Abstract
Individuals with anorexia nervosa (AN) restrict eating and become emaciated. AN tend to have an aversion to foods rich in fat. Because Epoxide Hydrolase 2 (EPHX2) was identified as a novel AN susceptibility gene, and because its protein product, soluble epoxide hydrolase (sEH), converts bioactive epoxides of polyunsaturated fatty acid (PUFA) to the corresponding diols, lipidomic and metabolomic targets of EPHX2 were assessed to evaluate the biological functions of EPHX2 and their role in AN.
Epoxide substrates of sEH and associated oxylipins were measured in ill AN, recovered AN, and gender- and race-matched controls. PUFA and oxylipin markers were tested as potential biomarkers for AN. Oxylipin ratios were calculated as proxy markers of in vivo sEH activity. Several free- and total PUFAs were associated with AN diagnosis and with AN recovery. AN displayed elevated n-3 PUFAs and may differ from controls in PUFA elongation and desaturation processes. Cytochrome P450 pathway oxylipins from arachidonic acid, linoleic acid, alpha-linolenic acid, and docosahexaenoic acid PUFAs are associated with AN diagnosis. The diol:epoxide ratios suggest the sEH activity is higher in AN compared to controls. Multivariate analysis illustrates normalization of lipidomic profiles in recovered ANs.
EPHX2 influences AN risk through in vivo interaction with dietary PUFAs. PUFA composition and concentrations as well as sEH activity may contribute to the pathogenesis and prognosis of AN. Our data support the involvement of EPHX2-associated lipidomic and oxylipin dysregulations in AN, and reveal their potential as biomarkers to assess responsiveness to future intervention or treatment.
INTRODUCTION
Anorexia nervosa (AN) is a serious eating disorder with a reported prevalence ranging from 0.24 to 4.3%.[1] While it is one of the less prevalent major psychiatric diseases, it is the deadliest of all psychiatric illnesses.[2, 3] Patients with AN restrict eating and become emaciated, yet the relentless behavior to achieve thinness by restrictive eating continues together with distorted body image and profound fear of weight gain. Although it has traditionally been speculated that factors contributing to AN are social and psychosocial in origin, recent findings demonstrated that genetic factors account for approximately 50 to 80% of the AN risk[4], suggesting that the pathophysiology of AN involves the complex interplay of biological, biochemical, and environmental risk factors.
Patients with AN have long been known to experience fat aversion and avoidance of fat-rich foods.[5, 6] The diet preferred by AN is typically low in total proteins, carbohydrates, polysaccharides, and fats.[7, 8] Additionally, pre-meal anxiety in AN was found to be correlated with consumption of high fat food like macaroni and cheese (42.2% fat) but not for a low-fat yogurt snack (12.9% fat). [9] The illness duration of AN is negatively correlated with the amount of dietary fat in both acute and chronic patients.[8] However, the reason for fat avoidance in AN patients is not well understood.
Fat-rich foods serve as one of the primary sources of essential fatty acids (EsFA), including omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids, which are critical for brain development, aging and general health.[10, 11] Frank EsFA deficiency, though rare from a purely dietary perspective, is associated with a number of cardio-metabolic, neurological, immunological and psychiatric diseases.[12] While essential fatty acids are influenced by diet, their biological functions and health consequences are also dependent on a number of diverse enzyme families for biosynthesis and modulation of resulting lipid mediators that goes far beyond being structural building blocks. Earlier studies in AN populations have found the presence of altered fatty lipid levels[13] that have been attributed to malnutrition partly based on findings from the pediatric population studies that showed associations between malnutrition and abnormalities in fatty acid profile[14, 15]. However, an earlier study in AN suggested that fatty acid alterations in AN differ from simple nutritional essential fatty acid deficiency or chronic malnutrition.[16] To account for the effect malnutrition specific to ill AN (active disease) has on lipidomic disturbances and to determine by what extent AN recovery normalizes these disturbances, we studied both ill AN and recovered AN together with healthy controls.
Through our recent targeted exomic individual and pooled sequencing discovery and multiple stage replication study, the Epoxide Hydrolase 2 gene (EPHX2) was found to harbor several common and rare risk variants for AN.[17] EPHX2 codes for soluble epoxide hydrolase (sEH), a key gate-keeper enzyme that affects lipid signaling functions of a broad range of metabolites by the catabolism of epoxy-fatty acids to their corresponding diols.[18] [19, 20] This sEH activity reduces the potency of anti-inflammatory epoxy-fatty acids (e.g. epoxy-eicosatrienoic acids [EpETrEs] from arachidonic acid [ARA]), thereby affecting human health through modulation of the biologically active lipid mediators.[21] Epoxy-fatty acid substrates of sEH include the epoxides derived from n-3 lipid, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and from n-6 lipid, such as arachidonic acid (ARA), linoleic acid (LA), and stearic acid (SA).[22] The sEH activity is highest in the liver and kidney and it is also found to be highly expressed in multiple other organs and tissues including the brain [23] and adipose tissue.[24] It has been suggested that since sEH is localized in neurons of the central amygdala, it may play a role in neuronal firing.[25] Thus, it is proposed that sEH acts in the brain through the metabolism of lipid mediators to modulate the release of neurotransmitters that influence cognition, affect, and eating behaviors that are key characteristics for AN. The discovery of EPHX2 as a risk gene for AN raised the possibility that fat-avoidance in AN could in part be due to some intrinsic biological process that is associated with sEH lipid regulatory functions.
Taken together, our discovery of EPHX2 variant association with AN[17] and current literature support the hypothesis that sEH, through biological interaction with polyunsaturated fatty acid substrates, plays a role in fat aversion in AN and the pathophysiology of disordered eating. We developed an integrative study to investigate downstream lipidomic targets of sEH to understand the mechanisms by which EPHX2 influences AN pathophysiology.
SUBJECTS AND METHODS
Study Design
This is a cross-sectional study designed to characterize plasma n-6 and n-3 PUFAs and their oxylipin derivatives in two groups of AN (ill and recovered) and healthy controls for two purposes: 1) to explore functional consequences of sEH in the PUFA pathway and assess if they contribute to AN risk, and 2) to test the feasibility of targeted (sEH associated) PUFA and oxylipin markers as biomarkers for AN and associated key temperament traits.
Subjects
We selected 30 ill AN (median age: 20 years; age range: 16–34 years), 30 recovered AN (median age: 22 years; age range: 18–38 years), and 36 controls (median age: 20 years; age range: 18–26 years) to test our hypothesis that sEH modulation of dietary polyunsaturated fat and oxylipins may result in dysregulated metabolism and increased AN risk. The cases and controls were chosen from the original Price Foundation Genetic Study biorepository[26] based on subjects’ AN disease subtype (restricting AN), extreme low body weight for ill AN (to maximize study power by contrasting the severely illness to recovered AN), and bio specimen availability. Cases selected for this study were women of European decent, selected from the Price Foundation AN Trio Study. [26] All cases were required to meet the following criteria: (a) modified DSM-IV lifetime diagnosis of AN, i.e., with or without amenorrhea; (b) onset before the age of 25 years; (c) age between 13 years and 40 years; (d) self-identified European ancestry; (e) no lifetime history of binge eating; and (f) study diagnostic criteria were met for at least 3 years before study entry. A diagnostic hierarchy was then applied to define AN subtypes: restricting AN, AN with purging but no binge eating, AN with binge eating with or without purging, and a lifetime history of both AN and BN (ANBN).[27] We selected only restricting AN subtype to ensure a homogeneous sample of cases given the hypothesized relationship between restrictive eating behavior and sEH pathway. The restricting AN subtype is characterized by fiercely limiting the food consumed that is well below their body’s caloric needs, essentially an act of chronic starvation. The ill AN cases (IAN) in this study are defined as having ongoing ED symptoms and BMI of equal or less than 17.5 kg/m2 in the past year; whereas the recovered AN cases (RecAN) are defined as having an absence of ED symptoms in the past year including maintenance of a BMI of 18 kg/m2 or greater. The healthy control women were chosen from the control group[26] and were (a) between the ages of 18–40 years; (b) at normal weight with lifetime adult BMI between 19 kg/m2 and 27 kg/m2; and (c) gender and ethnicity matched to the cases. The exclusion criteria for controls were: (a) reported history of an eating disorder or eating disordered behaviors; (b) had a first degree relative with an eating disorder; or (c) had any psychiatric, alcohol or drug use disorder defined by the presence of an Axis I disorder on the SCID Screen Patient Questionnaire.[28] Informed consent was obtained from all study participants, and all sites received approval from their local Institutional Review Board.
Isolation of Plasma
Polyunsaturated fatty acid and oxylipin assays were performed using banked plasma from selected subjects. Whole blood was collected in EDTA vacutainer tubes and plasma was separated by centrifugation at 1350 rpm for 10 minutes and removed with a pipet and then frozen at −80C.
Polyunsaturated Fatty Acid Measurement
Both free (non-esterified) and total (esterified) forms of fatty acids were measured in plasma of all AN cases (30 IAN, 30 RecAN) and 36 controls collected from their baseline visit. The free fatty acids were purified and enriched with the application of a bi-phasic solution of acidified methanol and isooctane. A set of deuterated fatty acids was added to the samples to serve as internal standards for quantitation and to compensate for any losses during the analytical procedure. Gas chromatography coupled with mass spectrometry (GC/MS) was used to quantify each fatty acid, as previously described.[29] For the total fatty acid measurement, a hydrolysis step with KOH was used to release the fatty acids from the triglyceride and phospholipid backbone.[29] While the readout of the PUFA assay include markers consisting of saturated-, monounsaturated-, and polyunsaturated-fatty acids, we analyzed only the omega 6 (n-6) and omega 3 (n-3) fatty acids and prioritized the well-established sEH PUFA substrates (n-3 [EPA, DHA, ALA] and n-6 [ARA, LA] fatty acids) as the primary variables for association analysis. These markers reflect the nutritional-based PUFA profile and their metabolites which are biologically active lipid mediators, and also serve as proxy markers for in vivo sEH activity. Concentration of each fatty acid was measured in the unit of µmol/L and reported as a percentage of total plasma fatty acid as described in Table 2 of our previously published work.[29]
Table 2.
Polyunsaturated fatty acids ratio markers: Non-esterified fatty acids and esterified fatty acids.
| AN - ALL mean |
IAN mean |
RecAN mean |
Controls mean |
Statistics: IAN to Controls |
Statistics: RecAN to Controls |
Statistics: IAN to RecAN |
|
|---|---|---|---|---|---|---|---|
| Ratios | Non-Esterified Fatty Acid | ||||||
| (ARA + Adrenic acid)/(EPA+DPA+DHA) | 1.17 | 1.17 | 1.17 | 1.32 | 0.396 | 0.161 | 0.942 |
| LA/ALA* | 139.5 | 126.7 | 152.3 | 195.7 | 0.001 | 0.018 | 0.266 |
| ARA/EPA* | 11.4 | 7.9 | 14.9 | 17.1 | 1.82E-09 | 0.333 | 0.0002 |
| ARA/DPA | 9.0 | 9.5 | 8.4 | 7.8 | 0.183 | 0.534 | 0.399 |
| ARA/DHA | 1.38 | 1.44 | 1.32 | 1.53 | 0.727 | 0.153 | 0.693 |
| EPA/DPA* | 1.07 | 1.39 | 0.76 | 0.50 | 8.16E-07 | 0.117 | 0.0007 |
| EPA/DHA* | 0.151 | 0.197 | 0.105 | 0.090 | 0.0003 | 0.396 | 0.0012 |
| ARA/DGLA | 6.58 | 5.87 | 7.28 | 7.17 | 0.077 | 0.866 | 0.039 |
| DHA/DPA* | 7.23 | 7.50 | 6.97 | 5.56 | 0.0007 | 0.284 | 0.186 |
| Ratios | Esterified Fatty Acid | ||||||
| (ARA + Adrenic acid)/(EPA+DPA+DHA) | 2.05 | 2.06 | 2.05 | 2.35 | 0.258 | 0.382 | 0.846 |
| LA/ALA | 204.2 | 197.2 | 211.2 | 232.9 | 0.427 | 0.205 | 0.794 |
| ARA/EPA* | 12.3 | 10.2 | 14.4 | 16.3 | 0.0002 | 0.325 | 0.0016 |
| ARA/DPA | 19.0 | 20.8 | 17.2 | 17.0 | 0.046 | 0.753 | 0.132 |
| ARA/DHA | 2.89 | 3.06 | 2.71 | 3.39 | 0.546 | 0.299 | 0.356 |
| EPA/DPA* | 2.11 | 2.72 | 1.50 | 1.33 | 0.003 | 0.87 | 0.006 |
| EPA/DHA* | 0.301 | 0.389 | 0.214 | 0.254 | 0.139 | 0.561 | 0.01 |
| ARA/DGLA | 3.96 | 3.70 | 4.22 | 4.05 | 0.757 | 0.409 | 0.323 |
| DHA/DPA* | 7.31 | 7.67 | 6.95 | 5.26 | 0.003 | 0.431 | 0.345 |
Note: Entries are ratios formed by concentration of the individual fatty acid. Concentration of each fatty acid was measured in the unit of µmol/L and reported as percentage of total plasma fatty acid. Statistical comparisons (among three groups and for each pair-wise comparison) were tested by age-adjusted ANOVA.
Statistics: * = p-value equal or less than 0.05 comparing among three groups.
LA: linoleic acid; ALA: alpha-linolenic acid; ARA: arachidonic acid; EPA: eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: Docosahexaenoic acid; DGLA: dihomo-γ-linolenic acid; IAN:Ill anorexia nervosa; RecAN: recovered anorexia nervosa.
Targeted Metabolomics - Oxylipin Measurement
A targeted metabolomics assay was carried out using plasma of 10 ill AN, 10 recovered AN, and 38 controls to identify sEH associated metabolomic biomarkers. The targeted oxylipin assay was designed based on the pathway of n-3 and n-6 polyunsaturated fatty acid precursors as described previously.[30, 31] The assay results in read-out of 80 individual oxylipins, of which 12 are direct substrates of sEH (epoxy fatty acids: EpOMEs from linoleic acid, EpETrEs from arachidonic acid etc.), 12 are catalyzed products of sEH (DiHOMEs from linoleic acid, DiHETrEs from arachidonic acid etc.). The remaining 9 oxylipins are metabolites associated with actions of a suite of enzymes, including those in the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) families (Supplement Table 2). In brief, these metabolite markers were prepared by solid phase extraction using Oasis HLB cartridges followed by reversed phase HPLC analysis utilizing C18 columns.[32] The analytes elute according to their polarity with the most polar analytes, prostaglandins and leukotrienes, eluting first followed by the hydroxy and epoxy fatty acids. The separated analytes were then quantified by tandem mass spectrometry in multiple-reaction monitoring mode utilizing negative electrospray ionization for the oxylipin profiling. Surrogate analytes and internal and external standards will be used to monitor extraction efficiency and ensure accurate quantitation of analytes. A random selection of 10% of the samples was replicated for validity testing. Quality control samples were analyzed at a minimum frequency of 10 hours to ensure stability of the analytical calibration throughout a given analysis.
Data Processing and Statistical Analysis
All polyunsaturated fatty acid (PUFA) and oxylipin data were assessed for normality with histograms. When non-normality was detected, values were log transformed prior to analysis. Significance was set at P<0.05; no adjustments were made for multiple testing due to the exploratory and hypothesis-driven nature of the analyses. PUFA and oxylipins with >30% missing observations were excluded, observations that were >4 SD from the mean were excluded. Change-detection plots were used to determine whether the observed signal was greater than that which could be expected by chance (noise). Absolute concentrations of oxylipin are presented as nmol/L; percentage of total PUFA in unit measure of umol/L is used to report PUFA concentration.
Tests of univariate association were conducted using Pearson's product moment correlation coefficient test and ANOVA using the statistical analysis tools in R (version 2.14.2).[33] Multivariate statistical analysis was employed to explore patterns in the overall PUFA (n-3 and n-6 classes) and oxylipin profiles of two group of AN subjects (Ill AN and recovered AN) and healthy controls; partial least-squares discriminant analysis (PLS-DA) was used as the classification method for modeling the discrimination between the groups using SIMCA-P software (version 12.0.1; Umetrics, Umea, Sweden) as previously described. [34] Briefly, data were mean centered and unit variance scaled. Unsupervised principal component analysis (PCA) was applied to all samples (Total n=96 for PUFA data and 58 for oxylipin data) and scores plots were visually inspected for trends or outliers in the data. Partial least-squares discriminant analysis (PLS-DA) was then used to explore variations in PUFA and oxylipin levels between different classes (IAN versus RecAN versus controls). A scores plot was created to visualize the PLS-DA model, and the corresponding loadings provided information on the contribution of metabolites to the separation of classes. The principal component that explains the best phenotype variance per PCA graph was tested by ANOVA to determine if this component is significantly different among and between groups.
Quantitative trait phenotype (subjects characteristics, Table 1) and oxylipins (Figure 2 and Supplement Table 2) were log-transformed for the standardized parametric inferential statistic tests. The PUFA and oxylipin markers were reformulated as ratios and used as markers of essential fatty acid balance (e.g.: n-6:n-3 fatty acid ratios) and sEH in vivo enzyme activity (metabolites/substrates), respectively. The correlation between the free form of fatty acids and matrix-bound total fatty acids was tested using Pearson's product moment correlation coefficient. PUFA and oxylipins were tested for association with the primary phenotypes of interest (AN risk, An recovery status) with ANOVA with age as covariate. Individual and ratio markers that were found to be significantly associated with AN risk were tested for association with eating disorder relevant temperament phenotypes using Pearson’s correlation coefficient.
Table 1.
Study subject characteristics.
| Characteristic | IAN (N=30) | RecAN (N=30) |
Controls (N=36) |
Statistics: IAN to Controls |
Statistics: RecAN to Controls |
Statistics: IAN to RecAN |
|---|---|---|---|---|---|---|
| Age, year | 22.2 ± 4.8 | 24.2 ± 5.7 | 19.92 ± 1.6 | 0.31 | 0.12 | 0.32 |
| Age Range | 16 – 34 | 18 – 38 | 18 – 26 | NA | NA | NA |
| BMI*, kg/m2 | 14.3 ± 1.4 | 21.0 ± 1.763 | 20.9 ± 0.9 | <2e-16 | 0.0001 | <2e-16 |
| Lowest reported BMI*, kg/m2 | 12.3 ± 1.6 | 14 ± 1.8 | 19.9 ± 0.9 | < 2e-16 | < 2e-16 | 0.001 |
| BDI* | 23.0 ± 13.2 | 12.5 ± 9.4 | 2.1 ± 2.8 | < 2e-16 | < 2e-16 | 6.4e-07 |
| STAI State Anxiety* | 54.6 ± 14.1 | 43.5 ± 12.6 | 25.5 ± 6.9 | <2e-16 | <2e-16 | 3.6e-06 |
| STAI Trait Anxiety* | 56.8 ± 14.1 | 47.6 ± 13.7 | 27.4 ± 6.6 | <2e-16 | <2e-16 | 3.1e-08 |
| TCI Novelty Seeking* | 12.3 ± 3.8 | 17.2 ± 6.2 | 20.3 ± 4.9 | < 2.2e-16 | 0.086 | 0.0008 |
| TCI Harm Avoidance* | 21.7 ± 6.9 | 21.1 ± 7.9 | 9.4 ± 4.9 | < 2.2e-16 | < 2.2e-16 | 0.005 |
| Total Cholesterol*, mg/dL | 162.6 ± 37.6 | 155.7 ± 31.9 | 154.3 ± 31.8 | 0.016 | 0.8 | 0.123 |
| HDL*, mg/dL | 55.0 ± 20.6 | 54.9 ± 13.8 | 51.6 ± 15.8 | 0.0037 | 0.309 | 0.59 |
| LDL, mg/dL | 88.4 ± 25.9 | 83.2 ± 22.8 | 83.8 ± 23.9 | 0.41 | 0.335 | 0.195 |
| Triglyceride, mg/dL | 81.7 ± 52.9 | 87.6 ± 43.4 | 94.2 ± 32.2 | 0.92 | 0.78 | 0.787 |
Note: Entries are of the form mean +/− SD. Statistical comparisons (among three groups and for each pair-wise comparison) were tested by age-adjusted ANOVA.
Statistics: * = p-value (nominal p-value) equal or less than 0.05 comparing among three groups. All variables except for age, BDI, TCI Novelty Seeking and TCI Harm Avoidance were log-transformed for inferential statistical analyses.
BMI: body mass index; BDI: Beck Depression Inventory; STAI: State-Trait Anxiety Inventory; TCI: Temperament and Character Inventory; HDL: High-density lipoprotein; LDL: Low-density lipoprotein. IAN: Ill anorexia nervosa with BMI<=17.5; RecAN: recovered anorexia nervosa with BMI >=18 for longer than one year.
Figure 2.
RESULTS
Participants and Phenotypes
Demographic and phenotypic characteristics of the 60 AN women (all restricting anorexia nervosa consisting of 30 ill AN [IAN], 30 recovered AN [RecAN]), and 36 healthy control women are summarized in Table 1. Body mass index (BMI) was significantly different between AN and controls (p-values: <0.00001) and between IAN and RecAN (p-value <0.00001). Beck Depression Inventory (BDI), state anxiety and trait anxiety scores from the State-Trait Anxiety Inventory (STAI), and “harm avoidance” and “novelty seeking” from the Temperament and Character Inventory (TCI) were also significantly different between AN and controls (p-values: < 0.0001, Table 1) and between IAN and RecAN (p-values: 0.005 to <0.0001, Table 1). Subjects with AN were significantly more depressed, having higher anxiety, higher harm avoidance, and lower novelty seeking scores than controls. Total cholesterol and HDL levels were significantly higher in IAN compared to controls (p-values= 0.016 and 0.004), consistent with the literature.[35, 36]
Non-esterified and Esterified Polyunsaturated Fatty Acids
Plasma specimens of all subjects were used to measure concentrations of non-esterified fatty acids (NEFA) and esterified fatty acids (EFA - fatty acids covalently bound to complex lipids) in two major structural isomer categories, omega 6 (n-6) and omega 3 (n-3). Of the eight n-6 fatty acids measured (LA, GLA, EDA, DGLA, ARA, 13/16-DA, ADA and OBA), four (LA, GLA, ADA and OBA) were significantly correlated between the NEFA and EFA (r=0.23 – 0.77, p-values: 0.02 – 7.7E-10). Out of the six n-3 fatty acids measured (ALA, SDA, ETE, EPA, DPA and DHA), five (ALA, SDA, EPA, DPA, and DHA) were significantly correlated between the NEFA and EFA (r=0.32 – 0.63, p-values: 0.001 – 2.2E-16) (Supplement Table 1).
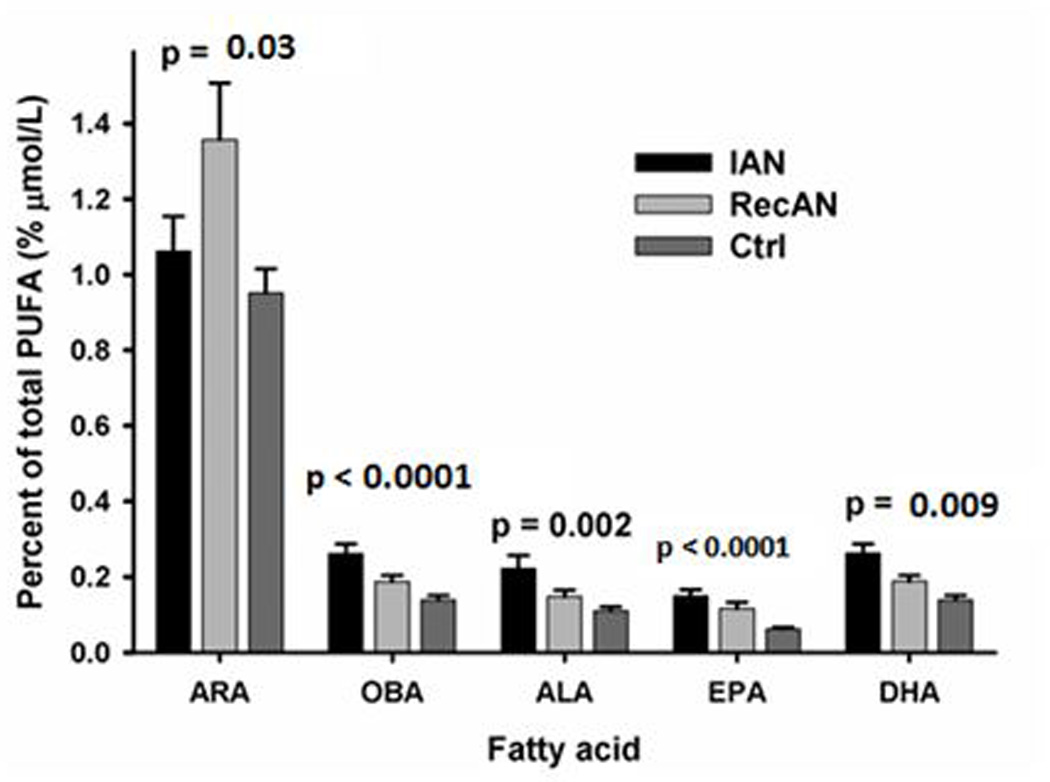
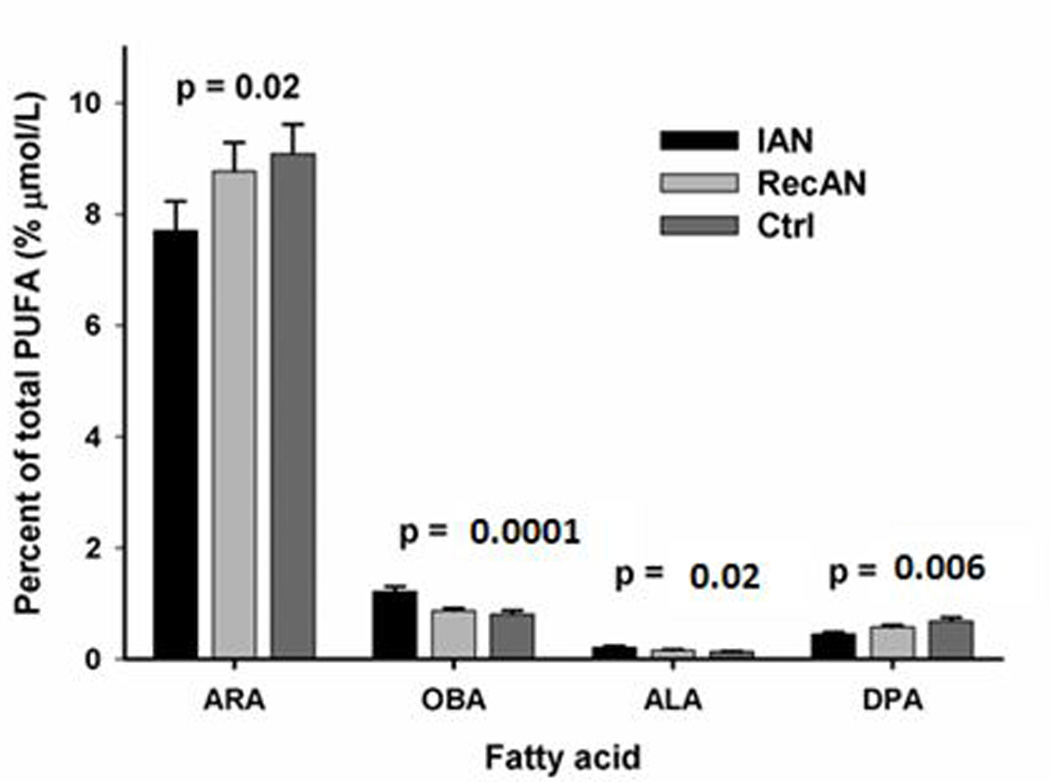
The concentrations of three n-6 (DGLA, ARA and OBA) and four n-3 (ALA, SDA, EPA and DHA) NEFAs were significantly different among three groups (IAN, RAN, Controls) (Figure 1A and Supplement Table 3). NEFA forms of GLA, DGLA, OBA, ALA, SDA, and EPA were significantly different between IAN and controls (p-values: <0.0001 to 0.03, Supplement Table 3). OBA and SDA concentrations were significantly higher in IAN compared to RecAN (p-values: 0.042 and 0.006, Supplement Table 3). OBA and SDA were correlated with BMI only in the AN group. In EFA form, only two n-6 (ARA and OBA) and two n-3 (ALA and DPA) EFAs were significantly different among IAN, RecAN, and controls (Figure 1B). EFA OBA concentration was significantly higher in IAN than RecAN (p-value= 0.002) whereas DPA was higher in RecAN than IAN (p-value= 0.009)(data not shown). EFA OBA and DPA were associated with BMI only in subjects with AN but not in the controls.
Figure 1.
Fatty Acid Ratio Associations
Nine PUFA ratio markers were formulated to infer the balance between n-6 and n-3 PUFAs and enzymatic activity in PUFA synthesis. Among NEFAs, LA:ALA and ARA:EPA ratios were lowest in IAN, followed by RecAN, and highest in controls (three-group comparison p-values: 0.0016 and 0.001, pair-wise comparison p-values please see Table 2). In comparison, EPA:DPA, EPA:DHA and DHA:DPA were significantly higher in both IAN and RecAN compared to controls, with IAN showing the highest ratios and controls the lowest (three-group comparison p-values: <0.001, pair-wise comparison p-values please see Table 2). None of the IAN-associated ratios were significantly different in RecAN compared to controls except for LA:ALA (p-value= 0.018, Table 2 Upper). Similar patterns of association were identified in EFA ratios, except that none of the EFA PUFA ratios was significantly different between RecAN and controls (Table 2 Lower).
Oxylipin Quantification and Association
Oxylipins are produced from PUFA precursors and are derived from the actions of a suite of enzymes, including those in the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) families, as well as by non-enzymatic oxidation.[30] Oxylipins were measured in the plasma of all subjects. The descriptive statistics of all oxylipins assayed, which consist of 24 sEH-associated oxylipins and their ratio markers (n=12) and 9 non-CYP450 pathway oxylipins, are presented in Supplement Table 2. To assess data validity, we confirmed that ARA oxylipins 14.15.EpETrE and 14.15.DiHETr fall within the range that has been validated as potential clinical trial biomarker.[37]
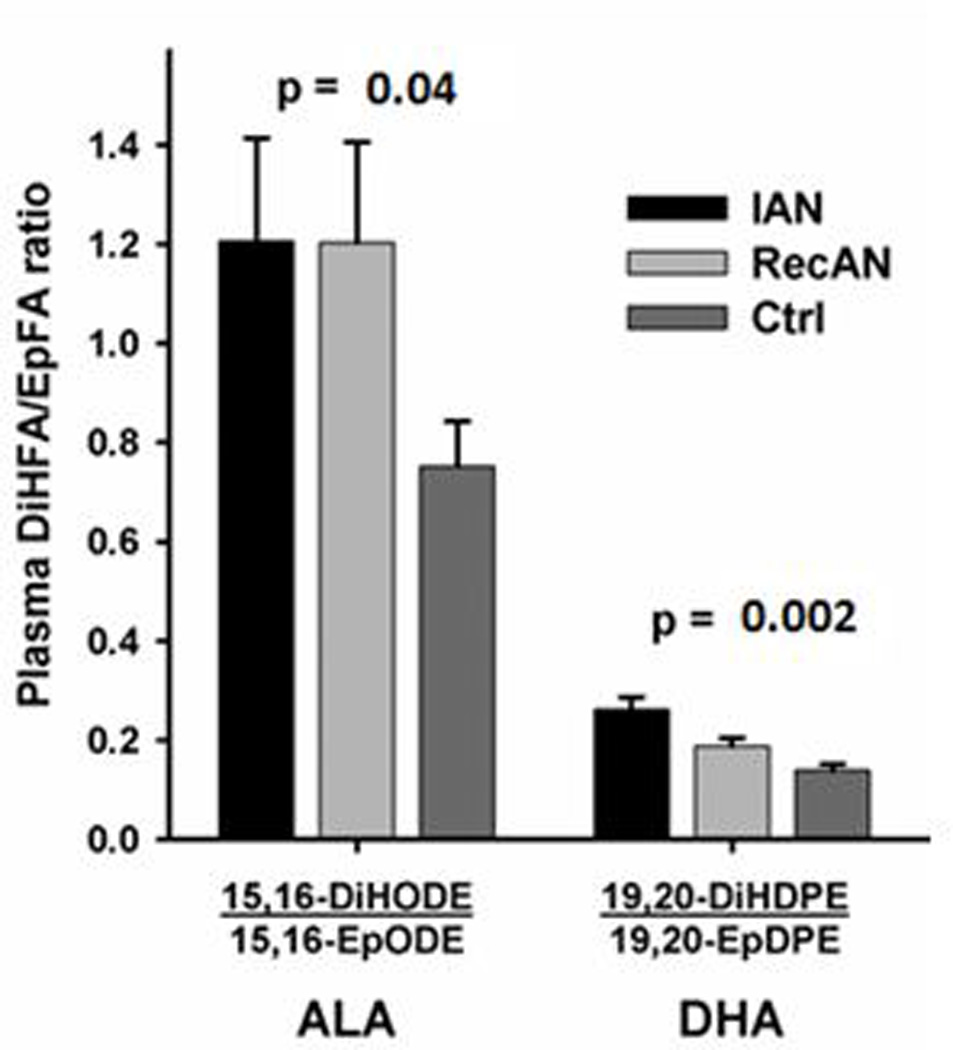
Focusing in on oxylipins that are sEH substrates and catalyzed products, significant differences between ANs and controls were observed both in individual oxylipins and ratios of oxylipins (metabolites/substrates) which mark in vivo sEH efficiency and activity.[38] AN-associated (IAN compared to controls) oxylipins are DHA metabolites 10.11.EpDPE (p-value = 0.049), 13.14.EpDPE (p-value = 0.039) and ARA’s metabolite 9.10.EpOME (p-value = 0.05)(Supplement Table 2). The oxylipin ratios from n-3 (ALA and DHA) and n-6 (ARA and LA) PUFAs were significantly different among three groups and were higher in IAN compared to controls (Figure 2 and Supplement Table 2), suggesting a higher in vivo sEH activity, concentration, or efficiency in AN.
To compare and contrast the sEH- associated oxylipins (Cyp450 pathway) with those that are modulated by other major enzymatic pathways, we examined the levels of oxylipins from the COX pathway (TXB2, PGE2, PGD2) and the LOX pathways (LTB4 and various HETEs), and found no evidence of differences between cases and controls (Supplement Table 2).
Multivariate Analysis
Multivariate statistical procedures were employed to compare total PUFA (n-3 and n-6 markers combined) and oxylipin profiles between IAN, RecAN and controls. The most informative two components of the PCA accounted for 31% and 27% of the variation in the PUFA data and oxylipin data, respectively (Figures 3A and 3B). Visual inspection of the PUFA scores plot indicates separation of IAN subjects from controls; whereas the recovered AN appeared to overlap equally well with both the IAN and controls (Figure 3A). The top 10 predicted lipidomic (PUFA) biomarkers are: non-esterified (NE) EPA:DPA, NE ARA:EPA, NE SDA (18:4n3), NE EPA:DHA, NE OBA (22:5n6), NE EPA (20:5n3), esterified OBA (22:5n6), esterified ARA:EPA, NE ALA (18:3n3), and NE LA:ALA.
Figure 3.
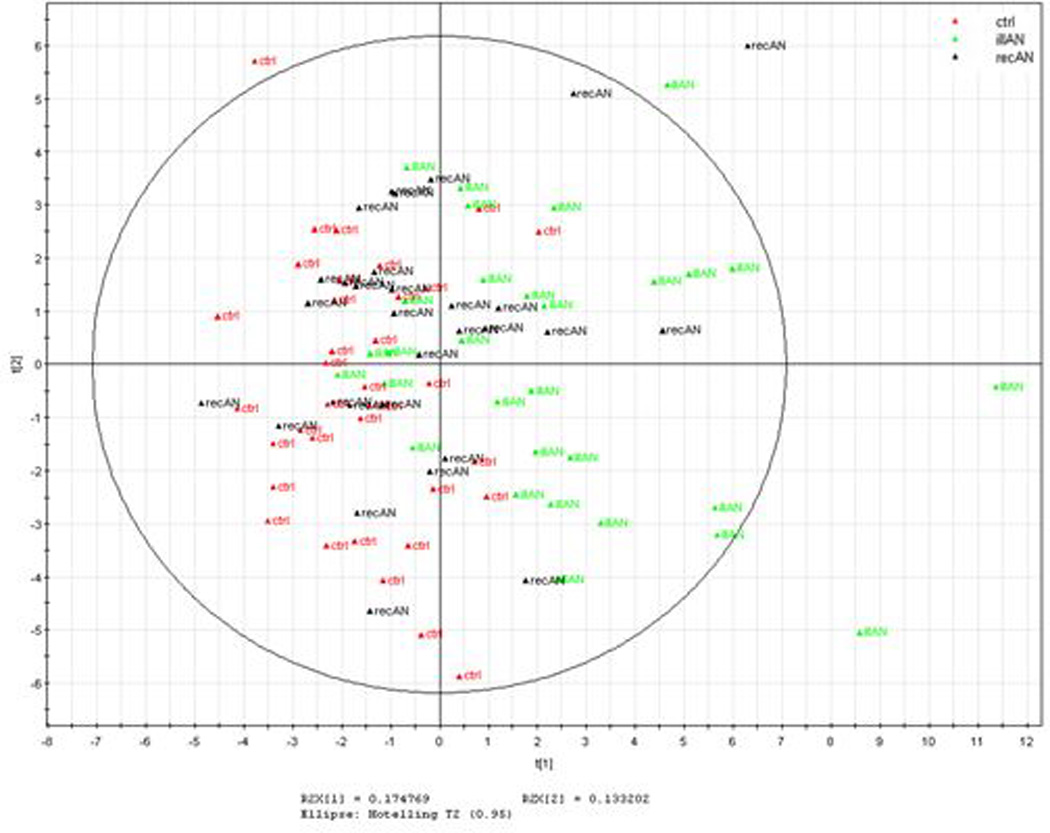
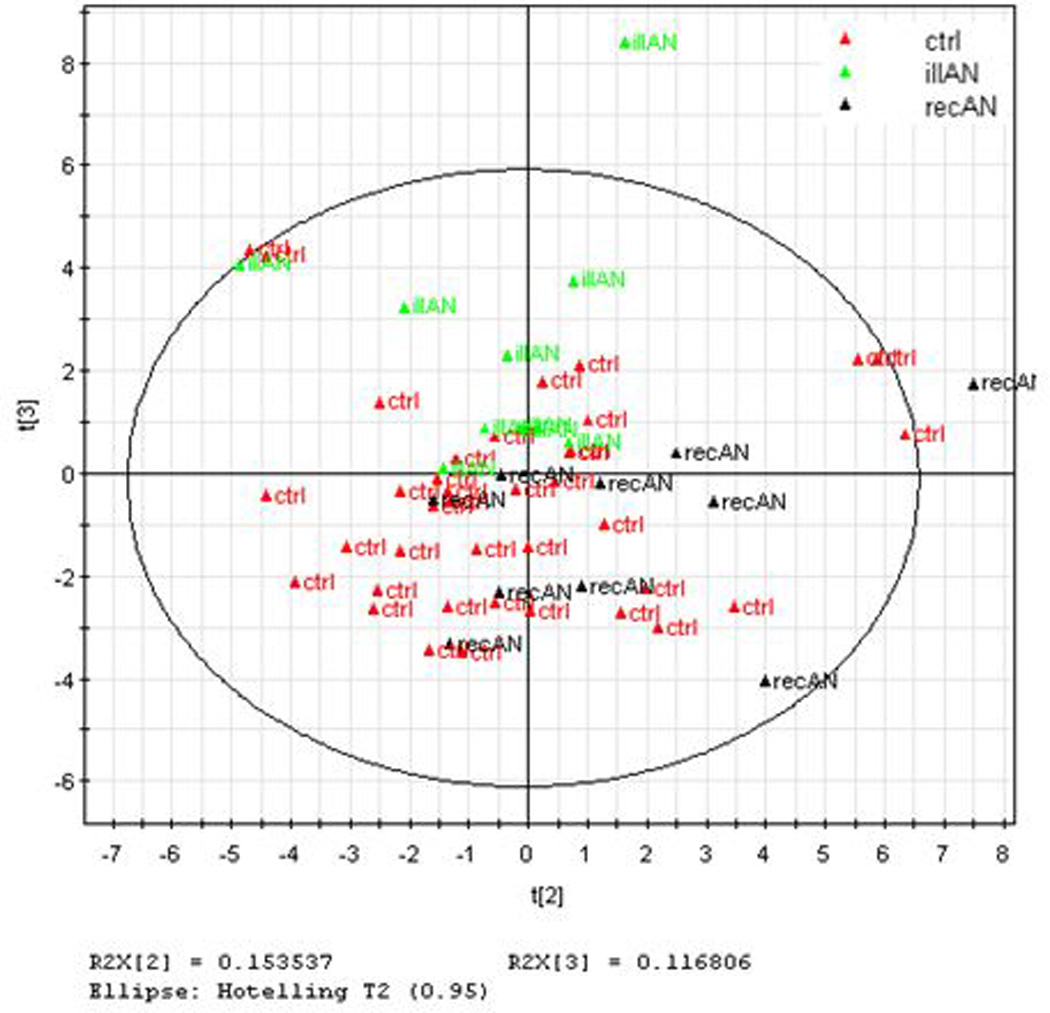
The oxylipin PCA plot showed IAN subjects separate clearly from controls, whereas RecAN showed compelling overlap with controls and limited overlap with IAN (Figure 3B). The top 10 predicted oxylipin biomarkers are: 19(20)-DiHDPE:EpDPE, 19(20)-DiHDPE, 10(11)-DiHDPE:EpDPE, 12(13)-DiHOME, 15(16)-DiHODE, 8(9)-EpETrE, 9(10)-DiHOME, 14(15)-DiHETrE:EpETrE, 5(6)-DiHETrE:EpETrE and 13(14)-EpDPE. The PCA scores plots illustrated a closer clustering between RecAN with controls in oxylipins than in PUFA profiles.
PUFA and oxylipin marker association with anorexia nervosa temperament and lipid phenotypes
PUFA ratios and oxylipin markers that were significantly associated with AN risk, were tested for associations with AN-specific phenotypes: BMI, depression score, anxiety, novelty seeking and harm avoidance scores. Both non-esterified (NE) and esterified forms of ARA:EPA ratios were significantly correlated with state anxiety (r= −0.363 and −0.378, p-values=0.029 and 0.022, respectively for NEFA and EFA) and trait anxiety (r= −0.358 and −0.525, p-values=0.032 and 0.001, respectively) in controls but not in ANs. In ANs, the NE ARA:EPA and LA:ALA were associated with state anxiety (r= −0.261 and −0.268, p-values=0.04 and 0.04, respectively). While ARA:EPA ratio showed no association with BMI in controls, both NE and esterified ARA:EPA significantly correlated with BMI in AN (r= 0.415 and 0.371, p-values=0.0009 and 0.0035, respectively).
NEFA and esterified EPA:DPA and EPA:DHA ratios significantly correlated with BMI in AN but not in controls (For EPA:DPA: r= −0.392 and −0.293, p-values=0.002 and 0.022. For EPA:DHA: r= −0.433 and −0.318, p-values=0.0005 and 0.013). NE form of the DHA:DPA ratio was associated with state anxiety and trait anxiety in controls only (r= 0.393 and 0.404, p-values=0.017 and 0.014).
DISCUSSION
Twin and discordant sister pair studies found evidence of not only individual environmental and genetic factors[39], but also gene-environment interactions[40] contributing to AN risk. Dieting is a common practice in adolescents and dietary behaviors always precede the onset of AN. However, only a very small fraction of dieters become sick with AN,[41, 42] supporting the heritability study finding that genetic predisposition is necessary for AN risk (first hit). Additional insults from non-genetic factors are also required for the disease onset (second hit).
AN differs from other eating disorders by a homogeneous clinical presentation of restricting food intake. The determination to maintain low body weight and reported food aversion are attributed to high levels of chronic anxiety, which does not seem to improve with weight restoration.[43] Anxiety and extreme eating behavior are thought to be affected by a combination of genetic, biological, psychological, and social factors.
Soluble epoxide hydrolase is the protein product of EPHX2. It is known for a key role in fat metabolism by modulation of bioactive lipids.[44] sEH catalyzes multiple epoxy-fatty acids and transforms anti-inflammatory epoxides to pro-inflammatory metabolites.[21] The transformative role sEH plays in the inflammation cascade through oxylipin modulation may therefore contribute to the “aversive sensation” associated with consumption of fat-rich foods reported by AN. In parallel, restrictive eating from dieting that precede the AN diagnosis, could lead to depletion of essential nutrition including PUFA that the brain needs for neuro-circuitry formation, leading to “switch-off” of protective neuronal- and biochemical-based mechanisms in those genetically susceptible.[45] Moreover, depletion of PUFA through insufficient dietary intake could also adversely affect metabolic regulatory function of sEH by limiting the substrate access, or skewing the optimum n6:n3 substrate ratio. To better understand how genetic and non-genetic risk factors work together to influence risk for AN, here we utilize metabolomics data that infer the in vivo activity of sEH, fatty acids markers that reflect relative nutritional status, and two groups of AN and controls to explore the evidence of gene-by-environment interaction and to clarify the roles dietary PUFA and sEH have on AN.
PUFA associating with AN status, BMI, and temperament traits
Fat-rich foods serve as one of the primary sources of two major classes of essential fatty acids, n-3 and n-6 polyunsaturated fatty acids. The alpha-linolenic acid (ALA) and linoleic acid (LA) are parental compounds for the n-3 and n-6 fatty acid metabolic families. Several groups have previously reported decreased dietary consumption of PUFA in AN,[46] and association of AN with abnormal PUFA levels and desaturase activity.[13, 16, 47–51] Data from a small number of pilot treatment studies indicate that n-3 supplement may have a beneficial effect on AN symptoms.[52, 53]
In our samples, the AN show elevated concentration in plasma n-3 fatty acids (ALA, SDA, EPA, DHA) compared to controls (Figure 1A and Supplement Table 3). The step-wise decrease of n-3 EsFA parental compound (ALA) from IAN to RecAN to controls coupled to no difference in n-6 EsFA parental compound (LA) may be explained in part by patients’ general preference of foods rich in n-3 PUFA such as leafy greens and fish. In humans, ALA and LA are metabolized by desaturase enzymes to long-chain unsaturated fatty acids through a series of elongation and desaturation steps.[54] The n-3 and n-6 fatty acids compete for the desaturation and elongation enzymes, and the Delta 5 and Delta 6 desaturases tend to “favor“ the n-3 fatty acids.[55] Therefore, any consideration given to health effects of one fatty acid class should take into account the presence and relative abundance of the other families of fatty acids, as well as the metabolic relations between them. The AN who are recovered may get there by increasing meat-based foods thereby improving the n6:n3 ratio and ALA concentration.
The desaturated and elongated versions of long-chain n-3 PUFAs, EPA and DHA, were also elevated in AN compared to controls (Figure 1A and Supplement Table 3). Elevated EPA:DPA and DHA:DPA ratios (Table 2) in AN suggest altered efficiency in elongation and desaturase activities in AN, consistent with Holman et al’s 1995 proposal of a deficiency in the elongation step and delta-5 desaturation step of EFA conversion in AN.[16] Other possible factors that contribute to the long-chain n-3 PUFA ratio differences include an increased precursor ALA in AN, and differential rate and expenditure of EPA and DHA during the enzymatic catalysis process. Together, the striking elevation of n-3 PUFAs reveal that contrary to expectation, enrichment of EPA and DHA in patients with AN should not be assumed to be protective as reported for other neuropsychiatric illness such as Alzheimer’s and major depression.[56, 57]
The concentration of esterified arachidonic acid (ARA) was significantly different among three groups (p=0.02, Figure 1B) and was shown to be lowest in IAN compared to controls; whereas the NE form of ARA was also significantly different among three groups (p=0.03, Figure 1A) but has the highest level observed in the RecAN group (Figure 1A). This striking pattern difference between esterified ARA and NE ARA might be attributed to the preferential agonist-induced ARA release mediated by cytosolic phospholipase A2 (cPLA2).[58] While NEFA may reflect acute changes in the metabolic profile, EFA provide a less dynamic, more chronic read-out of metabolic state. Our data show that NEFA and EFA species of each major FA were significantly correlated except for ARA (Supplement Table 1). The differential profile observed in NE and esterified forms of ARA suggests that the preferential substrate selectivity of the cPLA2 [59] may influence the variability of circulating NE ARA better than its esterified form.
The findings that both esterified ARA:EPA and LA:ALA ratios were lowest in IAN, followed by RecAN and were highest in controls (Table 2) highlight the imbalance of n-6:n-3 ratio in AN. A recent study in healthy adults showed that fish oil intervention resulted in increased appetite.[60] Given the observed significant increase of n-3 PUFA concentrations in AN, potential beneficial effects of fish oil to improve appetite for AN needs to be rigorously studied before conclusion can be drawn. While previous AN studies have already shown correlations between n-3 PUFA and body weight[47, 48, 50] or depression[46, 47, 49], no report of direct association between PUFA and key temperament traits in AN was available. Temperament is the biologically based characteristic that contributes to major psychological processes through the expression of consistent patterns of feeling, thinking, and behavior. Specific temperament traits such as increased anxiety[61, 62], increased harm avoidance[63], and decreased novelty seeking[43, 62] are tightly linked to AN (Table 1).[43] PUFA ratio markers were found to be differentially associated with a number of temperament phenotypes such as state anxiety and trait anxiety in AN compared to controls. Furthermore, a number of ratio markers show striking associations with BMI in AN only but not in controls, suggesting the dysregulated PUFA markers to be specific to AN.
Multivariate PCA analysis of all PUFA markers revealed distinct patterns in IAN, RecAN, and controls. Consistent with the individual marker results, IAN and controls are clustered with clear separation, whereas RecAN tend to cluster with both IAN and controls, demonstrating the process of PUFA profile normalization during the recovery phase of AN (Figure 3A) that may be attributed to dietary improvement, increased body fat, or changes in PUFA elongase and desaturase enzymes and metabolic rate. Taken together, the PUFAs may not only be useful markers of disease itself but may also contribute to temperament traits that drive persistent restrictive eating and prognosis.
Oxylipin markers reveal differential sEH activity in AN and controls
Among all the oxylipin markers examined, sEH-product to substrate ratios (diol:epoxide ratios) derived from the ARA, LA, ALA and DHA PUFA were all associated with AN status, but the most striking ratio predictors were those of ALA and DHA (Figure 2 and Supplement Table 2). These ratios are proxy markers of sEH activity.[64] The ratios of most PUFA metabolites are significantly different among groups and are higher in AN (Figure 2 and Supplement Table 2), which suggests elevated in vivo sEH activity in AN patients. These biomarkers validate the functional significance of the EPHX2 gene with AN and demonstrate that the epoxy-fatty acid-modulatory action of sEH may be a key mechanism by which EPHX2 affects AN risk. [17]
ARA is a substrate for three major enzymatic routes of metabolism by cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes. The AN-associated oxylipin markers identified in this study were ratio markers that serve as surrogate for sEH activity within the cytochrome P450 pathway. Given the possibility that oxylipin-disease associations may also be attributed to the upstream fatty acids, we analyzed oxylipin markers of ARA that are derivatives of cyclooxygenase and lipoxygenase actions as “controls” to assess the specificity of EPHX2/cytochrome P450 pathway in AN biology. Across all cyclooxygenase and lipoxygenase associated oxylipins measured, including thromboxane, prostaglandins and leukotriene, none were significantly different between AN and controls (Supplement Table 2). Taken together, our data indicate that sEH activity is elevated in AN, and may increase disease risk through sEH-modulated mechanism of transforming anti-inflammatory epoxy to unstable diol forms to induce cellular inflammation.
The inflammation link to AN observed by elevated sEH action and dysregulated oxylipin markers was re-assessed using multivariate analysis. In principle, if the inflammatory state is associated with or precedes the disease onset, the recovery status in subjects with AN should also link to normalization of the dysregulated biomarkers. Indeed, the oxylipin PCA profile (Figure 3B) showed a differential clustering of IAN and controls while RecAN appears to be overlapping with controls and not with IAN. By contrast, RecAN show equal overlap with both IAN and controls in the PUFA PCA model (Figure 3A), indicating a “slower” normalization of the PUFA profile in the RecAN group. Taken together, the interconnected lipidomic and metabolomic profiles reveal the biology of evolving disease state in AN.
We identified that sEH activity within the cytochrome P450 pathway plays a key role both in AN risk and recovery status. In the presence of increased n-3 PUFA in AN, the metabolite/substrate ratios derived from both n-3 and n-6 PUFA oxylipins indicate that in vivo sEH activity is an independent risk factor for AN risk and prognosis. The comparison of total lipidomic and oxylipin profiles further suggests that the resolution of presumed sEH-associated inflammation is apparent in AN who are recovered, even if the PUFA profile has not normalized completely. This process could occur in several ways including alterations of sEH, P450 pathway produced fatty acid epoxides, PUFA from membranes, or dysregulation from other metabolic pathways.
Prior EPHX2 variant associations and implications
EPHX2 variants found to be associated with AN risk were in the 3’ region of the gene as the 5’ region of EPHX2 was not sequenced due to limitations in the method.[17] Some of the AN-associated SNPs were also associated with increased depression and anxiety in AN, and decreased longitudinal increase in BMI over cholesterol trajectories in controls.[17] However, common 3’ EPHX2 SNPs associated with AN (rs2291635, rs1042064 and rs4149259) are found in variable linkage disequilibrium with two non-synonymous EPHX2 SNPs that have been the subject of numerous enzyme activity and cardiovascular association studies (rs41507953 [Lys55Arg] and rs751141 [Arg287Gln]) with pairwise r2 of 0.01 and 1, 0.21 and 0.33, and 0.41 and 0.02, respectively, in HapMap CEU (Supplement Figures 1A and 1B).
In the first in vitro EPHX2 expression study, the Arg 55 and Gln 287 alleles were found to be associated with elevated and reduced hydrolase activity, respectively.[65] Based on the reported under-representation of the rs2291635 minor allele in AN[17] and the pairwise r2 of 1 between rs2291635 and rs751141 from the HapMap CEU sample, we infer that the Gln 287 allele with reduced hydrolase activity is under-represented in AN cases, or that the Arg 287 allele with reference hydrolase activity is over-represented in AN cases, which is consistent with our finding that in vivo sEH activity is elevated in AN.
EPHX2 SNPs have been found to associate with multiple metabolic or cardiovascular phenotypes. rs41507953 (Lys55Arg) has been associated with elevated in vivo sEH and coronary heart disease[66] [66], vasoconstriction[67], SBP and male ischemic stroke[68] in European ancestry populations. rs751141 (Arg287Gln) has been associated with coronary artery calcification[69], atherosclerosis[70], and vasodilation in response to endothelial regulators of blood pressure in African American ancestry individuals.[67] rs751141 was also associated with reduced insulin resistance in Japanese [71] and protection against kidney failure in Koreans.[38] In European ancestry populations, rs751141 has been found to associate with coronary artery calcified plaque[72], stroke[73], blood pressure in men[68], atrial fibrillation recurrence[74], and insulin sensitivity.[75] The 3’ variants that are associated with AN have been less studied although associations were found between rs2291635 and stroke[73] whereas rs1042032 was associated with blood pressure with gender-interaction effect (significantly increased in males, non-significantly decreased in females)[68], kidney failure[38], and expression.[76] Furthermore, Wei et al in the CARDIA cohort identified that a significantly associated haplotype in African Americans was tagged by a different SNP than the haplotype-tagging SNP in European ancestry individuals.[70]
Together, these studies point to effects of functional EPHX2 variants on sEH activity and of resulting metabolites’ biological functions to be under the influence of gender and race, however, little is known about the direct influence of gender and race on the oxylipin biomarkers. To bridge the gap in this knowledge, our study took the first step to comprehensively characterize the metabolomic profile (oxylipin) of the EPHX2 pathway in European ancestry female psychiatric patients and population-based samples.
Unique strength of this study
Twin and discordant sister pairs studies found evidence of not only individual environmental and genetic factors[39] contributing to AN but also gene-environment interactions.[77] Taking the PUFA and oxylipin data together, we demonstrated the contributions from gene, diet, and their interaction to AN: while the oxylipin biomarkers implicate elevated sEH activity as a risk factor for AN as hypothesized through an established genetic susceptibility[17], the dysregulated EsFA and PUFAs observed in both ill and recovered AN suggest the adverse effect of poor diet on AN.[78] We provide evidence that the activity of the sEH, the substrates of the sEH (epoxy fatty acids), and the metabolomic derivatives (oxylipins) and their collective readout reflect changes in disease state and risk of AN. This finding has unraveled evidence of one specific gene-environment interaction and highlighted the importance of fatty acid lipid metabolism in AN risk and prognosis. From the clinical point of view, this study provides a comprehensive assessment of dietary fatty acid profile in which confirms the importance of malnutrition in disease risk. Taken together, pathway-specific biological targets, both nutritional and pharmaceutical, may be investigated further to develop more effective prevention and treatment strategies.
Limitation of this study
Our study reveals the contributing roles a deregulated lipidomic pathway and a functional gene have on AN. While the differences in PUFA profiles in AN and controls may be partially explained by differences in dietary behavior, in the absence of food intake history we could only use EsFA levels as proxy markers to infer the influence of the diet. We were also unable to directly assess the impact the dysregulated PUFA and oxylipin have on food aversion and anxiety in this cross-sectional study. We will address these limitations by a future longitudinal study and a blinded randomized placebo-controlled intervention trial in order to further identify the specific dietary or nutritional factors that may be targeted to reverse the metabolic dysregulation in AN.
Nutraceutical strategy implication
The primary treatment goal for AN covers three broad areas: restoration to a healthy weight, treatment of psychological issues related to the eating disorder, and reducing behaviors or thoughts that can lead to continued pathological eating and relapse. The current treatment strategies [79, 80] unfortunately have been proven to be ineffective and the death rate and relapse rate remain high.[2] Based on our finding, alternative or adjunct treatments may be developed through a rigorous randomized controlled trial targeting lipid metabolism and PUFA supplementation. If a specific formulation of n-6:n-3 ratio supplement can be shown to decrease anxiety and food aversion, improve eating behavior while restoring weight, such supplementation may lead to effective intervention for at risk individuals or adjunct treatment for patients. This study identified one of EPHX2’s biological mechanism in AN and revealed that in vivo sEH activity, dietary PUFAs, and cytochrome P450 metabolites all contribute to risk. We identified unique lipidomic and metabolomic signatures that may serve as AN prognostic biomarkers to aid in the development of predictive diagnostics, preventative strategies and nutraceutical or pharmaceutical interventions and treatments.
Supplementary Material
Acknowledgements
This work was supported, in part, by National Institutes of Health (NIH/NIDDK) Career Development Award Grant (1K01DK087813-01A1 - Shih); NIH West Coast Metabolomics Center Pilot Grant (Shih); Price Foundation of Geneva, Switzerland; NIH U24 DK097154/DK/NIDDK; NIEHS R01 ES002710 and NIEHS/Superfund Research Program P42 ES004699. We also wish to acknowledge the special help with polyunsaturated fatty acid data management provided by Yan Wang, and the Price Foundation study collaborators for providing the clinical data and biospecimens for this study.
Footnotes
Disclosures of Conflict of Interest: None of the authors has any conflict of interest to report.
REFERENCES
- 1.Smink FR, van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Current psychiatry reports. 2012;14:406–414. doi: 10.1007/s11920-012-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Archives of general psychiatry. 2011;68:724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry. 2009;194:10–17. doi: 10.1192/bjp.bp.108.054742. [DOI] [PubMed] [Google Scholar]
- 4.Thornton LM, Mazzeo SE, Bulik CM. The heritability of eating disorders: methods and current findings. Current topics in behavioral neurosciences. 2011;6:141–156. doi: 10.1007/7854_2010_91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatry. 1994;36:696–702. doi: 10.1016/0006-3223(94)91179-7. [DOI] [PubMed] [Google Scholar]
- 6.Drewnowski A, Pierce B, Halmi KA. Fat aversion in eating disorders. Appetite. 1988;10:119–131. doi: 10.1016/0195-6663(88)90063-3. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Prieto I, Bolanos-Rios P, Jauregui-Lobera I. Diet choice in weight-restored patients with eating disorders; progressive autonomy process by nutritional education. Nutricion hospitalaria : organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2013;28:1725–1731. doi: 10.3305/nh.2013.28.5.6725. [DOI] [PubMed] [Google Scholar]
- 8.Jauregui Lobera I, Bolanos Rios P. Choice of diet in patients with anorexia nervosa. Nutricion hospitalaria : organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2009;24:682–687. [PubMed] [Google Scholar]
- 9.Steinglass JE, Sysko R, Mayer L, Berner LA, Schebendach J, Wang Y, Chen H, Albano AM, Simpson HB, Walsh BT. Pre-meal anxiety and food intake in anorexia nervosa. Appetite. 2010;55:214–218. doi: 10.1016/j.appet.2010.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 11.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutrition reviews. 2006;64:S24–S33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72-91. [DOI] [PubMed] [Google Scholar]
- 12.Pelliccia F, Marazzi G, Greco C, Franzoni F, Speziale G, Gaudio C. Current evidence and future perspectives on n-3 PUFAs. International journal of cardiology. 2013;170:S3–S7. doi: 10.1016/j.ijcard.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Zak A, Vecka M, Tvrzicka E, Hruby M, Novak F, Papezova H, Lubanda H, Vesela L, Stankova B. Composition of plasma fatty acids and non-cholesterol sterols in anorexia nervosa. Physiol Res. 2005;54:443–451. [PubMed] [Google Scholar]
- 14.Squali Houssaini FZ, Foulon T, Payen N, Iraqi MR, Arnaud J, Groslambert P. Plasma fatty acid status in Moroccan children: increased lipid peroxidation and impaired polyunsaturated fatty acid metabolism in protein-calorie malnutrition. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2001;55:155–162. doi: 10.1016/s0753-3322(01)00041-5. [DOI] [PubMed] [Google Scholar]
- 15.Holman RT, Johnson SB, Mercuri O, Itarte HJ, Rodrigo MA, De Tomas ME. Essential fatty acid deficiency in malnourished children. Am J Clin Nutr. 1981;34:1534–1539. doi: 10.1093/ajcn/34.8.1534. [DOI] [PubMed] [Google Scholar]
- 16.Holman RT, Adams CE, Nelson RA, Grater SJ, Jaskiewicz JA, Johnson SB, Erdman JW., Jr Patients with anorexia nervosa demonstrate deficiencies of selected essential fatty acids, compensatory changes in nonessential fatty acids and decreased fluidity of plasma lipids. J Nutr. 1995;125:901–907. doi: 10.1093/jn/125.4.901. [DOI] [PubMed] [Google Scholar]
- 17.Scott-Van Zeeland AA, Bloss CS, Tewhey R, Bansal V, Torkamani A, Libiger O, Duvvuri V, Wineinger N, Galvez L, Darst BF, et al. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Molecular psychiatry. 2014;19:724–732. doi: 10.1038/mp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Progress in lipid research. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Koeners MP, Wesseling S, Ulu A, Sepulveda RL, Morisseau C, Braam B, Hammock BD, Joles JA. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2011;300:E691–E698. doi: 10.1152/ajpendo.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morisseau C, Sahdeo S, Cortopassi G, Hammock BD. Development of an HTS assay for EPHX2 phosphatase activity and screening of nontargeted libraries. Anal Biochem. 2013;434:105–111. doi: 10.1016/j.ab.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sura P, Sura R, Enayetallah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2008;56:551–559. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, Murphy SB, Friedman DB, Hammock BB, Vaughan DE. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity (Silver Spring, Md) 2010;18:489–498. doi: 10.1038/oby.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163:646–661. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Pinheiro AP, Bulik CM, Thornton LM, Sullivan PF, Root TL, Bloss CS, Berrettini WH, Schork NJ, Kaye WH, Bergen AW, et al. Association study of 182 candidate genes in anorexia nervosa. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2010;153B:1070–1080. doi: 10.1002/ajmg.b.31082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloss CS, Berrettini W, Bergen AW, Magistretti P, Duvvuri V, Strober M, Brandt H, Crawford S, Crow S, Fichter MM, et al. Genetic association of recovery from eating disorders: the role of GABA receptor SNPs. Neuropsychopharmacology. 2011;36:2222–2232. doi: 10.1038/npp.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Gibbon M, Spitzer RL, Williams JBW. Users guide for the structured clinical interview for DSM-IV Axis I disorders- research versio (SCID-I, version 2.0, February 1996 FINAL VERSION) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 29.Quehenberger O, Armando AM, Dennis EA. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochimica et biophysica acta. 2011;1811:648–656. doi: 10.1016/j.bbalip.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zivkovic AM, Yang J, Georgi K, Hegedus C, Nording ML, O'Sullivan A, German JB, Hogg RJ, Weiss RH, Bay C, Hammock BD. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics : Official journal of the Metabolomic Society. 2012;8:1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Dong H, Hammock BD. Profiling the regulatory lipids: another systemic way to unveil the biological mystery. Curr Opin Lipidol. 2011;22:197–203. doi: 10.1097/MOL.0b013e3283468c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical chemistry. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Team RDC. R A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 34.Yang J, Zhao X, Liu X, Wang C, Gao P, Wang J, Li L, Gu J, Yang S, Xu G. High performance liquid chromatography-mass spectrometry for metabonomics: potential biomarkers for acute deterioration of liver function in chronic hepatitis B. Journal of proteome research. 2006;5:554–561. doi: 10.1021/pr050364w. [DOI] [PubMed] [Google Scholar]
- 35.Rigaud D, Tallonneau I, Verges B. Hypercholesterolaemia in anorexia nervosa: frequency and changes during refeeding. Diabetes & metabolism. 2009;35:57–63. doi: 10.1016/j.diabet.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Weinbrenner T, Zuger M, Jacoby GE, Herpertz S, Liedtke R, Sudhop T, Gouni-Berthold I, Axelson M, Berthold HK. Lipoprotein metabolism in patients with anorexia nervosa: a case-control study investigating the mechanisms leading to hypercholesterolaemia. The British journal of nutrition. 2004;91:959–969. doi: 10.1079/BJN20041151. [DOI] [PubMed] [Google Scholar]
- 37.Zhu P, Peck B, Licea-Perez H, Callahan JF, Booth-Genthe C. Development of a semi-automated LC/MS/MS method for the simultaneous quantitation of 14,15-epoxyeicosatrienoic acid, 14,15-dihydroxyeicosatrienoic acid, leukotoxin and leukotoxin diol in human plasma as biomarkers of soluble epoxide hydrolase activity in vivo. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2487–2493. doi: 10.1016/j.jchromb.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 38.Lee JP, Yang SH, Kim DK, Lee H, Kim B, Cho JY, Yu KS, Paik JH, Kim M, Lim CS, Kim YS. In vivo activity of epoxide hydrolase according to sequence variation affects the progression of human IgA nephropathy. Am J Physiol Renal Physiol. 2011;300:F1283–F1290. doi: 10.1152/ajprenal.00733.2010. [DOI] [PubMed] [Google Scholar]
- 39.Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Archives of general psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 40.Karwautz AF, Wagner G, Waldherr K, Nader IW, Fernandez-Aranda F, Estivill X, Holliday J, Collier DA, Treasure JL. Gene-environment interaction in anorexia nervosa: relevance of non-shared environment and the serotonin transporter gene. Molecular psychiatry. 2011;16:590–592. doi: 10.1038/mp.2010.125. [DOI] [PubMed] [Google Scholar]
- 41.Hsu LK. Can dieting cause an eating disorder? Psychol Med. 1997;27:509–513. doi: 10.1017/s0033291797004753. [DOI] [PubMed] [Google Scholar]
- 42.Isomaa R, Isomaa AL, Marttunen M, Kaltiala-Heino R, Bjorkqvist K. Psychological distress and risk for eating disorders in subgroups of dieters. Eur Eat Disord Rev. 2010;18:296–303. doi: 10.1002/erv.1004. [DOI] [PubMed] [Google Scholar]
- 43.Wagner A, Barbarich-Marsteller NC, Frank GK, Bailer UF, Wonderlich SA, Crosby RD, Henry SE, Vogel V, Plotnicov K, McConaha C, Kaye WH. Personality traits after recovery from eating disorders: do subtypes differ? Int J Eat Disord. 2006;39:276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- 44.Morisseau C. Role of epoxide hydrolases in lipid metabolism. Biochimie. 2013;95:91–95. doi: 10.1016/j.biochi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford MA, Doyle W, Leaf A, Leighfield M, Ghebremeskel K, Phylactos A. Nutrition and neurodevelopmental disorders. Nutrition and health. 1993;9:81–97. doi: 10.1177/026010609300900205. [DOI] [PubMed] [Google Scholar]
- 46.Allen KL, Mori TA, Beilin L, Byrne SM, Hickling S, Oddy WH. Dietary intake in population-based adolescents: support for a relationship between eating disorder symptoms, low fatty acid intake and depressive symptoms. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2012 doi: 10.1111/jhn.12024. [DOI] [PubMed] [Google Scholar]
- 47.Swenne I, Rosling A. Omega-3 essential fatty acid status is improved during nutritional rehabilitation of adolescent girls with eating disorders and weight loss. Acta paediatrica (Oslo, Norway : 1992) 2012;101:858–861. doi: 10.1111/j.1651-2227.2012.02684.x. [DOI] [PubMed] [Google Scholar]
- 48.Caspar-Bauguil S, Montastier E, Galinon F, Frisch-Benarous D, Salvayre R, Ritz P. Anorexia nervosa patients display a deficit in membrane long chain poly-unsaturated fatty acids. Clin Nutr. 2012;31:386–390. doi: 10.1016/j.clnu.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 49.Swenne I, Rosling A, Tengblad S, Vessby B. Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta paediatrica (Oslo, Norway : 1992) 2011;100:1610–1615. doi: 10.1111/j.1651-2227.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 50.Swenne I, Rosling A, Tengblad S, Vessby B. Essential fatty acid status in teenage girls with eating disorders and weight loss. Acta paediatrica (Oslo, Norway : 1992) 2011;100:762–767. doi: 10.1111/j.1651-2227.2011.02153.x. [DOI] [PubMed] [Google Scholar]
- 51.Langan SM, Farrell PM. Vitamin E, vitamin A and essential fatty acid status of patients hospitalized for anorexia nervosa. Am J Clin Nutr. 1985;41:1054–1060. doi: 10.1093/ajcn/41.5.1054. [DOI] [PubMed] [Google Scholar]
- 52.Ayton AK, Azaz A, Horrobin DF. A pilot open case series of ethyl-EPA supplementation in the treatment of anorexia nervosa. Prostaglandins Leukot Essent Fatty Acids. 2004;71:205–209. doi: 10.1016/j.plefa.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Ayton AK, Azaz A, Horrobin DF. Rapid improvement of severe anorexia nervosa during treatment with ethyl-eicosapentaenoate and micronutrients. European psychiatry : the journal of the Association of European Psychiatrists. 2004;19:317–319. doi: 10.1016/j.eurpsy.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Catala A. Five Decades with Polyunsaturated Fatty Acids: Chemical Synthesis, Enzymatic Formation, Lipid Peroxidation and Its Biological Effects. Journal of lipids. 2013;2013:710290. doi: 10.1155/2013/710290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brenner RR. The oxidative desaturation of unsaturated fatty acids in animals. Molecular and cellular biochemistry. 1974;3:41–52. doi: 10.1007/BF01660076. [DOI] [PubMed] [Google Scholar]
- 56.Dacks PA, Shineman DW, Fillit HM. Current evidence for the clinical use of long-chain polyunsaturated n-3 fatty acids to prevent age-related cognitive decline and Alzheimer's disease. The journal of nutrition, health & aging. 2013;17:240–251. doi: 10.1007/s12603-012-0431-3. [DOI] [PubMed] [Google Scholar]
- 57.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. Journal of affective disorders. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 58.Syrbu SI, Waterman WH, Molski TF, Nagarkatti D, Hajjar JJ, Sha'afi RI. Phosphorylation of cytosolic phospholipase A2 and the release of arachidonic acid in human neutrophils. Journal of immunology. 1999;162:2334–2340. [PubMed] [Google Scholar]
- 59.Diez E, Chilton FH, Stroup G, Mayer RJ, Winkler JD, Fonteh AN. Fatty acid and phospholipid selectivity of different phospholipase A2 enzymes studied by using a mammalian membrane as substrate. The Biochemical journal. 1994;301(Pt 3):721–726. doi: 10.1042/bj3010721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damsbo-Svendsen S, Ronsholdt MD, Lauritzen L. Fish oil-supplementation increases appetite in healthy adults. A randomized controlled cross-over trial. Appetite. 2013;66:62–66. doi: 10.1016/j.appet.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Dellava JE, Kendler KS, Neale MC. Generalized anxiety disorder and anorexia nervosa: evidence of shared genetic variation. Depression and anxiety. 2011;28:728–733. doi: 10.1002/da.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klump KL, Strober M, Bulik CM, Thornton L, Johnson C, Devlin B, Fichter MM, Halmi KA, Kaplan AS, Woodside DB, et al. Personality characteristics of women before and after recovery from an eating disorder. Psychol Med. 2004;34:1407–1418. doi: 10.1017/s0033291704002442. [DOI] [PubMed] [Google Scholar]
- 63.Bailer UF, Frank GK, Price JC, Meltzer CC, Becker C, Mathis CA, Wagner A, Barbarich-Marsteller NC, Bloss CS, Putnam K, et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 2013;211:160–168. doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Podolin PL, Bolognese BJ, Foley JF, Long E, 3rd, Peck B, Umbrecht S, Zhang X, Zhu P, Schwartz B, Xie W, et al. In vitro and in vivo characterization of a novel soluble epoxide hydrolase inhibitor. Prostaglandins & other lipid mediators. 2013;104–105:25–31. doi: 10.1016/j.prostaglandins.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 65.Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, Bradbury JA, Enayetallah AE, Zeldin DC, Grant DF. Polymorphisms in human soluble epoxide hydrolase. Molecular pharmacology. 2003;64:482–490. doi: 10.1124/mol.64.2.482. [DOI] [PubMed] [Google Scholar]
- 66.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, Zeldin DC. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006;15:1640–1649. doi: 10.1093/hmg/ddl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee CR, Pretorius M, Schuck RN, Burch LH, Bartlett J, Williams SM, Zeldin DC, Brown NJ. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension. 2011;57:116–122. doi: 10.1161/HYPERTENSIONAHA.110.161695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fava C, Montagnana M, Danese E, Almgren P, Hedblad B, Engstrom G, Berglund G, Minuz P, Melander O. Homozygosity for the EPHX2 K55R polymorphism increases the long-term risk of ischemic stroke in men: a study in Swedes. Pharmacogenet Genomics. 2010;20:94–103. doi: 10.1097/FPC.0b013e3283349ec9. [DOI] [PubMed] [Google Scholar]
- 69.Fornage M, Boerwinkle E, Doris PA, Jacobs D, Liu K, Wong ND. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2004;109:335–339. doi: 10.1161/01.CIR.0000109487.46725.02. [DOI] [PubMed] [Google Scholar]
- 70.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DR, Jr, Siscovick DS, Fornage M. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 71.Ohtoshi K, Kaneto H, Node K, Nakamura Y, Shiraiwa T, Matsuhisa M, Yamasaki Y. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochemical and biophysical research communications. 2005;331:347–350. doi: 10.1016/j.bbrc.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 72.Burdon KP, Lehtinen AB, Langefeld CD, Carr JJ, Rich SS, Freedman BI, Herrington D, Bowden DW. Genetic analysis of the soluble epoxide hydrolase gene, EPHX2, in subclinical cardiovascular disease in the Diabetes Heart Study. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2008;5:128–134. doi: 10.3132/dvdr.2008.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Muller-Myhsok B, Wichmann HE, Meitinger T, Dichgans M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke; a journal of cerebral circulation. 2008;39:1593–1596. doi: 10.1161/STROKEAHA.107.502179. [DOI] [PubMed] [Google Scholar]
- 74.Wutzler A, Kestler C, Perrot A, Loehr L, Huemer M, Parwani AS, Attanasio P, Ozcelik C, Schunck WH, Gollasch M, et al. Variations in the human soluble epoxide hydrolase gene and recurrence of atrial fibrillation after catheter ablation. International journal of cardiology. 2013;168:3647–3651. doi: 10.1016/j.ijcard.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 75.Ramirez CE, Shuey MM, Milne GL, Gilbert K, Hui N, Yu C, Luther JM, Brown NJ. Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans. Prostaglandins & other lipid mediators. 2014 doi: 10.1016/j.prostaglandins.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L. Master’s thesis. 2011. Association Of MiRNA-146a Polymorphism With Risk Of Cardiovascular Disease And Ischemia Stroke And The Mechamisms. [Google Scholar]
- 77.Campbell IC, Mill J, Uher R, Schmidt U. Eating disorders, gene-environment interactions and epigenetics. Neuroscience and biobehavioral reviews. 2011;35:784–793. doi: 10.1016/j.neubiorev.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Laboratory of Physiological Hygiene., Keys AB: The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. University of Minnesota. [Google Scholar]
- 79.Marzola E, Nasser J, Hashim S, Shih P, Kaye W. Nutritional Rehabilitation in Anorexia Nervosa: Implications for Treatment. BMC Psychiatry. 2013 Nov 7;13:290. doi: 10.1186/1471-244X-13-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorla K, Mathews M. Pharmacological treatment of eating disorders. Psychiatry (Edgmont) 2005;2:43–48. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


