pediatric cardiovascular trials registered on ClinicalTrials.gov
Using the same database as Pasquali and Califf, we evaluated the subset of pediatric cardiovascular trials.17 Overall 213 pediatric cardiovascular trials (ages < 18 years) were registered between September 2005 and October 2010. After manual review, we identified an additional 71 trials that also included adult subjects (age ≥ 18 years) but that we judged to have a primary pediatric cardiovascular focus (examples include the U.S. Medtronic Melody valve trial and the Pediatric Heart Network trial of losartan versus atenolol for Marfan syndrome). Pediatric cardiovascular trials had a median (interquartile range, IQR) trial enrollment of 65 (36, 186) subjects and only four registered trials had a projected enrollment of > 1000 study subjects. The median trial duration was 2.2 (IQR: 1.4, 3.3) years. Overall 68% of trials used a drug/biologic intervention, 12% were device interventions, and 10% were behavioral interventions.
In terms of specific trial focus, collectively hypertension, dyslipidemia, obesity and pulmonary hypertension trials comprised >40% of all trials (Figure 4). These trial subsets also appeared to dominate funding priorities; hypertension and obesity trials represented 61% of all National Institutes of Health-funded trials while hypertension, pulmonary hypertension and dyslipidemia trials accounted for 49% of all industry funded trials. Device trials accounted for an additional 15% of industry sponsored trials. Overall, 44% of all registered pediatric cardiovascular trials identified industry or National Institutes of Health-sponsorship; over a three-year span (09/2007–10/2010) 24 pediatric cardiovascular trials were sponsored by the U.S. National Institutes of Health and 69 were sponsored by industry. In contrast, over the same time period 295 adult cardiovascular trials and 149 pediatric mental health trials were sponsored by the National Institutes of Health and 2,365 adult cardiovascular trials and 708 pediatric infectious disease trials were sponsored by industry. Importantly, pediatric cardiovascular trials generally did well in terms of quality metrics with 75% reporting randomization, 51% using blinding, and 54% reporting the use of a data monitoring committee. Similar to adult trials, the most important factor associated with the conduct of a high quality trial (randomized and blinded design) was sponsorship by the U.S. National Institutes of Health (multivariable odds ratio, 1.9 [95% CI, 1.5–2.4] when compared to industry sponsorship).17
Figure 4. Pediatric cardiovascular trial focus and funding sources.
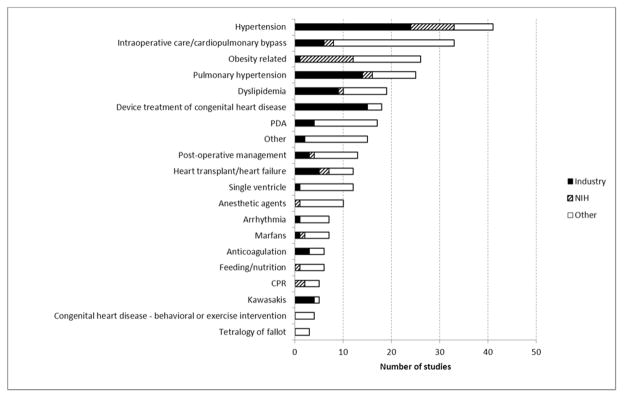
NIH = National Institutes of Health, PDA = patent ductus arteriosus, CPR = cardiopulmonary resuscitation
Heart failure trials
With the primary focus on pediatric heart failure and heart transplant at the 2015 Daicoff summit we subsequently used the search terms “heart failure” or “heart transplant” to interrogate ClinicalTrials.gov for all pediatric trials (enrolling subjects age < 18 years). These trials were downloaded and manually reviewed to identify trials with a primary pediatric cardiovascular focus. A search from July 1st, 2005 to January 1st, 2015 yielded 212 trials; however, the majority were excluded due to a primary adult focus or because the trials were withdrawn or terminated. We identified only 10 active (n=6) or completed (n=4) pediatric heart failure / heart transplant trials in this ten year period. These trials are summarized in Table 1. The 10 trials were sponsored by industry (N=8) or the National Institutes of Health (N=2); nine represent drug trials including five with a safety endpoint and five with a pharmacokinetic endpoint. Only two of the drug trials assessed efficacy endpoints and both were National Institutes of Health-sponsored trials: the Pediatric Heart Network Infant Single Ventricle trial and an National Institutes of Health-sponsored trial of diltiazem in subjects ages 5 years to 39 years with early signs of hypertrophic cardiomyopathy. One trial, the EXCOR® Pediatric Ventricular Assist Device trial, was an industry sponsored trial with a safety and pseudo-efficacy endpoint (comparison with historical controls). For these 10 trials, trial enrollment ranged from 10 to 230 subjects with only two enrolling > 100 subjects. Of the four completed trials, listed completion dates ranged from 01/2006 to 09/2013. Despite all trials being completed for over a year, only two of these trials have published results identified on ClinicalTrials.gov or Pubmed.
Table 1.
Pediatric heart failure or heart transplant trials registered on clinicaltrials.gov 2005–2014
| Trial (CT.gov ID) | Sponsor | N | Intervention | Patient population | Primary outcome | Design | Start–completion date | Trial status/Results |
|---|---|---|---|---|---|---|---|---|
| Infant Single Ventricle trial (NCT00113087) | NIH (PHN) | 230 | Enalapril | Infants (< 45 days) with single ventricle | Weight for age z-score | RCT | 08/2003 – 06/2008 | Completed, No change in primary outcome |
| Safety of twice daily carvedilol – extension of prior carvedilol trial (NCT00129363) | Industry | 75 | Carvedilol | Children (< 18 yrs) with systemic ventricular systolic dysfunction | Safety events | Open label | 01/2002 – 01/2006 | Completed, not published |
| Excor® pediatric ventricular assist device(NCT00583661) | Industry | 48 | Ventricular assist device | Children < 17 yrs with heart failure requiring circulatory support | Safety and probable benefit | Open label | 12/2007 – 12/2011 | Completed, Improved survival vs historical controls |
| Valgancyclovir PK (NCT01165580) | Industry | 17 | Valgancyclovir | Pediatric heart transplant recipients < 4 months | PK | Open label | 05/2011 – 09/2013 | Completed, not published |
| Daclizumab for Prevention of Allograft Rejection in Pediatric Heart Transplant (NCT00284531) | Industry | 10 | Daclizumab | Patients (< 18 yrs) undergoing a first cardiac allograft transplant | PK and safety | Open label | 10/2003 - | No status update, not published |
| Treatment of Preclinical Hypertrophic Cardiomyopathy With Diltiazem (NCT00319982) | NIH | 50 | Diltiazem | Patients (5–39 yrs) with preclinical hypertrophic cardiomyopathy | Diastolic function (Echo) | RCT | 01/2006 – 12/2013 | Completed, not published |
| Pharmacokinetics of Tacrolimus in Pediatric Allograft Recipients Converted From Prograf® to Advagraf® (NCT01294020) | Industry | 72 | Tacrolimus | Children (5–16 yrs) s/p solid organ transplant | PK/safety | Open label | 05/2011 - | Enrolling |
| Pharmacokinetics of Children Receiving Modigraf Following Solid Organ Transplantation (NCT01371331) | Industry | 60 | Tacrolimus | Children up to 12 years with liver, kidney or heart transplant | PK | Open label | 06/2011 - | Enrolling |
| A Paediatric, Open, Follow up Study With Modigraf (NCT01371344) | Industry | 60 | Tacrolimus | Children up to 12 years with liver, kidney or heart transplant | Safety | Open label | 06/2011 - | Enrolling |
| Pharmacokinetics & Safety of Serelaxin in Pediatric Patients with Acute Heart Failure (NCT02151383) | Industry | 36 | Seralexin | Pediatric patients (< 18 yrs) hospitalized with acute heart failure | PK/Safety | Open label | 09/2014 - | Enrolling |
The Food and Drug Administration Pediatric Drug Labeling Database
ClinicalTrials.gov provides a meaningful overview of the clinical trials landscape. However, a major limitation is that it is difficult to use this database to assess the impact of trials on the overall evidence base. While there is no perfect metric for evaluating this outcome, changes to the drug label represent a reasonable surrogate of new evidence. The U.S. Food and Drug Administration Labeling database tracks labeling changes for pediatric drugs.22 As of November 14th 2014, the drug database documents 489 studies that have been completed for pediatric agents. Of these, 28 (5.1%) represent cardiovascular agents including 16 anti-hypertensive agents and eight lipid-lowering agents (including seven statins). The remaining four drugs are carvedilol, sildenafil, sotalol and clopidogrel (Table 2). All eight of the lipid lowering agents have been studied for heterozygous familial hypercholesterolemia in adolescents and all have resulted in a new labeled indication for the drug (i.e. have demonstrated efficacy). The anti-hypertensive trials have led to ten new labeled indications (63% success rate). None of the remaining four drugs have demonstrated efficacy in the pediatric trials; three were negative trials while the sotalol trials24,25 did not evaluate an efficacy endpoint, instead focusing on drug kinetics.
Table 2.
Cardiovascular drugs with studies performed for pediatric exclusivity that have resulted in labeling changes
| Drug name, trade (generic) | Indication studied | Trial “N” | Safety extension phase | Label date | New pediatric information on drug label | ||||
|---|---|---|---|---|---|---|---|---|---|
| PK | Dose | Safety | Liquid suspension | Efficacy demonstrated | |||||
|
| |||||||||
| Betapace (sotalol) | Arrhythmia | 25 | − | 10/01 | + | + | + | + | Not evaluated |
|
| |||||||||
| Vasotec (enalapril) | Hypertension | 110 | − | 02/01 | + | + | + | + | Yes |
|
| |||||||||
| Monopril (fosinopril) | Hypertension | 252 | + (1 yr) | 05/03 | + | + | + | − | No |
|
| |||||||||
| Prinivil (Lisinopril) | Hypertension | 115 | − | 05/03 | + | + | + | + | Yes (6–16 yrs) |
|
| |||||||||
| Zestril (Lisinopril) | Hypertension | 115 | − | 07/03 | + | + | + | + | Yes (6–16 yrs) |
|
| |||||||||
| Norvasc (amlodipine) | Hypertension | 268 | − | 01/04 | + | + | + | − | No |
|
| |||||||||
| Lotensin (benazepril) | Hypertension | 144 | − | 03/04 | + | − | + | + | No |
|
| |||||||||
| Cozaar (losartan) | Hypertension | 177 | − | 03/04 | + | + | + | + | Yes (6–16 yrs) |
|
| |||||||||
| Avapro (irbesartan) | Hypertension | ? | − | 03/06 | − | − | − | − | No |
|
| |||||||||
| Diovan (valsartan) | Hypertension | 351 | − | 11/07 | + | + | + | + | Yes |
|
| |||||||||
| Inspira (eplerenone) | Hypertension | 304 | + (1 yr) | 01/08 | + | − | + | − | No |
|
| |||||||||
| Atacand (candesartan) | Hypertension | 333 | + (1 yr) | 10/09 | + | + | + | + | Yes |
|
| |||||||||
| Benicar (olmesartan) | Hypertension | 302 | − | 02/10 | + | + | + | + | Yes (> 6yrs) |
|
| |||||||||
| Corlopam (fenoldopam) | Hypertension | 77 | − | 04/04 | + | + | + | − | Yes |
|
| |||||||||
| Nitropress (Na nitroprusside) | Hypertension | 266 | − | 11/13 | + | + | + | − | Yes |
|
| |||||||||
| Toprol XL (metoprolol) | Hypertension | 144 | − | 11/13 | + | − | + | − | No |
|
| |||||||||
| Mevacor (lovastatin) | Heterozygous familial hypercholesterolemia | 180 | + (48 wk) | 02/02 | − | + | + | − | Yes (10–17yrs) |
|
| |||||||||
| Lipitor (atorvastatin) | Heterozygous familial hypercholesterolemia | 187 | + (1 yr) | 10/02 | − | + | + | − | Yes (10–17yrs) |
|
| |||||||||
| Pravachol (pravastatin) | Heterozygous familial hypercholesterolemia | 214 | + (2yr) | 10/02 | − | + | + | − | Yes (8–18yrs) |
|
| |||||||||
| Zocor (simvastatin) | Heterozygous familial hypercholesterolemia | 175 | + (48 wk) | 10/02 | − | + | + | − | Yes (10–17 yrs) |
|
| |||||||||
| Lescol (fluvastatin) | Heterozygous familial hypercholesterolemia | 114 | + (2 yr) | 04/06 | − | + | + | − | Yes (10–16yrs) |
|
| |||||||||
| Zetia and Vytorin (ezetimibe ± simvastatin) | Heterozygous familial hypercholesterolemia | 248 | + (33 wk) | 06/08 | − | + | + | − | Yes (10–17yrs) |
|
| |||||||||
| Welchol (colesevelam) | Heterozygous familial hypercholesterolemia | 194 | + (29 wk) | 10/09 | − | + | + | + | Yes (10–17yrs) |
|
| |||||||||
| Crestor (rosuvastatin) | Heterozygous familial hypercholesterolemia | 176 | + (1 yr) | 10/09 | − | + | + | − | Yes (10–17yrs) |
|
| |||||||||
| Revatio (sildenafil) | Pulmonary arterial hypertension | 184 | + (2 yr) | 08/12 | − | − | + | + | No |
|
| |||||||||
| Plavix (clopidogrel) | Prevention of shunt thrombosis | 1006 | − | 05/11 | − | − | − | − | No |
|
| |||||||||
| Coreg (carvedilol) | Heart failure | 161 | + (8 mo) | 02/07 | − | − | + | − | No |
? Irbesartan trials remain unpublished and details are not readily available
What can we learn from the negative trials?
Hypertension trials: In an insightful analysis, Benjamin et al. found that the successful hypertension trials used large differences in the dose ranges of the drugs studied (20–32-fold), with little or no overlap between low, medium, and high doses whereas failed trials used overlapping dose ranges which made it more difficult to detect a dose-response effect.26 Successful trials also provided pediatric formulations and used diastolic blood pressure (which tends to demonstrate less variability than systolic blood pressure) as the primary study endpoint.
Carvedilol: These studies failed to demonstrate improved outcomes in children with cardiomyopathy with either low or high dose carvedilol when compared to placebo.27 The investigators noted several important factors that may have contributed to the negative trial outcome and can serve as valuable lessons for future trials: 1) relative to adults, pediatric subjects (particularly younger subjects) with heart failure had a higher than anticipated rate of spontaneous improvement. As a result, the trial was relatively underpowered; 2) the study end-point was a composite heart failure outcome with three levels of assessment (improved, no change, worsened). Assessing three levels requires more power and compounded the noted problems with sample size; 3) there were several signals suggesting a mixed response; children with a systemic right ventricle demonstrated a trend towards worsening function with carvedilol whereas those with a single left ventricle demonstrated a trend towards improvement, and younger children were more likely to improve than older children. Finally, relative to adults, children demonstrated more rapid drug clearance and consequently trough concentrations of carvedilol were relatively lower. Taken together, the findings from this trial are often viewed as inconclusive in patients with a systemic left ventricle. A show of hands at the Daicoff summit suggests that most heart failure providers still use carvedilol off-label in children with left heart failure.
Sildenafil: These trials have been the source of much debate in the pediatric literature. Initial pharmacokinetics trials were completed but to our knowledge the data have not been published in the medical literature and are available only through the Food and Drug Administration’s clinical pharmacology reviews.28 The initial efficacy trial did not demonstrate improved exercise tolerance when comparing low dose sildenafil to placebo in children with pulmonary hypertension.29 In the long-term extension trial, children randomized to high dose sildenafil had increased mortality when compared to low dose sildenafil.29,30 Similar to carvedilol, there was a mixed signal with worse outcomes in children with idiopathic pulmonary hypertension when compared to those with congenital heart disease associated pulmonary hypertension, and the increased mortality effect seen primarily in older children. The European Medicines Agency interpreted the trial results in the context of historically poor outcomes for children with pulmonary hypertension, noting that children receiving low dose sildenafil had improved survival when compared to historical controls.31 They approved low dose sildenafil for the treatment of pediatric pulmonary hypertension. In contrast, the Food and Drug Administration did not approve any dose of sildenafil and went one step further, placing a safety warning on the drug label.32
Clopidogrel: This trial evaluated clopidogrel in addition to standard therapy (aspirin for 87% of trial participants) for the prevention of shunt thrombosis and associated morbidity in neonates and infants with a systemic to pulmonary artery shunt.33 There was no additive benefit with clopidogrel and therefore there is no pediatric indication for the use of clopidogrel in children. However, the drug label specifically notes that the negative study may reflect that the majority of study subjects were receiving concomitant aspirin therapy.
Conclusions
When analyzed together, ClinicalTrials.gov and the Food and Drug Administration Labelling Information Database provide important insight into the pediatric clinical trials landscape. First, it is clear that pediatric cardiovascular trials are relatively under-represented; this is the case when compared to adult cardiovascular trials and also to other pediatric subspecialties registered on ClinicalTrials.gov. This is also reflected in the Food and Drug Administration pediatric labeling database where only 28/489 listed agents represent cardiovascular agents. In comparison the labeling database lists studies that have been performed for 31 anti-asthmatic agents, 32 antihistamines, 16 agents to treat gastroesophageal reflux disease, 20 stimulant or non-stimulant attention deficit-hyperactivity disorder agents and 11 anti-acne agents. The relative lack of pediatric cardiovascular studies is also reflected in the relative paucity of both United States National Institutes of Health- and industry-funded trials. Of note, National Institutes of Health-funded trials are consistently the highest quality and highest impact trials, yet in a five year period there were only 13 National Institutes of Health-funded pediatric cardiovascular trials that did not focus on either obesity or hypertension. Strategies are needed to increase funding and also the overall volume of pediatric cardiovascular trials, particularly larger trials focused on efficacy endpoints.
Second, the focus of pediatric cardiovascular trials appears to be driven by the financial incentive structure which does not always align perfectly with actual need within the field. This conclusion stems from the fact that the Food and Drug Administration typically requires a minimum of two to three trials in order to satisfy pediatric exclusivity requirements (e.g. a pharmacokinetics trial, short term efficacy trial and a longer term safety extension). Therefore, it is reasonable to assume that many of the trials of anti-hypertensive agents registered on ClinicalTrials.gov represent trials being completed for one of the 14 different anti-hypertensive agents that have gained patent extension under the exclusivity program. It is likely that the same logic can be applied to the dyslipidemia and perhaps also the pulmonary hypertension trials. It is certainly beneficial that we now have five angiotensin receptor blockers labeled for pediatric hypertension, and seven different statins for familial heterozygous hypercholesterolemia. However, there might have been greater child health benefit if only one or two agents in each drug class were studied for these specific indications and the other agents perhaps could have been evaluated in other important patient populations. As an example, one needs only to consider pediatric heart failure or heart transplantation. These patients typically require an arsenal of medications yet our evaluation of ClinicalTrials.gov indicates that very few trials over the past eight years have focused specifically on these high risk patient cohorts. One could easily rationalize a trial of an antihypertensive agent or lipid lowering agent in the heart transplant patient population. Moreover, three trials for pediatric exclusivity have been completed to evaluate transplant rejection agents however all three have been performed in kidney transplant recipients and none have included heart transplant recipients.
Finally, there are patterns that emerge when evaluating outcomes of our trials. Our experiences with the hypertension trials have demonstrated the value of child-centric dosing strategies including the use of weight based dosing and the use of liquid formulations for younger children.26 Successful trials in our field have focused on homogeneous patient populations with more restrictive age ranges. For example, the dyslipidemia trials (100% success rate) all enrolled adolescents with familial heterozygous hypercholesterolemia. Similarly the successful hypertension trials have typically performed separate trials in the 1–6 year versus 6–17 year age ranges. In contrast, interpretation of the carvedilol and sildenafil trials was significantly hampered by differing effects in the heterogeneous diagnostic cohorts and when comparing younger to older patients. The carvedilol trial also demonstrated the value of preliminary pharmacokinetic data to optimize trial drug dosing as well as the importance of pilot data in children (not extrapolated from adults) to guide power calculations. Looking forward, further work is needed to evaluate optimal outcome measures, particularly for heart failure trials where outcomes are inherently difficult to ascertain. Adult trials have recently considered the global rank endpoint, a mechanism for increasing power by considering the full range of different outcomes (death, transplant, clinical worsening, rehospitalization etc). This may be something to consider in children given the inherent difficulties studying these relatively rare conditions.34
In conclusion, recent legislative initiatives have led to a complete metamorphosis in the pediatric clinical trials landscape. Clinical trials represent the best means to improve outcomes for our patients and it is critical that we continue to evaluate ways to improve upon the existing clinical trials emphasis, infrastructure and processes. While significant advances have been made to date in pediatric hypertension and hyperlipidemia, in order to improve the health for children with heart disease, future work should focus on these processes for children with heart failure, congenital heart disease, and pulmonary hypertension.
Footnotes
Disclosures: Drs. Hornik and Li receive grant support from the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117. Dr. Hill receives grant support from the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR001115-02), the Gilead Cardiovascular Scholars Program, the Mend-A-Heart Foundation and the United States Department of health and Human Services Food and Drug Administration (1UO1FD004858-01). None of the authors have any conflicts of interest to report.
References
- 1.Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104(3 Pt 2):585–590. [PubMed] [Google Scholar]
- 2.Shah SS, Hall M, Goodman DM, et al. Off-label drug use in hospitalized children. Archives of pediatrics & adolescent medicine. 2007;161(3):282–290. doi: 10.1001/archpedi.161.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Sutherell JS, Hirsch R, Beekman RH., 3rd Pediatric interventional cardiology in the United States is dependent on the off-label use of medical devices. Congenital heart disease. 2010;5(1):2–7. doi: 10.1111/j.1747-0803.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 4.Breslow LH. The Best Pharmaceuticals for Children Act of 2002: the rise of the voluntary incentive structure and congressional refusal to require pediatric testing. Harvard J Legis. 2003;40(1):133–193. [PubMed] [Google Scholar]
- 5.Field M, Boat TF, editors. Safe and Effective Medicines for Children. Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. 1. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 6.Ward RM, Kauffman R. Future of pediatric therapeutics: reauthorization of BPCA and PREA. Clin Pharmacol Ther. 2007;81(4):477–479. doi: 10.1038/sj.clpt.6100109. [DOI] [PubMed] [Google Scholar]
- 7.United States Food and Drug Administration. [December 30th, 2014];Status Report to Congress on the Pediatric Exclusivity Provision. Accessed at: http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm049915.pdf.
- 8.United States Food and Drug Administration. [December 30th, 2014];Full text of Best Pharmaceuticals for Children Act. Accessed at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148011.htm.
- 9.United States Food and Drug Administration. [December 30th, 2014];Full Text of the Food and Drug Administration Amendments Act of 2007. 2007 Accessed at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FoodandDrugAdministrationAmendmentsActof2007/FullTextofFDAAALaw/default.htm.
- 10.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill KDS, PB, Cohen-Wolkowiez M, Benjamin DK, Li JS. Pediatric Exclusivity and Other Contemporary Regulatory Changes: Impact on Pediatric Drug Study, Labeling and Safety. Clinical Investigation. 2013;3(3):227–239. [Google Scholar]
- 12.United States Food and Drug Administration. [December 24th, 2013];List of dugs granted pediatric exclusivity. Accessed at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM223058.pdf.
- 13.United States Food and Drug Administration. [December 30th, 2014];Breakdown of Food and Drug Administration Amendments Act (FDAAA) Completed Pediatric Studies. Accessed at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm190622.htm.
- 14.European Medicines Agency. [December 27, 2014];Five-year Report to the European Commission: General report on the experience acquired as a result of the application of the Paediatric Regulation. Accessed online at: http://ec.europa.eu/health/files/paediatrics/2012-09_pediatric_report-annex1-2_en.pdf.
- 15.Li JS, Eisenstein EL, Grabowski HG, et al. Economic return of clinical trials performed under the pediatric exclusivity program. JAMA. 2007;297(5):480–488. doi: 10.1001/jama.297.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquali SK, Lam WK, Chiswell K, Kemper AR, Li JS. Status of the pediatric clinical trials enterprise: an analysis of the US ClinicalTrials.gov registry. Pediatrics. 2012;130(5):e1269–1277. doi: 10.1542/peds.2011-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill KD, Chiswell K, Califf RM, Pearson G, Li JS. Characteristics of pediatric cardiovascular clinical trials registered on ClinicalTrials.gov. American heart journal. 2014;167(6):921–929. e922. doi: 10.1016/j.ahj.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Status Report to Congress on the Pediatric Exclusivity Provision. 2001 Accessed at: http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm049915.pdf.
- 19.Aisenberg AC. Problems in Hodgkin’s disease management. Blood. 1999;93(3):761–779. [PubMed] [Google Scholar]
- 20.United States Food and Drug Administration. [December 24, 2014];Full text of Food and Drug Administration Modernization Act. Accessed at: http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDAMA/FullTextofFDAMAlaw/default.htm.
- 21.De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351(12):1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- 22.United States Food and Drug Administration. [December 30th, 2014];New Pediatric Drug Labeling Information Database. Accessed at http://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase.
- 23.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307(17):1838–1847. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 24.Saul JP, Ross B, Schaffer MS, et al. Pharmacokinetics and pharmacodynamics of sotalol in a pediatric population with supraventricular and ventricular tachyarrhythmia. Clin Pharmacol Ther. 2001;69(3):145–157. doi: 10.1067/mcp.2001.113795. [DOI] [PubMed] [Google Scholar]
- 25.Saul JP, Schaffer MS, Karpawich PP, et al. Single-dose pharmacokinetics of sotalol in a pediatric population with supraventricular and/or ventricular tachyarrhythmia. Journal of clinical pharmacology. 2001;41(1):35–43. doi: 10.1177/00912700122009818. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin DK, Jr, Smith PB, Jadhav P, et al. Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension. 2008;51(4):834–840. doi: 10.1161/HYPERTENSIONAHA.107.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298(10):1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 28.United States Food and Drug Administration. [April 14th, 2015];Medical, Statistical, and Clinical Pharmacology Reviews of Pediatric Studies Conducted under Section 505A and 505B of the Federal Food, Drug, and Cosmetic Act, as amended by the FDA Amendments Act of 2012 (FDASIA) Accessed online at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm316937.htm.
- 29.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125(2):324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 30.Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014;129(19):1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 31.European Medicines Agency. [December 26, 2014];Assessment Report for Revatio. Accessed online at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000638/WC500107804.pdf.
- 32.United States Food and Drug Administration. [April 14th, 2015];Drug Safety Communication. Accessed online at: http://www.fda.gov/Drugs/DrugSafety/ucm390876.htm.
- 33.Wessel DL, Berger F, Li JS, et al. Clopidogrel in infants with systemic-to-pulmonary-artery shunts. N Engl J Med. 2013;368(25):2377–2384. doi: 10.1056/NEJMoa1114588. [DOI] [PubMed] [Google Scholar]
- 34.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circulation. Heart failure. 2010;3(5):643–646. doi: 10.1161/CIRCHEARTFAILURE.109.926030. [DOI] [PubMed] [Google Scholar]