Abstract
The purpose of the study is to define AroER tri-screen's utility for identifying endocrine-disrupting chemicals (EDCs) that target aromatase and/or estrogen receptor (ER), and to measure the total estrogenic activity in biological specimens. ER-positive, aromatase-expressing MCF-7 breast cancer cells were stably transfected with an estrogen responsive element (ERE)-driven luciferase reporter plasmid to yield a new high-throughput screening platform—the AroER tri-screen. AroER tri-screen was capable of identifying estrogen precursors, such as cortodoxone, which function as estrogens through a two-step conversion process in aromatase-expressing tissue. Furthermore, the system proved useful for assessing EDC activity in biologically relevant samples. Estimating these activities is critical because natural estrogens and estrogenic EDCs are important factors in ER-positive breast cancer risk. As our research demonstrates, incorporating functionally active aromatase into the AroER tri-screen produces a powerful and unique tool to (1) identify new EDCs targeting aromatase and/or ER; (2) discover novel EDCs activated by aromatase; and (3) estimate overall estrogenic activities in biological samples as a potential intermediate risk factor for breast cancer.
Keywords: AroER tri-screen, Endocrine-disrupting chemicals (EDCs), Aromatase, Estrogenic
Introduction
By interfering with hormone biosynthesis, metabolism, or action, EDCs can cause deviations in both normal homeostatic control and reproduction [1]. Through multiple mechanisms, EDC exposure (during development or in later life) may perturb the physiological actions of hormones [2]. We are exposed to thousands of chemicals in food, water, and the general environment every day; and some of them can act as EDCs. Therefore, high-throughput screening systems must be established to identify EDCs in these locales. With this in mind, we developed the AroER tri-screen assay: estrogen receptor (ER)-positive, aromatase-expressing MCF-7 breast cancer cells were stably transfected with an estrogen responsive element (ERE)-driven luciferase reporter plasmid. The AroER tri-screen can identify several types of EDCs: estrogenic ones, which increase luciferase signal without 17β-estradiol (E2); antiestrogenic EDCs, which inhibit the E2-induced luciferase signal; and aromatase inhibitor (AI)-like EDCs, which suppress a testosterone (T)-induced luciferase signal. This high quality assay has an excellent signal-to-background ratio (6.9-fold); a low coefficient of variation (5.4 %); and an impressive screening window coefficient (Z-factor of 0.78) [3]. The technique was first optimized in a 96-well plate format; it was then miniaturized into a 1536-well plate format to screen both the NIH Clinical Collection [Evotec (US) Inc, San Francisco, CA] and the Tox-21 10 K library [4]. The Food and Drug Administration (FDA) announced the availability of a draft guidance for industry entitled “Endocrine Disruption Potential of Drugs: Nonclinical Evaluation” on September 20, 2013. As stated in this draft guidance, additional studies are needed to characterize potential endocrine-disrupting properties of currently used drugs. AroER tri-screen identified novel EDCs from 446 drugs in the NIH Clinical Collection, which included FDA-approved drugs [3]. Since these drugs (with hitherto unknown estrogen-disrupting function) are currently used in the clinic or serve as precursors for the development of new drugs, people can be exposed to their endocrine-disrupting activities. In this regard, in vitro high-throughput screening of EDCs can help inform decisions about both drug development and treatment, provided the screen is highly reproducible and renders a minimal number of false-positives (for example through rescreening and validation) [5]. Herein, we disclose positive-hit chemicals identified by the AroER tri-screen and show novel mechanistic actions of chemicals as estrogenic compounds.
In most cases during an EDC exposure event, multiple compounds are simultaneously encountered (i.e., most materials are mixtures). In this regard, the effects of different classes of EDCs may be additive or even synergistic [1]. In biological samples, direct analytical chemistry approaches can (with high sensitivity) quantify levels of known EDCs. However, using this approach, contributions from compounds not typically considered EDCs cannot be anticipated and included in the analysis. Furthermore, the combined activities of a mixture of EDCs can be overlooked. To compensate for these disadvantages, a functional assay must be used to determine the sample's overall biological activity. Using mammalian cells transfected with ERE-luciferase reporter vector, conventional ER bioassays have been used to detect bioactiviy in human samples [6–9]. Especially for breast cancer studies in postmenopausal women, ER bioassays can predict an increased risk of postmenopausal breast cancer independently from hormone levels [10–12]. In this study, we demonstrated directly that the detectable estrogenic activity in tested human serum samples correlated with the sample's ability to promote the proliferation of breast cancer cell line MCF-7aro. Furthermore, by employing both inhibitors [ICI 182,780 (ICI) and Letrozole (Let)] and estrogenic chemicals (including both natural and synthetic chemicals) during the assay, we can also define the action of the active constituents. To isolate compounds for this assay, steroid hormones and potential EDCs are extracted using a C-18 solid-phase extraction (SPE) column; they are then subjected to our AroER tri-screen procedure. Below, we present data indicating that the unique AroER tri-screen can assess both estrogenic activity and (aromatizable) estrogen precursor activity simultaneously in tested biological samples. As such, we demonstrate the extended utility of the AroER tri-screen: the presence of functionally active aromatase makes it a unique tool to identify novel EDCs targeting aromatase and/or ER; it is also a versatile method to estimate the overall activity of female hormones in biological samples, which can be correlated to risks associated with developing breast cancer.
Materials and methods
Samples
Whole blood was centrifuged and serum was aliquoted and frozen at −80 °C until analysis. The study protocol was approved by the Committee for the Protection of Human Subjects (CPHS).
Luciferase assay
Prior to the assay, AroER tri-screen cells were cultured in phenol red-free MEM containing 10 % charcoal-treated FBS for 2 days and then seeded into 96-well plates at 2 × 105 cells per well. After 24 h, the cells were individually treated with the extract or compound alone and/or with ICI (100 nM) or Let (200 nM) in a total volume of 100 μL. After an overnight incubation at 37 °C, 100 μL of 1X One-Glo reagent (Promega Corporation) was added to each well and incubated for 5 min. Luciferase signal was measured using SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA) and normalized by protein concentration. Each assay was performed in triplicate. For the transient transfection experiment using C4-12/ERα or C4-12/ERβ stable cell line, cells were seeded into 24-well plates at 1 × 105 cells per well. After 24 h of seeding, the cells were individually transfected with pGL4.26 [luc2/minP/Hygro] (ERE)3 plasmid [3]. An additional 24 h later, cells were treated with each compound. After an overnight incubation at 37 °C, cells were lysed with 200 μL of passive lysis buffer. 20 μL of supernatant was mixed with 20 μL of luciferase reagent (Promega Corporation). The luciferase signal was measured using a TD-20/20 luminometer (Turner designs, Sunnyvale, CA) and normalized by protein concentration. Each assay was performed in triplicate.
Reagents and cell culture
Generation of the C4-12/ERα and C4-12/ERβ cells
Extraction by solid-phase extraction (SPE) column
Cell proliferation assay
Liquid chromatography and mass spectrometry
Please see supplementary materials for procedures.
Statistical analysis
Data are presented as mean ± SD. One-way analysis of variance (ANOVA) with Dunnett's post hoc test was used to determine significant difference in human sample analysis. Unpaired t tests were used for the rest of the analysis. We used GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, CA) to perform statistical analysis.
Results
Validation of novel EDCs exhibiting ER agonistic activity
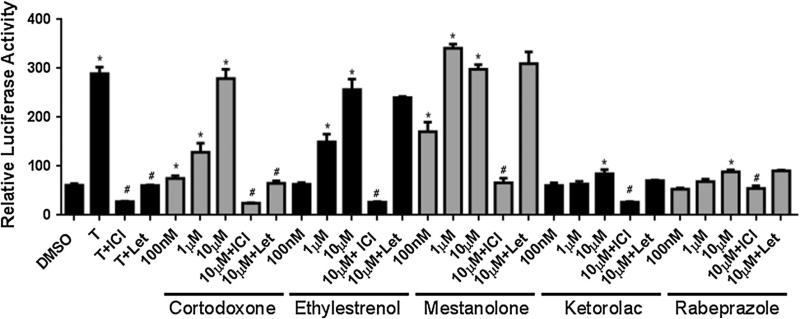
Screening 446 drugs in the National Institutes of Health Clinical Collection revealed 67 compounds exhibiting estrogenic activity. Using the Estrogenic Activity Database (EADB) developed by the National Center for Toxico-logical Research (NCTR) [13], we found that 13 out of the 67 compounds had been reported already for their ER agonistic action (Supplemental Table 1). For the remaining 54 compounds, we have used the ERE-luciferase reporter to validate six compounds so far as estrogenic. One of the six substances is the anti-depressant drug paroxetine [3]. The pure ER antagonist, ICI, significantly reduced to baseline levels the reporter-induced activity of five other compounds: cortodoxone, ethylestrenol, mestanolone, ketolorac, and rabeprazole (Fig. 1).
Fig. 1.
Validation of screening results for estrogenic compounds. AroER tri-screen cells were treated with each of the chemicals and inhibitors [ICI (ER-agonist) 100 nM and/or Let (aromatase inhibitor) 200 nM] for 24 h, and the luciferase activity was measured. Each assay was performed in triplicate. Asterisks indicate p < 0.05 compared with DMSO, number signs indicate p < 0.05 compared with the activity without inhibitor treatment (ICI or Let) by t test
Cortodoxone functionally acting like estrogens through a two-step conversion process
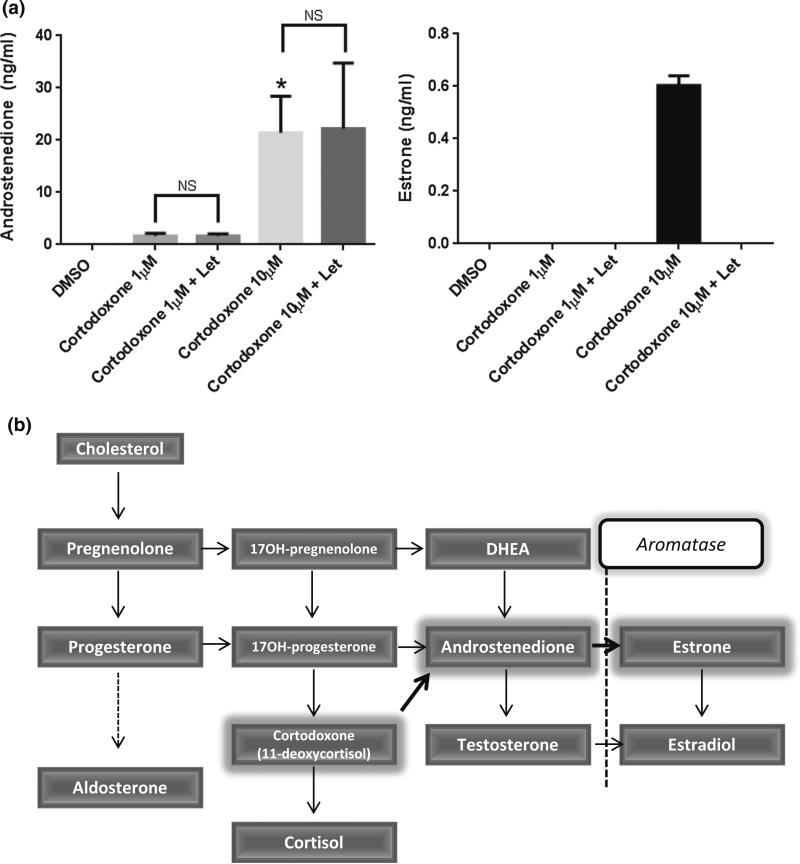
Among these drugs, cortodoxone, ethylestrenol, and mestanolone maximized luciferase activity at 10 μM. While Let should not affect the activities of ER agonists, cortodoxone activity was nevertheless inhibited by Let. A report by Azueby et al. suggests that cortodoxone can be converted into androstenedione by an uncharacterized enzyme [14]. To account for our observed sensitivity of cortodoxone to Let, we hypothesize that cortodoxone (through the unidentified enzyme) is first converted to androstenedione; this is subsequently converted by aromatase (in the AroER tri-screen) to estrone. To test our hypothesis, analysis using LC-tandem mass spectrometry revealed that androstenedione and estrone were produced and detected in cell culture media when the cells were treated with cortodoxone; furthermore, production of estrone was inhibited by Let treatment (Fig. 2a). The percent yields of both androstenedione from cortodoxone and estrone from androstenedione are shown in Table 1. Summary of this steroid biosynthesis pathway is shown in Fig. 2b.
Fig. 2.
Cortodoxone functionally acts like estrogens through a two-step conversion process. Androstenedione and estrone levels in supernatant treated with cortodoxone were measured using liquid chromatography and mass spectrometry (a). Summarized steroid biosynthesis pathway (b). All experiments were performed in triplicate. Asterisks indicate p < 0.05 compared with DMSO, number signs indicate p < 0.05 compared with the activity without inhibitor treatment (ICI or Let) by t test
Table 1.
In vitro biosynthesis of androstenedione from cortodoxone
| % Androstenedione | % Estrone | |
|---|---|---|
| DMSO | 0 | 0 |
| 10 μM cortodoxone | 0.75 ± 0.24 | 3.19 ± 1.04 |
| 1 μM cortodoxone | 0.06 ± 0.01 | 0 |
| 10 μM cortodoxone + Let | 0.78 ± 0.43 | 0 |
| 1 μM cortodoxone + Let | 0.06 ± 0.01 | 0 |
Results (mean ± SD of triplicate) are given as a percentage of the precursor
Validation using ERα- and ERβ-specific expression systems: ER-transduced C4-12 cells
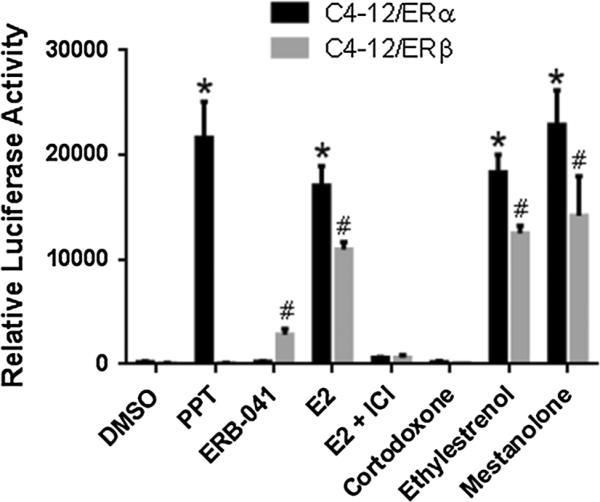
Cortodoxone, ethylestrenol, and mestanolone induced greater luciferease activity among the five tested compounds when AroER tri-screen cells were treated with each compound individually. We therefore evaluated those three chemicals in an ERα- and ERβ-specific luciferase assay using ER-expressing C4-12 cells that are deficient in endogeneous ER expression. As shown in Fig. 3, ERα-specific ligand (PPT) only induced ERE reporter activity in ERα-expressing cells; ERβ-specific ligand (ERB-041) only induced the ERE reporter activity in ERβ-expressing cells. E2 induced ERE reporter activity in both C4-12 ERα- and ERβ-expressing cells. A similar pattern of ERE reporter activity was observed in the cells treated with ethylesterenol and mestanolone. Cortodoxone did not increase any ERE reporter activity in either C4-12 ERα- or ERβ-expressing cells. This result is consistent with our finding that cortodoxone functionally acts like estrogens through a conversion process by aromatase: ERE reporter activity was not significantly induced in the ER-expressing C4-12 cells because they do not overexpress aromatase (Fig. 3).
Fig. 3.
Validation using ERα- and ERβ-specific systems: an MCF-7 variant—C4-12—that is deficient in endogenous ER expression. C4-12/ERα or C4-12/ERβ stable cell lines were seeded into 24-well plates at 1 × 105 cells per well. After 24 h of seeding, the cells were individually transfected with ERE reporter plasmid (pGL4.26 [luc2/ minP/Hygro] (ERE)3). An additional 24 h later, cells were treated with each compound. After an overnight incubation at 37 °C, the luciferase signal was measured using the supernatants and normalized by protein concentration. All experiments were performed in triplicate. Asterisks indicate p < 0.05 compared with DMSO in C4-12/ERα cells, number signs indicate p < 0.05 compared with DMSO in C4-12/ERβ cells
Since the enzyme that converts cortodoxone to androstenedione remains undefined, it is difficult to comment on the efficiency of this reaction. However, we were surprised by the low yielding conversion of androstenedione to estrone by aromatase expressed in the AroER tri-screen. To better understand the catalytic efficiency of expressed aromatase, we performed a dose–response study of the T-induced and E2-induced luciferase reporter assay (Supplemental Fig. 1). The activity reached maximum at 1 nM for both T- and E2-treated cells. The T-induced activity profile was similar to the E2-induced profile. Importantly, the results demonstrate that the expressed aromatase is highly efficient when the concentrations of androgen are kept lower than 1 nM. Such findings for the assay in a 96-well format were similar to those reported in the 1536-well format [3]. Therefore, the yield of aromatization by AroER tri-screen is significantly reduced when the concentration of androgen is higher than 1 nM.
SPE column extraction of EDC-containing specimens
Since the AroER tri-screen effectively detected T or E2 at concentrations less than 1 nM, we evaluated the system's capacity to isolate and detect these hormones in biological samples. As proof-of-principle experiments, solutions (10 mL) of T (100 fM–1 nM) or E2 (100 fM–1 nM) were loaded onto SPE columns. After a 5 % methanol wash, the 100 % methanol-eluted fractions were collected, concentrated, and reconstituted in DMSO. AroER tri-screen cells were treated with these reconstituted fractions for 24 h and the luciferase activity was measured. An average of 91 % of T was recovered from T samples. Similarly, an average of 90 % of E2 was recovered from E2 samples (Supplemental Fig. 1). To quantify the recovery rate of the SPE column, an experiment was performed using androst-4-ene-3, 17-dione [1-β-3H(N)] (NEN-Dupont, Boston, MA). 10 μL of 10 mM androstenedione and 20 μL of 0.19 pmol androst-4-ene-3, 17-dione [1-β-3H(N)] were added into 10 mL double distilled water; this mixture was then loaded to the SPE column. 200 μL of each step (flow through, wash and elution) was added into 3 mL scintillation fluid, and they were quantified by a liquid scintillation counter. The wash and flow-through samples had minimal counts per minute (CPM) activities. Over 96 % of samples were recovered using an elution volume of 4, 6, 8, or 10 mL. The 6 mL elution could achieve a recovery rate of 98.26 %; therefore, we standardized our extraction procedures with 6 mL of 100 % methanol (Table 2).
Table 2.
Recovery rate of SPE column extraction
| Elution volume (mL) | Total CPM |
Recovery rate (%) | |||
|---|---|---|---|---|---|
| Loading | Flow-through | Wash | Eluate | ||
| 4 | 408,978 ± 8821 | 320 ± 75 | 290 ± 69 | 397,829 ± 6590 | 97.3 |
| 6 | 414,384 ± 9924 | 260 ± 75 | 220 ± 62 | 407,181 ± 9584 | 98.3 |
| 8 | 411,158 ± 17,796 | 460 ± 62 | 400 ± 17 | 399,661 ± 8578 | 97.2 |
| 10 | 431,651 ± 6332 | 350 ± 180 | 240 ± 30 | 416,106 ± 12,302 | 96.4 |
Analysis of the samples containing multiple estrogenic substances
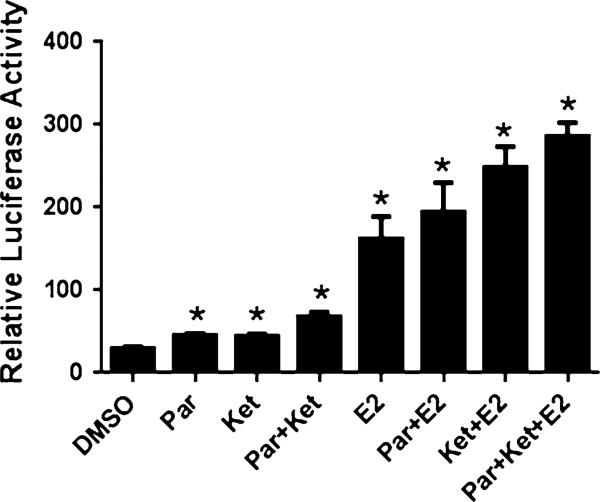
In biological samples, EDCs (including natural and synthetic chemicals) are present in a mixture. Experiments were performed to determine whether the presence of a weak estrogenic EDC could impact the total estrogenic activity in samples containing endogenous estrogen. Ketolorac showed weak estrogenic activity in a dose-dependent manner from 100 nM to 10 μM (Fig. 1a). In the presence of ICI, this activity was suppressed. AroER tri-screen cells were treated with ketolorac, paroxetine, and 50 pM of estradiol. The estradiol concentration was picked to mimic physiological levels in blood level of post-menopausal women [15]. In this analysis, paroxetine increased luciferase activity (compared DMSO treatment) by 15.91 RLU/μg; ketolorac by 14.75 RLU/μg; and 50 pM of E2 by 132.34 RLU/μg. When the cells were treated with paroxetine together with ketolorac, the expected activity was 30.66 RLU/lg, and the actual measured activity was 38.19 RLU/μg. Similarly, when cells were treated with a combination of paroxetine and 50 pM of E2, the expected activity was 148.24 RLU/μg, and the measured activity was 164 RLU/μg. When ketolorac was treated with 50 pM of E2, the expected induced RLU/μg was 147.09, and actual induced activity was 218.15 RLU/μg. When all three (paroxetine, ketolorac and 50 pM E2) were treated together in a combination, expected activity was 163 RLU/μg, and actual activity was 256.12 RLU/μg (Fig. 4). These results suggest that complex activation of ER can occur when humans are exposed to multiple hormonal substances.
Fig. 4.
Analysis of the samples containing multiple estrogenic substances. AroER tri-screen cells were treated with each chemical and its combination [10 μM paroxetine (Par), 10 μM ketolorac (Ket), and/or 50 pM E2] for 24 h. Luciferase signal was measured using a microplate reader and normalized by protein concentration. Values are expressed as mean and standard deviation. All experiments were performed in triplicate. Asterisks indicate p < 0.05 compared with DMSO by t test
The luciferase activity of the extract correlated with its ability to promote proliferation of estrogen-responsive breast cancer cells
Luciferase activity was compared to cell proliferation activity to determine the physiological significance of the AroER tri-screen assay results. T or E2 samples, at different concentrations in 6 mL of Milli-Q water, were loaded and eluted from an SPE column. Importantly, eluant samples, but not wash samples, could increase cell proliferation of AroER tri-screen cells. Using eluants from samples containing T or E2, luciferase activity strongly correlated with cell proliferation (R values of 0.815 or 0.716) (Fig. 5a). These results suggest that luciferase activities of eluants are associated with their biological activities (i.e., their impact on cell proliferation).
Fig. 5.
Significant correlation between the luciferase activity of the extract and its ability to promote the proliferation of estrogen-responsive breast cancer cells. AroER tri-screen cells were treated with eluates for 24 h and the luciferase activity was measured. We also performed an MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) assay to determine the effect on cell proliferation. There are significant correlations between luciferase activity and cell proliferation using the eluates from samples containing testosterone or estrogen (a). The AroER tri-screen cells were treated with eluates and ICI (ER-agonist: 100 nM) or Let (aromatase inhibitor: 200 nM) to determine whether the estrogenic activity results from androgen and/or estrogen (b). Values are expressed as mean and standard deviation. All experiments were performed in triplicate. Asterisks indicate p < 0.05 compared with DMSO, number signs indicate p < 0.05 compared with the activity without inhibitor treatment (ICI or Let) by t test
Evaluation of estrogenic and/or estrogen precursor activity of extracts
To determine whether samples’ estrogenic activity resulted from estrogen and/or estrogenic precursor constituents, the AroER tri-screen cells were treated with eluates and ICI or Let simultaneously. As expected, ICI significantly inhibited estrogen-induced luciferase activity for all concentrations of E2 (100 fM–1 nM). ICI and Let significantly reduced T-induced luciferase activity down to baseline levels for all concentrations of T (100 fM–1 nM) (Fig. 5b). This reduction is a consequence of Let's ability to inhibit the conversion of T to estrogen. These results indicate that our assay can determine both estrogenic and androgenic activities in test samples in a sensitive and reproducible manner.
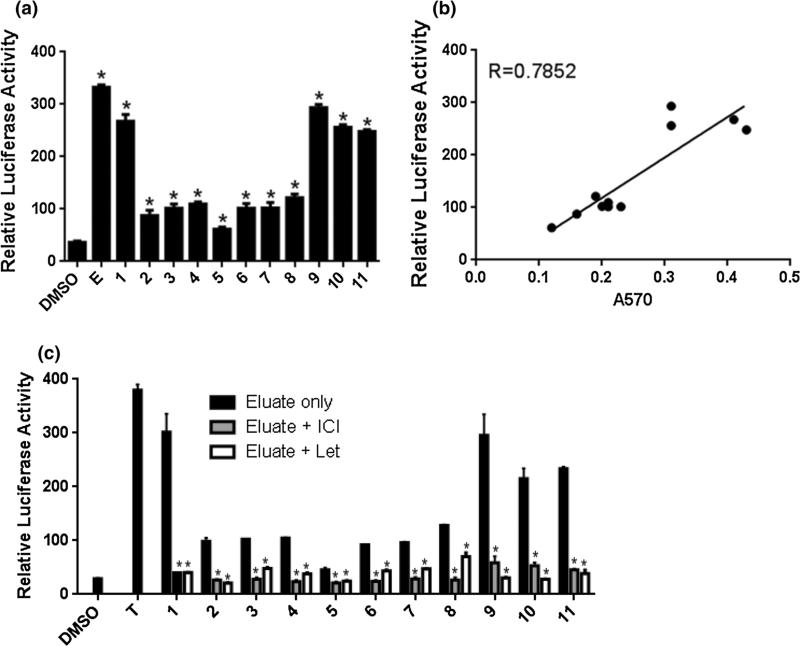
Human serum samples had estrogenic activity, which correlated with cell proliferation
To test our assay's usefulness in detecting estrogenic and androgenic activities in biospecimens, we evaluated 11 human serum samples. All 11 eluted samples had significantly higher luciferase activity compared to the DMSO control (Fig. 6a). Moreover, we performed cell proliferation assays using these extracts. Eleven eluted samples induced the cell proliferation. Importantly, luciferase activity significantly correlated with cell proliferation (Fig. 6b). These results suggest that the assay's range in which estrogenic and/or estrogenic precursor materials can be detected has biological significance—i.e., these concentrations can stimulate cell growth.
Fig. 6.
Analysis of human serum samples using AroER tri-screen cells. All 11 eluted samples had significantly higher luciferase activity compared to the DMSO control (a). Moreover, we performed cell proliferation assays using these extracts. 11 eluted samples induced cell proliferation. There is a significant correlation between luciferase activity and cell proliferation (b). Extracts from human sera were treated with ICI (ER-agonist: 100 nM) or Let (aromatase inhibitor: 200 nM) to elucidate if luciferase activity of human serum samples results from estrogen or/and androgen (c). Values are expressed as mean and standard deviation. All experiments were performed in triplicate. E; estrogen (0.5 nM), T; testosterone (0.3 nM) asterisks indicate p < 0.05 compared with DMSO (a); with treatment without ICI or Let (c); by one-way ANOVA with Dunnett's post hoc test
Aromatizable androgens are present at significant levels in human serum samples
To elucidate whether luciferase activity of human serum samples comes from estrogen or/and estrogenic precursors (converted to estrogen by the expressed aromatase), we treated human serum extracts with ICI and/or Let. All samples’ activities was decreased by ICI treatment—this suggests that luciferase activities were a consequence of ER pathway operations (Fig. 6c). Let treatment of extract could also inhibit luciferase activity of all samples; these results imply that estrogen precursor activity is the main source of luciferase signal in human serum samples.
Discussion
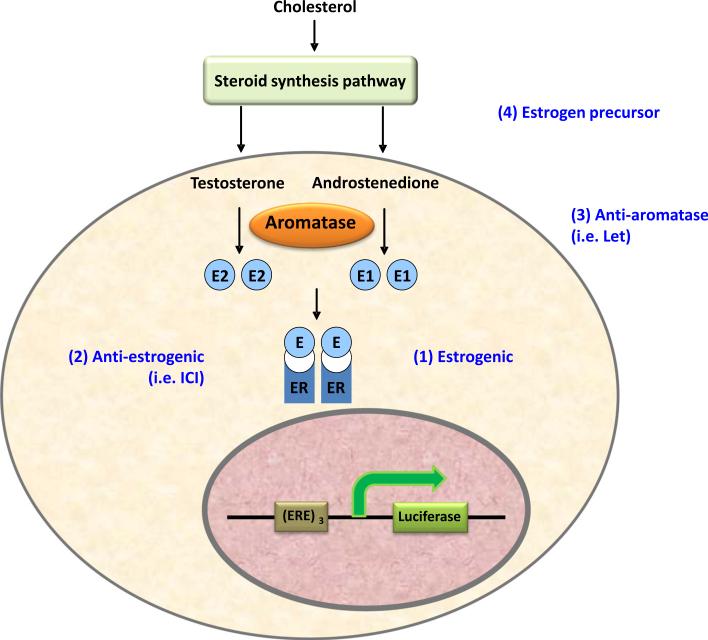
In vitro screening systems can help identify chemicals that potentially disrupt the endocrine system. The AroER tri-screen differs from other ER bioactivity systems because it uses cells that overexpress the aromatase enzyme; and the assay can screen three different types of EDCs (estrogenic, antiestrogenic, and AI-like) [3]. In a previous study applying the assay to the NIH Clinical Collection, we showed that paroxetine (Paxil), a commonly prescribed selective serotonin reuptake inhibitor, is a weak ER agonist [3]. This activity of paroxetine may be responsible, in part, for the observed reduction in the effectiveness of tamoxifen in some women with breast cancer [16]. In building upon this work, we demonstrate herein that the screen also identifies indirect estrogenic EDCs (Fig. 7). Four compounds—ethylestrenol, mestanolone, ketorolac, and rabeprazole—were newly identified as estrogenic (without antiestrogenic and AI-like activities). In addition, cortodoxone appears to impact ER activity through a conversion process to androstenedione, which is subsequently transformed by aromatase to estrone. Whereas traditional ER bioassays are not expected to capture this mode of action, it is detected by the AroER tri-screen. Discovering such classes of EDCs—i.e., those that require the catalytic activity of aromatase—could have important physiological implications. We believe this is a conceptually important example of a new mechanism for promoting ER-positive breast cancer. Most of breast cancer cells are known to express higher levels of aromatase than non-cancerous cells [17]. Therefore, drugs like cortodoxone could be preferentially converted to estrogen in breast cancer cells, promoting the proliferation of cancerous cells.
Fig. 7.
AroER tri-screen can detect chemicals with four different mechanisms of action
The Interagency Breast Cancer & Environmental Research Coordinating Committee (IBCERCC) made two important recommendations in February 2013: (1) pharmacological agents with steroidal hormone-like activity should be identified and (2) by developing better pre-clinical screening tests, the potential risks of these materials should be evaluated before they are brought to market. Mestanolone, an androgenic EDC, binds with high affinity to nuclear androgen receptors [18]. In our assay, luciferase activity was significantly increased upon treatment with mestanolone at several concentrations (100 nM, 1 and 10 μM); 50 % of maximum activity was observed at approximately 100 nM. These results suggest that high concentrations of mestanolone act like estrogenic compounds. ICI could significantly reduce compound induced activity, suggesting that the luciferase activity produced by mestanolone was a result of it acting though an ER pathway. Moreover, activity was not inhibited by Let; thus, our results are consistent with the report that mestanolone is a nonaromatizable androgen [19]. Ethylestrenol is an anabolic steroid which potentially affects autoimmune disorders [20] and/or postoperative venous thrombosis [21]. Recently, the compound has been illegally used as an anabolic steroid in cattle to increase their lean mass [22]. Our results showed ethylestrenol-induced luciferase activity was inhibited by ICI treatment but not by Let. This suggests that luciferase activity was induced through the ER pathway, not through conversion by aromatase.
We also examined compound binding to particular estrogen receptor subtypes. While role of ERβ in breast cancer is not well elucidated, it is usually considered a tumor suppressor in estrogen-sensitive breast cancer. ERα and ERβ are transcribed from two genes located on different chromosomes. Two ERs share ~97 % homology in their DNA-binding domain and 59 % in ligand binding domain [23]. AroER tri-screen cells were generated from MCF7aro, which dominantly express ERα and minimally express ERβ. The luciferase activity detected using AroER tri-screen is thought to be mainly from ERα; however, the system cannot completely dissect which ERs (such as ERα, ERβ, GPR30 and membrane-associated ER) induce luciferease activity. We therefore defined each compound's ability to bind ERα and/or ERβ by generating ERα or ERβ expressing cells using a MCF-7 variant—C4-12—that is deficient in endogenous ER expression. As shown in our results, mestanolone and ethylestrenol did not show any specificity for ERα or ERβ; rather, it bound to both receptors in a similar manner as E2.
Ketorolac is a nonsteroidal anti-inflammatory drug (NSAID), widely used for the short-term relief of moderate to severe pain. During early stages of development, inhibiting cyclooxygenase by NSAIDs may disrupt heart development [24, 25]. As such, routine use of ketolorac in neonates is not recommended. Rabeprazole is an example of a proton pump inhibitor (PPIs) to treat acid-related conditions such as stomach and duodenal ulcers; gastroesophageal reflux disease (GERD); and Helicobactor pylori. PPIs are some of the most frequently prescribed medications for adults and children, including newborns. These drugs have also been used for pediatric patients chronically (over years) [26]. Importantly, this is the first report of the EDC activities of ketolorac and rabeprazole—specifically, weak estrogenic activity.
Since many of these drugs (NSAIDs, PPIs, etc.) are currently used in the clinic or serve as precursors for the development of new drugs, people can be exposed to their hitherto unknown endocrine-disrupting activities inadvertently. The newly identified estrogenic chemical ketolorac and previously identified paroxetine were used as a model of multiple exposures. According to our results, the activity of a combination of ketolorac, paroxetine, and 50 pM of E2 (equivalent to the blood concentration found in post-menopausal women) [15] does not simply reflect the additive activities of the three individual components. Rather, the mixture activated the ER pathway in a more complicated manner. Congruently, there are a few reports that demonstrate the complexity of multi-component exposure [27, 28]. This emphasizes the importance of determining the total biological activity of a sample properly (i.e., the combined effects of all constituents).
Testosterone, estrone, and high levels of androgens (e.g., androstenedione) have been associated with increased risk of breast cancer [11, 15, 29]. When evaluating individual and summed contributions to risk, it can be challenging to isolate and quantify these steroidal hormones in biospecimens [30]. Toward clarifying the complex interplay between the steroids and EDCs, the AroER tri-screen is the first biological assay that can simultaneously assess both direct estrogenic activity and indirect (aromatizable) estrogen precursor activity. By using ICI and/or an AI, the system can also distinguish functional contributions of the steroids. The results demonstrate that luciferase signal significantly correlates with breast cancer cell proliferation. Thus, the biological activity of individual samples appears to directly correlate with breast cancer growth; and the total estrogenic activity may potentially serve as an intermediate risk factor for breast cancer. In terms of its utility for assessing serum samples, the screen's expressed aromatase was highly efficient when the concentration of androgen was as low as 1 nM. Physiological levels of estrogen in blood levels of postmenopausal women [15] and premenopausal women [31] are approximately 20–130 and 150–300 pM, respectively. Therefore, the sensitive AroER tri-screen can assess the activity of estrogen in blood at physiological levels.
In summary, we identified five novel compounds that exhibit estrogenic activity. Our experiments provide important confirmation that EDCs, including both known and unknown chemicals, can work in a synergistic manner. The mixtures of natural estrogens, estrogen precursors, and EDCs can promote the proliferation of breast cancer cell lines, i.e., MCF-7aro. Our results show that estrogen precursor activity in human sera can be a major source of ERE-luciferase activity, which is undetectable by other ER-only assays. Overall, the AroER tri-screen is a novel functional assay to estimate a sample's overall estrogenic bioactivity; to distinguish the activities contributed by estrogens versus aromatizable estrogen precursors in breast cancer-relevant samples; and to probe the action of drugs with endocrine-disrupting activity. It is therefore a useful approach to evaluate bioactivities in specimens as important predictive markers for the onset of breast cancer.
Supplementary Material
Acknowledgments
The authors would like to thank Talisman, Ian, PhD and Kelly Yeo for assistance in editing the manuscript and luciferase assay, respectively. We also would like to thank Susan Markel in the the Analytical Pharmacology Core for her performance of liquid chromatography and mass spectrometry analysis. This work was supported by California Breast Cancer Research Program [Grant number 17UB-8701] and the National Cancer Institute (P30 CA033572), NIH/NIEHS (ESO8258). In addition, we would like to thank Dr. Joanne Mortimer for her support and comments.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-015-3398-z) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. doi:10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. doi:10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Zhou D, Hsin LY, Kanaya N, Wong C, Yip R, Sakamuru S, Xia M, Yuan YC, Witt K, Teng C. AroER Tri-screen is a biologically relevant assay for endocrine disrupting chemicals modulating the activity of aromatase and/or the estrogen receptor. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu023. doi:10.1093/toxsci/kfu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tox-21 10K library. 2012 http://www.epa.gov/ncct/dsstox/sdf_tox21s.html.
- 5.Rotroff DM, Dix DJ, Houck KA, Knudsen TB, Martin MT, McLaurin KW, Reif DM, Crofton KM, Singh AV, Xia M, Huang R, Judson RS. Using in vitro high throughput screening assays to identify potential endocrine-disrupting chemicals. Environ Health Perspect. 2013;121(1):7–14. doi: 10.1289/ehp.1205065. doi:10.1289/ehp.1205065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hjelmborg PS, Ghisari M, Bonefeld-Jorgensen EC. SPEHPLC purification of endocrine-disrupting compounds from human serum for assessment of xenoestrogenic activity. Anal Bioanal Chem. 2006;385(5):875–887. doi: 10.1007/s00216-006-0463-9. doi:10.1007/s00216-006-0463-9. [DOI] [PubMed] [Google Scholar]
- 7.Kruger T, Spano M, Long M, Eleuteri P, Rescia M, Hjelmborg PS, Manicardi GC, Bizzaro D, Giwercman A, Toft G, Bonde JP, Bonefeld-Jorgensen EC. Xenobiotic activity in serum and sperm chromatin integrity in European and inuit populations. Mol Reprod Dev. 2008;75(4):669–680. doi: 10.1002/mrd.20747. doi:10.1002/mrd.20747. [DOI] [PubMed] [Google Scholar]
- 8.Kanno Y, Okada H, Kobayashi T, Takenaka T, Suzuki H. Effects of endocrine disrupting substance on estrogen receptor gene transcription in dialysis patients. Ther Apher Dial. 2007;11(4):262–265. doi: 10.1111/j.1744-9987.2007.00472.x. doi:10.1111/j.1744-9987.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- 9.Lim VW, Li J, Gong Y, Yuan JM, Wu TS, Hammond GL, Jin A, Koh WP, Yong EL. Serum free estradiol and estrogen receptor-alpha mediated activity are related to decreased incident hip fractures in older women. Bone. 2012;50(6):1311–1316. doi: 10.1016/j.bone.2012.03.006. doi:10.1016/j.bone.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widschwendter M, Lichtenberg-Frate H, Hasenbrink G, Schwarzer S, Dawnay A, Lam A, Menon U, Apostolidou S, Raum E, Stegmaier C, Jacobs IJ, Brenner H. Serum oestrogen receptor alpha and beta bioactivity are independently associated with breast cancer: a proof of principle study. Br J Cancer. 2009;101(1):160–165. doi: 10.1038/sj.bjc.6605106. doi:10.1038/sj.bjc.6605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fourkala EO, Zaikin A, Burnell M, Gentry-Maharaj A, Ford J, Gunu R, Soromani C, Hasenbrink G, Jacobs I, Dawnay A, Widschwendter M, Lichtenberg-Frate H, Menon U. Association of serum sex steroid receptor bioactivity and sex steroid hormones with breast cancer risk in postmenopausal women. Endocr Relat Cancer. 2012;19(2):137–147. doi: 10.1530/ERC-11-0310. doi:10.1530/ERC-11-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim VW, Li J, Gong Y, Jin A, Yuan JM, Yong EL, Koh WP. Serum estrogen receptor bioactivity and breast cancer risk among postmenopausal women. Endocr Relat Cancer. 2014;21(2):263–273. doi: 10.1530/ERC-13-0233. doi:10.1530/ERC-13-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Xu L, Fang H, Richard AM, Bray JD, Judson RS, Zhou G, Colatsky TJ, Aungst JL, Teng C, Harris SC, Ge W, Dai SY, Su Z, Jacobs AC, Harrouk W, Perkins R, Tong W, Hong H. EADB: an estrogenic activity database for assessing potential endocrine activity. Toxicol Sci. 2013;135(2):277–291. doi: 10.1093/toxsci/kft164. doi:10.1093/toxsci/kft164. [DOI] [PubMed] [Google Scholar]
- 14.Auzeby A, Bogdan A, Touitou Y. Evidence for a new biologic pathway of androstenedione synthesis from 11-deoxycortisol. Steroids. 1991;56(1):33–36. doi: 10.1016/0039-128x(91)90112-9. [DOI] [PubMed] [Google Scholar]
- 15.Key T, Appleby P, Barnes I, Reeves G, Endogenous H, Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, Paszat LF. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. doi:10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To SQ, Knower KC, Cheung V, Simpson ER, Clyne CD. Transcriptional control of local estrogen formation by aromatase in the breast. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.05.004. doi:10.1016/j.jsbmb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Sperry TS, Thomas P. Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities. Endocrinology. 1999;140(4):1602–1611. doi: 10.1210/endo.140.4.6631. doi:10.1210/endo.140.4.6631. [DOI] [PubMed] [Google Scholar]
- 19.Piferrer F, Baker IJ, Donaldson EM. Effects of natural, synthetic, aromatizable, and nonaromatizable androgens in inducing male sex differentiation in genotypic female chinook salmon (Oncorhynchus tshawytscha). Gen Comp Endocrinol. 1993;91(1):59–65. doi: 10.1006/gcen.1993.1104. doi:10.1006/gcen.1993.1104. [DOI] [PubMed] [Google Scholar]
- 20.Verheul HA, Schot LP, Deckers GH, Schuurs AH. Effects of tibolone, lynestrenol, ethylestrenol, and desogestrel on autoimmune disorders in NZB/W mice. Clin Immunol Immunopathol. 1986;38(2):198–208. doi: 10.1016/0090-1229(86)90138-8. [DOI] [PubMed] [Google Scholar]
- 21.Allenby F, Jeyasingh K, Calnan J. Ethyloestrenol and postoperative venous thrombosis. Lancet. 1973;2(7819):38–39. doi: 10.1016/s0140-6736(73)91966-1. [DOI] [PubMed] [Google Scholar]
- 22.Van Puymbroeck M, Kuilman ME, Maas RF, Witkamp RF, Leyssens L, Van Miert AS, Hendriks L, Vanderzande D, Adriaensens P, Jacobs MP, Raus J. 17alpha-ethyl-5beta-estrane-3alpha, 17beta-diol, a biological marker for the abuse of norethandrolone and ethylestrenol in slaughter cattle. J Chromatogr B Biomed Sci Appl. 1999;728(2):217–232. doi: 10.1016/s0378-4347(99)00091-2. [DOI] [PubMed] [Google Scholar]
- 23.Dey P, Barros RP, Warner M, Strom A, Gustafsson JA. Insight into the mechanisms of action of estrogen receptor beta in the breast, prostate, colon, and CNS. J Mol Endocrinol. 2013;51(3):T61–T74. doi: 10.1530/JME-13-0150. doi:10.1530/JME-13-0150. [DOI] [PubMed] [Google Scholar]
- 24.Cappon GD, Cook JC, Hurtt ME. Relationship between cyclooxygenase 1 and 2 selective inhibitors and fetal development when administered to rats and rabbits during the sensitive periods for heart development and midline closure. Birth Defects Res B. 2003;68(1):47–56. doi: 10.1002/bdrb.10008. doi:10.1002/bdrb.10008. [DOI] [PubMed] [Google Scholar]
- 25.Maitra S, Baidya DK, Khanna P, Ray BR, Panda SS, Bajpai M. Acute perioperative pain in neonates: an evidence-based review of neurophysiology and management. Acta Anaesthesiol Taiwanica. 2014;52(1):30–37. doi: 10.1016/j.aat.2014.02.004. doi:10.1016/j.aat.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Ward RM, Kearns GL. Proton pump inhibitors in pediatrics : mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 2013;15(2):119–131. doi: 10.1007/s40272-013-0012-x. doi:10.1007/s40272-013-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katchy A, Pinto C, Jonsson P, Nguyen-Vu T, Pandelova M, Riu A, Schramm KW, Samarov D, Gustafsson JA, Bondesson M, Williams C. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol Sci. 2014;138(1):21–35. doi: 10.1093/toxsci/kft271. doi:10.1093/toxsci/kft271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SP, Li J, Shen P, Gong Y, Yap SP, Yong EL. Ultrasensitive cell-based bioassay for the measurement of global estrogenic activity of flavonoid mixtures revealing additive, restrictive, and enhanced actions in binary and higher order combinations. Assay Drug Dev Technol. 2007;5(3):355–362. doi: 10.1089/adt.2007.056. doi:10.1089/adt.2007.056. [DOI] [PubMed] [Google Scholar]
- 29.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, Dossus L, Lukanova A, Bingham S, Khaw KT, Allen NE, Buenode-Mesquita HB, van Gils CH, Grobbee D, Boeing H, Lahmann PH, Nagel G, Chang-Claude J, Clavel-Chapelon F, Fournier A, Thiebaut A, Gonzalez CA, Quiros JR, Tormo MJ, Ardanaz E, Amiano P, Krogh V, Palli D, Panico S, Tumino R, Vineis P, Trichopoulou A, Kalapothaki V, Trichopoulos D, Ferrari P, Norat T, Saracci R, Riboli E. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. doi: 10.1677/erc.1.01038. doi:10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 30.Ziegler RG, Faupel-Badger JM, Sue LY, Fuhrman BJ, Falk RT, Boyd-Morin J, Henderson MK, Hoover RN, Veenstra TD, Keefer LK, Xu X. A new approach to measuring estrogen exposure and metabolism in epidemiologic studies. J Steroid Biochem Mol Biol. 2010;121(3–5):538–545. doi: 10.1016/j.jsbmb.2010.03.068. doi:10.1016/j.jsbmb.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Dowsett M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoffman-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, Peeters PH, Riboli E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–1019. doi: 10.1016/S1470-2045(13)70301-2. doi:10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.