Abstract
Objectives
Response to epidermal growth factor receptor inhibitors is poorer among Stage IV colorectal cancer (CRC) patients with KRAS mutations, thus KRAS testing is recommended prior to treatment. KRAS testing was collected by Surveillance, Epidemiology, and End Results (SEER) registries for 2010 CRC cases, and our goal was to provide the first population-based estimates of testing in the U.S.
Methods
SEER CRC cases diagnosed in 2010 were evaluated (n=30,351). Chi-square tests and logistic regression were conducted to determine patient characteristics associated with KRAS testing, stratified by Stages I-III vs. Stage IV. Log-rank tests were used to examine survival by testing status.
Results
KRAS testing among Stage IV cases ranged from 39% in New Mexico to 15% in Louisiana. In the model, younger age, being married, living in a metropolitan area, and having primary site surgery were associated with greater odds of receiving KRAS testing. Those who received testing had significantly better survival then those who did not (p<0.0001). Among those who received testing, there was no significant difference in survival by mutated vs. wild type KRAS. Five percent of Stage I-III cases received testing.
Conclusions
Wide variation in documented KRAS testing for Stage IV CRC patients exists among SEER registries. Age remained highly significant in multivariate models, suggesting it plays an independent role in the patient and/or provider decision to be tested. Further research is needed to determine drivers of variation in testing, as well as reasons for testing in Stage I-III cases where it is not recommended.
Keywords: colorectal cancer, epidemiology, KRAS, Surveillance, Epidemiology, and End Results Program
Introduction
Approximately 136,830 new cases of invasive colorectal cancer (CRC) were diagnosed in 2014 in the United States (U.S.).1 It is the third most commonly diagnosed cancer and third leading cause of cancer death in the U.S.1 Twenty percent of CRC cases are Stage IV at diagnosis.2 Anti-epidermal growth factor receptor (anti-EGFR) drugs have proven effective at slowing progression of Stage IV CRC.3-6 However, studies have demonstrated the presence of KRAS mutations, which occur in approximately 40% of Stage IV patients,7,8 make response to anti-EGFR therapy less likely.9-16
In 2009, both the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) published updated CRC treatment guidelines.9,17 NCCN recommends KRAS testing for all patients upon diagnosis of metastatic CRC, and that only those with tumors characterized by the wild-type KRAS gene should receive anti-EGFR therapy, while ASCO recommends KRAS testing in all patients for whom anti-EGFR therapy is being considered.9,17 Consequently, in 2010, KRAS was included as a site-specific factor (SSF) to be collected by the National Cancer Institute's (NCI) Surveillance, Epidemiology and End Results (SEER) program.
While KRAS testing for Stages I-III CRC is not recommended, it has been suggested that KRAS status can be used as a prognostic indicator in all stages.18-20 Our objectives were to document the U.S. population estimates of KRAS testing using data from the SEER Program for both Stage IV and Stages I-III CRC patients, as well as to determine factors associated with receipt of testing, including survival. In particular, geographic variation of KRAS testing was examined to highlight issues potentially related to differential diffusion, adherence or access to KRAS testing.
Materials and Methods
Data Source and Study Population
Cases of invasive CRC diagnosed in 2010 in any of the SEER 18 Registries: the states of California (including Los Angeles, San Francisco, San Jose and Greater California Registries), Connecticut, Georgia (including Atlanta, Rural Georgia and Greater Georgia Registries), Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah, as well as the metropolitan areas of Detroit and Seattle, were extracted using SEER*Stat (version 8.1.2). Cases were included in the analysis if they had diagnostic confirmation by positive histology and were of the histologic types included in the Colon & Rectal Cancer Collaborative Stage (CS) Schema v0204.21 Cases were excluded if they had an American Joint Committee on Cancer (AJCC) 7th Edition22 Stage equal to ‘not applicable’ (n=45) or ‘unknown’ (n=2093), if they were diagnosed on autopsy or death certificate only (n=28), if they were not microscopically confirmed (n=524), or if they had a histology of squamous cell neoplasms (n=161) or cloacogenic carcinoma (n=1). Furthermore, cases in the Alaska Natives registry (n=65) were excluded due to extremely small numbers. Special access to CRC SSF 9 (KRAS Testing) was granted by the NCI. This study was granted human subject exemption status by the University of Iowa Institutional Review Board.
Variables
The primary outcome variable was receipt of KRAS testing. Testing was considered done if the SSF 9 variable had a value of ‘abnormal (mutated)’ or ‘normal (wild type)’; otherwise KRAS testing was not considered to be done if coded as ‘test ordered, results not in chart’, ‘not done’ or ‘unknown’. Results of KRAS testing were also examined.
Patient demographic variables included age, gender, race, marital status, health insurance, SEER registry, and metropolitan (metro)/urban vs. non-metro/rural county of residence. Clinical variables included tumor location (colon vs. rectum and left/rectum vs. right side), histology, grade, first vs. subsequent primary cancer, and presence of other primary cancer(s). Treatment variables included primary site surgery and recommendation for, or receipt of, radiation therapy. The number of months survived post-diagnosis was also examined by stage and KRAS testing, with a maximum of 11 months of follow-up.
Statistical Analyses
Analyses were stratified by Stage IV vs. Stages I-III. In order to address the effect of incomplete documentation and/or incomplete clinical work-up on KRAS testing as recorded by the SEER registries, a missing category for variables with large numbers of missing values was included in analyses. Differences in characteristics between those with and without KRAS testing were assessed using chi-square tests. Multivariate logistic regression analyses were conducted to determine patient characteristics associated with KRAS testing, with all variables listed above (except AJCC T and N stage as they are main components in determining overall stage) considered for inclusion in the models. Variables that were not significant predictors (p>0.10) of KRAS testing were removed in a manual backward selection process. Kaplan Meier curves and log-rank tests were used to evaluate the association between KRAS testing and survival. All tests of statistical significance were two-sided. Analyses were conducted using SAS software (version 9.3, SAS Institute, Cary, NC).
Results
The study population included 6,119 cases of Stage IV and 24,232 cases of Stages I-III CRC. Variables with missing case information included race (n=229), insurance (n=871), marital status (n=1,614), county of residence (n=5), grade (n=2,761), T stage (n=1,512), nodal status (n=890).
Table 1 summarizes KRAS values by stage and location (colon vs. rectum). The overall proportion of KRAS testing captured by SEER registries was 22.7% among Stage IV cases and 5.3% among Stages I-III cases. Of the 1,390 Stage IV cases who received KRAS testing, 58% were classified as wild type and 42% were classified as mutated. Among the 1,277 cases of Stages I-III who received testing, 64% were classified as wild type and 36% were classified as mutated. There was no significant difference in KRAS testing or mutation rates between colon and rectum sites for stage IV or I-III (results not shown).
Table 1. Number (%) with Site Specific Factor 9: KRAS Testing by Colorectal Cancer Stage and Site, 2010.
| Overall | Colon | Rectum | ||||
|---|---|---|---|---|---|---|
| N (column %) | Stage IV | Stages I-III | Stage IV | Stages I-III | Stage IV | Stages I-III |
| KRAS Values | ||||||
| 010: Abnormal (mutated) | 588 (9.6%) | 462 (1.9%) | 447 (9.9%) | 331 (1.9%) | 141 (8.8%) | 131 (1.9%) |
| 020: Normal (wild type) | 802 (13.1%) | 815 (3.4%) | 586 (12.9%) | 606 (3.5%) | 216 (13.6%) | 209 (3.1%) |
| 997: Test ordered, results not in chart | 72 (1.2%) | 87 (0.4%) | 54 (1.2%) | 76 (0.4%) | 18 (1.1%) | 11 (0.2%) |
| 998: Test not done | 2718 (44.4%) | 13365 (55.1%) | 2008 (44.4%) | 9729 (55.7%) | 710 (44.6%) | 3636 (53.8%) |
| 999: Unknown | 1939 (31.7%) | 9503 (39.2%) | 1431 (31.6%) | 6726 (38.5%) | 508 (31.9%) | 2777 (41.0%) |
| Total | 6119 (100%) | 24232 (100%) | 4526 (100%) | 17468 (100%) | 1593 (100%) | 6764 (100%) |
Stage IV Cases
Among those with Stage IV CRC, for whom KRAS testing is recommended either upon diagnosis or prior to initiating anti-EGFR therapy, the following demographic characteristics were associated with higher proportions of KRAS testing (Table 2): younger age, white, other, or missing race (marginal association, p=0.06), being married, and living in an urban/metro area. Substantial variation between registries was detected, ranging from 15% receiving KRAS testing in Louisiana to 39% in New Mexico. The following tumor and treatment characteristics were also associated with higher proportions of KRAS testing (Table 3): adenoma/adenocarcinoma and cystic/mucinous/serous histology, non-missing grade, more advanced T stage, nodal involvement, single primary cancer, no prior cancer history, and primary site surgery. Cases with ‘not otherwise specified’ (NOS), ‘other’ or ‘unknown’ tumor characteristics had a lower proportion of KRAS testing compared to cases with specific information.
Table 2. KRAS testing for colorectal cancer by stage and demographics, 2010.
| Stage IV, n (row %) | Stages I-III, n (row %) | ||||||
|---|---|---|---|---|---|---|---|
| No KRAS testing | KRAS testing | p-value | No KRAS testing | KRAS testing | p-value | ||
| TOTAL | 4729 (77.3%) | 1390 (22.7%) | 22955 (94.7%) | 1277 (5.3%) | |||
| Age (years) | <40 | 130 (63.7%) | 74 (36.3%) | <.0001 | 493 (89.5%) | 58 (10.5%) | <.0001 |
| 40-49 | 430 (66.3%) | 219 (33.7%) | 1674 (92.7%) | 132 (7.3%) | |||
| 50-59 | 923 (72.7%) | 346 (27.3%) | 4226 (94.3%) | 256 (5.7%) | |||
| 60-69 | 1205 (77.1%) | 357 (22.9%) | 5416 (94.0%) | 344 (6.0%) | |||
| 70-79 | 1034 (79.1%) | 273 (20.9%) | 5756 (95.4%) | 279 (4.6%) | |||
| 80+ | 1007 (89.3%) | 121 (10.7%) | 5390 (96.3%) | 208 (3.7%) | |||
| Gender | Female | 2177 (77.2%) | 643 (22.8%) | 0.88 | 11110 (94.8%) | 616 (5.2%) | 0.91 |
| Male | 2552 (77.4%) | 747 (22.6%) | 11845 (94.7%) | 661 (5.3%) | |||
| Registry | Californiaa | 1798 (77.6%) | 518 (22.4%) | <.0001 | 9194 (95.4%) | 441 (4.6%) | <.0001 |
| Connecticut | 212 (77.9%) | 60 (22.1%) | 1053 (94.8%) | 58 (5.2%) | |||
| Detroit | 234 (73.8%) | 83 (26.2%) | 1215 (96.1%) | 50 (3.9%) | |||
| Georgiab | 554 (78.9%) | 148 (21.1%) | 2226 (92.6%) | 179 (7.4%) | |||
| Hawaii | 81 (68.1%) | 38 (31.9%) | 465 (95.7%) | 21 (4.3%) | |||
| Iowa | 212 (81.2%) | 49 (18.8%) | 1166 (98.3%) | 20 (1.7%) | |||
| Kentucky | 327 (79.8%) | 83 (20.2%) | 1444 (89.5%) | 169 (10.5%) | |||
| Louisiana | 378 (84.6%) | 69 (15.4%) | 1600 (96.9%) | 51 (3.1%) | |||
| New Jersey | 554 (79.0%) | 147 (21.0%) | 2588 (96.6%) | 91 (3.4%) | |||
| New Mexico | 87 (61.3%) | 55 (38.7%) | 494 (93.2%) | 36 (6.8%) | |||
| Seattle | 211 (66.6%) | 106 (33.4%) | 1080 (88.7%) | 138 (11.3%) | |||
| Utah | 81 (70.4%) | 34 (29.6%) | 430 (94.9%) | 23 (5.1%) | |||
| Race | White | 3604 (77.1%) | 1069 (22.9%) | 0.06 | 18415 (94.7%) | 1029 (5.3%) | 0.49 |
| Black | 719 (80.1%) | 179 (19.9%) | 2489 (94.9%) | 134 (5.1%) | |||
| Other | 383 (73.9%) | 135 (26.1%) | 1867 (95.0%) | 99 (5.0%) | |||
| Missing | 23 (76.7%) | 7 (23.3%) | 184 (92.5%) | 15 (7.5%) | |||
| Insurance | Insured | 3671 (76.9%) | 1102 (23.1%) | 0.58 | 19258 (94.7%) | 1080 (5.3%) | 0.0006 |
| Medicaid | 687 (79.0%) | 183 (21.0%) | 2279 (94.0%) | 145 (6.0%) | |||
| Uninsured | 249 (77.6%) | 72 (22.4%) | 717 (95.1%) | 37 (4.9%) | |||
| Missing | 122 (78.7%) | 33 (21.3%) | 701 (97.9%) | 15 (2.1%) | |||
| Marital Status | Married | 2319 (74.3%) | 801 (25.7%) | <.0001 | 12256 (94.8%) | 676 (5.2%) | <.0001 |
| Divorced/Separated | 529 (76.8%) | 160 (23.2%) | 2235 (92.9%) | 172 (7.1%) | |||
| Widowed | 785 (84.3%) | 146 (15.7%) | 4131 (95.9%) | 176 (4.1%) | |||
| Single/Never married | 845 (78.8%) | 227 (21.2%) | 3061 (93.4%) | 218 (6.6%) | |||
| Missing | 251 (81.8%) | 56 (18.2%) | 1272 (97.3%) | 35 (2.7%) | |||
| Area of Residence | Metro area | 4003 (76.7%) | 1219 (23.3%) | 0.004 | 19750 (94.5%) | 1143 (5.5%) | 0.0005 |
| Non-Metro area/Rural/Alaska | 726 (81.0%) | 170 (19.0%) | 3201 (96.0%) | 134 (4.0%) | |||
| Missing | 0 (0%) | 1 (100%) | 4 (100%) | 0 (0%) | |||
Los Angeles, San Francisco, San Jose, and Greater California registries combined;
Atlanta, Rural Georgia, and Greater Georgia registries combined
Table 3. KRAS testing for colorectal cancer by stage and tumor/treatment characteristics, 2010.
| Stage IV, n (row %) | Stages I-III, n (row %) | ||||||
|---|---|---|---|---|---|---|---|
| No KRAS testing | KRAS testing | P-value | No KRAS testing | KRAS testing | P-value | ||
| TOTAL | 4729 (77.3%) | 1390 (22.7%) | 22955 (94.7%) | 1277 (5.3%) | |||
| CS Schema | Colon | 3493 (77.2%) | 1033 (22.8%) | 0.74 | 16531 (94.6%) | 937 (5.4%) | 0.29 |
| Rectum | 1236 (77.6%) | 357 (22.4%) | 6424 (95.0%) | 340 (5.0%) | |||
| Sub-site | Right Side* | 1846 (78.0%) | 520 (22.0%) | 0.27 | 9961 (94.7%) | 563 (5.3%) | 0.63 |
| Left Side | 2883 (76.8%) | 870 (23.2%) | 12994 (94.8%) | 714 (5.2%) | |||
| Histology | Epithelial neoplasm, NOS | 121 (89.6%) | 14 (10.4%) | <.0001 | 80 (94.1%) | 5 (5.9%) | 0.0006 |
| Adenoma/adenocarcinoma | 4106 (77.0%) | 1228 (23.0%) | 20841 (94.9%) | 1115 (5.1%) | |||
| Cystic/Mucinous/Serous | 459 (75.9%) | 146 (24.1%) | 1943 (92.8%) | 150 (7.2%) | |||
| Other/Unspecified | 43 (95.6%) | 2 (4.4%) | 91 (92.9%) | 7 (7.1%) | |||
| Grade | Well (I) to Moderately (II) differentiated | 2541 (75.5%) | 824 (24.5%) | <.0001 | 17626 (95.0%) | 924 (5.0%) | <.0001 |
| Poor (III) to Undifferentiated (IV) | 1119 (75.9%) | 356 (24.1%) | 3892 (92.7%) | 308 (7.3%) | |||
| Missing | 1069 (83.6%) | 210 (16.4%) | 1437 (97.0%) | 45 (3.0%) | |||
| Stage | I | -- | -- | -- | 7638 (97.7%) | 180 (2.3%) | <.0001 |
| II | -- | -- | 7715 (95.3%) | 381 (4.7%) | |||
| III | -- | -- | 7602 (91.4%) | 716 (8.6%) | |||
| T Stage | ≤ T3 | 2275 (76.9%) | 682 (23.1%) | <.0001 | 20495 (95.3%) | 1009 (4.7%) | <.0001 |
| ≥ T4a | 1204 (73.0%) | 446 (27.0%) | 2460 (90.2%) | 268 (9.8%) | |||
| TX (Unknown) | 1250 (82.7%) | 262 (17.3%) | 0 (0%) | 0 (0%) | |||
| N Stage | N0 | 1478 (81.2%) | 343 (18.8%) | <.0001 | 15353 (96.5%) | 561 (3.5%) | <.0001 |
| ≥ N1a | 2502 (73.4%) | 906 (26.6%) | 7602 (91.4%) | 716 (8.6%) | |||
| NX (Unknown) | 749 (84.2%) | 141 (15.8%) | 0 (0%) | 0 (0%) | |||
| Primaries | Single primary | 3709 (76.2%) | 1161 (23.8%) | <.0001 | 17122 (94.6%) | 972 (5.4%) | 0.22 |
| Multiple primaries | 1020 (81.7%) | 229 (18.3%) | 5833 (95.0%) | 305 (5.0%) | |||
| Prior Cancer | No prior cancer | 3863 (76.2%) | 1207 (23.8%) | <.0001 | 18194 (94.6%) | 1045 (5.4%) | 0.03 |
| Prior cancer | 866 (82.6%) | 183 (17.4%) | 4761 (95.4%) | 232 (4.6%) | |||
| Surgery | Yes | 2595 (73.8%) | 921 (26.2%) | <.0001 | 21885 (94.6%) | 1242 (5.4%) | 0.001 |
| No or Unknown | 2134 (82.0%) | 469 (18.0%) | 1070 (96.8%) | 35 (3.2%) | |||
| Radiation | Yes or recommended | 572 (78.5%) | 157 (21.5%) | 0.42 | 3259 (93.7%) | 218 (6.3%) | 0.004 |
| No/Unknown/Refused | 4157 (77.1%) | 1233 (22.9%) | 19696 (94.9%) | 1059 (5.1%) | |||
Right-sided sites include cecum, ascending colon, hepatic flexure, transverse colon; left-sided sites included splenic flexure, descending and sigmoid colon, rectosigmoid junction, rectum and the classification of ‘large intestine NOS’.
In multivariate analyses (Table 4), younger age, residing in New Mexico, Seattle, Hawaii, and having primary site surgery were associated with greater odds of receiving KRAS testing. Being single (never married) or missing marital status, having histology of epithelial neoplasm NOS or other/unspecified, missing grade information, receipt or recommendation for radiation, and residing in Louisiana or in non-metro areas were associated with lower odds of receiving KRAS testing.
Table 4. Odds Ratios (95% Confidence Intervals) from Stage-stratified (IV vs. I-III) Logistic Regression Models Examining the Association between KRAS Testing and Colorectal Cancer Patient Demographic and Clinical Characteristics,2010.
| Stage IV | Stages I-III | ||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Odds Ratio* (95% CI) | Variable | Odds Ratio* (95% CI) | ||
| Age (years) | ≤ 39 | 5.20 (3.62-7.49) | Age (years) | ≤ 39 | 2.25 (1.63-3.12) |
| 40-49 | 4.35 (3.32-5.70) | 40-49 | 1.64 (1.28-2.09) | ||
| 50-59 | 3.29 (2.57-4.20) | 50-59 | 1.45 (1.18-1.78) | ||
| 60-69 | 2.52 (1.98-3.19) | 60-69 | 1.57 (1.30-1.90) | ||
| 70-79 | 2.20 (1.73-2.80) | 70-79 | 1.23 (1.02-1.49) | ||
| 80+ | 1.00 (Referent) | 80+ | 1.00 (Referent) | ||
|
| |||||
| SEER Registry | California | 1.00 (Referent) | SEER Registry | California | 1.00 (Referent) |
| Connecticut | 1.11 (0.81-1.51) | Connecticut | 1.26 (0.95-1.68) | ||
| Detroit | 1.32 (1.00-1.74) | Detroit | 0.90 (0.67-1.22) | ||
| Georgia | 0.93 (0.75-1.15) | Georgia | 1.86 (1.53-2.25) | ||
| Hawaii | 1.71 (1.13-2.59) | Hawaii | 1.02 (0.63-1.64) | ||
| Iowa | 1.02 (0.72-1.45) | Iowa | 0.48 (0.30-0.76) | ||
| Kentucky | 0.91 (0.69-1.21) | Kentucky | 3.33 (2.72-4.09) | ||
| Louisiana | 0.64 (0.48-0.85) | Louisiana | 0.78 (0.58-1.06) | ||
| New Jersey | 0.98 (0.80-1.22) | New Jersey | 0.77 (0.61-0.98) | ||
| New Mexico | 2.67 (1.83-3.89) | New Mexico | 1.83 (1.28-2.63) | ||
| Seattle | 1.87 (1.44-2.43) | Seattle | 2.84 (2.31-3.50) | ||
| Utah | 1.26 (0.83-1.93) | Utah | 1.14 (0.73-1.76) | ||
|
| |||||
| Marital Status | Married | 1.00 (Referent) | Marital Status | Married | 1.00 (Referent) |
| Divorced/Separated | 0.89 (0.73-1.09) | Divorced/Separated | 1.30 (1.08-1.55) | ||
| Single (Never Married) | 0.70 (0.59-0.84) | Single (Never Married) | 1.26 (1.07-1.49) | ||
| Widowed | 0.87 (0.71-1.08) | Widowed | 0.90 (0.74-1.08) | ||
| Missing | 0.62 (0.45-0.85) | Missing | 0.61 (0.43-0.88) | ||
|
| |||||
| Area of Residence | Metro/Urban | 1.00 (Referent) | Area of Residence | Metro/Urban | 1.00 (Referent) |
| Non-Metro/Rural | 0.75 (0.61-0.92) | Non-Metro/Rural | 0.57 (0.46-0.69) | ||
|
| |||||
| Histology | Adenomas/Adenocarcinomas | 1.00 (Referent) | Histology | Adenomas/Adenocarcinomas | 1.00 (Referent) |
| Epithelial | 0.48 (0.27-0.86) | Epithelial | 1.11 (0.44-2.83) | ||
| Cystic/Mucinous/Serous | 1.02 (0.83-1.26) | Cystic/Mucinous/Serous | 1.24 (1.03-1.48) | ||
| Other | 0.19 (0.05-0.78) | Other | 1.18 (0.53-2.60) | ||
|
| |||||
| Grade | Well (I) to Moderately (II) differentiated | 1.00 (Referent) | Grade | Well (I) to Moderately (II) differentiated | 1.00 (Referent) |
| Poor (III) to Undifferentiated (IV) | 1.02 (0.88-1.19) | Poor (III) to Undifferentiated (IV) | 1.24 (1.08-1.43) | ||
| Missing | 0.80 (0.66-0.97) | Missing | 0.72 (0.53-0.98) | ||
|
| |||||
| Surgery | Performed | 1.41 (1.22-1.62) | Insurance | Uninsured | 0.63 (0.45-0.90) |
| Not Performed/Unknown | 1.00 (Referent) | Medicaid | 1.02 (0.84-1.23) | ||
| Missing | 0.39 (0.23-0.66) | ||||
| Insured (Other Than Medicaid) | 1.00 (Referent) | ||||
|
| |||||
| Radiation | Yes or recommended | 0.81 (0.67-0.99) | Race | White | 1.00 (Referent) |
| No/Unknown/Refused | 1.00 (Referent) | Black | 0.89 (0.73-1.09) | ||
| Other | 0.94 (0.75-1.19) | ||||
| Missing | 2.19 (1.26-3.83) | ||||
|
| |||||
| Stage | I | 1.00 (Referent) | |||
| II | 2.00 (1.66-2.40) | ||||
| III | 3.59 (3.02-4.26) | ||||
Adjusted for all variables listed within this stage column
Stage I-III Cases
Among those with Stages I-III, for whom KRAS testing is not explicitly recommended, the following demographic characteristics were associated with higher proportions of KRAS testing (Table 2): younger age, being single or divorced, having insurance or Medicaid, and living in a metropolitan area. Again, there was substantial variation among registries. Earlier stage CRC cases residing in Seattle and Kentucky had the highest proportions of testing (11.3% and 10.5%, respectively), whereas those in Louisiana or Iowa had the lowest (3.1% and 1.7%, respectively). The following tumor and treatment characteristics were associated with higher receipt of KRAS testing (Table 3): tumors with histology of cystic/mucinous/serous or other/unspecified, poor to undifferentiated grade, higher stage, more advanced T and N stages, no prior cancer, primary site surgery and radiation therapy.
In multivariate analyses (Table 4), younger age, being single (never married) or divorced, missing race cystic/mucinous/serous histology, poor to undifferentiated tumor grade, and higher stage (II or III) were associated with greater odds of receiving KRAS testing, while missing marital status, living in a non-metro area, missing grade, and being uninsured or missing insurance information were associated with lower odds of KRAS testing. In addition, those residing in Seattle, New Mexico, Kentucky or Georgia had greater odds of receiving KRAS testing, whereas those in Iowa, and New Jersey had lower odds of receiving KRAS testing.
Survival Time
Survival months by KRAS testing status and stage (IV vs. I-III) are displayed in Table 5. Those with Stage IV CRC who survived the longest had higher receipt of testing compared to those who survived a shorter time. Thirty percent of Stage IV cases surviving at least 11 months received KRAS testing, compared to 14% of Stage IV cases surviving less than one month. Figure 1 shows the Kaplan Meier curves for Stage IV cases who received KRAS testing compared to those who did not. A log-rank test indicated curves were significantly different (p<0.0001). Among Stage IV cases who received KRAS testing, there was no significant difference in survival by mutated vs. wild type KRAS status (Supplemental Digital Content Figure 1).
Table 5. KRAS testing in colorectal cancer cases by number of months survived and cause of death, stratified by stage, 2010.
| Stage IV, n (%) | Stages I-III, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Survival Months | N | No KRAS testing | KRAS testing | p | N | No KRAS testing | KRAS testing | p |
| 0 | 972 | 835 (85.9%) | 137 (14.1%) | <.0001 | 2659 | 2544 (95.7%) | 115 (4.3%) | 0.07 |
| 1 | 768 | 623 (81.1%) | 145 (18.9%) | 2179 | 2057 (94.4%) | 122 (5.6%) | ||
| 2 | 688 | 538 (78.2%) | 150 (21.8%) | 2053 | 1958 (95.4%) | 95 (4.6%) | ||
| 3 | 551 | 427 (77.5%) | 124 (22.5%) | 2009 | 1882 (93.7%) | 127 (6.3%) | ||
| 4 | 553 | 423 (76.5%) | 130 (23.5%) | 2031 | 1917 (94.4%) | 114 (5.6%) | ||
| 5 | 452 | 338 (74.8%) | 114 (25.2%) | 1878 | 1794 (95.5%) | 84 (4.5%) | ||
| 6 | 433 | 311 (71.8%) | 122 (28.2%) | 2185 | 2071 (94.8%) | 114 (5.2%) | ||
| 7 | 361 | 265 (73.4%) | 96 (26.6%) | 1829 | 1717 (93.9%) | 112 (6.1%) | ||
| 8 | 365 | 260 (71.2%) | 105 (28.8%) | 1822 | 1727 (94.8%) | 95 (5.2%) | ||
| 9 | 368 | 276 (75.0%) | 92 (25.0%) | 2094 | 1986 (94.8%) | 108 (5.2%) | ||
| 10 | 309 | 224 (72.5%) | 85 (27.5%) | 1806 | 1715 (95.0%) | 91 (5.0%) | ||
| 11 (or still alive) | 299 | 209 (69.9%) | 90 (30.1%) | 1687 | 1587 (94.0%) | 100 (6.0%) | ||
|
| ||||||||
| Status at 11 months | ||||||||
| Alive | 4308 | 3131 (72.7%) | 1177 (27.3%) | <.0001 | 22578 | 21354 (94.6%) | 1224 (5.4%) | <.0001 |
| Deceased | 1811 | 1598 (88.2%) | 213 (11.8%) | 1654 | 1601 (96.8%) | 53 (3.2%) | ||
| Cause of Death | ||||||||
| Colon, rectum or rectosigmoid | 1443 | 1267 (87.8%) | 176 (12.2%) | 0.19 | 871 | 835 (95.9%) | 36 (4.1%) | 0.08 |
| Miscellaneous malignant cancer | 89 | 77 (86.5%) | 12 (13.5%) | 39 | 39 (100%) | 0 (0%) | ||
| Heart or cerebrovascular disease | 64 | 55 (85.9%) | 9 (14.1%) | 275 | 271 (98.6%) | 4 (1.4%) | ||
| Other or Unknown | 215 | 199 (92.6%) | 16 (7.4%) | 469 | 456 (97.2%) | 13 (2.8%) | ||
Figure 1. Kaplan Meier survival curves for Stage IV colorectal cancer cases who received KRAS testing compared to those who did not, 2010.
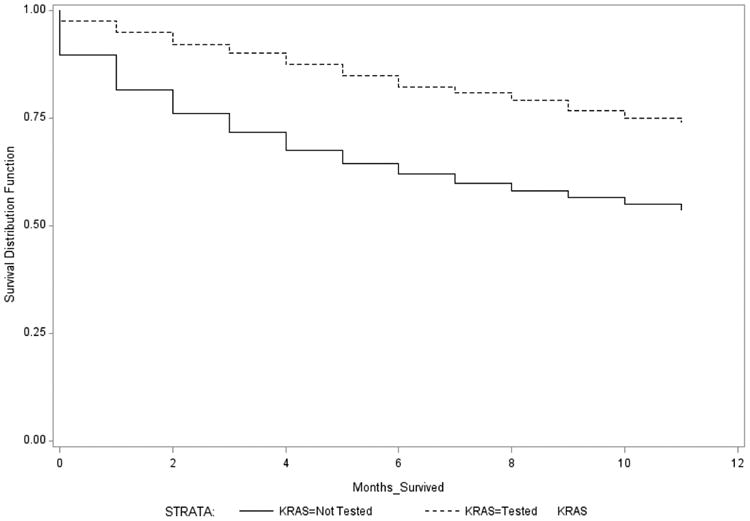
The relationship between survival months and receipt of KRAS testing was much less pronounced among Stages I-III cases (Table 5). Four percent of those surviving less than one month received KRAS testing compared to 6% of those surviving at least 11 months, but proportions of testing increased and decreased between those time points. Supplemental Digital Content Figure 2 shows the Kaplan Meier curves for Stage I-III cases who received KRAS testing compared to those who did not. A log-rank test indicated the curves were significantly different (p<0.0001).
Discussion
Despite the recommendation by NCCN that all patients diagnosed with Stage IV CRC undergo KRAS testing for treatment planning purposes, SEER data indicate that only 23% of Stage IV cases received testing in 2010, and that testing rates vary substantially by geographic region and patient characteristics Our estimate is similar to the 29% of cases found to be tested for KRAS mutations within 90 days of diagnosis of metastatic CRC in seven Health Maintenance Organizations (HMOs) across the U.S. in 2009.23 The proportion tested also falls within the range found on medical record review of 3,820 patients seen by participating physicians for Stage IV CRC in 2010 across 14 countries in Europe, Latin America, and Asia (40%, 27%, and 12%, respectively).24 A variety of factors may explain the low proportion and geographic variation of KRAS testing in our national, population-based estimates.
First, the recommendation by ASCO differs from that of NCCN in that KRAS testing is recommended for those who are being considered for anti-EGFR therapy, not all stage IV CRC patients.9,17 For patients diagnosed with very advanced disease with short life expectancies, the oncologist and/or patient may decide to forego treatment with chemotherapy, hence eliminating the need for KRAS testing.9 A recent nationwide study of stage IV CRC patients who received chemotherapy between January 2004 and March 2011 found 26% of these patients had received anti-EGFR therapy, with its use falling by 18% after the US Food and Drug Administration limited anti-EGFR use to patients with wild-type KRAS expression.25 Although a benefit has been established for anti-EGFR treatment in patients with wild-type KRAS, response rates to anti-EGFR therapy can be modest and even nonexistent in certain patients,13 perhaps causing some providers to be reluctant to offer anti-EGFR therapy/KRAS testing. Furthermore, some clinicians may be more comfortable with other recommended, more established chemotherapy regimens.
Second, SEER only collects incident cases of cancer, so it is possible the rate of KRAS testing among those initially diagnosed with Stage IV would be lower than would be anticipated among all metastatic cases. Those initially diagnosed with earlier stage cancer that later progressed to metastatic disease may have been under more frequent surveillance. Therefore, their metastasis may have been detected at an earlier and potentially more treatable point, making them more likely to receive anti-EGFR therapy and consequently be KRAS tested. Conversely, those who were initially diagnosed as Stage IV may have had more advanced disease and less interest in pursuing treatment with chemotherapy. There were a number of cases with stage or site information categorized in a ‘NOS’ related category, which suggests they may not have undergone thorough staging procedures due to their general health. A recent nationwide study of patients with metastatic cancer at diagnosis found close to 16% of both colon and rectum cases received no form of anticancer therapy.26 Another recent study which examined treatment in Medicare patients (≥ 66 years of age) with Stage IV CRC at diagnosis found only 45% received systemic chemotherapy.27
There is wide variation in KRAS testing in Stage IV patients by SEER registry. The fact that the patterns of KRAS testing within registries differed somewhat between Stages I-III and Stage IV suggests that the variation between registries is not simply due to differences in data capture. Louisiana, Iowa and Kentucky had low KRAS testing in Stage IV cases. These states contain large, rural areas located long distances from academic medical centers. It is possible that KRAS testing and use of anti-EGFR therapy in these states is most commonly done in larger medical centers, but has not yet diffused out into smaller community hospitals and practices where CRC cancer cases are frequently treated. This was supported by the finding that patients residing in urban areas had higher rates of KRAS testing after controlling for other key factors.
Alternatively, it could be that providers in states such as Louisiana, Iowa and Kentucky conduct KRAS testing as frequently as providers in other states, but more frequently send their specimens to out-of-state private pathology laboratories, thus impeding the ability of cancer registrars to abstract the information. Future studies will focus on validating the KRAS SSF values to determine if instances of KRAS testing were missed. In addition, the Iowa and Louisiana registries will be classifying the treating facilities into academic medical centers vs. community hospitals with accredited cancer programs vs. community hospitals with no accredited cancer program to determine if KRAS testing patterns are substantially different by type of treatment facility.
A missing category was included in the analyses of variables with missing information (race, insurance, marital status, grade, AJCC T and N stage) in order to address the effect of incomplete documentation and/or clinical work-up on KRAS testing as recorded by the registries. In multivariate analysis, missing marital status and grade were associated with non-receipt of KRAS testing in stage IV cases, while missing marital status, grade, and insurance were associated with no KRAS testing for stages I-III. While this may be indicative of incomplete documentation, especially for stage I-III cases where both missing marital status and insurance were significant, missing grade information may also indicate an incomplete clinical work-up, perhaps due to patient preference for limited/no work-up or treatment, and/or advanced disease/comorbidities making treatment unfeasible. Interestingly, for stage I-III cases, having missing race information was associated with KRAS testing. While not significant, the black and other stage I-III race categories had lower odds of testing, suggesting many of those missing race information were white.
After controlling for registry site, it appears that several patient characteristics are important in the decision to order KRAS testing. Younger patients, for example, underwent KRAS testing at a more frequent rate. This may be due to better general health and longer life expectancy or due to seeking treatment at medical centers where KRAS testing is more frequently performed. In the aforementioned study of HMO Stage IV CRC cases, non-receipt of chemotherapy, older age, higher Charlson co-morbidity index score, and mortality within six months of Stage IV CRC diagnosis were all associated with not receiving KRAS testing.23 Interestingly, in this current analysis, having health insurance was not associated with KRAS testing among Stage IV patients, but was among Stages I-III patients.
Those with Stage IV disease who were single (never married) had lower odds of KRAS testing, which suggests that having a spouse or other family support was important in a patient or provider's decision to recommend testing and anti EGFR therapy. Conversely, being divorced or single (never married) was actually associated with higher odds of having KRAS testing among patients with Stage I-III disease, which suggests a different decision-making process among patients and providers when dealing with earlier vs. later stage disease. Stage was clearly an important consideration in the non-metastatic population given that Stage III patients had more than three times greater odds of KRAS testing compared to Stage I patients, and Stage II patients had twice the odds of KRAS testing compared to Stage I patients. While KRAS testing is not recommended for earlier stage cancers, studies suggest KRAS mutations may have prognostic significance, and thus may be used for reasons beyond anti-EGFR therapy.28 A recent CRC chemotherapy trial of stage III cases found KRAS mutations were independently associated with disease free survival, suggesting KRAS testing should be expanded to stage III CRC.29 In addition, some patients might have clinically diagnosed metastatic CRC and therefore were KRAS tested, but were down-staged after more diagnostic procedures were performed.
In terms of survival, a clear advantage was demonstrated among those who received testing. As there was no significant survival difference detected between those with KRAS mutations and those with wild-type status, it appears the survival advantage was more likely related to the selection of patients for KRAS testing rather than treatment with anti-EGFR therapy. Poor prognostic factors negatively associated with receipt of testing, including older age, more advanced T and N stage, and prior cancer, may have influenced the decision to forego testing at the time of diagnosis. In fact, more than 50% of Stage IV CRC cases who had no KRAS testing died less than 3 months after diagnosis, compared with 30% who had KRAS testing, suggesting those not receiving KRAS testing were generally in poorer health.
The survival advantage could also be related to those living longer having more of an opportunity to be tested. Anti-EGFR therapy may have only been considered after the patient failed other chemotherapeutic agents. Therefore, patients would have had to live long enough to fail one regimen and be considered for a second regimen involving anti-EGFR therapy in order to be tested. While this approach is not totally consistent with NCCN guidelines for KRAS testing, it is in accordance with ASCO guidelines.9,17
Limitations of this study must be considered when interpreting results. SEER does not collect information about specific KRAS testing methods, and it is not possible to assess rates of false positives or negatives. However, our estimate of KRAS mutations occurring in 42% of Stage IV patients is consistent with previously published estimates of approximately 40%.7,8 This analysis was based on a new variable collected for the first time in 2010. The primary source of information used by cancer registrars to collect information across all cancer cases is the hospital medical record. The primary source of KRAS testing information is the hospital pathology report. KRAS results are part of the College of American Pathologists (CAP) protocol, but reporting is optional; partly because this result is not always available when the pathology report is issued.30 Thus, KRAS testing may not always be documented in the pathology report, or it may be added as an addendum at a later time, potentially after the report was abstracted by the cancer registrar. In addition, physician offices, or even hospitals, may send specimens to private out-of-state pathology laboratories for KRAS testing, and some of these laboratories may not be a case finding source for the registries. Thus, KRAS results not incorporated into the patient's hospital medical record could be missed by registries.
Furthermore, SEER only collects information related to first-course therapy. If anti-EGFR therapy was offered as a second line option and KRAS testing was deferred until that point, it is possible the testing occurred outside the registry chart abstraction window. SEER also only captures cases who reside within SEER areas, but the current SEER 18 Registries cover 28% of the population,31 and the cancer cases included in SEER are widely considered to be representative of the U.S. cancer population.32 Finally, SEER data do not capture patient or provider preferences or reasons for refusal of testing.
In conclusion, this is the first U.S. study to provide both population estimates and patient characteristics associated with KRAS testing among CRC patients, and provides the oncology community with baseline testing rates for stage IV patients for whom targeted treatments are available. The results show that just over one in five Stage IV colorectal cancer patients receives KRAS testing, and substantial variation exists by geographic region and patient characteristics. Somewhat surprisingly, it was also found that approximately 5% (and in certain geographic areas up to 10%) of non-metastatic patients received KRAS testing despite it not being recommended in the guidelines. Our findings suggest the need to clarify KRAS testing protocols and improve the uptake of these guidelines across all geographic areas of the U.S., as it is possible that testing and/or anti-EGFR therapy are not uniformly accessible to those who could benefit. The results can also guide the development of future studies to evaluate variations in KRAS testing and its impact on patient outcomes.
Our analyses also highlight the need for more complete reporting and standardization of data included in medical records and registries, as important clinical variables can only be analyzed when they are accurately documented and captured. Complete capture of KRAS testing information will provide an opportunity to evaluate therapy options and quality of care, and to assess survival outcomes. This could be facilitated by inclusion of KRAS testing in CAP protocols for all stage IV CRC cases. Given increased interest in tumor biomarkers and their impact on prognosis and prediction of cancer treatment response, it is highly likely that researchers will be interested in using KRAS and other similar prognostic and predictive factors in their analyses. It is anticipated that data capture and quality will improve with time and will allow researchers to assess the pattern of dissemination and diffusion in clinical practice of KRAS testing in different regions of the country.
Supplementary Material
Supplemental Digital Content Figure 1. Kaplan Meier survival curves for mutated vs. wild type KRAS status among stage IV colorectal cancer cases who received KRAS testing, 2010
Supplemental Digital Content Figure 2. Kaplan Meier survival curves for Stage I-III colorectal cancer cases who received KRAS testing compared to those who did not, 2010
Acknowledgments
Source of Funding: This work was supported in part under NIH/NCI contract number HHSN261201000030C with Louisiana State University Health Sciences Center (VWC); NIH/NCI contract number HHSN261201000032C with University of Iowa (MEC, JAS, CFL). This work was supported by the University of Iowa Holden Comprehensive Cancer Center, which is funded in part by NIH/NCI P30 CA086862.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.American Cancer Society. Colorectal Cancer Facts & Figures 2014-2016. [Accessed July 16, 2014]; [American Cancer Society Web site]. Available at: http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf.
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: Apr, 2013. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 4.Keating GM. Panitumumab: a review of its use in metastatic colorectal cancer. Drugs. 2010;70:1059–1078. doi: 10.2165/11205090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein SD, Sayegh R, Christensen S, et al. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer. 1993;71:3827–3838. doi: 10.1002/1097-0142(19930615)71:12<3827::aid-cncr2820711207>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27(12):2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 10.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304(16):1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 11.Lievre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 12.Morton RF, Hammond EH. ASCO Provisional Clinical Opinion: KRAS, Cetuximab, and Panitumumab-Clinical Implications in Colorectal Cancer. J Oncol Pract. 2009;5:71–72. doi: 10.1200/JOP.0924603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vale CL, Tierney JF, Fisher D, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38:618–625. doi: 10.1016/j.ctrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 15.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 16.Tejpar S, Peeters M, Humblet Y, et al. Relationship of efficacy with KRAS status (wild type versus mutant) in patients with irinotecan-refractory metastatic colorectal cancer, treated with irinotecan and escalating doses of cetuximab: the EVEREST experience (preliminary data) J Clin Oncol. 2008;26(Suppl 1) Abstract 4001. [Google Scholar]
- 17.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 18.Ahnen DJ, Feigl P, Quan G, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res. 1998;58:1149–1158. [PubMed] [Google Scholar]
- 19.Abubaker J, Bavi P, Al-Haqawi W, et al. Prognostic significance of alterations in KRAS isoforms KRAS-4A/4B and KRAS mutations in colorectal carcinoma. J Pathol. 2009;219:435–445. doi: 10.1002/path.2625. [DOI] [PubMed] [Google Scholar]
- 20.Nash GM, Gimbel M, Cohen AM, et al. KRAS mutation and microsatellite instability: two genetic markers of early tumor development that influence the prognosis of colorectal cancer. Ann Surg Oncol. 2010;17:416–424. doi: 10.1245/s10434-009-0713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Joint Committee on Cancer. CS Collaborative Stage Data Collection System. [Accessed July 16, 2014]; [American Joint Committee on Cancer Web site]. Available at: https://cancerstaging.org/cstage/schema/Pages/version0204.aspx.
- 22.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, editors. AJCC Cancer Staging Manual. 7th. New York: Springer; 2009. [Google Scholar]
- 23.Webster J, Kauffman TL, Feigelson HS, et al. KRAS testing and epidermal growth factor receptor inhibitor treatment for colorectal cancer in community settings. Cancer Epidemiol Biomarkers Prev. 2013;22:91–101. doi: 10.1158/1055-9965.EPI-12-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciardiello F, Tejpar S, Normanno N, et al. Uptake of KRAS mutation testing in patients with metastatic colorectal cancer in Europe, Latin America and Asia. Target Oncol. 2011;6:133–145. doi: 10.1007/s11523-011-0181-x. [DOI] [PubMed] [Google Scholar]
- 25.Abrams TA, Meyer G, Schrag D, et al. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106:371. doi: 10.1093/jnci/djt371. [DOI] [PubMed] [Google Scholar]
- 26.Small AC, Tsao CK, Moshier EL, et al. Prevalence and characteristics of patients with metastatic cancer who receive no anticancer therapy. Cancer. 2012;118:5947–5954. doi: 10.1002/cncr.27658. [DOI] [PubMed] [Google Scholar]
- 27.Vargas GM, Sheffield KM, Parmar AD, et al. Trends in treatment and survival in older patients presenting with stage IV colorectal cancer. J Gastrointest Surg. 2014;18:369–377. doi: 10.1007/s11605-013-2406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraus S, Nabiochtchikov I, Shapira S, et al. Recent advances in personalized colorectal cancer research. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Yoon HH, Tougeron D, Shi Q, et al. KRAS Codon 12 and 13 Mutations in Relation to Disease-Free Survival in BRAF-Wild-Type Stage III Colon Cancers from an Adjuvant Chemotherapy Trial (N0147 Alliance) Clin Cancer Res. 2014;20:3033–3043. doi: 10.1158/1078-0432.CCR-13-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartley A, Hamilton SR, Alsabeh R, et al. Template for Reporting Results of Biomarker Testing of Specimens From Patients With Carcinoma of the Colon and Rectum [The College of American Pathologists Web site] [Accessed July 16, 2014];2013 Oct; doi: 10.5858/arpa.2013-0231-CP. Available at: http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2013/ColorectalBiomarker_13Template_1100.pdf. [DOI] [PubMed]
- 31.National Cancer Institute. Surveillance, Epidemiology, and End Results Program Brochure [The National Cancer Institute, SEER Program Web site] [Accessed January 14, 2015];2012 Mar; Available at: http://seer.cancer.gov/about/factsheets/SEER_brochure.pdf.
- 32.Yu JB, Gross CP, Wilson LD, et al. NCI SEER public-use data: applications and limitations in oncology research. Oncology. 2009;23:288–95. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content Figure 1. Kaplan Meier survival curves for mutated vs. wild type KRAS status among stage IV colorectal cancer cases who received KRAS testing, 2010
Supplemental Digital Content Figure 2. Kaplan Meier survival curves for Stage I-III colorectal cancer cases who received KRAS testing compared to those who did not, 2010


