Abstract
Objective
The objective was to conduct a systematic review and meta-analysis of studies that examined the effects of structured exercise on depressive symptoms in stroke patients.
Methods
We searched for published randomized controlled trials which evaluated the effect of structured exercise programs (e.g., functional, resistance or aerobic training) on depressive symptoms. The mean effect size, a 95% confidence interval (CI) and I-squared (I2) for heterogeneity were estimated. Sensitivity analyses were conducted.
Results
Thirteen studies (n=1022) were included in the meta-analysis. Exercise resulted in less depressive symptoms immediately after the exercise program ended, SMD=−0.13 [95%CI = −0.26, −0.01], I2 =6%, p=0.03, but these effects were not retained with longer term follow-up. Exercise appeared to have a positive effect on depressive symptoms across both the subacute (≤ 6 months post-stroke) and chronic stage of recovery (> 6 months). There was a significant effect of exercise on depressive symptoms when higher intensity studies were pooled, but not for lower intensity exercise protocols. Antidepressant medication use was not documented in the majority of studies and thus, its potential confounding interaction with exercise could not be assessed.
Conclusions
Exercise may be a potential treatment to prevent or reduce depressive symptoms in individuals with sub-acute and chronic stroke.
Keywords: Stroke, depression, exercise, meta-analysis, systematic review
Introduction
Depressive symptoms are a common sequel after a stroke. The prevalence of depression among stroke survivors is between 29 and 36%.1 The risk of onset seems to be similar for the early, medium, and late stage of stroke recovery. Post-stroke depression has been related to longer hospitalization and reduced functional recovery,2,3 suicidal ideas,4 poor communicative function,5 impaired memory, visual perception and language,6 reduced social activities,7 and higher risks for mortality.8
A Cochrane Review found no clear effect of pharmacological treatments on the prevention of depression in stroke patients while psychotherapy was related to small effect sizes.9 Another Cochrane Review on treatments found evidence that antidepressants reduced the proportion of stroke patients who were classified with depression.10 However, there was also an associated increase in adverse events of the central nervous and gastrointestinal systems. There was no evidence of a benefit of psychotherapy as a treatment for depression after stroke.10 These results demonstrate the need to explore additional interventions for stroke survivors experiencing depressive symptoms. Physical exercise may provide a complementary treatment to improve depressive symptoms.
A recent Cochrane review reported a large clinical effect with a standardized mean difference (SMD) of −0.82 of physical exercise on depressive symptoms in adults with depression, but who did not have a stroke.11 In a meta-analysis, Sjösten and Kivelä12 reported that physical exercise was effective in treating depression, and especially in individuals with high baseline levels of depression. A meta-analysis of 90 studies involving patients with a chronic illness found that exercise had a small effect on reducing depressive symptoms, but stroke patients were not included.13
Only two studies have included exercise trials with a stroke population; Graven et al.14 meta-analyzed two exercise studies for depressive symptoms in individuals living with stroke and found a large effect (SMD: −2.03). The low number of studies in their review increases the potential for bias and reduces the generalizability of their results. Saunders et al.15 reviewed the effect of eight exercise trials on depressive symptoms (and meta-analyzed three of these studies), and concluded that the results were inconsistent among the trials.
The objective of this study was to examine the evidence on the effects of physical exercise for depressive symptoms in stroke patients by performing a systematic review and meta-analysis of relevant RCTs.
Methods
We applied the PRISMA guidelines for systematic reviews of RCTs.16 We accessed MEDLINE (1950 to January 10, 2014), Cochrane Database of Systematic Reviews (2005 to January 10, 2014), Cumulated Index to Nursing and Allied Health Literature (CINAHL) (1982 to January 10, 2014), and Physical Therapy Evidence Database (PEDro) (1952 to January 10, 2014). An electronic database search using the terms “stroke” or “cerebrovascular accident” or “hemiparesis” paired with “exercise,” or “physical exercise” or “rehabilitation” and “depression” or “depressive symptoms” or “mood” or “dysthymia”. We used no language or date restriction in our search. A sample of the MEDLINE search strategy is shown in the Online Supplementary Appendix 1. Experts in the field were contacted and asked for information on unpublished trials to avoid publication bias. Hand searches of reference lists from retrieved papers were completed.
Eligibility criteria were RCTs that evaluated the effect of physical exercise on depressive symptoms. Studies which evaluated an exercise intervention against a control group with a lesser intensity were included (e.g., 60 minutes versus 30 minutes daily exercise). Depressive symptoms were defined pragmatically as a range of symptoms detected by a validated scale of depressive symptoms used in established mental health research. Exercise was defined as a subset of physical activity that is planned, structured, repetitive, and purposeful with the objective of improvement or maintenance of physical fitness,17 as well as for optimizing functioning. Additional inclusion criteria were: 1) confirmed diagnosis of stroke by medical records, imaging, or clinical examination; 2) adult patients over 18 years of age; 3) intervention and control group treatments clearly defined, and 4) baseline and follow-up of at least 4 weeks available for depressive symptoms. Exclusion criteria were studies utilizing electrical stimulation, passive movement, or exercise only with the upper extremities if they had a focus on motor skill learning or fine motor skills. Studies were also excluded if they used a mood instead of depressive symptom scale or if they evaluated an exercise intervention against a different type of exercise of similar intensity. For each study, the scale’s cut-off point for the diagnosis of a clinical depression is reported in the Online supplementary of Appendix 2. Studies which utilized populations with mixed diagnoses (e.g., stroke and traumatic brain injury18) were also excluded.
Both authors independently extracted the estimates on the basis of the inclusion and exclusion criteria. If there was disagreement regarding eligibility, an independent adjudicator was available. We used the Physiotherapy Evidence Database (PEDro) scale19 (maximum score of 10) to assess the methodology of the trials for potential bias. We utilized the official scores posted on the PEDro website as these have been verified by two trained experts.
If several follow-up end-points were available from one study (for example, follow-up after 3, 6, and 9 months later), we used the first end-point following the completion of the intervention for the immediate effects, and the last follow-up after the completion of the intervention for long-term retention effects. If the median and range were reported, we converted them to the mean and standard deviation.20 If an interquartile range was reported, we converted to a standard deviation.21 All studies reported continuous data that we meta-analyzed (Review Manager 5.222) with a standardized mean difference (SMD)20 and illustrated with Forest plots. A negative SMD indicated that the treatment group resulted in less depressive symptoms than the control group. The strength of the effect sizes were interpreted as small, moderate or large effect sizes, referring to 0.20, 0.50, and 0.80 respectively.23 We also explored publication bias using Funnel plots.
Fixed effect models were utilized if statistical heterogeneity was low, while random effect models were utilized in all other cases. Statistical heterogeneity was quantified with the I-squared value (I2).24 I2 values of 25%, 50%, and 75% have been related to low, moderate, and high heterogeneity, respectively.
Planned subgroup analysis was performed for time since stroke (subacute ≤ 6 months versus chronic > 6 months), and the intensity of the exercise program. The definition of intensity considered the weekly frequency of the sessions, as well as the content of the intervention. We defined “higher intensity” if sessions were administered at least three times per week, for a minimum of four weeks, and specifically stated that graded strengthening, repetitive functional training (e.g., circuit training) or aerobic exercise was undertaken. All other interventions were considered “lower intensity”. Sensitivity analysis was used to assess the robustness of our results, i.e., we tested the effect of deleting studies of poor quality (less than 4 on the PEDro scale) and studies that analyzed according to protocol instead of intention to treat.
Results
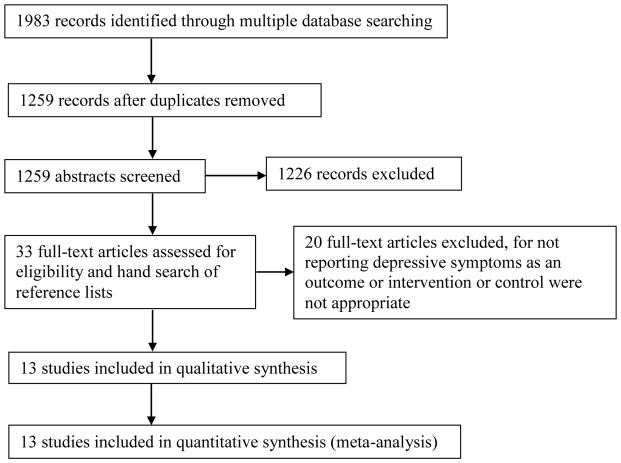
Thirteen articles met the inclusion criteria (Figure 1).25–37 Mead et al.30 and Ryan et al.32 did not report the required data but we received it upon contacting the authors, leaving a total of 1022 patients from 13 trials. Four studies required some conversion of the data; all four of these studies reported median values,25–26,29,32 one study reported range values29 and three studies reported interquartile ranges25–26,32 and these were converted to mean and standard deviation.
Figure 1.
Selection process of individual trials examining the effect of exercise on depressive1 symptoms
Only two studies29,34 confirmed the stroke by imaging, 2 studies28,36 confirmed by imaging or clinical presentation, and the rest did not explicitly state the requirements for stroke diagnosis beyond medical records, registries or clinical presentation. The time since stroke ranged from 30 days to six years and the patients’ age ranged from 21 to 93 years.
The study characteristics are summarized in the online supplementary of Appendix 2. Physical exercise included progressive resistance training,28,30,33,37 functional,26,36 aerobic exercises,29 treadmill exercises,34,37 Bobath exercises,31 individualized exercises with education,27 and community-based rehabilitation services including physical therapy and occupational therapy.25,32,35 The majority of studies (6/13) utilized usual care as the control group.26,28–29,31–33 Four studies utilized an attention control intervention in the form of a relaxation protocol,30 discussion group,27 weekly phone call,34 or cognitive training37, however, only one study30 provided equivalent minutes of attention between groups. In one study, control subjects were put on a wait list but received the same treatment later.35 A control group with no treatment was utilized in two studies.25,36
Eleven of the 13 studies had a frequency of at least two sessions per week for a minimal duration of 4 weeks.26–34,36–37 The least frequent intervention comprised three sessions within a 13 week period.25 The length of the intervention ranged from 4 weeks34 to 12 weeks.28,30,32,36,37
Most studies (8/13)25–26,29–32,35,37assessed the patients’ depressive symptoms using the Hospital Anxiety and Depression Scale.38 Two studies27,28 used the Geriatric Depression Scale,39 and two studies34,36 used the Beck Depression Scale.40 The Centre for Epidemiology Scale for Depression41 was used in one study.33
The mean baseline depressive symptoms of the study samples were below established thresholds for clinically relevant depressive symptoms in the majority of studies (12/13).25–30,32–37 Mean depressive symptoms for one study31 was above the depressive symptom threshold, i.e., abnormal or symptomatic. While mean depressive symptoms were relatively low, there were a substantial proportion of participants who had clinically relevant symptoms or were taking antidepressant medication. The proportion of patients who had clinically relevant depressive symptoms (i.e., above established cut-points) was reported in three studies and ranged from 18–34%.25,28,31 The percentage of patients taking antidepressants was reported in two studies and ranged from 21 to 40%.27,28 Lai et al.28 found that drop-outs were more depressed at baseline and were more likely to report antidepressant use compared to the rest of the sample.
Funnel plots showed that trials with non-significant results were underrepresented. The quality of the included studies on the PEDro scale ranged from three34 to eight25–26 (Online supplementary Appendix 3). Four studies did not perform an intention to treat analysis.31,34–36 Lack of blinding of the assessor undertaking the outcome measures was a potential for bias in six studies.27–30,33,34 More than 15% of loss to follow-up occurred in four studies.32,34,36–37
Only two studies reported whether any adverse events took place,30,33 while the other eleven did not state whether any adverse events occurred or not. Sims et al.33 reported there were no adverse events. Mead et al.30 found that eight of the 32 patients in the exercise group reported 11 falls and four of 34 patients in the control group reported five falls. All falls occurred outside the sessions.
Overall, physical exercise resulted in less depressive symptoms over 13 studies involving 1022 patients (SMD=−0.13 [95%CI = −0.26, −0.01], I2=6%, p=0.03) with low heterogeneity (Figure 2).25–37 Ten studies evaluated the effect of depressive symptoms after a period of time had elapsed following the exercise sessions (range from 10 weeks to 9 months). Physical exercise did not change depressive symptoms over these 10 follow-up studies involving 889 patients (SMD=−0.04 [95%CI = −0.17, 0.09], I2=1%, p=0.53).25–28,30–31,33,35–37
Figure 2.
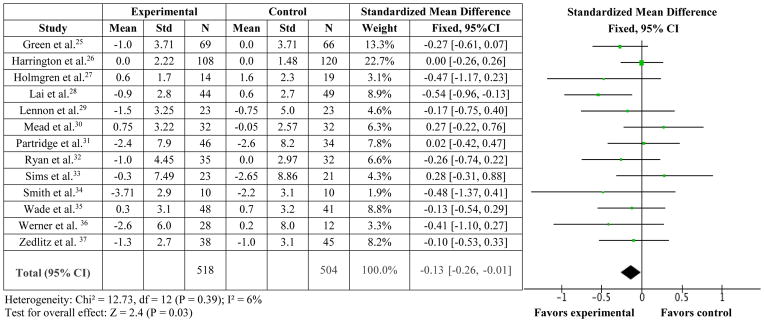
Standardized mean difference for experimental (exercise) versus control trials on depressive symptoms.
Restricting the analysis to studies which utilized an intent to treat protocol (n=10)25–30,32–34,37 produced a near-significant model (SMD=−0.14 [95%CI = −0.28, 0.0], p=0.05, I2=22%). Removing the one study34 which had a PEDro score less than of 4 out of 10 (i.e., considered poor quality)42 also produced a significant model (SMD=−0.13 [95%CI = −0.25, 0.0, p=0.04, I2=9%). Of importance, only one study30 matched for equivalent minutes of attention between the control and treatment groups, and this study did not find that exercise affected depressive symptoms over relaxation training.
Physical exercise resulted in less depressive symptoms upon restricting the data to the four studies involving 273 patients undertaken during the subacute phase of stroke (≤ 6 months since stroke) (SMD=−0.30 [95%CI = −0.54, −0.05], p=0.02, I2 =17%).27–28,31–32 There was also a tendency for exercise to result in less depressive symptoms in the five trials involving 393 patients in the chronic phase (> 6 months since stroke) (SMD=−0.20 [95%CI = −0.40, 0.0], p=0.05, I2=0%).25,29,35–37 Four studies included both subacute and chronic patients and were not part of these subgroup analyses.26,30,33–34
Six studies involving 333 patients utilized a high intensity program according to our pre-defined criteria.27–28,30,34,36–37 These studies produced a significant model: SMD=−0.24 [95%CI = −0.46, −0.02], p=0.03, I2=33% using a fixed (p=0.03), but not random model (p=0.07). A model utilizing the seven studies involving 689 patients with lower intensity treatments,25–26,29,31–33,35 was not significant (SMD=−0.08 [95%CI = −0.23, 0.07], p=0.27, I2 = 0%).
Discussion
From a meta-analysis of thirteen studies, we found that depressive symptoms after stroke were lower immediately after 4 or more weeks of exercise (SMD=−0.13 [−0.26, −0.01], I2=6%, p=0.03). Exercise appeared to have a positive effect on depressive symptoms across both the sub-acute and chronic stages of stroke recovery, but these effects were not retained after the exercise was terminated. While one study30 did report that twice as many individuals in the exercise group experienced falls compared to the control group, it is difficult to draw conclusions about adverse events when eleven of the thirteen studies did not state whether any adverse events occurred or not.
Given that the studies included subjects who had a clinically relevant level of depressive symptoms, as well as those that did not, the results of the meta-analysis may be relevant to the prevention as well as treatment of depressive symptoms. There was a significant fixed effects model when higher intensity studies were pooled, but not for lower intensity exercise protocols. Our definition of “higher intensity” adheres to adult exercise guidelines (e.g., American College of Sports Medicine recommends 150 weekly minutes of exercise which includes cardiorespiratory, resistance, and neuromotor activities43) and aligns with current guidelines for depression (e.g., United Kingdom Institute for Clinical Systems Improvement recommends physical activity for 30 minutes 3–5 days per week to decrease symptoms of a major depression44). The study by Lai et al.28 is typical of a high intensity protocol and was administered three times a week for 36 sessions and consisted of progressive exercise targeting strength, balance, endurance and upper extremity function. In our study, determining the dose effect of exercise on depressive symptoms was limited by the utilization of highly diverse types of exercise without any reporting of exertion. Only four studies reported the exertional intensity of the training protocol (50–60% maximal heart rate29; RPE 13–1630, RPE ≤ 1334; 40–70% maximal heart rate37) and no studies reported the actual exertional levels of the participants during the training. Several studies utilized progressive, functional exercises which might not have had a planned aerobic component but it is possible that participants reached high levels of intensity as it is known that energy expenditure per covered distance is almost double in stroke patients.45 In the future, it would be useful for studies to report common metrics of training intensity (e.g., heart rate, ratings of perceived exertion) to facilitate analyses on the dosage of exercise.
These studies do not identify the mechanisms behind the link between exercise and depressive symptoms. Among the suggested pathways are psycho-endocrinological effects of exercise such as a decline in stress hormones and an increase in the concentrations of circulating beta-endorphins and monoamines as well as an increase in body temperature and fitness level,46 and a stimulation of new nerve cells and release of proteins that supports the survival of nerve cells such as brain-derived growth neurotrophic factor.47 Further, interactions with peers during some of the group exercise interventions may have functioned as a distraction from negative thoughts. As well, it is not known whether exercise itself improved depressive symptoms, or whether this was secondary to the functional gains.
The present systematic review has some limitations. It is possible that the effects were over-estimated as trials with non-significant results were under-represented, possibly as a result of publication bias. There was heterogeneity in the study characteristics (e.g., varying exercise protocols, low and high quality studies), however, we undertook sensitivity analysis to explore potential effects of time since stroke, exercise intensity and study quality. Four of the studies required some conversion of the data (e.g., reported median values) and this could inherently increase the error especially if the sample size was small and the data distribution was not normal. Two of these studies25,26 had large sample sizes (n=170; n=243), while the other two studies had moderately sized samples of 8932 and 48.29 The studies in this systematic review assessed depressive symptoms, rather than depression as diagnosed using the Diagnostic and Statistical Manual of Mental Disorders. Since only two studies27,28 reported whether participants were using antidepressants or not, we cannot assess the potential confounding interactions of exercise and pharmacological management of depressive symptoms. We recommend that future studies consider reporting the number of subjects taking depressive medication, the number of subjects with clinically relevant depressive symptoms, the prescribed training intensity, and the actual exertional levels during training. Future studies should include a control group matched for the minutes of attention. Furthermore, studies should be sufficiently powered to determine the effect of exercise on depressive symptoms as a primary outcome in samples of individuals who are clinically depressed who may have the most to gain from exercise. As well, the rigor of future trials can be improved by ensuring concealed allocation, assessor blinding and the reporting of adverse events.
In summary, our meta-analysis based on all the available evidence shows a small benefit of exercise for stroke patients in terms of their depressive symptoms. Screening for depressive symptoms should occur along the continuum of stroke recovery and health care providers may consider prescribing a structured exercise program as one element of treatment for stroke survivors with depressive symptoms.
Supplementary Material
Clinical message.
Based on the available evidence, exercise may be a potential treatment to prevent or reduce depressive symptoms in individuals with stroke
Acknowledgments
Funding acknowledgements
The work of the study was supported by the Canadian Institutes of Health Research (111183). The authors would like to thank the Canadian Institutes of Health Research [CIHR MSH-63617] and the Michael Smith Foundation of Health Research for salary support to JJE.
Footnotes
Declaration of conflicting interests
The Author(s) declare(s) that there is no conflict of interest
References
- 1.Hackett ML, Yapa C, Parag V, et al. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 2.Kotila M, Numminen H, Waltimo O, et al. Post-stroke depression and functional recovery in a population-based stroke register. The Finnstroke study. Eur J Neurol. 1999;6:309–312. doi: 10.1046/j.1468-1331.1999.630309.x. [DOI] [PubMed] [Google Scholar]
- 3.Pohjasvaara T, Leskela M, Vataja R, et al. Post-stroke depression, executive dysfunction and functional outcome. Eur J Neurol. 2002;9:269–275. doi: 10.1046/j.1468-1331.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 4.Pohjasvaara T, Vataja R, Leppävuori A, et al. Suicidal ideas in stroke patients 3 and 15 months after stroke. Cerebrovasc Dis. 2001;12:21–26. doi: 10.1159/000047676. [DOI] [PubMed] [Google Scholar]
- 5.Kauhanen M, Korpelainen JT, Hiltunen P, et al. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke. 1999;30:1875–1880. doi: 10.1161/01.str.30.9.1875. [DOI] [PubMed] [Google Scholar]
- 6.Nys GM, van Zandvoort MJ, van der Worp HB, et al. Early depressive symptoms after stroke: neuropsychological correlates and lesion characteristics. J Neurol Sci. 2005;228:27–33. doi: 10.1016/j.jns.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Landreville P, Desrosiers J, Vincent C, et al. The role of activity restriction in poststroke depressive symptoms. Rehabil Psychol. 2009;54:315–322. doi: 10.1037/a0016572. [DOI] [PubMed] [Google Scholar]
- 8.Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161:1090–1095. doi: 10.1176/appi.ajp.161.6.1090. [DOI] [PubMed] [Google Scholar]
- 9.Hackett ML, Anderson CS, House A, et al. Interventions for preventing depression after stroke. Cochrane Database Syst Rev. 2008;3:CD003689. doi: 10.1002/14651858.CD003689.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Hackett ML, Anderson CS, House A, et al. Interventions for treating depression after stroke. Cochrane Database Syst Rev. 2008;4:CD003437. doi: 10.1002/14651858.CD003437.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database Syst Rev. 2009;3:CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- 12.Sjösten N, Kivelä SL. The effects of physical exercise on depressive symptoms among the aged: a systematic review. Int J Geriatr Psychiatry. 2006;21:410–418. doi: 10.1002/gps.1494. [DOI] [PubMed] [Google Scholar]
- 13.Herring MP, Puetz TW, O’Connor PJ, et al. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:101–111. doi: 10.1001/archinternmed.2011.696. [DOI] [PubMed] [Google Scholar]
- 14.Graven C, Brock K, Hill K, et al. Are rehabilitation and/or care co-ordination interventions delivered in the community effective in reducing depression, facilitating participation and improving quality of life after stroke? Disabil Rehabil. 2011;33:1501–1520. doi: 10.3109/09638288.2010.542874. [DOI] [PubMed] [Google Scholar]
- 15.Saunders DH, Sanderson M, Brazzelli M, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2013 Oct 21;10:CD003316. doi: 10.1002/14651858.CD003316.pub5. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tezlaff J, et al. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology and the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 18.Bateman A, Culpan FJ, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Arch Phys Med Rehabil. 2001;82:174–182. doi: 10.1053/apmr.2001.19744. [DOI] [PubMed] [Google Scholar]
- 19.Physicaltherapy Evidence Database (PEDro) [accessed January 10, 2014];2013 http://www.pedro.org.au/english/downloads/pedro-scale.
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limpert E, Stahel WA, Abbt M. Log-normal distributions across the sciences: Keys and Clues. Biosciences. 2001;51:341–352. [Google Scholar]
- 22.The Cochrane Collaboration, Oxford, England. [accessed January 10, 2014];Revman 5.2. 2012 http://ims.cochrane.org/revman.
- 23.Cohen J. Statistical power analysis for the behavioral sciences. New York, NY: Academic Press; 1988. [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green J, Forster A, Bogle S, et al. Physiotherapy for patients with mobility problems more than 1 year after stroke: a randomised controlled trial. Lancet. 2002;359:199–203. doi: 10.1016/S0140-6736(02)07443-3. [DOI] [PubMed] [Google Scholar]
- 26.Harrington R, Taylor G, Hollinghurst S, et al. A community-based exercise and education scheme for stroke survivors: a randomized controlled trial and economic evaluation. Clin Rehabil. 2010;24:3–15. doi: 10.1177/0269215509347437. [DOI] [PubMed] [Google Scholar]
- 27.Holmgren E, Gosman-Hedström G, Lindström B, et al. What is the benefit of a high-intensive exercise program on health-related quality of life and depression after stroke? A randomized controlled trial. Adv Physiother. 2010;12:125–133. doi: 10.3109/14038196.2010.488272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai SM, Studenski S, Richards L, et al. Therapeutic exercise and depressive symptoms after stroke. J Am Geriatr Soc. 2006;54:240–247. doi: 10.1111/j.1532-5415.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- 29.Lennon O, Carey A, Gaffney N, et al. A pilot randomized controlled trial to evaluate the benefit of the cardiac rehabilitation paradigm for the non-acute ischaemic stroke population. Clin Rehabil. 2008;22:125–133. doi: 10.1177/0269215507081580. [DOI] [PubMed] [Google Scholar]
- 30.Mead GE, Greig CA, Cunningham I, et al. Stroke: A randomized trial of exercise or relaxation. J Am Geriatr Soc. 2007;55:892–899. doi: 10.1111/j.1532-5415.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 31.Partridge C, Mackenzie M, Edwards S, et al. Is dosage of physiotherapy a critical factor in deciding patterns of recovery from stroke: a pragmatic randomized controlled trial. Physiother Res Int. 2000;5:230–240. doi: 10.1002/pri.203. [DOI] [PubMed] [Google Scholar]
- 32.Ryan T, Enderby P, Rigby AS. A randomized controlled trial to evaluate intensity of community-based rehabilitation provision following stroke or hip fracture in old age. Clin Rehabil. 2006;20:123–131. doi: 10.1191/0269215506cr933oa. [DOI] [PubMed] [Google Scholar]
- 33.Sims J, Galea M, Taylor N, et al. Regenerate: assessing the feasibility of a strength-training program to enhance the physical and mental health of chronic post stroke patients with depression. Int J Geriatr Psychiatry. 2009;24:76–83. doi: 10.1002/gps.2082. [DOI] [PubMed] [Google Scholar]
- 34.Smith PS, Thompson M. Treadmill training post stroke: are there any secondary benefits? A pilot study. Clin Rehabil. 2008;22:997–1002. doi: 10.1177/0269215508088988. [DOI] [PubMed] [Google Scholar]
- 35.Wade DT, Collen FM, Robb GF, et al. Physiotherapy intervention late after stroke and mobility. BMJ. 1992;304:609–613. doi: 10.1136/bmj.304.6827.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner RA, Kessler S. Effectiveness of an intensive outpatient rehabilitation program for postacute stroke patients. Am J Phys Med Rehabil. 1996;75:114–120. doi: 10.1097/00002060-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Zedlitz AM, Rietveld TC, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke. 2012;43:1046–1051. doi: 10.1161/STROKEAHA.111.632117. [DOI] [PubMed] [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 39.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 40.Beck AT, Ward CH, Mock J, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 41.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 42.Foley NC, Bhogal SK, Teasell RW, et al. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther. 2006;86:817–824. [PubMed] [Google Scholar]
- 43.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 44.Institute for Clinical Systems Improvement (ICSI) Major depression in adults in primary care. Bloomington MN: Institute for Clinical Systems Improvement (ICSI); 2010. [accessed 1 March 2013]. https://www.icsi.org/_asset/fnhdm3/Depr-Interactive0512b.pdf. [Google Scholar]
- 45.Stoquart G, Detrembleur C, Lejeune TM. The reasons why stroke patients expend so much energy to walk slowly. Gait Posture. 2012;36:409–413. doi: 10.1016/j.gaitpost.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Arent SM, Landers DM, Etnier JL. The effects of exercise on mood in older adults: A meta-analytic review. J Aging Phys Act. 2000;8:407–430. [Google Scholar]
- 47.Duclos M. Effects of physical training on endocrine functions. Ann Endocrinol. 2001;62:19–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.