Abstract
While protective measures have been taken to mitigate injury to the thorax during a blast exposure, primary blast lung injury (PBLI) is still evident in mounted/in vehicle cases during military conflicts. Moreover, civilians, who are unprotected from blast exposure, can be severely harmed by terrorist attacks that use improvised explosive devices (IEDs). Since the lungs are the most susceptible organ due to their air-filled nature, PBLI is one of the most serious injuries seen in civilian blast cases.
Determining lethality threshold for rodent studies is crucial to guide experimental designs centered on therapies for survival after PBLI or mechanistic understanding of the injury itself. Using an Advanced Blast Simulator, unprotected rats were exposed to a whole body blast to induce PBLI. The one-hour survival rate was assessed to determine operating conditions for a 50% lethality rate. Macroscopic and histological analysis of lung was conducted using hematoxylin and eosin staining. Results demonstrated lethality risk trends based on static blast overpressure (BOP) for rodent models, which may help standardized animal studies and contribute to scaling to the human level.
The need for a standardized method of producing PBLI is pressing and establishing standard curves, such as a lethality risk curve for lung blasts, is crucial for this condensing of BOP methods.
Keywords: Blast, lung injury, lethality risk, rats
INTRODUCTION
Significance of Lung Injury in Blast Settings
Primary blast lung injury (PBLI) is a major cause of immediate mortality following IED strikes [1]. PBLI is particularly more frequent when exposure occurs in enclosed spaces. Fatal injury percentage is significantly increased when soldiers were exposed to the blast wave in vehicle compared to on foot, in terms of PBLI [2]. In recent Israeli conflicts, half of all civilians injured in terrorist bombings suffered acute lung injury, which required immediate treatment. It is also known that currently used treatments for lung and brain injury following blast overpressure (BOP) exposure can often contradict each other, so finding new treatments for lung injury is necessary for improving clinical interventions in cases of blast polytrauma [3]. From 2003 to 2009 for U.K. soldiers involved in military conflicts, less than 50% of patients that exhibited evidence of blast lung injury survived to reach a medical facility. Out of those that did survive, 80% required immediate ventilation support. The need for a first responding treatment is vital in order to increase the chance patients with PBLI can survive to receive the needed intervention. The incidence of blast lung injuries seen in theatre increased from the Iraqi conflict to the war in Afghanistan for these U.K. troops, demonstrating a growing clinical burden [4]. In clinical treatment, physicians have the resources to adequately treat blast lung trauma, so having immediate acute treatment to prolong survival would allow patients the opportunity for full recovery through long-term hospital care [5]. Lung hemorrhage is a common clinical outcome, independent of blast exposure, so an animal model of induced lung hemorrhage could have broad impacts on mechanistic and pharmacological research.
Summary of Experimental Work in Blast Lung Injury
PBLI has been previously studied in animal models in order to analyze inflammatory, anti-oxidant, and other physiological characteristics of this injury [6–8]. Limited PBLI research has been conducted with pharmaceutical studies geared towards mitigating this injury [9]. Previous researchers induced PBLI in order to examine the effects of varied overpressures and lung recovery post-blast; however, methodologies have not been standardized [10, 11]. Recent efforts have also looked at the combined effects of blast and burn exposure on lethality and inflammatory cytokines [12, 13].
Determining the 50% Lethality Threshold
A thorough literature review was conducted in order to identify overpressure ranges previously used for rat experimentation. Surprisingly, published reports using rat models to investigate PBLI are few in number compared to other injury modalities [6–18, 20–23]. Moreover, the experimental set-up greatly varies between research groups, which leads to conflicting results. Collectively, overpressures ranging from 62 – 136 kPa have been investigated in a PBLI rat model with variable time points for survival [14–16]. However, there are reported results of exposing rats to extremely high overpressures in the range of 550 – 827 kPa [17, 18]. A general consensus is that between 60–100 kPa peak overpressure produces low level blast injury, while overpressures ranging between 100–140 kPa produces moderate level blast injury and overpressures >180 kPa produces severe blast injury. To further the confusion, there is a lack of adequate evidence documenting the exact pressure wave pulse across the entire range of published results. This is a critical element because the time duration of exposure is a well-known characteristic of most other injury phenomena and likely plays a role in tissue damage. In conclusion, there is not an established lung injury threshold for transitioning between the overpressure levels.
METHODS
Animals and Blast Overpressure (BOP) Exposure
The Virginia Tech Institutional Animal Care and Use Committee approved experimental protocols described herein. Prior to all experiments, male Sprague Dawley rats (~325 g, Harlan Labs, San Diego) were acclimated to a 12 hour light/dark cycle with food and water provided ad lib. Animals (n=26) were exposed to a single incident pressure profile resembling a ‘free-field’ blast exposure within the 130 to 190 kPa range.
Prior to blast exposure, rats are anesthetized with a ketamine/xylazine solution, in accordance with the rodent weight, to have sedation over the entire procedure (one hour). The shock front and blast overpressure were generated by a custom-built Advanced Blast Simulator (ABS) with an end wave eliminator (ORA Inc. Fredericksburg, VA) located at the Center for Injury Biomechanics at Virginia Polytechnic Institute and State University [19]. A peak static overpressure was produced with compressed helium and calibrated acetate sheets (Grafix Plastics, Cleveland, OH) that were varied to produce a range of BOP. Pressure measurements were collected at 250 kHz using a Dash 8HF data acquisition system (Astro-Med, Inc, West Warwick, RI) and shockwave profiles were verified to maintain consistent exposure pressures between subjects. Peak overpressures were calculated by determining wave speed (m/s) at specimen position. A mesh sling was used to hold the animal during the exposure that allowed for minimal hindrance of the wave through the tube in addition to holding the animal in a prone position with the right side of the thorax facing the shock wave driver. Peak overpressure is followed by an exponential decay (2.5 ms positive duration) and a short negative phase.
After the blast exposure, animals were removed, returned to a warm pad and injected with sterile phosphate buffered saline (500 μls) via tail vein injection, crucial for simulating any post-exposure injection. All large organs were immediately collected in animals that did not survive the blast exposure. Animals that survived the one-hour time assessment, which included monitoring the physiology of the rat, were sacrificed by transcardial perfusion with ice-cold phosphate buffered saline (PBS) and 4% paraformaldehyde.
Lung Injury Severity
Imaging of the lungs occurred immediately after removal from the specimen. Macroscopic damage and gross characteristics were noted to determine any landmarks associated with increased peak overpressure and to investigate if specific injury patterns could be determined.
Lung Histology
After collection, lungs were stored in a 4% paraformaldehyde fixative solution. After 48 hours in fixative, the lungs were placed in 30% sucrose solution for tissue sectioning preparation. Lungs were separated into cassettes with each lobe isolated for analysis. Samples were then cut (8 μm) and stained with hematoxylin and eosin (H&E) to predict injury extent. Images were taken of three regions of interest (ROI) in each lung tissue section at 10X magnification (Zeiss AxioCam ICc 1). These three images were converted to black and white and optical density readings were collected in order to determine the level of hemorrhaging in the lung tissue using Image J software (NIH, Bethesda, MD).
Statistical Analysis
A one-variable linear logistic regression was performed on the experimental data obtained in this study in terms of static overpressure (SOP). The model in equation form is:
where A is the probability of lethality, SOP is the static overpressure, and parameters β0 and β1 are calculated and optimized. Logistic regression was completed using Matlab (Version R2012a, MathWorks, Natick, MA).
The model was assessed using the C-statistic or the area under the receiver operating characteristic (ROC) curve (AUC), which compares sensitivity to (1-specificity) of the data obtained. Analysis was done similarly to other computations on lethality risk [25]. Statistical analysis of the coefficients was also performed in Matlab examining contribution to the model. Microsoft Excel was used to calculate a linear fit (R2) of the histological results.
RESULTS
Literature Studies Included for Lethality Risk Analysis
Choosing appropriate studies for comparison of lethality must be methodical and precisely done to construct an appropriate threshold. The different modes of injury procurement vary widely and the injury model intended is important for consideration. Many methods have been utilized to create blast-induced lung injury, including percussive nail guns, high-energy explosives, and compressed-air driven shock tubes [10, 12, 20]. Since the shock tube method has the most consistency and is most similar to the method used in the experimental study at hand, only reports using shock tubes and blast wave generators were included in analyzing lethality risk. Only blast-induced lung models were considered, since blast-induced brain injury models often utilize drastically different settings. Furthermore, the recorded values of overpressure needed to be static overpressure since that is the load bearing parameter commonly used in reporting of blast magnitude. All studies that utilized pharmacological interventions or other protective measures, such as vests covering the thorax, were excluded. Overall, thirteen additional studies that used around 330 rats were included in lethality risk comparison to the current experimental work [6, 7, 9–11, 13–16, 21–24]. All studies either used compressed air or helium to drive a shock tube for BOP administration.
Lung Injury Severity
Examining macroscopic images of the lungs after blast gave insight into mechanism of lung contusion after sustaining blast exposure to the thorax. It was observed that the median lobe in all animals (even noticeable macroscopic hemorrhaging. With increased overpressure magnitude, there was an increase in the number of lung lobes that were affected and appeared hemorrhaged. Figure 2 provides examples of macroscopic lung images.
Figure 2.
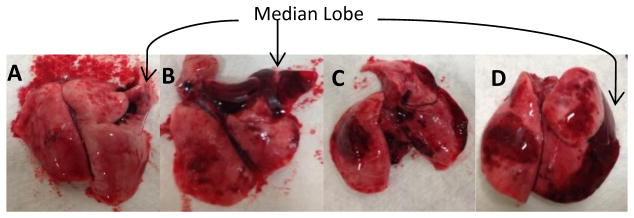
Macroscopic images of lungs after extraction. The following images correspond to increasing level of BOP: A -156 kPa, B – 162 kPa, C – 180 kPa, and D -182 kPa.
Results are consistent with other studies testing effects of blast peak pressure on lung injury severity. [9]
Lung Histology
Animals given varied degrees of BOP were analyzed and grouped according to outcome (survival and non-survival). The bronchiole shown in image 2 of Figure 3A was filled with blood indicated severe hemorrhaging and possible hemothorax.
Figure 3.
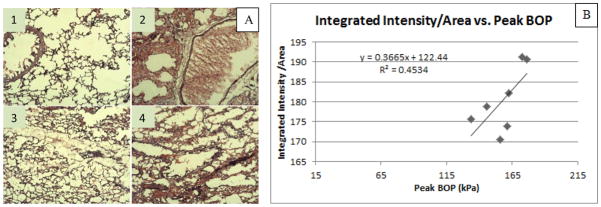
(A) HE staining of median lobe of animals exposed to blast. Images 1 and 3 are taken from an animal in a lower pressure group that remained alive following the blast. Images 2 and 4 are from an animal in a higher pressure group which died within one hour following blast. (B) Graph depicts the Integrated Intensity/Area vs. Peak BOP for animals which survived one hour. Integrated intensity normalized to the area of the image was used to characterize the amount of bleeding within the median lobe.
Lethality Risk for Blast Lung Injury
Even though it has been suggested that a non-linear form is needed for short-duration blasts, using unscaled rodent tests and a consistent method of BOP stimulus (shock tube) provides for linear analysis [25]. The value for the parameters was: β0 = −29.82 and β1 = 0.178 and the p-value to determine the contribution of each parameter was 0.018 and 0.0178, respectively. The value for AUC was 0.951 for the experimental data, which is considered a strong representation, and was 0.95 for the literature data.
DISCUSSION
Examining the mechanism of lung injury during the blast is useful in determining causes of mortality, namely hypoxia. Inability to prevent hemorrhaging in the lungs can cause mortality after the blast. Injury can often be caused by blunt impact with the rib cage or rapid compression/decompression of lung tissue [26, 27]. It is speculated that orientation of the body to the blast wave played a significant role in this injury progression, and BOP exposure directed towards the sternum of the rat would have a different cascade of macroscopic trauma. Examining macroscopic lung trauma showed that the median lobe was progressively injured as peak BOP was increased, while other lobes, such as the upper right lobe and the left lobe, sustain external trauma as peak BOP reaches higher levels. Continuing with the observation that the middle lobe on the right side was increasingly affected by overpressure, it was also observed that histological analysis of this median lobe showed a linear increase of hemorrhaging compared to peak BOP. Even though the correlation in Figure 3B had a low R2 value, this preliminary result coincides with macroscopic observations, while also providing a basis for further detailed studies on microscopic injury progression. Macroscopic and histological findings in this study resembled clinical symptoms seen in the human PBLI, such as hemothorax, pulmonary contusion, and interstitial emphysema. [28]
Injury severity can be portrayed in a variety of ways, one being lethality prevalence. According to previous studies, the span of 140 kPa to 180 kPa side exposure in rats marks the transition from moderate to severe PBLI, which corresponds to the entire range of lethality probability in the risk curve (Figure 4). The fifty percent lethality point is 168 kPa according to the developed risk curve with the ten percent lethality point at 155 kPa and the ninety percent lethality point at 180 kPa, so there is an operating range of 25 kPa between these marks. Orientation to the shock front of the blast wave is crucial in examining the mechanism of injury to the lungs. In Figure 4, there are some discrepancies in the threshold for injury in comparison to other researchers. This was to be expected since a variety of methods are used. In this study, the duration of the blast wave had a linear correlation with peak overpressure. With a standard primary blast wave that has consistency and obeys the general tendencies of a Friedlander waveform, duration is a function of the peak overpressure. Since these go hand-in-hand, there was not any examination of lethality risk based on duration and/or impulse. When creating a threshold for blast injuries, it is important to know the parameters that are crucial for creating the injury. In all studies concerning this injury mode, peak overpressure had the strongest correlation with the survival outcome.
Figure 4.
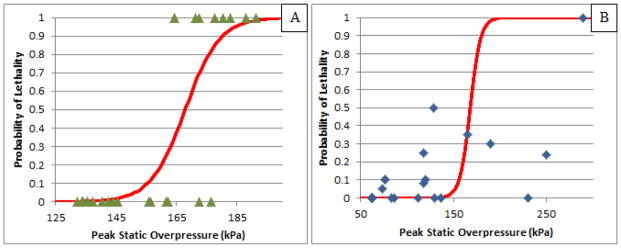
(A) Probability of Lethality versus Peak BOP for the experimental results obtained in this study. (B) The results from literature are included for comparison of the risk curve [6, 7, 9–11, 13–16, 21–24].
CONCLUSIONS
Lethality risk curves can serve to give guidance for further lung injury treatment studies. Scaling these results to the human body can establish new thresholds for clinical outcomes according to blast severity or can validate previous models. Also, observations on the development of gross trauma to lungs as corresponding to peak BOP could have implications to the response of larger specimens. Even though many experiments have pinpointed several markers associated with PBLI, there is a vast amount of effort that is needed to fully characterize the injury, as well as to develop therapeutics to combat sustained symptoms after initial trauma. Having a standard lethality risk curve is crucial for constancy among blast research and will be beneficial to all mechanistic or treatment-oriented blast-induced lung injury studies.
Figure 1.

ABS at Virginia Tech with a representative figure used for peak overpressure calculation.
Acknowledgments
The authors would like to thank Dr. Kristofer D. Kusano for aid in developing the lethality risk curves. This work was funded by DoD grant number W81XWH-11-2-0014.
References
- 1.Yeh DD, Schecter WP. Primary blast injuries--an updated concise review. World J Surg. 2012;36(5):966–72. doi: 10.1007/s00268-012-1500-9. [DOI] [PubMed] [Google Scholar]
- 2.Singleton JA, et al. Primary blast lung injury prevalence and fatal injuries from explosions: insights from postmortem computed tomographic analysis of 121 improvised explosive device fatalities. J Trauma Acute Care Surg. 2013;75(2 suppl 2):S269–74. doi: 10.1097/TA.0b013e318299d93e. [DOI] [PubMed] [Google Scholar]
- 3.Hicks RR, et al. Neurological effects of blast injury. J Trauma. 2010;68(5):1257–63. doi: 10.1097/TA.0b013e3181d8956d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JE. The epidemiology of blast lung injury during recent military conflicts: a retrospective database review of cases presenting to deployed military hospitals, 2003–2009. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):291–4. doi: 10.1098/rstb.2010.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritenour AE, Baskin TW. Primary blast injury: update on diagnosis and treatment. Crit Care Med. 2008;36(7 suppl):S311–7. doi: 10.1097/CCM.0b013e31817e2a8c. [DOI] [PubMed] [Google Scholar]
- 6.Gorbunov NV, et al. Inflammatory leukocytes and iron turnover in experimental hemorrhagic lung trauma. Exp Mol Pathol. 2006;80(1):11–25. doi: 10.1016/j.yexmp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Elsayed NM, et al. Antioxidant loading reduces oxidative stress induced by high-energy impulse noise (blast) exposure. Toxicology. 2000;155(1–3):91–9. doi: 10.1016/s0300-483x(00)00281-x. [DOI] [PubMed] [Google Scholar]
- 8.Irwin RJ, et al. Cardiopulmonary physiology of primary blast injury. J Trauma. 1997;43(4):650–5. doi: 10.1097/00005373-199710000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Chavko M, Prusaczyk WK, McCarron RM. Protection against blast-induced mortality in rats by hemin. J Trauma. 2008;65(5):1140–5. doi: 10.1097/TA.0b013e3181870a8c. [DOI] [PubMed] [Google Scholar]
- 10.Skotak M, et al. Rat injury model under controlled field-relevant primary blast conditions: acute response to a wide range of peak overpressures. J Neurotrauma. 2013;30(13):1147–60. doi: 10.1089/neu.2012.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavko M, Prusaczyk WK, McCarron RM. Lung injury and recovery after exposure to blast overpressure. J Trauma. 2006;61(4):933–42. doi: 10.1097/01.ta.0000233742.75450.47. [DOI] [PubMed] [Google Scholar]
- 12.Chai JK, et al. Role of neutrophil elastase in lung injury induced by burn-blast combined injury in rats. Burns. 2013;39(4):745–53. doi: 10.1016/j.burns.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Chai JK, et al. A novel model of burn-blast combined injury and its phasic changes of blood coagulation in rats. Shock. 2013;40(4):297–302. doi: 10.1097/SHK.0b013e3182837831. [DOI] [PubMed] [Google Scholar]
- 14.Elsayed NM, Gorbunov NV, Kagan VE. A proposed biochemical mechanism involving hemoglobin for blast overpressure-induced injury. Toxicology. 1997;121(1):81–90. doi: 10.1016/s0300-483x(97)03657-3. [DOI] [PubMed] [Google Scholar]
- 15.Gorbunov NV, et al. Air blast-induced pulmonary oxidative stress: interplay among hemoglobin, antioxidants, and lipid peroxidation. Am J Physiol. 1997;272(2 pt 1):L320–34. doi: 10.1152/ajplung.1997.272.2.L320. [DOI] [PubMed] [Google Scholar]
- 16.Gorbunov NV, et al. Assessment of inflammatory response and sequestration of blood iron transferrin complexes in a rat model of lung injury resulting from exposure to low-frequency shock waves. Crit Care Med. 2004;32(4):1028–34. doi: 10.1097/01.ccm.0000120051.79520.b6. [DOI] [PubMed] [Google Scholar]
- 17.Cooper GJ, et al. The role of stress waves in thoracic visceral injury from blast loading: modification of stress transmission by foams and high-density materials. J Biomech. 1991;24(5):273–85. doi: 10.1016/0021-9290(91)90346-o. [DOI] [PubMed] [Google Scholar]
- 18.Irwin RJ, et al. Global primary blast injury: a rat model. J Okla State Med Assoc. 1998;91(7):387–92. [PubMed] [Google Scholar]
- 19.Hockey KS, et al. A new model for mild blast injury utilizing Drosophila melanogaster. Biomed Sci Instrum. 2013;49:134–40. [PubMed] [Google Scholar]
- 20.Badami CD, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63(3):596–600. doi: 10.1097/TA.0b013e318142d231. discussion 600–2. [DOI] [PubMed] [Google Scholar]
- 21.Gorbunov NV, et al. Electron paramagnetic resonance analysis of transferrin-bound iron in animal models of blunt trauma. J Trauma. 2003;54(3):574–83. doi: 10.1097/01.TA.0000043922.40376.38. [DOI] [PubMed] [Google Scholar]
- 22.Seitz DH, et al. Pulmonary contusion induces alveolar type 2 epithelial cell apoptosis: role of alveolar macrophages and neutrophils. Shock. 2008;30(5):537–44. doi: 10.1097/SHK.0b013e31816a394b. [DOI] [PubMed] [Google Scholar]
- 23.Flierl MA, et al. The role of C5a in the innate immune response after experimental blunt chest trauma. Shock. 2008;29(1):25–31. doi: 10.1097/shk.0b013e3180556a0b. [DOI] [PubMed] [Google Scholar]
- 24.Bauman RA, et al. Exposure to sublethal blast overpressure reduces the food intake and exercise performance of rats. Toxicology. 1997;121(1):65–79. doi: 10.1016/s0300-483x(97)03656-1. [DOI] [PubMed] [Google Scholar]
- 25.Bass CR, Rafaels KA, Salzar RS. Pulmonary injury risk assessment for short-duration blasts. J Trauma. 2008;65(3):604–15. doi: 10.1097/TA.0b013e3181454ab4. [DOI] [PubMed] [Google Scholar]
- 26.Clemedson CJ. Blast injury. Physiol Rev. 1956;36(3):336–54. doi: 10.1152/physrev.1956.36.3.336. [DOI] [PubMed] [Google Scholar]
- 27.Sasser SM, et al. Blast lung injury. Prehosp Emerg Care. 2006;10(2):165–72. doi: 10.1080/10903120500540912. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie IM, Tunnicliffe B. Blast injuries to the lung: epidemiology and management. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):295–9. doi: 10.1098/rstb.2010.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]


