Abstract
Background
The findings that α-crystallins are multi-functional proteins with diverse biological functions have generated considerable interest in understanding their role in health and disease. Recent studies have shown that chaperone peptides of α-crystallin could be delivered into cultured cells and in experimental animals with beneficial effects against protein aggregation, oxidation, inflammation and apoptosis.
Scope of Review
In this review, we will summarize the latest developments on the therapeutic potential of α-crystallins and their functional peptides.
Major conclusions
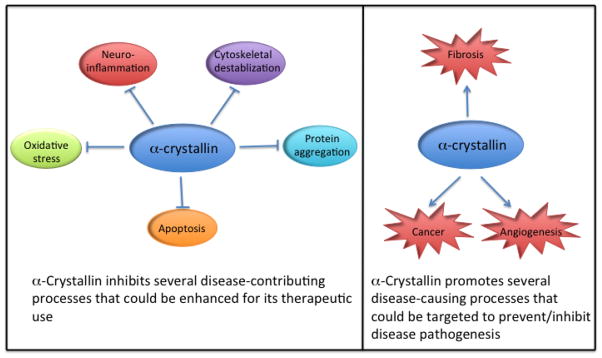
α-Crystallins and their functional peptides have shown significant favorable effects against several diseases. Their targeted delivery to tissues would be of great therapeutic benefit. However, α-crystallins can also function as disease-causing proteins. These seemingly contradictory functions must be carefully considered prior to their therapeutic use.
General significance
αA and αB-Crystallin are members of the small heat shock protein family. These proteins exhibit molecular chaperone and anti-apoptotic activities. The core crystallin domain within these proteins is largely responsible for these prosperities. Recent studies have identified peptides within the crystallin domain of both α- and αB-crystallins with remarkable chaperone and anti-apoptotic activities. Administration of α-crystallin or their functional peptides have shown substantial inhibition of pathologies in several diseases. However, α-crystallins have been shown to promote disease-causing pathways. These two sides of the proteins are discussed in this review.
1. Introduction
α-Crystallin is a major protein in the lens, and it consists of two subunits, αA (HspB4) and αB (HspB5), which possess nearly 55% sequence homology between them [1]. It belongs to the family of small heat shock proteins (sHSPs). All sHSPs contain a core “α-crystallin domain” (ACD) that is approximately 90 amino acids and that is flanked by a variable hydrophobic N-terminal domain and a hydrophilic C-terminal extension [2]. Both αA and αB are polydisperse oligomeric proteins, and their oligomeric size depends on their environment. The average molecular weights for the αA and αB homooligomers are 660 kDa and 620 kDa, respectively [3, 4]. α-Crystallin constitutes approximately 40% of the protein in the human lens, with the αA and αB subunits present in approximately a 3:1 molar ratio that is proposed to be optimal for protecting the β- and γ-crystallins of the lens [5]. αA- and αB-crystallins spontaneously exchange subunits with each other [6]. In the lens, α-crystallin is both a structural and a functional protein. The αA- and αB-crystallin oligomer is polydisperse in nature, with a molecular weight ranging from 300 to 1200 kDa [7]. In addition to being a major protein in the lens, α-crystallin is present in other tissues. αA is predominantly present in the lens, although small amounts are present in the retina, thymus and spleen [8, 9]. However, αB-crystallin is present in relatively large quantities in the retina, skeletal muscles, kidneys and heart [10, 11].
α-Crystallin exhibits molecular chaperone-like activity. Numerous studies have shown that both αA- and αB-crystallins bind structurally perturbed proteins and prevent their aggregation in an ATP-independent manner. This property has been projected to help cells in coping with various stresses. In addition to the chaperone-like activity, both αA- and αB-crystallins are strongly anti-apoptotic. Under stress conditions, an up-regulation of αB-crystallin protects against cell death. In a similar manner, cells that are genetically manipulated to overexpress these proteins are more resistant to stress conditions. Given that α-crystallins protect cells against the undesirable consequences of cellular stress and protein denaturation, it seems reasonable to hypothesize that they can be used therapeutically. In this review, we will summarize the use of α-crystallin as a therapeutic agent to block protein aggregation and apoptosis.
1.1. Chaperone activity of α-crystallin
In 1992, Horwitz reported that α-crystallin inhibited the thermal aggregation of β-crystallin in the lens and proposed that α-crystallin possesses chaperone-like activity [12]. This was in line with the findings on another prominent small heat shock protein, Hsp27, which was established at that time as a molecular chaperone. Subsequent studies confirmed this initial finding and expanded the repertoire of client proteins, which included randomly selected proteins that aggregated by chemical or thermal insults in addition to physiological client proteins. The physiological client proteins originated from virtually all parts of the cell. It has been shown that α-crystallin binds to intermediate filament glial fibrillary acidic protein, desmin [13], filensin, phakinin, vimentin [14] and actin [15–17] and that it prevents their aggregation. A recent study using the HuProt microarray system showed that more than 100 proteins could bind to αA-crystallin [18].
α-Crystallin’s chaperone activity increases with temperature [19] and is accompanied by an increased hydrophobicity of the protein. This behavior is especially true for αA-crystallin [20]. However, the question of whether the increased hydrophobicity is a prerequisite for the increase in the chaperone activity remains unclear, as some studies show a direct relationship between hydrophobicity and the chaperone activity, while others do not [21]. Subunit exchange occurs actively in α-crystallin between homooligomers and heterooligomers [6, 22]. The subunit exchange rate has been linked to the chaperone activity; however, the subunit dimers and potentially the larger structures appear more important to the chaperone activity than does monomer exchange [6, 23, 24].
1.2. Influence of post-translational modifications on the chaperone activity of α-crystallin
The chaperone activity of α-crystallin is affected by posttranslational modifications, with the most widely studied posttranslational modification being phosphorylation. One proteomic analysis of human fiber cells detected 22 residues in αA and 17 residues in αB that were phosphorylated [25]. In vivo αA-crystallin is phosphorylated at serine residues at 45, 122 and 148, and αB- at serines residues 19, 45 and 59 [26–31]. The phosphorylation at these residues reduces the oligomeric size of the proteins [29] and enhances the chaperone activity [32]. However, in some studies, phosphorylation reduced the chaperone activity [29], and the reduction in oligomeric size has also been disputed [33]. Phosphorylation is required for αB-crystallin’s binding to actin during cellular stress [17]. Phosphorylation of αB-crystallin may also modulate anti-apoptotic activity, as phosphorylation enhances the nuclear translocation of αB-crystallin [34] and appears to be necessary for its anti-apoptotic activity [35]. However, some have found evidence against this hypothesis as well [36, 37]. In addition to phosphorylation, α-crystallin also undergoes deamidation [38], which decreases the chaperone activity [39]. C-terminal truncation is another modification that reduces the chaperone activity [40], but this type of modification can be cleared by the ubiquitin system [41]. Diabetic lenses have exhibited increased C-terminal truncation [42], which could lead to enhanced protein aggregation, resulting in diabetic cataracts. A recent study showed that the O-GlcNAcylation of αB-crystallin regulates its nuclear translocation and cytoprotection [43]. Glycation is another major modification [44]; glycation by sugars has been shown to decrease the chaperone activity of α-crystallin [45], while glycation by methylglyoxal enhances chaperone activity [46]. The specific arginine residue modification responsible for this increase in activity has been mapped [47]. Additionally, these glycation pathways appear to be mutually exclusive because the methylglyoxal modification has been shown to prevent a glycation-mediated loss of chaperone function [48]. Acetylation of lysine residues is another modification [49, 50] that improves chaperone activity [51]. Acetylation at K92 in αB-crystallin was shown to increase both chaperone and anti-apoptotic activities [52]. Taking these findings together, these observations suggest a discordant effect of post-translational modification on the functions of α-crystallin.
1.3. Anti-apoptotic activity
In addition to the chaperone activity, α-crystallins inhibit apoptosis induced by various factors such as UV light, TNFα, high glucose, okadaic acid and staurosporine in several different cell types [53–59]. One study showed that αA-crystallin is better than αB-crystallin with regard to the anti-apoptotic function [58]. α-Crystallins inhibit apoptosis in both mitochondrial and death receptor-mediated pathways. αB-Crystallin has been shown to interact with procaspase-3 and to inhibit its maturation to caspase-3 [60, 61]. Other studies have shown that both αA and αB directly interact with caspase-3, Bcl-X(S) and Bax [62–64]. Additionally, αA-crystallin has been shown to increase PI3 kinase activity by inactivating PTEN [65]. In lens epithelial cells, α-crystallins inhibit UVA-induced apoptosis through the regulation of PKCα, RAF/MEK/ERK and AKT signaling pathways [66]. αB-Crystallin has also been shown to inhibit cytochrome c release from mitochondria and the downregulation of Bcl-2 in H2O2-treated cells [67]. The anti-apoptotic activity of αB-crystallin has also been linked to its translocation to mitochondria during oxidative stress and to its binding to cytochrome c, mitochondrial voltage-dependent anion channels, caspase-3 and caspase-12 [68]. Furthermore, a recent study showed that a reduction in αB-crystallin leads to an increase in endoplasmic reticulum stress-mediated apoptosis in retinal pigment epithelial cells [69]. Together, these observations demonstrate that α-crystallin is a robust anti-apoptotic protein that can prevent cell death under various conditions of cellular stress.
1.4. Copper binding and ROS inhibition
α-Crystallin binds to transitional metal ions such as Cu2+ and renders them redox inactive [70]. Raju et al. identified a peptide sequence in αA-crystallin that binds to Cu2+ and prevents ROS formation from ascorbate oxidation [71]. Whether this property of α-crystallins endows them with the ability to reduce oxidative stress and to inhibit apoptosis in vivo has yet to be established.
1.5. Intracellular translocation
An interesting property of the normally cytoplasmic αB-crystallin is that it translocates to the nucleus in stressed cells [72]. αB-Crystallin does not contain a nuclear localization signal (NLS), unlike many proteins that translocate to nucleus. The mechanism of nuclear translocation in the absence of NLS is not known. It is possible that αB-crystallin is transported to the nucleus after it binds to other proteins that contain an NLS. It is believed that phosphorylation is essential for such translocation [34]. The reason that αB translocates to the nucleus is not entirely clear, but studies suggest that it binds to proteins such as intranuclear lamin A/C and the splicing factor SC-35 [72]. αB-crystallin can also translocate to the mitochondria under stress conditions. Phosphorylated αB-crystallin has been found in the mitochondria of cardiac myocytes during ischemic/reperfusion injury [73, 74]. The function of αB-crystallin in mitochondria is not clear, but the prevention of mitochondria-mediated apoptosis is likely.
1.6. Chaperone activity sequences in α-crystallin
After the initial discovery of the chaperone function, numerous studies have attempted to map the chaperoning sequences in α-crystallin. Sharma and colleagues have conducted extensive work in this area. For example, they used protein crosslinking methods to determine the interaction sites between α-crystallin and target proteins. Their methods resulted in the identification of peptides within the ACD domain of α-crystallin. These peptides corresponded to the sequence 70FVIFLDVKHFSPEDLTVK88 in αA [75] and 73DRFSVNLDVKHFSPEELKVK92 in αB-crystallin [76], both of which reside within the ACD of α-crystallin. For the sake of simplicity, these two peptides are henceforth referred to as “mini alpha-crystallin chaperones”, or “MACs”. These two peptides were shown to possess chaperone activity against various target proteins that was similar to the full-length parent molecules. It is important to note that the chaperoning sequences in α-crystallin need not be client protein binding sites. Using protein pin array, Clark and colleagues have also identified a number of peptides within the N-terminus, C-terminus and the ACD region that possess substrate-binding properties [77], even though some of those segments of the protein may not function as chaperones. Several other studies have identified interaction sequences in α-crystallin [78, 79]. Several binding sites in client proteins have also been identified [80, 81]. Finally, anti-chaperone peptides that bind to α-crystallin and that decrease chaperone activity have also been identified in human lenses [82].
2. Therapeutic use of α-crystallins and MACs
2.1. For eye diseases
The ability of α-crystallin to inhibit protein aggregation and apoptosis has been exploited for its therapeutic use. For example, the intravenous injection of α-crystallin protected both retinal ganglion cells against apoptosis and ganglion cell axons after optic nerve crush in rats [83, 84]. Intravitreally injected α-crystallin also promoted axonal regeneration after optic nerve crush in rats [85]. The intravitreally injected α-crystallin also promoted axonal regeneration after optic nerve crush in rats [85]. In addition, the direct delivery of α-crystallin to retinal ganglion cells was shown to increase their survival after optic nerve axotomy [86]. In the early phase of autoimmune uveoretinitis in mice, αA-crystallin is upregulated in the retina through Toll-like receptor 4 [87], but in the absence of αA-crystallin, retinal degeneration is enhanced in this animal model. Such retinal degeneration can be inhibited by the systemic administration of αA-crystallin [88]. This effect has been ascribed to the reduction in the synthesis of pro-inflammatory cytokines and the expression of Toll-like receptors. Exogenous administration of αB-crystallin rescued optic nerve oligodendrocytes through the inhibition of microglial activation in an experimental model of anterior ischemic optic neuropathy in mice [89]. Another study showed that adenovirus-mediated delivery of αA-crystallin reduced vascular leakage and pericyte apoptosis in experimental diabetes, suggesting its usefulness in halting early lesions in diabetic retinopathy [90]. The exogenous administration of αA-crystallin reduced suture- and burn-induced corneal neovascularization in mice, which was proposed to be through the upregulation of VEGFR-1 [91]. In the absence of α-crystallins, retinal degeneration was enhanced in chemically induced hypoxia [92]. The absence of αB-crystallin enhanced retinal apoptosis during bacterial endophthalmitis [93] and following sodium iodate injection [94]. Whether administration of αB-crystallin would have protected against such apoptosis is not known. Because of the benefits of α-crystallin in cells, attempts are being made to deliver α-crystallin tagged with a cell penetration peptide [95]. This strategy to deliver α-crystallin into cells has shown improved protection against heat and oxidative stress in lens epithelial cells [96].
In addition to the whole protein, the administration of MACs has also shown promising results. A recent study showed that the intraperitoneal injection of MACs or of their acetyl derivatives inhibited selenite-induced cataracts in rats, which was accompanied by the inhibition of protein aggregation and lens epithelial cell apoptosis [97]. A MAC derived from αB-crystallin has been shown to inhibit oxidative stress-mediated apoptosis in cultured retinal pigmented epithelial cells [98]; this study also showed that MAC entry into cells was mediated by sodium-coupled oligopeptide transporters. The inhibition of amyloid fibrillogenesis and toxicity of the αA-crystallin MAC [99] could be further exploited for therapy against Alzheimer’s disease.
2.2. For other diseases
The administration of human αB-crystallin during the first week following a contusion injury to the central nervous system led to an improvement in locomotor skills and inhibited secondary tissue damage in mice [100]. In a mouse model for cerebral ischemia, the absence of αB-crystallin resulted in increased lesion size and diminished neurologic function, which was partially inhibited by the systemic administration of αB-crystallin [101]. The absence of αB-crystallin led to a worsening of experimental autoimmune encephalomyelitis (EAE) in mice, which was reversed by the exogenous administration of αB-crystallin [102]. The therapeutic benefit of αB-crystallin in animal models of multiple sclerosis and ischemia is thought to be due αB-crystallin’s capacity to bind pro-inflammatory molecules [103]. A recent study from Steinman’s group has also shown that a hexameric peptide of αB-crystallin (residues corresponding to 76–81 and 89–94) can inhibit neuro-inflammation in an experimental model for EAE [104]. A later study showed that the chaperone activity of αB-crystallin is necessary for this inhibitory action [105]. However, in contrast to these findings, one study did not find any beneficial effect from the overexpression of αB-crystallin in spinal motor neurons in a mouse model for paralysis [106]. The administration of αB-crystallin has also improved cardiac function after ischemia-reperfusion injury in mice, possibly by blocking the apoptosis of endothelial cells [107]. Recently, it was shown that the administration of αB-crystallin prevented ventricular arrhythmia by attenuating inflammation and oxidative stress associated with autoimmune myocarditis in mice [108].
2.3. The other side of α-crystallin
Even though α-crystallin administration proves to be beneficial in many diseases, in other diseases, inhibition of its expression could be beneficial to prevent the disease pathology. For example, in idiopathic pulmonary fibrosis, αB-crystallin is overexpressed, and pulmonary fibrosis is curtailed in its absence [109]. αB-Crystallin is highly expressed in basal-like breast tumors, and it independently predicts a shorter survival of patients [110]. Remarkably, a recent study has identified a small molecule inhibitor of αB-crystallin that inhibits tumor growth in human breast cancer xenografts in mice [111]. The binding of the inhibitor to the ACD domain of αB-crystallin has been proposed as the mechanism for this property. αB-Crystallin has also been thought to regulate breast cancer metastasis in the brain [112]. Similarly, α-crystallin is overexpressed in non-small cell lung cancer, colorectal cancer and retinoblastoma [113–115]. In addition, αB-crystallin has been shown to be a chaperone for VEGF-A during angiogenesis [116], which could have implications for angiogenesis-associated pathologies.
3. Concluding remarks
It is remarkable that α-crystallins exhibit beneficial effects through multiple mechanisms. The major attribute for such effects appears to be anti-apoptotic, at least in diseases where apoptosis is a contributory factor and in which α-crystallin supplementation has shown disease amelioration. However, its chaperone activity could also be working hand in hand with its anti-apoptotic activity to cause such favorable effects. The binding of plasma anti-inflammatory proteins is one example in which chaperone activity seems to be essential for αB-crystallin to work against multiple sclerosis [103]. Additionally, binding and inhibiting denatured cytoskeletal proteins during cellular stress may also be important. Further evidence for such codependency of chaperone and anti-apoptotic activities has stemmed from the work of Pasupuleti et al. [65], who have shown that the chaperone activity is directly related to the anti-apoptotic activity. Other attributes such as its ability to bind to copper and quench ROS formation also could contribute to the beneficial effects.
The posttranslational modifications of α-crystallin appear to modulate the anti-apoptotic and chaperone activities, thus it remains to be determined whether the introduction of modifications that enhance these activities (such as methylglyoxal-mediated modification and acetylation) would further improve the beneficial effects of the exogenously administered proteins. Tagging the protein with cell-permeable peptides for better delivery of the protein is another exciting area needing further research. As described above, some studies have shown phosphorylation and nuclear translocation appear to be necessary for α-crystallin’s anti-apoptotic functions. However, it is unclear how α-crystallin gains entry into the nucleus when it lacks an NLS peptide and why translocation is necessary for its anti-apoptotic function. If these aspects are better understood, then α-crystallin’s efficacy against diseases could be further improved. Systemic administration has limitations such as, rapid clearance of the protein and off-target effects. The targeted delivery of the protein to the intended tissues and improvements in the half-life of the protein warrant further research.
The demonstrations of beneficial effects of the whole protein are in itself very promising and exciting areas for future research, but even more exciting are the discovery of MACs. These peptides show remarkably similar activities to the whole proteins. Furthermore, unlike the whole proteins, peptides are easier to produce and are more amenable to manipulations for better delivery and for improvements in activities. The acetylation of αB-crystallin-derived MAC has already shown improved activity compared to the nonacetylated peptide, and such acetylation of lysine residues could also improve the pharmacokinetics by thwarting protease digestion. Remarkably, Sharma and colleagues have recently shown that the MAC of αA-crystallin substituted with D-amino acids in the place of L-amino acids retains its chaperone activity [117]. Because this peptide is expected to be refractory to proteases as well, it might be retained longer in the system and thus might better protect cells. The discovery of oligopeptide transporters for α-crystallin [98] is another exciting area. If such transportation can be improved and engineered for selected delivery to tissues, the functionality of the MACs could not only be improved but could also be directed toward specific tissues. In conclusion, α-crystallin appears to function both as a disease-causing and disease-inhibiting protein (Fig. 1); thus, these two seemingly contradictory functions must be carefully considered prior to its therapeutic use.
Fig. 1.
Acknowledgments
Work in RHN’s laboratory is supported by NIH grants EY022061, EY023286 and JMP’s laboratory by EY005856 and EY021498.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kappe G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slingsby C, Wistow GJ, Clark AR. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013;22:367–380. doi: 10.1002/pro.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santhoshkumar P, Murugesan R, Sharma KK. Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-crystallin. Biochemistry. 2009;48:5066–5073. doi: 10.1021/bi900085v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallur LS, Aziz A, Abraham EC. C-Terminal truncation affects subunit exchange of human alphaA-crystallin with alphaB-crystallin. Mol Cell Biochem. 2008;308:85–91. doi: 10.1007/s11010-007-9615-2. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas PN, Reddy PY, Reddy GB. Significance of alpha-crystallin heteropolymer with a 3:1 alphaA/alphaB ratio: chaperone-like activity, structure and hydrophobicity. Biochem J. 2008;414:453–460. doi: 10.1042/BJ20080544. [DOI] [PubMed] [Google Scholar]
- 6.Bova MP, Ding LL, Horwitz J, Fung BK. Subunit exchange of alphaA-crystallin. J Biol Chem. 1997;272:29511–29517. doi: 10.1074/jbc.272.47.29511. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 8.Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deretic D, Aebersold RH, Morrison HD, Papermaster DS. Alpha A- and alpha B-crystallin in the retina. Association with the post-Golgi compartment of frog retinal photoreceptors. J Biol Chem. 1994;269:16853–16861. [PubMed] [Google Scholar]
- 10.Iwaki T, Kume-Iwaki A, Goldman JE. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990;38:31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- 11.Bhat SP, Nagineni CN. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott JL, Der Perng M, Prescott AR, Jansen KA, Koenderink GH, Quinlan RA. The specificity of the interaction between alphaB-crystallin and desmin filaments and its impact on filament aggregation and cell viability. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120375. doi: 10.1098/rstb.2012.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su SP, McArthur JD, Friedrich MG, Truscott RJ, Aquilina JA. Understanding the alpha-crystallin cell membrane conjunction. Mol Vis. 2011;17:2798–2807. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown Z, Ponce A, Lampi K, Hancock L, Takemoto L. Differential binding of mutant (R116C) and wildtype alphaA crystallin to actin. Curr Eye Res. 2007;32:1051–1054. doi: 10.1080/02713680701769989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennardini F, Wrzosek A, Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 17.Singh BN, Rao KS, Ramakrishna T, Rangaraj N, Rao Ch M. Association of alphaB-crystallin, a small heat shock protein, with actin: role in modulating actin filament dynamics in vivo. J Mol Biol. 2007;366:756–767. doi: 10.1016/j.jmb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Fan Q, Huang LZ, Zhu XJ, Zhang KK, Ye HF, Luo Y, Sun XH, Zhou P, Lu Y. Identification of proteins that interact with alpha A-crystallin using a human proteome microarray. Mol Vis. 2014;20:117–124. [PMC free article] [PubMed] [Google Scholar]
- 19.Datta SA, Rao CM. Differential temperature-dependent chaperone-like activity of alphaA- and alphaB-crystallin homoaggregates. J Biol Chem. 1999;274:34773–34778. doi: 10.1074/jbc.274.49.34773. [DOI] [PubMed] [Google Scholar]
- 20.Kumar MS, Kapoor M, Sinha S, Reddy GB. Insights into hydrophobicity and the chaperone-like function of alphaA- and alphaB-crystallins: an isothermal titration calorimetric study. J Biol Chem. 2005;280:21726–21730. doi: 10.1074/jbc.M500405200. [DOI] [PubMed] [Google Scholar]
- 21.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 22.Bova MP, McHaourab HS, Han Y, Fung BK. Subunit exchange of small heat shock proteins. Analysis of oligomer formation of alphaA-crystallin and Hsp27 by fluorescence resonance energy transfer and site-directed truncations. The Journal of biological chemistry. 2000;275:1035–1042. doi: 10.1074/jbc.275.2.1035. [DOI] [PubMed] [Google Scholar]
- 23.Augusteyn RC. Dissociation is not required for alpha-crystallin’s chaperone function. Experimental eye research. 2004;79:781–784. doi: 10.1016/j.exer.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg GK, Ecroyd H, Liu C, Cox D, Cascio D, Sawaya MR, Collier MP, Stroud J, Carver JA, Baldwin AJ, Robinson CV, Eisenberg DS, Benesch JL, Laganowsky A. The structured core domain of alphaB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc Natl Acad Sci U S A. 2014;111:E1562–1570. doi: 10.1073/pnas.1322673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Han J, David LL, Schey KL. Proteomics and phosphoproteomics analysis of human lens fiber cell membranes. Investigative ophthalmology & visual science. 2013;54:1135–1143. doi: 10.1167/iovs.12-11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer H, Marcus K, Sickmann A, Herrmann M, Klose J, Meyer HE. Identification of phosphorylation and acetylation sites in alphaA-crystallin of the eye lens (mus musculus) after two-dimensional gel electrophoresis. Analytical and bioanalytical chemistry. 2003;376:966–972. doi: 10.1007/s00216-003-1983-1. [DOI] [PubMed] [Google Scholar]
- 27.Takemoto LJ. Differential phosphorylation of alpha-A crystallin in human lens of different age. Experimental eye research. 1996;62:499–504. doi: 10.1006/exer.1996.0060. [DOI] [PubMed] [Google Scholar]
- 28.Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of alphaB-crystallin in response to various types of stress. The Journal of biological chemistry. 1997;272:29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- 30.Voorter CE, Mulders JW, Bloemendal H, de Jong WW. Some aspects of the phosphorylation of alpha-crystallin A. European journal of biochemistry/FEBS. 1986;160:203–210. doi: 10.1111/j.1432-1033.1986.tb09958.x. [DOI] [PubMed] [Google Scholar]
- 31.Chiesa R, Gawinowicz-Kolks MA, Spector A. The phosphorylation of the primary gene products of alpha-crystallin. The Journal of biological chemistry. 1987;262:1438–1441. [PubMed] [Google Scholar]
- 32.Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JL, Robinson CV, Macphee CE, Carver JA. Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aquilina JA, Benesch JL, Ding LL, Yaron O, Horwitz J, Robinson CV. Phosphorylation of alphaB-crystallin alters chaperone function through loss of dimeric substructure. J Biol Chem. 2004;279:28675–28680. doi: 10.1074/jbc.M403348200. [DOI] [PubMed] [Google Scholar]
- 34.den Engelsman J, Bennink EJ, Doerwald L, Onnekink C, Wunderink L, Andley UP, Kato K, de Jong WW, Boelens WC. Mimicking phosphorylation of the small heat-shock protein alphaB-crystallin recruits the F-box protein FBX4 to nuclear SC35 speckles. Eur J Biochem. 2004;271:4195–4203. doi: 10.1111/j.1432-1033.2004.04359.x. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Reiser G. Phosphorylation of Ser45 and Ser59 of alphaB-crystallin and p38/extracellular regulated kinase activity determine alphaB-crystallin-mediated protection of rat brain astrocytes from C2-ceramide- and staurosporine-induced cell death. J Neurochem. 2011;118:354–364. doi: 10.1111/j.1471-4159.2011.07317.x. [DOI] [PubMed] [Google Scholar]
- 36.Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92:203–211. doi: 10.1161/01.res.0000052989.83995.a5. [DOI] [PubMed] [Google Scholar]
- 37.Launay N, Tarze A, Vicart P, Lilienbaum A. Serine 59 phosphorylation of {alpha}B-crystallin down-regulates its anti-apoptotic function by binding and sequestering Bcl-2 in breast cancer cells. J Biol Chem. 2010;285:37324–37332. doi: 10.1074/jbc.M110.124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemoto L, Boyle D. Deamidation of alpha-A crystallin from nuclei of cataractous and normal human lenses. Mol Vis. 1999;5:2. [PubMed] [Google Scholar]
- 39.Gupta R, Srivastava OP. Deamidation affects structural and functional properties of human alphaA-crystallin and its oligomerization with alphaB-crystallin. J Biol Chem. 2004;279:44258–44269. doi: 10.1074/jbc.M405648200. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi N, Ouchida A, Kamei A. C-terminal truncation of alpha-crystallin in hereditary cataractous rat lens. Biol Pharm Bull. 2004;27:308–314. doi: 10.1248/bpb.27.308. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Dudek EJ, Liu B, Ding L, Fernandes AF, Liang JJ, Horwitz J, Taylor A, Shang F. Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci. 2007;48:4200–4208. doi: 10.1167/iovs.07-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thampi P, Hassan A, Smith JB, Abraham EC. Enhanced C-terminal truncation of alphaA- and alphaB-crystallins in diabetic lenses. Invest Ophthalmol Vis Sci. 2002;43:3265–3272. [PubMed] [Google Scholar]
- 43.Krishnamoorthy V, Donofrio AJ, Martin JL. O-GlcNAcylation of alphaB-crystallin regulates its stress-induced translocation and cytoprotection. Mol Cell Biochem. 2013;379:59–68. doi: 10.1007/s11010-013-1627-5. [DOI] [PubMed] [Google Scholar]
- 44.Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012;42:1205–1220. doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- 45.Datta P, Kallur L, Abraham EC. Reversal of chaperone activity loss of glycated alphaA-crystallin by a crosslink breaker. Mol Cell Biochem. 2008;315:137–142. doi: 10.1007/s11010-008-9797-2. [DOI] [PubMed] [Google Scholar]
- 46.Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 47.Nagaraj RH, Panda AK, Shanthakumar S, Santhoshkumar P, Pasupuleti N, Wang B, Biswas A. Hydroimidazolone modification of the conserved Arg12 in small heat shock proteins: studies on the structure and chaperone function using mutant mimics. PLoS One. 2012;7:e30257. doi: 10.1371/journal.pone.0030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puttaiah S, Biswas A, Staniszewska M, Nagaraj RH. Methylglyoxal inhibits glycation-mediated loss in chaperone function and synthesis of pentosidine in alpha-crystallin. Exp Eye Res. 2007;84:914–921. doi: 10.1016/j.exer.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Lin PP, Barry RC, Smith DL, Smith JB. In vivo acetylation identified at lysine 70 of human lens alphaA-crystallin. Protein Sci. 1998;7:1451–1457. doi: 10.1002/pro.5560070622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lapko VN, Smith DL, Smith JB. In vivo carbamylation and acetylation of water-soluble human lens alphaB-crystallin lysine 92. Protein Sci. 2001;10:1130–1136. doi: 10.1110/ps.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, Biswas A. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta. 2012;1822:120–129. doi: 10.1016/j.bbadis.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nahomi RB, Huang R, Nandi SK, Wang B, Padmanabha S, Santhoshkumar P, Filipek S, Biswas A, Nagaraj RH. Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human alphaB-crystallin. Biochemistry. 2013;52:8126–8138. doi: 10.1021/bi400638s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kannan R, Sreekumar PG, Hinton DR. Novel roles for alpha-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31:576–604. doi: 10.1016/j.preteyeres.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43:3575–3582. [PubMed] [Google Scholar]
- 55.Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- 56.Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- 57.Li DW, Xiang H, Mao YW, Wang J, Fass U, Zhang XY, Xu C. Caspase-3 is actively involved in okadaic acid-induced lens epithelial cell apoptosis. Exp Cell Res. 2001;266:279–291. doi: 10.1006/excr.2001.5223. [DOI] [PubMed] [Google Scholar]
- 58.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 59.Liu B, Bhat M, Nagaraj RH. AlphaB-crystallin inhibits glucose-induced apoptosis in vascular endothelial cells. Biochem Biophys Res Commun. 2004;321:254–258. doi: 10.1016/j.bbrc.2004.06.151. [DOI] [PubMed] [Google Scholar]
- 60.Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 61.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, Oshita S, Wilkinson JC, Yu C, Oliver PG, Duckett CS, Buchsbaum DJ, LoBuglio AF, Jordan VC, Cryns VL. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 62.Hu WF, Gong L, Cao Z, Ma H, Ji W, Deng M, Liu M, Hu XH, Chen P, Yan Q, Chen HG, Liu J, Sun S, Zhang L, Liu JP, Wawrousek E, Li DW. alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med. 2012;12:177–187. doi: 10.2174/156652412798889036. [DOI] [PubMed] [Google Scholar]
- 63.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 64.Hamann S, Metrailler S, Schorderet DF, Cottet S. Analysis of the cytoprotective role of alpha-crystallins in cell survival and implication of the alphaA-crystallin C-terminal extension domain in preventing Bax-induced apoptosis. PLoS One. 2013;8:e55372. doi: 10.1371/journal.pone.0055372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasupuleti N, Matsuyama S, Voss O, Doseff AI, Song K, Danielpour D, Nagaraj RH. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Liu L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. doi: 10.1016/j.exer.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 67.Xu F, Yu H, Liu J, Cheng L. alphaB-crystallin regulates oxidative stress-induced apoptosis in cardiac H9c2 cells via the PI3K/AKT pathway. Mol Biol Rep. 2013;40:2517–2526. doi: 10.1007/s11033-012-2332-2. [DOI] [PubMed] [Google Scholar]
- 68.Chis R, Sharma P, Bousette N, Miyake T, Wilson A, Backx PH, Gramolini AO. alpha-Crystallin B prevents apoptosis after H2O2 exposure in mouse neonatal cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;303:H967–978. doi: 10.1152/ajpheart.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, Hinton DR. Deficiency of alphaB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012;53:1111–1122. doi: 10.1016/j.freeradbiomed.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmad MF, Singh D, Taiyab A, Ramakrishna T, Raman B, Rao Ch M. Selective Cu2+ binding, redox silencing, and cytoprotective effects of the small heat shock proteins alphaA- and alphaB-crystallin. J Mol Biol. 2008;382:812–824. doi: 10.1016/j.jmb.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 71.Raju M, Santhoshkumar P, Henzl TM, Sharma KK. Identification and characterization of a copper-binding site in alphaA-crystallin. Free Radic Biol Med. 2011;50:1429–1436. doi: 10.1016/j.freeradbiomed.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, Mohan Rao C. Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell Res. 2004;299:393–403. doi: 10.1016/j.yexcr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 73.Jin JK, Whittaker R, Glassy MS, Barlow SB, Gottlieb RA, Glembotski CC. Localization of phosphorylated alphaB-crystallin to heart mitochondria during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H337–344. doi: 10.1152/ajpheart.00881.2007. [DOI] [PubMed] [Google Scholar]
- 74.Whittaker R, Glassy MS, Gude N, Sussman MA, Gottlieb RA, Glembotski CC. Kinetics of the translocation and phosphorylation of alphaB-crystallin in mouse heart mitochondria during ex vivo ischemia. Am J Physiol Heart Circ Physiol. 2009;296:H1633–1642. doi: 10.1152/ajpheart.01227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 76.Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghosh JG, Houck SA, Clark JI. Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol. 2007;39:1804–1815. doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukuhara S, Nishigaki T, Miyata K, Tsuchiya N, Waku T, Tanaka N. Mechanism of the chaperone-like and antichaperone activities of amyloid fibrils of peptides from alphaA-crystallin. Biochemistry. 2012;51:5394–5401. doi: 10.1021/bi3004236. [DOI] [PubMed] [Google Scholar]
- 79.Houck SA, Landsbury A, Clark JI, Quinlan RA. Multiple sites in alphaB-crystallin modulate its interactions with desmin filaments assembled in vitro. PLoS One. 2011;6:e25859. doi: 10.1371/journal.pone.0025859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santhoshkumar P, Sharma KK. Identification of a region in alcohol dehydrogenase that binds to alpha-crystallin during chaperone action. Biochim Biophys Acta. 2002;1598:115–121. doi: 10.1016/s0167-4838(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 81.Gupta R, Srivastava OP. Identification of interaction sites between human betaA3- and alphaA/alphaB-crystallins by mammalian two-hybrid and fluorescence resonance energy transfer acceptor photobleaching methods. J Biol Chem. 2009;284:18481–18492. doi: 10.1074/jbc.M109.013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kannan R, Santhoshkumar P, Mooney BP, Sharma KK. The alphaA66-80 peptide interacts with soluble alpha-crystallin and induces its aggregation and precipitation: a contribution to age-related cataract formation. Biochemistry. 2013;52:3638–3650. doi: 10.1021/bi301662w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu N, Yu J, Chen S, Xu J, Ying X, Ye M, Li Y, Wang Y. alpha-Crystallin protects RGC survival and inhibits microglial activation after optic nerve crush. Life Sci. 2014;94:17–23. doi: 10.1016/j.lfs.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 84.Ying X, Zhang J, Wang Y, Wu N, Wang Y, Yew DT. Alpha-crystallin protected axons from optic nerve degeneration after crushing in rats. J Mol Neurosci. 2008;35:253–258. doi: 10.1007/s12031-007-9010-1. [DOI] [PubMed] [Google Scholar]
- 85.Wang YH, Wang DW, Wu N, Wang Y, Yin ZQ. alpha-Crystallin promotes rat axonal regeneration through regulation of RhoA/rock/cofilin/MLC signaling pathways. J Mol Neurosci. 2012;46:138–144. doi: 10.1007/s12031-011-9537-z. [DOI] [PubMed] [Google Scholar]
- 86.Munemasa Y, Kwong JM, Caprioli J, Piri N. The role of alphaA- and alphaB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest Ophthalmol Vis Sci. 2009;50:3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- 87.Saraswathy S, Nguyen AM, Rao NA. The role of TLR4 in photoreceptor {alpha}a crystallin upregulation during early experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2010;51:3680–3686. doi: 10.1167/iovs.09-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao NA, Saraswathy S, Pararajasegaram G, Bhat SP. Small heat shock protein alphaA-crystallin prevents photoreceptor degeneration in experimental autoimmune uveitis. PLoS One. 2012;7:e33582. doi: 10.1371/journal.pone.0033582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pangratz-Fuehrer S, Kaur K, Ousman SS, Steinman L, Liao YJ. Functional rescue of experimental ischemic optic neuropathy with alphaB-crystallin. Eye (Lond) 2011;25:809–817. doi: 10.1038/eye.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim YH, Park SY, Park J, Kim YS, Hwang EM, Park JY, Roh GS, Kim HJ, Kang SS, Cho GJ, Choi WS. Reduction of experimental diabetic vascular leakage and pericyte apoptosis in mice by delivery of alphaA-crystallin with a recombinant adenovirus. Diabetologia. 2012;55:2835–2844. doi: 10.1007/s00125-012-2625-y. [DOI] [PubMed] [Google Scholar]
- 91.Zhu W, Qi X, Ren S, Jia C, Song Z, Wang Y. alphaA-crystallin in the pathogenesis and intervention of experimental murine corneal neovascularization. Exp Eye Res. 2012;98:44–51. doi: 10.1016/j.exer.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Yaung J, Kannan R, Wawrousek EF, Spee C, Sreekumar PG, Hinton DR. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp Eye Res. 2008;86:355–365. doi: 10.1016/j.exer.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whiston EA, Sugi N, Kamradt MC, Sack C, Heimer SR, Engelbert M, Wawrousek EF, Gilmore MS, Ksander BR, Gregory MS. alphaB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76:1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou P, Kannan R, Spee C, Sreekumar PG, Dou G, Hinton DR. Protection of retina by alphaB crystallin in sodium iodate induced retinal degeneration. PLoS One. 2014;9:e98275. doi: 10.1371/journal.pone.0098275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mueller NH, Ammar DA, Petrash JM. Cell penetration peptides for enhanced entry of alphaB-crystallin into lens cells. Invest Ophthalmol Vis Sci. 2013;54:2–8. doi: 10.1167/iovs.12-10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Christopher KL, Pedler MG, Shieh B, Ammar DA, Petrash JM, Mueller NH. Alpha-crystallin-mediated protection of lens cells against heat and oxidative stress-induced cell death. Biochim Biophys Acta. 2014;1843:309–315. doi: 10.1016/j.bbamcr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH. Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem. 2013;288:13022–13035. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR. Antiapoptotic properties of alpha-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci. 2013;54:2787–2798. doi: 10.1167/iovs.12-11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santhoshkumar P, Sharma KK. Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem. 2004;267:147–155. doi: 10.1023/b:mcbi.0000049373.15558.b8. [DOI] [PubMed] [Google Scholar]
- 100.Klopstein A, Santos-Nogueira E, Francos-Quijorna I, Redensek A, David S, Navarro X, Lopez-Vales R. Beneficial effects of alphaB-crystallin in spinal cord contusion injury. J Neurosci. 2012;32:14478–14488. doi: 10.1523/JNEUROSCI.0923-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arac A, Brownell SE, Rothbard JB, Chen C, Ko RM, Pereira MP, Albers GW, Steinman L, Steinberg GK. Systemic augmentation of alphaB-crystallin provides therapeutic benefit twelve hours post-stroke onset via immune modulation. Proc Natl Acad Sci U S A. 2011;108:13287–13292. doi: 10.1073/pnas.1107368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 103.Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, Chen R, Fathman CG, Robinson WH, Steinman L. Therapeutic effects of systemic administration of chaperone alphaB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem. 2012;287:9708–9721. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kurnellas MP, Adams CM, Sobel RA, Steinman L, Rothbard JB. Amyloid fibrils composed of hexameric peptides attenuate neuroinflammation. Sci Transl Med. 2013;5:179ra142. doi: 10.1126/scitranslmed.3005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman L, Rothbard JB. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:36423–36434. doi: 10.1074/jbc.M112.371229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu G, Fromholt S, Ayers JI, Brown H, Siemienski Z, Crosby KW, Mayer CA, Janus C, Borchelt DR. Substantially elevating the levels of alphaB-crystallin in spinal motor neurons of mutant SOD1 mice does not significantly delay paralysis or attenuate mutant protein aggregation. J Neurochem. 2014 doi: 10.1111/jnc.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Velotta JB, Kimura N, Chang SH, Chung J, Itoh S, Rothbard J, Yang PC, Steinman L, Robbins RC, Fischbein MP. alphaB-crystallin improves murine cardiac function and attenuates apoptosis in human endothelial cells exposed to ischemia-reperfusion. Ann Thorac Surg. 2011;91:1907–1913. doi: 10.1016/j.athoracsur.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 108.Park H, Park H, Hwang HJ, Hwang HS, Kim H, Choi BR, Pak HN, Lee MH, Chung JH, Joung B. Alpha B-crystallin prevents ventricular arrhythmia by attenuating inflammation and oxidative stress in rat with autoimmune myocarditis. Int J Cardiol. 2014;182C:399–402. doi: 10.1016/j.ijcard.2014.12.152. [DOI] [PubMed] [Google Scholar]
- 109.Bellaye PS, Burgy O, Colas J, Fabre A, Marchal-Somme J, Crestani B, Kolb M, Camus P, Garrido C, Bonniaud P. Anti-fibrotic Role of alphaB-crystallin Inhibition in Pleural and Subpleural Fibrosis. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2014-0011OC. [DOI] [PubMed] [Google Scholar]
- 110.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Z, Ruan Q, Han S, Xi L, Jiang W, Jiang H, Ostrov DA, Cai J. Discovery of structure-based small molecular inhibitor of alphaB-crystallin against basal-like/triple-negative breast cancer development in vitro and in vivo. Breast Cancer Res Treat. 2014;145:45–59. doi: 10.1007/s10549-014-2940-8. [DOI] [PubMed] [Google Scholar]
- 112.Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, Miller CR, Ugolkov A, Livasy C, Fritchie K, Hamilton E, Blackwell K, Geradts J, Ewend M, Carey L, Shusta EV, Anders CK, Cryns VL. alphaB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res. 2014;20:56–67. doi: 10.1158/1078-0432.CCR-13-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qin H, Ni Y, Tong J, Zhao J, Zhou X, Cai W, Liang J, Yao X. Elevated expression of CRYAB predicts unfavorable prognosis in non-small cell lung cancer. Med Oncol. 2014;31:142. doi: 10.1007/s12032-014-0142-1. [DOI] [PubMed] [Google Scholar]
- 114.Shi C, He Z, Hou N, Ni Y, Xiong L, Chen P. Alpha B-crystallin correlates with poor survival in colorectal cancer. Int J Clin Exp Pathol. 2014;7:6056–6063. [PMC free article] [PubMed] [Google Scholar]
- 115.Kase S, Parikh JG, Rao NA. Expression of alpha-crystallin in retinoblastoma. Arch Ophthalmol. 2009;127:187–192. doi: 10.1001/archophthalmol.2008.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhattacharyya J, Sharma KK. Conformational specificity of mini-alphaA-crystallin as a molecular chaperone. J Pept Res. 2001;57:428–434. doi: 10.1034/j.1399-3011.2001.00871.x. [DOI] [PubMed] [Google Scholar]


