Abstract
Background
“Probable active syphilis,” is defined as seroreactivity in both non-treponemal and treponemal tests. A correction factor of 65%, namely the proportion of pregnant women reactive in one syphilis test type that were likely reactive in the second, was applied to reported syphilis seropositivity data reported to WHO for global estimates of syphilis during pregnancy.
Objectives
To identify more accurate correction factors based on test type reported.
Search Strategy
Medline search using: “Syphilis [Mesh] and Pregnancy [Mesh],” “Syphilis [Mesh] and Prenatal Diagnosis [Mesh],” and “Syphilis [Mesh] and Antenatal [Keyword].
Selection Criteria
Eligible studies must have reported results for pregnant or puerperal women for both non-treponemal and treponemal serology.
Data collection and analysis
We manually calculated the crude percent estimates of subjects with both reactive treponemal and reactive non-treponemal tests among subjects with reactive treponemal and among subjects with reactive non-treponemal tests. We summarized the percent estimates using random effects models.
Main results
Countries reporting both reactive non-treponemal and reactive treponemal testing required no correction factor. Countries reporting non-treponemal testing or treponemal testing alone required a correction factor of 52.2% and 53.6%, respectively. Countries not reporting test type required a correction factor of 68.6%.
Conclusions
Future estimates should adjust reported maternal syphilis seropositivity by test type to ensure accuracy.
Keywords: Congenital syphilis, Diagnostics, Pregnancy, Prenatal diagnosis, Syphilis
1. Background
In 2088, WHO estimated that, worldwide, approximately 1.4 million pregnant women had “probable active syphilis” (PAS) or syphilis infections sufficiently active to result in mother-to-child transmission (MTCT) and with the potential of subsequent adverse pregnancy outcomes [1]. Syphilis in pregnancy can be devastating and is associated with poor fetal or infant outcomes in the majority of cases, with an estimated 52% of PAS cases resulting in an adverse perinatal outcome attributable to syphilis [2]. PAS (defined as seroreactivity for both non-treponemal and treponemal tests) is used as the reporting measure by WHO since surveillance data typically do not include clinical information.
Currently, no single test or combination of tests accurately predicts the extent to which maternal syphilis infection in pregnancy will affect the fetus. However, serologic tests can be suggestive; the combination of a reactive non-treponemal test (e.g. rapid plasma regain [RPR], venereal disease research laboratory [VDRL]) and a reactive treponemal test (e.g. Treponema pallidum particle agglutination [TP-PA], T. pallidum hemagglutination assay), defined in the 2008 WHO estimates as PAS, is compelling evidence for an infection that may result in MTCT. Neither type of test is both sensitive and specific on its own. A reactive, but unconfirmed, non-treponemal test may represent a biological false-positive result, whereas a reactive treponemal test alone may represent an old or previously treated infection that poses little exposure risk for the fetus. Considered schematically (Table 1), individuals with a positive result in both test types are likely to have syphilis (Cell A). Those with a single positive result in either test type could have syphilis, but might have false-positive or past-treated infection (Cells B and C). Those with negative results in both test types are unlikely to have syphilis (Cell D).
Table 1.
Schematic of syphilis testing by test type.
| Treponemal test
|
|||
|---|---|---|---|
| Reactive | Non-reactive | ||
| Non-treponemal test | Reactive | A (syphilis likely) | B (biologic false positive) |
| Non-reactive | C (possible past infection) | D (syphilis unlikely) | |
WHO estimated that untreated syphilis in pregnancy resulted in approximately 521 000 adverse perinatal outcomes globally in 2008, including an estimated 212 000 stillbirths, 92 000 neonatal deaths, 65 000 preterm or low birth weight infants, and 152 000 syphilis-infected newborns [1]. Health outcomes were modeled based on the published literature on MTCT risk of syphilis transmission [2] and national data reported to WHO from 147 countries on antenatal clinic (ANC) attendance (at least one visit) and from 97 countries on materna syphilis seropositivity among ANC attendees through the WHO/UNAIDS Global AIDS Response Progress Reporting System (GARPR, formerly known as HIV Universal Access Reporting: http://www.unaids.org/en/dataanalysis/knowyourresponse/globalaidsprogressreporting/). Maternal syphilis seropositivity data reported to WHO varied across countries, generally falling into four categories (Table 2). Category 1 included countries reporting the number of maternal syphilis cases reactive to both non-treponemal and treponemal syphilis tests (PAS); Category 2 included countries reporting cases reactive to non-treponemal syphilis tests only (i.e. no confirmatory treponemal testing reported); Category 3 included countries reporting cases reactive to treponemal tests only (i.e. no confirmatory non-treponemal testing reported); and Category 4 included countries for which the type of laboratory test used was not reported.
Table 2.
Syphilis seropositivity in antenatal women: WHO reporting categories based on syphilis test type, assumptions for new correction factors, and new correction factor estimates.
| Syphilis seropositivity | ||||
|---|---|---|---|---|
| WHO reporting categories | Category 1 (countries reporting based on both reactive non-treponemal and reactive treponemal testing) | Category 2 (countries reporting based on reactive non-treponemal testing only) | Category 3 (countries reporting based on reactive treponemal testing) | Category 4 (countries not reporting type of testing used) |
| Previous correction factor used for estimating probable active syphilis WHO [1] | 65% | 65% | 65% | 65% |
| Assumptions used for new correction factors | Additional correction factor not needed; reported data represent best estimate of probable active syphilis when only test type data are available | Proportion of pregnant women with reactive non-treponemal tests that also have reactive treponemal tests; A/(A + B) from Table 1 | Proportion of pregnant women with reactive treponemal tests that also have reactive non-treponemal tests; A/(A + C) from Table 1 | Non-reporting countries would be evenly distributed between Categories 1–3: average of the correction factors for Categories 1–3 |
| New correction factor estimate (95% CI) | 1.0 Actual data, no CI needed | 52.2% (38.0–66.6) | 53.6% (36.9–70.2) | 68.6% (61.3–78.9) |
In the 2008 estimates on burden of syphilis in pregnancy, WHO applied a correction factor assuming that 65% of all reported seropositive cases among pregnant women, regardless of test type, had infections that could lead to MTCT (PAS). A correction factor was necessary since 97% (188 of 193) of countries reporting to WHO had not reported on the test type used (Category 4), and many may have included only one test type (treponemal or non-treponemal) in their case definition. The correction factor was based on data from three ANC studies in which both non-treponemal and treponemal test results were reported [3–5], allowing calculation of the proportion of seropositive women in either test type expected to be reactive for both non-treponemal and treponemal tests (i.e. A/(A + B + C), Table 1). This estimation is best suited for Category 4 countries. However, for countries in Categories 1–3, more precise correction factors can be calculated. In this analysis, we sought to identify more accurate correction factors for future estimates of global burden of syphilis MTCT and resultant adverse pregnancy outcomes when test type data are available. Correction factors calculated were the estimated proportion of pregnant or puerperal women with reactive non-treponemal tests that had reactive treponemal tests (correction factor for Category 2 countries), or the proportion of pregnant or puerperal women with reactive treponemal tests that had reactive non-treponemal tests (correction factor for Category 3 countries).
2. Materials and methods
For this meta-analysis, we reviewed the published literature to identify country-level studies reporting maternal syphilis seropositivity results for both treponemal and non-treponemal tests on all patients in order to estimate the likelihood that a single unconfirmed syphilis test would also be positive for the alternative test type, had it been conducted.
To identify studies, we conducted a systematic Medline search using the terms: “Syphilis [Mesh] and Pregnancy [Mesh],” “Syphilis [Mesh] and Prenatal Diagnosis [Mesh],” and “Syphilis [Mesh] and Antenatal [Keyword]”, including observational studies (trials, cross-sectional serosurveys, and cohort and case-control studies) published between January 2000 and November 2013, and reporting both non-treponemal and treponemal syphilis testing results of any type in pregnant or puerperal women. We also looked at the three studies used in the original WHO correction factor estimate [3–5].
2.1. Inclusion criteria
To be included, eligible studies must have tested pregnant or puerperal women for both non-treponemal and treponemal serology and reported at least one of the following: the proportion of pregnant or puerperal women with reactive non-treponemal tests that had reactive treponemal tests (correction factor for Category 2 countries) or the proportion of pregnant or puerperal women with reactive treponemal tests that had reactive non-treponemal tests (correction factor for Category 3 countries). Studies were included regardless of type of non-treponemal (e.g. RPR, VDRL) or treponemal (e.g. fluorescent treponemal antibody absorption, TP-PA) test used, publication language, country, or age of subjects.
We used these data to estimate maternal syphilis seropositivity for countries reporting data to WHO based on a single test type (Categories 2 and 3), or that did not report the test type used (Category 4; Table 2). For Category 1 countries, we assumed that reported data should be used without correction since these are the best possible estimates for PAS cases in pregnancy when only test type (no clinical or titer) data are available. For Category 2 countries, we used the published literature to calculate estimates and 95% confidence intervals (CIs) for the proportion of pregnant women with reactive non-treponemal tests that also had reactive treponemal tests (i.e. A/(A + B) from Table 1). For Category 3 countries, we used the published literature to calculate estimates and CIs for the proportion of pregnant women with reactive treponemal tests that also had reactive non-treponemal tests (i.e. A/(A + C) from Table 1). For Category 4 countries, we assumed an equal probability of having used only non-treponemal, only treponemal, or a combined test strategy. Thus, we used the average of the estimates for the three correction factors for Categories 1 – 3 to estimate the number of PAS cases ((Category 1 correction factor + Category 2 correction factor + Category 3 correction factor)/3). The estimated proportions for each WHO reporting category represent the correction factors to be used for their respective categories.
2.2. Statistical analysis
For each study identified from the literature review, based on the reported data, we manually retrieved or calculated the crude percent estimates of subjects with both reactive treponemal and reactive non-treponemal tests among subjects with reactive treponemal (Category 2) and among subjects with reactive non-treponemal (Category 3) tests and corresponding 95% CIs for the assessed outcomes. We summarized the percent estimates using random effects models, which take into account the presence of between-study heterogeneity into the calculations. This approach was chosen over a fixed effects model since the underlying syphilis prevalence and other factors were different in each population studied.
3. Results
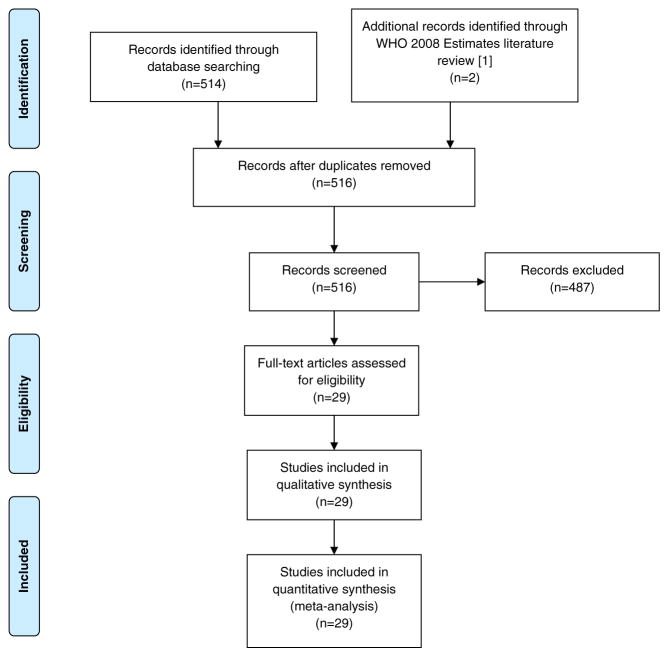
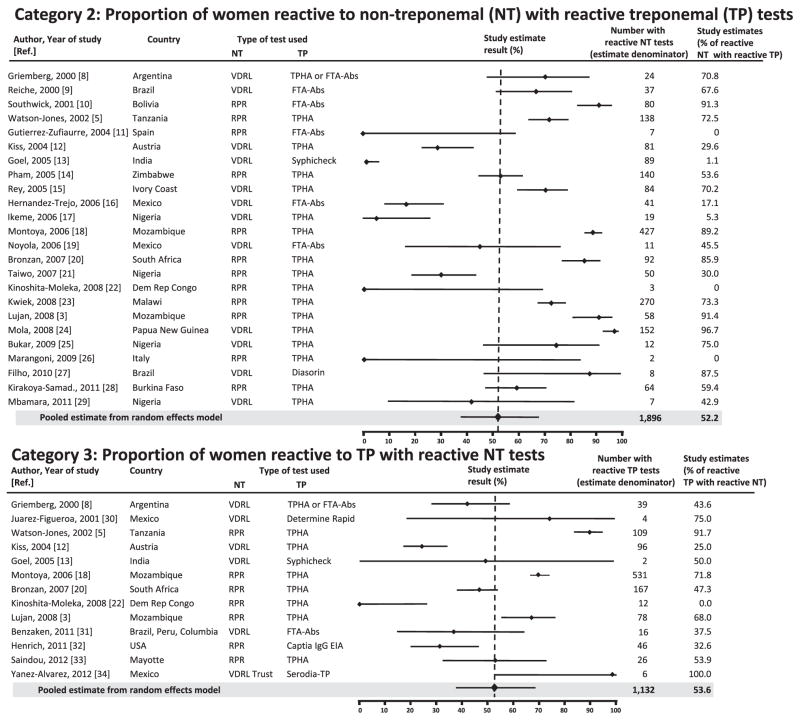
The MEDLINE search identified 514 studies along with two of the three studies identified in the WHO 2008 estimates literature search that met our inclusion criteria [1]. Of the 516 studies screened for eligibility, 29 met the criteria and were included in the analyses [3,5–32] (Fig. 1). Studies could be included in more than one analysis depending on what type of results were reported: once for estimating the correction factor for Category 2, once for Category 3, and all were included in the Category 4 estimate. In total, 24 of the 29 studies reported A/(A + B) results (Table 1) [3,5–25,33,34], representing 1896 women used to estimate the correction factor for Category 2 countries; and 13 of the 29 reported A/(A + C) results [3,5,8,9,14,16,18,26–30,33], representing 1132 women included for the estimate for Category 3 countries. The studies were conducted in various clinical settings (e.g. hospitals, ANC clinics, rural clinics, urban clinics) and represented 22 countries. The study estimates and CIs for Category 2 and 3 countries are shown in Fig. 2.
Fig. 1.
Flow diagram of study selection.
Fig. 2.
Meta-analysis of studies reporting non-treponemal (NT) and treponemal (TP) test results in pregnant women and correction factor estimates for the WHO reporting Categories 2 and 3 with 95% CIs. *Points represent reported study values for given Category; bars represent 95% CIs. EIA, Enzyme immunoassay; FTA-Abs, Fluorescent treponemal antibody absorption; RPR, Rapid plasma regain; TPHA, T. pallidum hemagglutination assay; TP, Treponema pallidum; VDRL, Venereal disease research laboratory.
Following pooling of the results from individual studies and accounting for within- and between-study variation using the random effects model, the correction factor for Category 2 countries was estimated to be 52.2% (95% CI, 38.0–66.6), indicating that an estimated 52.2% of the syphilis cases in pregnancy reported to WHO by these countries were likely to have PAS (Table 2). Using the random effects model, the pooled correction factor for Category 3 countries was quite similar, calculated as 53.6% (95% CI, 36.9–70.2; Table 2). As previously discussed, Category 1 countries reported the best possible estimates as data were based on both treponemal and non-treponemal testing results, and thus the correction factor was set as 1.0. For Category 4 countries, we used the average of the correction factors calculated for the first three categories, and the correction factor was calculated as 68.6% (95% CI, 61.3–78.9; Table 2). Thus, an estimated 68.6% of cases in pregnancy reported by these countries were likely to have been PAS.
4. Discussion
This analysis was conducted to improve future estimates of the global burden of syphilis in pregnancy and the related adverse outcomes. The meta-analysis results indicate that, among countries reporting maternal syphilis infections using a single test result, regardless of test type, an estimated 53% of cases represent sufficiently active infections to result in transmission of syphilis from mother to fetus. For countries not reporting test type, approximately 69% of cases are estimated to have sufficiently active infections to result in MTCT. Had the correction factors calculated herein been used in the 2008 WHO estimates, there would have been an increase in syphilis cases in pregnancy (1 408 811 vs 1 473 152 infections, or a 4.6% increase), and a proportionately similar increase in associated outcomes. Nevertheless, despite the difference between using a uniform or a variable correction factor based on reported test type not being significant in 2008, testing practices within countries may evolve over time and, thus, this may not always be the case. Furthermore, efforts are being made by WHO and UNAIDS to improve maternal syphilis seropositivity test type reporting, which will allow for improvements in the accuracy of estimates. Accurate estimates are important to evaluate progress in global and regional congenital syphilis elimination initiatives as well as for strategic planning [33].
Serologic testing is inherently imprecise in identifying infectious syphilis. Positive predictive values of tests vary according to population prevalence, clinical stage of disease, prior history of disease and treatment, and quality of laboratory testing. In pregnancy, MTCT risk can be infiuenced by co-infection with malaria or HIV [34]. Health systems with accurate laboratory testing and strong antenatal programs are likely to better identify true syphilis cases earlier in the course of pregnancy, leading to disease prevention. In settings with a stronger public health infrastructure, unconfirmed reactive treponemal tests are likely to represent previously treated syphilis infections; while in settings with weak testing and treatment infrastructures, unconfirmed reactive treponemal tests are likely to represent untreated syphilis. A clinical history can help distinguish previously treated from newly infected cases; however, these data are not available in WHO (or most national) surveillance systems, and their inclusion in routine surveillance is impractical.
It must be noted, however, that this study is not without limitations. First, the studies included in the meta-analysis varied in their setting (urban vs rural), underlying syphilis and other disease prevalence, and available health care and laboratory infrastructure. Further, despite having estimated the results using a random effects model, the correction factors are unlikely to be generalizable to every individual locale, country, or region. In particular, the underlying prevalence of syphilis in pregnant women will greatly affect the correction factor in Category 3. Second, relatively few studies in the published literature reported syphilis seropositivity in pregnancy for both treponemal and non-treponemal tests. It is hoped that, over time, more study data will be available to further refine the correction factor estimates. Third, although a structured search was performed, the possibility of unpublished studies showing different results leads to a likelihood of selection bias in the studies included in the meta-analysis.
Despite these limitations, our study describes how estimates of maternal syphilis can be improved by correcting for test type. While not perfect, the correction factors calculated herein represent a step toward improved accuracy in estimating the global burden of syphilis infections in pregnant women and resultant perinatal health outcomes. This updated methodology, along with improvements in global reporting of test types, development of more sensitive and specific syphilis tests, and improved access to syphilis diagnostics in resource-poor settings, are likely to improve the global estimates of syphilis in pregnancy and associated outcomes in the future. Although this study focuses on maternal syphilis, the methodology could be applied to other global disease estimates where biomarkers are used to measure burden of disease.
References
- 1.Newman LM, Kamb ML, Hawkes S, Gomez G, Lale S, Seuc A, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med. 2013;10:1–10. doi: 10.1371/journal.pmed.1001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217–26. doi: 10.2471/BLT.12.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lujan J, de Onate WA, Delva W, Claeys P, Sambola F, Temmerman M, et al. Prevalence of sexually transmitted infections in women attending antenatal care in Tete province, Mozambique. S Afr Med J. 2008;98:49–51. [PubMed] [Google Scholar]
- 4.Tinajeros F, Grossman D, Richmond K, Steele M, Garcia SB, Zegarra L, et al. Diagnostic accuracy of a point-of-care syphilis test when used among pregnant women in Bolivia. Sex Transm Infect. 2006;82:17–21. doi: 10.1136/sti.2006.022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson-Jones D, Changalucha J, Gumodoka B, Weiss H, Rusizoka M, Ndeki L, et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis. 2002;186:940–7. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]
- 6.Griemberg G, Ravelli MR, Etcheves PC, Orfus G, Pizzimenti MC. Syphilis and pregnancy: prenatal control, seroprevalence and false biological positives. Medicina (B Aires) 2000;60(3):343–7. [PubMed] [Google Scholar]
- 7.Reiche EM, Morimoto HK, Farias GN, Hisatsugu KR, Geller L, Gomes AC, et al. Prevalence of American trypanosomiasis, syphilis, toxoplasmosis, rubella, hepatitis B, hepatitis C, human immunodeficiency virus infection, assayed through serological tests among pregnant patients, from 1996 to 1998, of the Hospital Universitario Regional Norte do Parana. Rev Soc Bras Med Trop. 2000;33:519–27. doi: 10.1590/s0037-86822000000600002. [DOI] [PubMed] [Google Scholar]
- 8.Southwick KL, Blanco S, Santander A, Estenssoro M, Torrico F, Seoane E, et al. Maternal and congenital syphilis in Bolivia, 1996: prevalence and risk factors. Bull World Health Organ. 2001;79:33–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez-Zufiaurre N, Sanchez-Hernandez J, Munoz S, Marin R, Delgado N, Saenz N, et al. Seroprevalence of antibodies against Treponema pallidum, Toxoplasma gondii, rubella virus, hepatitis B and C virus, and HIV in pregnant women. Enferm Infecc Microbiol Clin. 2004;22:512–6. doi: 10.1016/s0213-005x(04)73152-3. [DOI] [PubMed] [Google Scholar]
- 10.Kiss H, Widhalm A, Geusau A, Husslein P. Universal antenatal screening for syphilis: is it still justified economically? A 10-year retrospective analysis. Eur J Obstet Gynecol Reprod Biol. 2004;112:24–8. doi: 10.1016/s0301-2115(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 11.Goel N, Sharma M, Gupta N, Sehgal R. Rapid immunochromatographic test for syphilis. Indian J Med Microbiol. 2005;23:142–3. doi: 10.4103/0255-0857.16061. [DOI] [PubMed] [Google Scholar]
- 12.Pham L, Woelk GB, Ning Y, Madzime S, Mudzamiri S, Mahomed K, et al. Seroprevalence and risk factors of syphilis infection in pregnant women delivering at Harare Maternity Hospital, Zimbabwe. Cent Afr J Med. 2005;51:24–30. [PubMed] [Google Scholar]
- 13.Rey JL, Coulibaly M, Noba V. Syphilis test proposed within the context of a programme to reduce mother/child HIV transmission: example of the Wassakara health care center in Abidjan. Bull Soc Pathol Exot. 2005;98:390–1. [PubMed] [Google Scholar]
- 14.Hernandez-Trejo M, Hernandez-Prado B, Uribe-Salas F, Juarez-Figueroa L, Conde-Gonzalez CJ. Maternal and congenital syphilis in two Mexican hospitals: evaluation of a rapid diagnostic test. Rev Invest Clin. 2006;58:119–25. [PubMed] [Google Scholar]
- 15.Ikeme AC, Okeke TC. The relevance of VDRL as routine test in pregnant women: a critical study. Niger J Clin Pract. 2006;9:65–7. [PubMed] [Google Scholar]
- 16.Montoya PJ, Lukehart SA, Brentlinger PE, Blanco AJ, Floriano F, Sairosse J, et al. Comparison of the diagnostic accuracy of a rapid immunochromatographic test and the rapid plasma regain test for antenatal syphilis screening in Mozambique. Bull World Health Organ. 2006;84:97–104. doi: 10.2471/blt.04.018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noyola DE, Malacara-Alfaro O, Lima-Rogel V, Torres-Montes A. Seroprevalence of syphilis in pregnant women in San Luis Potosi. Salud Publica Mex. 2006;48:151–4. doi: 10.1590/s0036-36342006000200008. [DOI] [PubMed] [Google Scholar]
- 18.Bronzan RN, Mwesigwa-Kayongo DC, Narkunas D. Onsite rapid antenatal syphilis screening with an immunochromatographic strip improves case detection and treatment in rural South African clinics. Sex Transm Dis. 2007;34:55–60. doi: 10.1097/01.olq.0000245987.78067.0c. [DOI] [PubMed] [Google Scholar]
- 19.Taiwo SS, Adesiji YO, Adekanle DA. Screening for syphilis during pregnancy in Nigeria: a practice that must continue. Sex Transm Infect. 2007;83:357–8. doi: 10.1136/sti.2006.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita-Moleka R, Smith JS, Atibu J, Tshefu A, Hemingway-Foday J, Hobbs M, et al. Low prevalence of HIV and other selected sexually transmitted infections in 2004 in pregnant women from Kinshasa, the Democratic Republic of the Congo. Epidemiol Infect. 2008;136:1290–6. doi: 10.1017/S0950268807009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kweik JJ, Mwapasa V, Alker A, Muula AS, Misiri HE, Molyneux ME, et al. Socio-demographic characteristics associated with HIV and syphilis seroreactivity among pregnant women in Blantyre, Malawi, 2000–2004. Malawi Med J. 2008;20:80–5. doi: 10.4314/mmj.v20i3.10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mola GD, Golpak A, Amoa AB. A case-control study of VDRL-positive antenatal clinic attenders at the Port Moresby General Hospital antenatal clinic and labour ward to determine outcomes, sociodemographic features and associated risk factors. PNG Med J. 2008;51:17–26. [PubMed] [Google Scholar]
- 23.Bukar M, Audu BM, Takai UI, Ajayi BB, Kullima AA. Is routine antenatal screening for syphilis in Nigeria still justified clinically and economically. Saudi Med J. 2009;30:1311–5. [PubMed] [Google Scholar]
- 24.Marangoni A, Moroni A, Accardo S, Cevenini R. Laboratory diagnosis of syphilis with automated immunoassays. J Clin Lab Anal. 2009;23:1–6. doi: 10.1002/jcla.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filho AC, Sardinha JF, Ponte RL, Costa EP, da Silva SS, Martinez-Espinosa FE. Prevalence of infection for HIV, HTLV, HBV and of syphilis and chlamydia in pregnant women in a tertiary health unit in the western Brazilian Amazon region. Rev Bras Ginecol Obstet. 2010;32:176–83. [PubMed] [Google Scholar]
- 26.Kirakoya-Samadoulougou F, Defer MC, Yaro S, Fao P, Illboudo F, Langani, et al. Low seroprevalence of syphilis in Burkina Faso. Sex Transm Infect. 2011;87:35–7. doi: 10.1136/sti.2010.042465. [DOI] [PubMed] [Google Scholar]
- 27.Mbamara SU, Obiechina NJ. Seroprevalence of venereal disease among pregnant women attending antenatal care (ANC) in Onitsha, Anambra State, Southeast, Nigeria. Niger J Med. 2011;20:57–60. [PubMed] [Google Scholar]
- 28.Juarez-Fiqueroa LA, Melendez-Betancourt LA, Conde-Gonzalez CJ. Syphilis at full term pregnancy in women from Cuernavaca, Mexico. Rev Invest Clin. 2001;53:375–7. [PubMed] [Google Scholar]
- 29.Benzaken AS, Sabido M, Galban E, Pedroza V, Araujo AJ, Peeling RW, et al. Field performance of a rapid point-of-care diagnostic test for antenatal syphilis screening in the Amazon region, Brazil. Int J STD AIDS. 2011;22:15–8. doi: 10.1258/ijsa.2010.010145. [DOI] [PubMed] [Google Scholar]
- 30.Henrich TJ, Yawetz S. Impact of age, gender, and pregnancy on syphilis screening using the captia syphilis-g assay. Sex Transm Dis. 2011;38:1126–30. doi: 10.1097/OLQ.0b013e31822e60e1. [DOI] [PubMed] [Google Scholar]
- 31.Saindou M, Benet T, Troalen D, Abaine A, Voirin N, Giard M, et al. Prevalence and risk factors for HIV, hepatitis B virus, and syphilis among pregnant women in Mayotte, Indian Ocean, 2008–2009. Int J Gynecol Obstet. 2012;119:61–5. doi: 10.1016/j.ijgo.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Yanez-Alvarez I, Conde-Gonzalez CJ, Uribe-Salas FJ, Olamendi-Portugal ML, Garcia-Cisneros S, Sanchez-Aleman MA. Maternal/child seroprevalence of antibodies against Treponema pallidum at four general hospitals in the state of Morelos, Mexico. Arch Med Res. 2012;43:571–7. doi: 10.1016/j.arcmed.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. [Accessed January 15, 2014];The Global Elimination of Congenital Syphilis: Rationale and Strategy for Action. http://www.who.int/reproductivehealth/publications/rtis/9789241595858/en/. Published 2007.
- 34.Mwapasa V, Rogerson SJ, Kwiek JJ, Wilson PE, Milner D, Molyneux ME, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. AIDS. 2006;20:1869–77. doi: 10.1097/01.aids.0000244206.41500.27. [DOI] [PubMed] [Google Scholar]