Abstract
Background
Minor histocompatibility (miHA) antigen vaccines have the potential to augment graft-versus-tumor effects without graft-versus-host disease (GVHD). We used mixed hematopoietic chimerism in the canine model of MHC-matched allogeneic hematopoietic cell transplantation (HCT) as a platform to develop a miHA vaccination regimen.
Methods
We engineered DNA plasmids and replication-deficient human adenovirus type 5 (rAd5) constructs encoding large sections of canine SMCY and the entire canine SRY gene.
Results
Priming with rAd5 constructs and boosting with ex vivo plasmid-transfected dendritic cells and cutaneous delivery of plasmids with a particle-mediated epidermal delivery device (PMED) in two female dogs induced antigen-specific T cell responses. Similar responses were observed following a prime-boost vaccine regimen in three female HCT donors. Subsequent donor lymphocyte infusion resulted in a significant change of chimerism in 1 of 3 male recipients without any signs of GVHD. The change in chimerism in the recipient occurred in association with the development of CD4+ and CD8+ T cell responses to the same peptide pools detected in the donor.
Conclusions
These studies describe the first in vivo response to miHA vaccination in a large, outbred animal model without using recipient cells to sensitize the donor. This model provides a platform for ongoing experiments designed to define optimal miHA targets, and develop protocols to directly vaccinate the recipient.
INTRODUCTION
Following the clinical application of major histocompatibility complex (MHC)-matched allogeneic hematopoietic cell transplantation (HCT), it became apparent that graft rejection (1), graft-versus-host disease (GVHD) (2), and curative graft-versus-tumor (GVT) responses (3) could occur due to non-MHC antigenic differences between the donor and recipient, referred to as minor histocompatibility antigens (miHAs). Additionally, clinical observations that cures of the underlying malignancies following allogeneic HCT (4) or donor lymphocyte infusions (DLI) (5) can occur in the absence of GVHD suggested that GVT effects could be separated from GVHD.
MiHAs are MHC-presented antigenic peptides derived from male-specific genes encoded on the Y chromosome in sex-mismatched transplants (HY), as well as disparate peptides derived from autosomally-encoded non-synonymous coding variations (6). Many miHAs are ubiquitously expressed, but subsets demonstrate a tissue- or cell type-restricted pattern of expression. This observation has led to the hypothesis that therapies targeting miHAs expressed preferentially on hematopoietic cells may promote GVT responses with little or no GVHD, providing a valuable therapeutic option to treat relapsed or refractory hematologic malignancies post-allogeneic HCT (7).
Strategies designed to produce GVT responses without GVHD can be broadly separated into protocols requiring ex vivo manipulation or those relying on direct in vivo sensitization. For ex vivo manipulation, phase I/II clinical trials of expanded donor-derived T cell clones specific for recipient miHAs were infused with some success, but also demonstrated the barriers of safety, efficacy, and feasibility that prevent broader application of this approach (8-11). Gene-engineered donor T cells targeting specific miHA/MHC combinations may prove even more efficacious (12), but ex vivo manipulation remains costly, time consuming, and potentially dangerous. In contrast, direct in vivo sensitization with a miHA vaccine may offer a safer and less costly approach. Importantly, adoptive transfer of miHA-sensitized donor T cells has been shown to eradicate implanted malignancies in congenic mouse models (13,14). We sought to build on these findings by establishing a safe and effective miHA sensitization regimen in the transplant donor of a large outbred animal model of allogeneic HCT.
The canine allogeneic HCT model has a remarkable track record of translating therapies into clinical protocols for human HCT (15), and we propose to use the canine model of mixed hematopoietic chimerism as a platform to develop a miHA vaccine (16). Stable mixed hematopoietic chimerism is achieved following 200 centigray (cGy) total body irradiation (TBI), MHC-identical marrow infusion, and a short course of post-grafting immunosuppression (16). In the mixed chimeric state, regulatory T cells induce donor T cell tolerance to residual recipient hematopoiesis, which we use as a surrogate of relapsed disease (17,18). Our group has performed 14 “unsensitized” donor lymphocyte infusions (DLIs) into stable mixed hematopoietic chimeras and has never observed an effect on chimerism (combination of published and unpublished observations) (19,20). However, if the donor is first “sensitized” against recipient miHAs using viable recipient-derived skin implants, organ transplantation, or injections of peripheral blood mononuclear cells (PBMCs), DLI reliably converts the recipient to full donor hematopoiesis, representing the experimental equivalent of a GVT response (19-21). Since recipient cells used for sensitization can present a large repertoire of miHA disparities expressed in many tissues, conversion is often accompanied by fatal GVHD (19-21). Our initial goal was to use this model to develop a miHA vaccine regimen that does not utilize recipient cells to sensitize the donor.
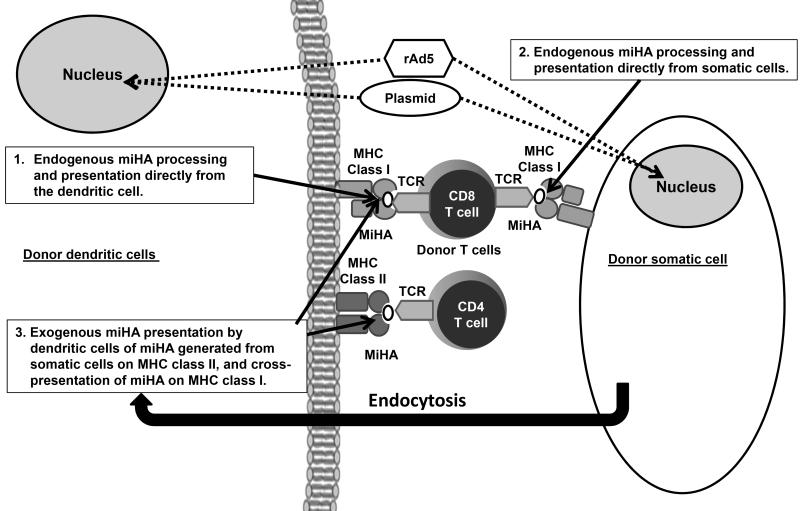
To develop a miHA vaccine, large sections of canine SMCY and the entire canine SRY gene were cloned into expression plasmids and replication-deficient human adenoviral vectors (rAd5; Figure 1 shows a diagram of how these constructs sensitize donor T cells). An overlapping peptide pool matrix covering the entire coding sequence of the cloned constructs was produced in order to evaluate immune responses using ELISpot and intracellular cytokine staining (ICS). Based on the antigen-specific responses that developed following the use of the rAd5 constructs and plasmids in two test females, we tested a prime-boost vaccination regimen in three female allogeneic HCT donors. These donors developed antigen-specific T cell responses and adoptive transfer of vaccine-“sensitized” donor T cells into their respective male mixed chimeric recipients resulted in a substantial increase in donor chimerism in one of three recipients.
Figure 1. Immunological mechanisms behind the miHA vaccine.
Donor T cell activation to the miHA-encoded in the vaccine constructs occurs by several mechanisms (55). Direct transfection with the expression plasmids or transduction with rAd5 of donor dendritic cells and somatic cells occurs following vaccination. The constructs are shuttled to the nucleus (dotted lines). This is followed by transcription, translation, peptide processing, and expression of miHAs on MHC class I via the endogenous pathway to donor CD8 T cells directly from the dendritic cells and somatic cells (solid arrows). The transfected or transduced somatic cells act as an antigen reservoir, producing miHAs that undergo endocytosis by dendritic cells that are then presented by the exogenous pathway on MHC class II or through cross-presentation on MHC class I (solid arrows).
MATERIALS AND METHODS
Experimental animals and DLA typing
Random-bred beagles and mini-mongrel cross breads were raised at the Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA or purchased commercially. Animals were housed in kennels certified by the American Association for Accreditation of Laboratory Animal Care. All study designs were approved by the Institutional Animal Care and Use Committee. Dog leukocyte antigen (DLA)-typing used highly polymorphic microsatellite markers within DLA class I (DLA 88) (22) and class II regions (DLA-DRB1) (23,24). Table 1 summarizes the DLA typing information.
Table 1.
DLA-typing summary
| Dog | Transplant | |
|---|---|---|
| Donor/Recipient | Dog LeukocyteAntigen | DRB1; DLA-88 |
| H619 | Test Female | 9/22; 01101/50101 |
| H581 | Test Female | 9/9; 01101/03801 |
| H380 | Female Donor 1 (D1) | 1/1; 50801/50801 |
| H382 | Male Recipient 1 (R1) | 1/1; 50801/50801 |
| H592 | Female Donor 2 (D2) | 9/15; 01101/01201 |
| H597 | Male Recipient 2 (R2) | 9/15; 01101/01201 |
| H353 | Female Donor 3 (D3) | 9/22; 03801/50101 |
| H519 | Male Recipient 3 (R3) | 9/22; 03801/50101 |
Minimal intensity DLA-identical HCT and chimerism analysis
On day 0, HCT recipients were treated with 200 cGy TBI, intravenous infusion of marrow from their respective DLA-identical siblings, and post-grafting immunosuppression consisting of oral cyclosporine at 15 mg/kg orally twice daily from days -1 to 35 and mycophenolate mofetil at 10 mg/kg twice daily injected subcutaneously from days 0 to 28 as previously described (25). Chimerism analyses were done on PBMCs and granulocytes following separation of blood on Ficoll (density =1.074), and quantified by fluorescent variable number of tandem repeat PCR analysis, as described (26).
Vaccine preparation and administration
Plasmids and rAd5 vectors expressing three domains of canine SMCY and the entire canine SRY gene were produced as described in Supplemental Digital Content (SDC) Table S1. The amino acid sequences are shown in Figure 2, and were injected into the dogs as follows:
(1) The rAd5 vectors were separated into three insulin syringes; one contained pooled rAd5 SMCY domains 1 and 2, another contained rAd5 SMCY domain 3, and the third contained rAd5 SRY. Each syringe injected 200 microliters intramuscularly (IM) into a separate muscle group (two flank muscles and one shoulder muscle).
(2) CD34-derived dendritic cells (DCs) were produced as described (27,28). DCs underwent transfection in P3 nucleoporation solution plus 4 μg of plasmid DNA (four plasmids mixed at equal concentrations) per 1×106 DCs per well in a 96-well shuttle using an AMAXA-96 well shuttle program set at FD-137 (Lonza, Basel, Switzerland). Post-transfection viability was assessed using Acridine Orange/Ethidium Bromide. DCs were suspended in 1 mL (1 mg) of Poly (I:C) HMW VacciGrade adjuvant (InvivoGen, San Diego, CA) and injected subcutaneously into the scruff of the neck. As a positive control for transfection efficiency, a small sample of DCs were transfected using 4 μg of the green fluorescent protein (GFP) control included in the kit, and analyzed following overnight culture by flow cytometry with data expressed as percent of cells expressing GFP.
(3) For in vivo transfection of plasmids, the four plasmids were mixed at equal concentrations, and 2 μg of plasmid DNA was coated onto 0.5 mg of 1 micron gold particles per cartridge, as described (29). The dogs underwent general anesthesia. Eight cartridges were then delivered to each side of the abdomen lateral to the mammary glands using the particle-mediated epidermal delivery device (PMED), Powderject XR1, set between 400 to 500 pounds per square inch (Powderject Vaccines, Inc., Middleton, WI), as described (30).
Figure 2. Protein sequences for SMCY and SRY.
Here is the alignment of the three cloned domains of canine SMCY with the X homologue SMCX, and the cloned SRY protein sequence, as there is no X chromosome homologue. Sequences of the domains were compared to the Canis Familiaris GenBank reference sequence DQ156494.1 for KDM5D (SMCY), and GenBank reference sequence AF107021.1 for SRY. The non-homologous disparities between SMCY and SMCX are underlined, and insertions or deletions between the homologues marked with gaps. As compared to the GenBank sequence for SMCY, the cloned domains contain two non-synonymous single nucleotide polymorphism changes: in domain 1, an arginine (R) to a glycine (G), and in domain 2, a methionine (M) to isoleucine (I), highlighted in light gray. SMCY domain 3 contained three intron sequences with the amino acid insertion site highlighted in dark gray.
Donor lymphocyte infusion (DLI)
Female donor dogs underwent general anesthesia, central venous catheter placement in the external jugular vein, and collection of 200 mL leukapheresis product, as described (COBE 2997; Blood Component Technology, Lakewood, CO) (31,32). Cell counts with differentials were obtained using an ADVIA 2120i (Siemens, Deerfield, IL). Recipients were pre-treated with diphenhydramine and the entire leukapheresis product was slowly infused.
Interferon-γ ELISpot and intracellular cytokine staining (ICS)
PBMCs were suspended in CTL-Test Medium (Cellular Technology Ltd, Shaker Heights, OH) and stimulated the same day with peptide or peptide pools prepared as described in SDC Table S2, or with the same amount of DMSO used to dissolve the peptide as a negative control. Phytohemagglutinin (PHA) at a final concentration of 25 μg/mL was used as a positive control. ELISpots were performed as described and counted with a Bioreader 5000 (Biosys, Miami, FL) (33). ICS was based on described methods using canine-specific antibodies for CD3, CD4, CD8, and IFN-γ (34,35). The samples were analyzed using a FACS Canto II (BD Biosciences, San Jose, CA). Data was analyzed using FlowJo Software (Treestar Ashland, OR).
RESULTS
IM prime injection of rAD5 constructs in two female dogs
Dog H619 was given an IM dose of 0.25×1010 rAd5 viral particles (VP) per construct resulting in a 1×1011 VP total dose (36). This dose was well tolerated and induced T cell responses that peaked at 4 weeks (Figure 3A), consistent with observations following rAd5 vaccination in humans (36). A second female dog, H581 was given a larger initial dose of 1×1012 rAd5 VP per construct (4×1012 VP total dose) with the rationale that a larger dose may produce a stronger response. The higher dose was also well tolerated but produced high background responses in the pooled peptide ELISpot assays at 2, 3, and even 4 weeks after the injection without an associated increase in either magnitude or length of a response to the encoded antigens (Figure 3B). At this stage of sensitization, we were unable to demonstrate a positive ELISpot to individual peptides within the positive peptide pools for either dog (not shown). At 6 weeks post rAd5 IM injection, responses against the peptide pools had declined to undetectable levels in both dogs (not shown).
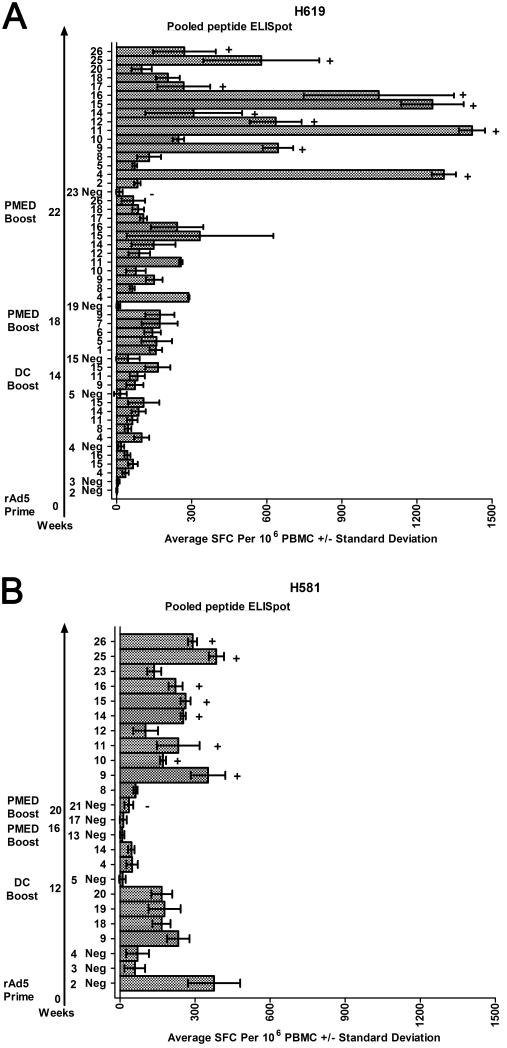
Figure 3.
Pooled peptide ELISpot results tracking an immune response to the encoded disparities following the vaccine series for H619 (A) and H581 (B). DMSO-negative (Neg) controls are shown for each time point tested. Only positive peptide pools are shown following the DMSO-negative control from that time point and defined as greater than 50 spot-forming cells (SFC) per 106 PBMCs on average, with non-overlapping standard deviations with the DMSO-negative control. The arrow plots out the time in weeks following the prime and boost vaccinations and the subsequent pooled peptide ELISpot assays. Specific pools underwent ICS confirmation. A plus sign (+) indicates a positive ICS response and a minus sign (−) indicates a negative ICS response (ICS examples shown in Figure 4 and data listed in Table 2).
DC boost of expression plasmids in two female dogs
We sought to determine whether marrow-derived DCs transfected with the expression plasmids could boost the immune response. The two female dogs initially treated with rAd5 constructs were boosted with sequential daily subcutaneous injections of transfected DCs along with the toll-like receptor 3 agonist poly(I:C) as an adjuvant similar to a previously reported method (37). Bilateral marrow aspiration, CD34+ selection, culture, and harvest produced sufficient DCs for a two day subcutaneous injection of DCs for H619 as follows: day 1, 2.75×106 DCs with 55% transfection efficiency (TE), and day 2, 2.3×106 DCs with 16% TE (week 14, Figure 3A). For H581, the same procedure produced a single injection of 1.82×106 DCs with 11% TE (week 12, Figure 3B). Positive pooled ELISpot responses were shown one week after this vaccination for H619 (week 15, Figure 3A). By two weeks after the injection in H619, the number and magnitude of the positive pools by ELISpot had decreased (not shown). No responses were observed for H581 (week 13, Figure 3B).
PMED boost of expression plasmids in two female dogs
We next tested the immune response following in vivo cutaneous delivery by PMED. Both H619 and H581 underwent PMED-DNA vaccination at weeks 18 and 16, respectively, after the rAd5 prime injections. H619 demonstrated a robust response with multiple positive peptide pools (week 19, Figure 3A). Positive responses were shown for individual peptides within the peptide pools and confirmed by ICS (not shown). No positive response was seen for H581 at week 17 (Figure 3B). To further confirm the PMED method of delivery and to demonstrate a response in a second female dog, both H619 and H581 underwent a second PMED vaccination at weeks 22 and 20, respectively. Both H619 and H581 demonstrated a robust response with multiple positive peptide pools at weeks 23 and 21, respectively. The responses were confirmed by ICS with the majority of these responses due to CD4+ T cells, although a CD8 component to the response was evident within pools 15 and 16 for H619 and within pool 15 for H581 (Figure 3, Figure 4, and Table 2).
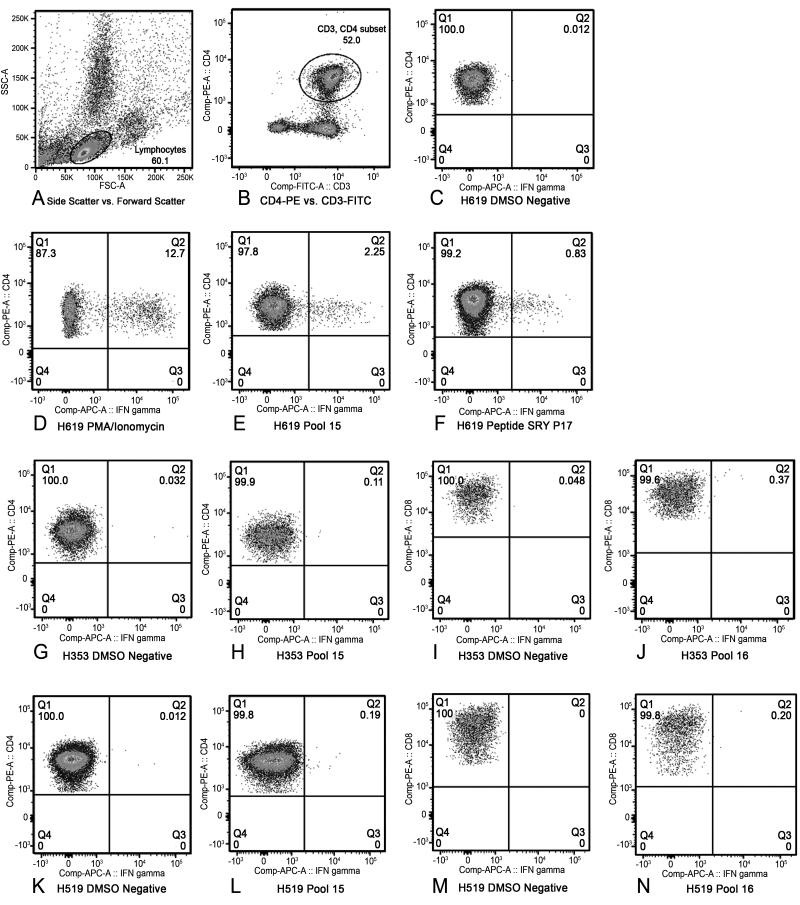
Figure 4. Three-color intracellular cytokine staining for interferon-γ to confirm ELISpot results.
For each sample, a lymphocyte gate was drawn in the forward scatter and side scatter plot (A). All samples were stained with CD3-FITC, and either CD4-PE or CD8-PE. The CD4 or CD8+ T cells were gated from the lymphocyte gate as shown (B). All samples underwent intracellular cytokine staining for interferon-γ (APC). The percentage of interferon-γ-expressing CD4+ or CD8+ T cells were then determined by placing a gate with an adequate proximity to the DMSO-negative control (C) and maintaining that same gate for all samples tested. A positive response was defined as greater than or equal to 0.1% interferon-γ-expressing CD3+CD4+ or CD3+CD8+ T cells as long as the DMSO negative control for that sample remained below 0.05%. Phorbol myristate acetate (PMA) at 100 ng/mL and Ionomycin at 1 μg/mL was used as a positive control (D). The percentage of interferon-γ CD4+ T cells of H619 responding to pool 15 (E) and peptide SRY P17 (F). The percentage of interferon-γ CD4+ T cells of D3 (H353) responding to pool 15 (G-H) and percentage of interferon-γ CD8+ T cells responding to pool 16 (I-J). The percentage of interferon-γ CD4+ T cells of R3 (H519) responding to pool 15 (K-L) and percentage of interferon-γ CD8+ T cells responding to pool 16 (M-N). The remainder of the data is listed in table format (Table 2).
Table 2.
Summary of the intracellular cytokine staining results.
| Dog | Corresponding ELISpot Figure |
Time Point (Week) |
Condition | CD3+CD4+ %IFN-γ |
CD3+CD8+ %IFN-γ |
|---|---|---|---|---|---|
| H619 | 3A | 23 | Negative | 0.01 | 0.04 |
| PMA/Ionomycin | 12.7 | 12 | |||
| Pool 4 | 0.42 | 0.08 | |||
| Pool 9 | 0.37 | 0.04 | |||
| Pool 11 | 1.86 | 0.05 | |||
| Pool 12 | 0.46 | 0.08 | |||
| Pool 14 | 0.41 | 0.06 | |||
| Pool 15 | 2.25 | 0.17 | |||
| Pool 16 | 1.94 | 0.11 | |||
| Pool 17 | 0.21 | 0.04 | |||
| Pool 25 | 0.9 | 0.04 | |||
| Pool 26 | 0.54 | 0.01 | |||
| 6A | SMCY D3P1 | 0.04 | 0.01 | ||
| SMCY D2P34 | 0.3 | 0.06 | |||
| SMCY D2P35 | 0.46 | 0.07 | |||
| SMCY D2P36 | 0.33 | 0.08 | |||
| SMCY D2P37 | 0.07 | 0.03 | |||
| SMCY D3P60 | 0.13 | 0.06 | |||
| SRY P2 | 0.1 | 0.04 | |||
| SRY P17 | 0.83 | 0.01 | |||
| SRY P18 | 0.80 | 0.07 | |||
| SRY P27 | 0.55 | 0.04 | |||
| H581 | 3B | 21 | Negative | 0.06 | 0.04 |
| PMA/Ionomycin | 3.29 | 2.66 | |||
| Pool 9 | 0.13 | 0.05 | |||
| Pool 10 | 0.1 | 0.03 | |||
| Pool 11 | 0.18 | 0.08 | |||
| Pool 14 | 0.17 | 0.05 | |||
| Pool 15 | 0.22 | 0.15 | |||
| Pool 16 | 0.13 | 0.05 | |||
| Pool 25 | 0.21 | 0.02 | |||
| Pool 26 | 0.08 | 0.08 | |||
| 6B | SMCY D3P60 | 0.14 | 0.01 | ||
| SMCY D3P61 | 0.16 | 0.02 | |||
| SRY P1 | 0.07 | 0.08 | |||
| SRY P2 | 0.13 | 0.1 | |||
| SRY P3 | 0.13 | 0.09 | |||
| SRY P4 | 0.06 | 0.04 | |||
| SRY P17 | 0.04 | 0.04 | |||
| H380 (D1) |
7A | 12 | Negative | 0.04 | 0.05 |
| PMA/Ionomycin | 13.44 | 15.09 | |||
| Pool 2 | 0.02 | 0.46 | |||
| Pool 6 | 0 | 0.13 | |||
| Pool 8 | 0.03 | 0.27 | |||
| Pool 20 | 0.03 | 0.37 | |||
| H592 (D2) |
7B | 12 | Negative | 0.02 | 0 |
| PMA/Ionomycin | 1.24 | 4.62 | |||
| Pool 10 | 0.03 | 0.13 | |||
| Pool 16 | 0.02 | 0.02 | |||
| Pool 17 | 0.04 | 0.05 | |||
| H353 (D3) |
7C | 12 | Negative | 0.03 | 0.05 |
| PMA/Ionomycin | 1.5 | 2.18 | |||
| Pool 9 | 0.11 | 0.04 | |||
| Pool 15 | 0.11 | 0.04 | |||
| Pool 16 | 0 | 0.37 | |||
| H519 (R3) |
7C | 26 | Negative | 0.01 | 0 |
| PMA/Ionomycin | 5.19 | 10.36 | |||
| Pool 15 | 0.19 | 0.07 | |||
| Pool 16 | 0.05 | 0.2 |
Confirming the pooled ELISpot results with individual peptide responses
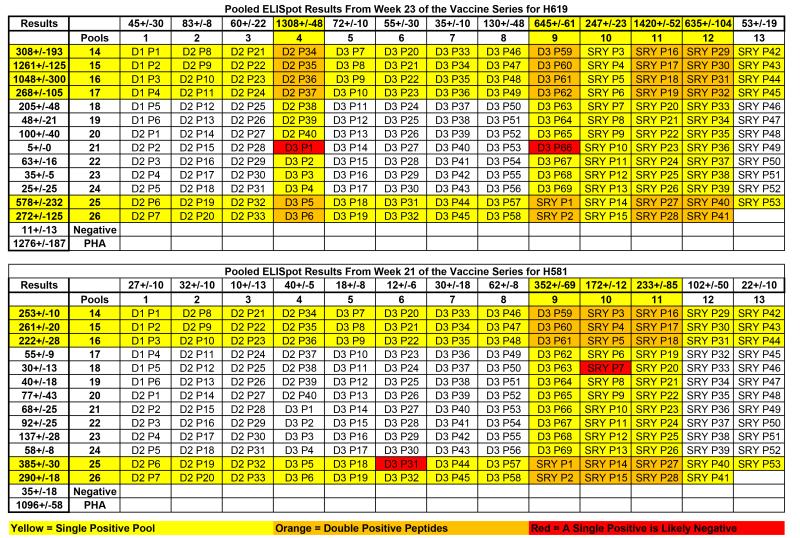
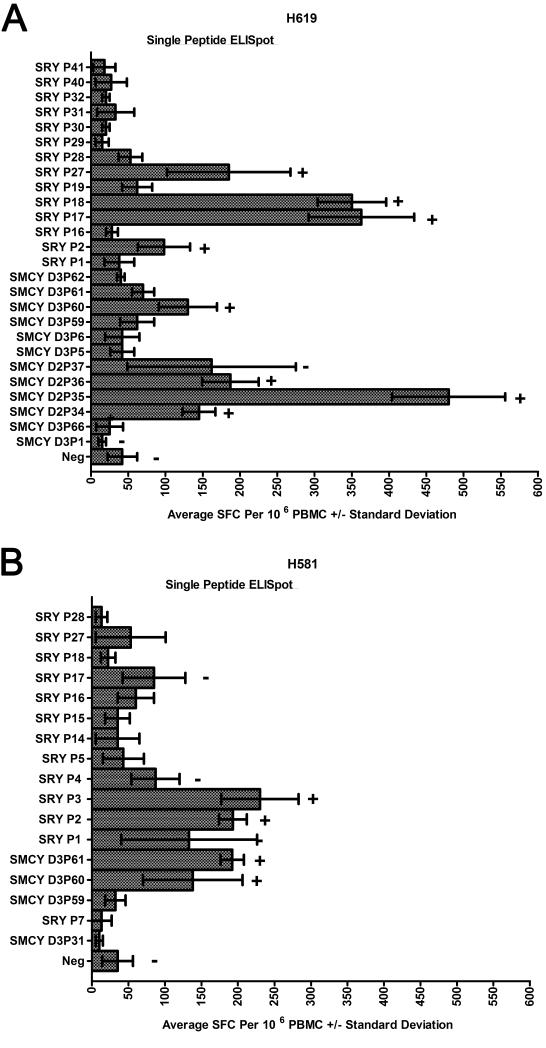
Next, we confirmed responses against individual peptides from within the positive peptide pools for both H619 and H581 at weeks 23 and 21, respectively. The data are presented in a 13 × 13 peptide pool matrix of overlapping peptides; each column and each row displays the peptides included in a pool (Figure 5). Strongly positive pools are highlighted in yellow. Individual peptides found at the intersections of two strongly positive pools (highlighted in orange) underwent single peptide ELISpot confirmation (Figure 6). Two peptides that overlapped a negative and positive pool, thus predicted not to elicit a single peptide response, are highlighted in red and included in the individual peptide analysis (Figure 6).
Figure 5.
ELISpot results from week 23 for H619 (Top), and week 21 for H581 (Bottom) are shown in context of the 13 × 13 peptide pool matrix of overlapping 15-mer peptides. Results are expressed as average spot-forming cells per 106 PBMC +/− standard deviation for pools 1-13 across and 14-26 down. The DMSO-negative control and a PHA-positive control were included as a reference. Extremely positive pools are highlighted in yellow. Overlapping peptides that were positive in both arms of the matrix are highlighted in orange and underwent single peptide ELISpot confirmation (see Figure 6). As further validation of the matrix, two peptides that are in a positive peptide pool, but are predicted to be non-responsive due to the presence in a negative pool, are highlighted in red and used as negative peptide controls in the single-peptide ELISpot assays (see Figure 6).
Figure 6.
Single peptide ELISpot results for week 23 of the vaccine series for H619 (A) and week 21 for H581 (B). The orange and red highlighted individual peptides from Figure 4 are shown following the DMSO-negative (Neg) control on the Y-axis. The X-axis presents the data as average SFCs per 106 PBMCs +/− standard deviation. Specific peptide responses underwent ICS confirmation. A plus sign (+) indicates a positive ICS response and a minus sign (−) indicates a negative ICS response (ICS examples shown in Figure 4 and data listed in Table 2).
Responses against individual peptides were defined for each targeted pool and verified by ICS (Figure 4, Table 2). As predicted, the red highlighted peptides were negative by individual peptide ELISpots in both dogs. In contrast, positive responses were shown from within the orange highlighted peptides (Figure 6). H619 responses to individual peptides mapped to SMCY domain 2 (D2) peptides 34-37 (P34-37), SMCY domain 3 (D3) peptide 60 (P60), SRY peptides 17 and 18 (P17-18), and SRY peptide 27 (P27) (Figure 6A). All of these individual peptides were confirmed by ICS and showed a CD3+CD4+IFN -γ response with the exception of SMCY D2P37 that was negative by ICS. In this manner, most peptide pool responses were accounted for by individual peptides highlighted in orange for H619 at week 23. In a similar fashion for H581 at week 21 (Figure 6B), responses against individual peptides were detected by ELISpot from within the orange highlighted peptides and confirmed by ICS (Table 2). From these experiments, responses were shown to individual peptides encoded within three of the four constructs including SMCY domain 2, SMCY domain 3, and SRY.
PMED prime viral boost vaccine study in female donors
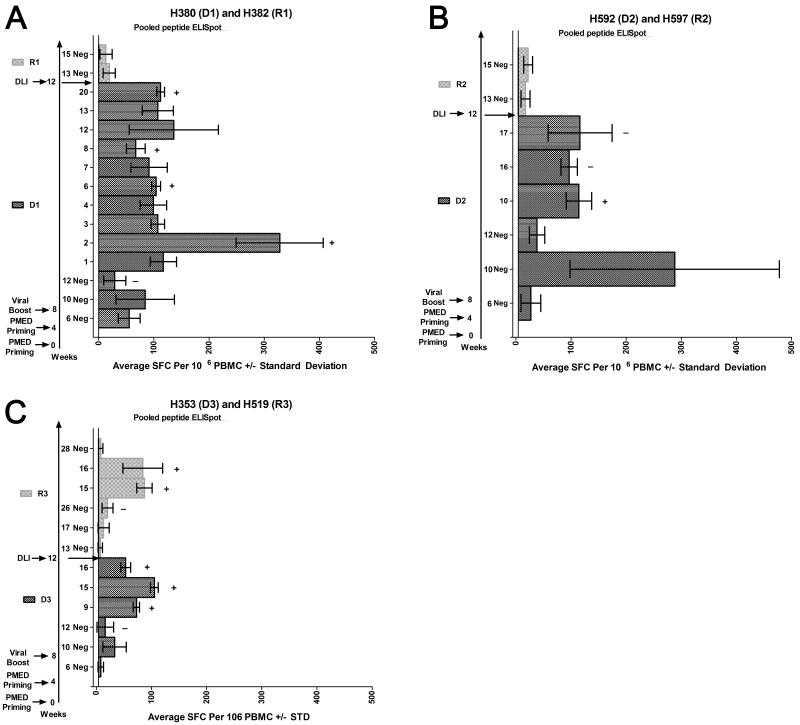
Next, we posed the question whether a prime-boost vaccine was an effective means to produce a sensitized DLI and whether injecting vaccine-sensitized female donor cells into a male mixed chimeric recipient could convert the recipient to full donor chimerism. To this end, three female HCT donors 39, 8, and 6 months following marrow donation underwent two rounds of PMED-plasmid DNA priming separated by four weeks, and then four weeks later underwent a rAd5 IM boost injection of 0.25×1010 VP per construct (1×1011 VP total dose). Pooled peptide ELISpot analyses were performed for each donor dog six weeks after the start of the vaccine regimen, corresponding to two weeks after the second PMED-DNA prime. No responses were detected at week 6. By week 10, two weeks after the rAd5 boost, only high backgrounds were observed for negative controls, consistent with the highly stimulatory capacity of an IM injection of 1×10^11 VP. However, by four weeks after the rAd5 boost, corresponding to 12 weeks after initiation of the vaccine regimen, significant and specific ELISpot responses were apparent for all three donor dogs. For the first donor (D1), ELISpot responses were shown against multiple pools, and there was sufficient sample to confirm pools 2, 6, 8, and 20 as CD8+ responses by ICS (Figure 7A and Table 2). For D2, pools 10, 16, and 17 were positive by ELISpot, and pool 10 was confirmed by ICS as a CD8+ response (Figure 7B, and Table 2). For D3, pools 9, 15, and 16 were positive by ELISpot. Pools 9 and 15 were confirmed as a CD4+ response and pool 16 as a CD8+ response by ICS (Figure 7C, Figure 4G-J, and Table 2).
Figure 7.
Pooled peptide ELISpot results following a heterologous prime boost vaccine regimen in female sibling donors and subsequent DLI into their respective male mixed chimeric recipients. DMSO-negative (Neg) controls were included for each time point tested. Positive peptide pools follow the DMSO-negative control from that time point and were defined as greater than 50 SFCs per 106 PBMCs on average with non-overlapping standard deviations with the DMSO-negative control. Specific pooled responses underwent ICS confirmation. A plus sign (+) indicates a positive ICS response and a minus sign (−) indicates a negative ICS response (ICS examples shown in Figure 4 and data listed in Table 2).
DLI into stable male mixed chimeric recipients
The male transplant recipients H382 (R1), H597 (R2), and H519 (R3) demonstrated stable mixed chimerism off all immunosuppression without any signs of GVHD for 42, 11, and 9 months after transplant at the time of the DLI, respectively. Following vaccine-mediated sensitization of the donors as described above, R1 received 1.2 ×108 leukocytes/kg, R2 received 3.2×108 leukocytes/kg, and R3 received 0.7×108 leukocytes/kg. Refer to SDC Table S3 for the cell differential.
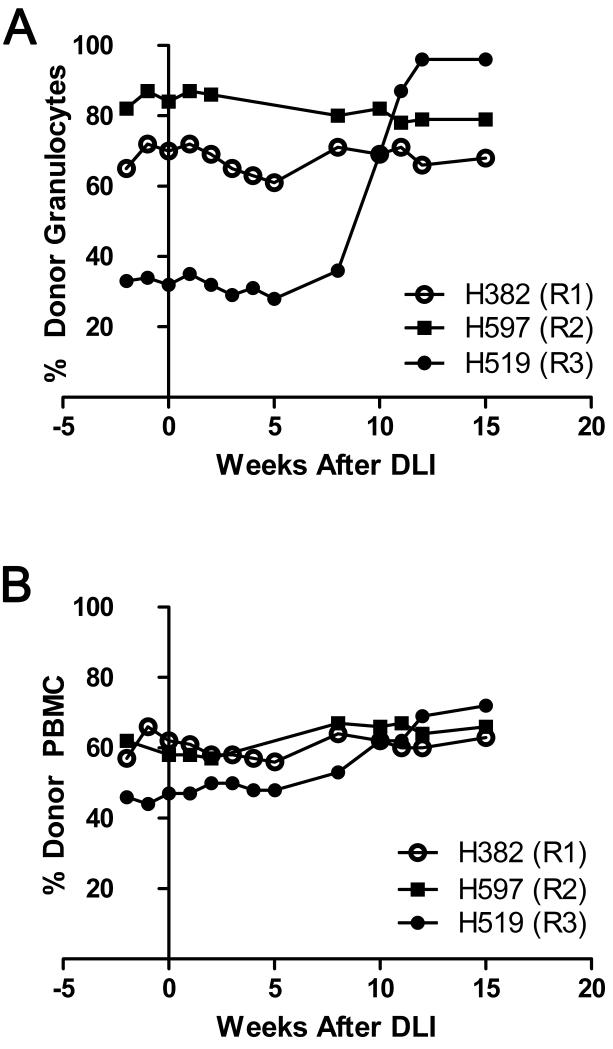
Following DLI, recipients were evaluated by pooled peptide ELISpot, analysis of donor chimerism, and monitored for development of GVHD. Pooled ELISpot analyses were performed at 1 and 5 weeks post-DLI, corresponding to weeks 13 and 17 from the beginning of the vaccine series in the donor. No responses were observed in any of the three recipients (Figure 7). However, R3 began to demonstrate a change in donor chimerism starting eight weeks and completed by 13 weeks after the DLI. For R3, donor granulocytes increased from 30% to 90%, and the donor PBMCs from 50% to 70%. Donor chimerism did not change in the other two recipients (Figure 8). A pooled peptide ELISpot was performed 14 weeks after the DLI in R3, corresponding to week 26 since the beginning of the vaccine series in the donor. Peptide pools 15 and 16 were positive by ELISpot. Pool 15 was confirmed as a CD4+ response and pool 16 was confirmed as a CD8+ response by ICS. Of note, R3’s female donor, D3, had the same CD4+ response to pool 15 and CD8+ response to pool 16 at the time of the DLI (Figure 7C, Figure 4KN, and Table 2). As responses in R3 were generally low, we were unable to show an ELISpot response against a single peptide contained within the positive pools from either R3 or D3 (not shown). No positive pooled ELISpot responses were seen in R3 by 16 weeks after the DLI, corresponding to 28 weeks since the beginning of the vaccine series (Figure 7C). No GVHD was observed in any of the recipients.
Figure 8. Chimerism results in the 3 male mixed chimeric recipients following DLI.
Percent donor granulocytes (A) and percent donor PBMCs (B) are shown on the Y-axis, with weeks after DLI shown on the X-axis.
DISCUSSION
MiHA vaccination of the allogeneic HCT recipient with antigens expressed only on hematopoietic cells represents an approach to produce GVT responses without GVHD for the treatment of relapsed or refractory hematologic malignancies after MHC-matched allogeneic HCT. However, this requires a vaccine regimen that can produce antigen-specific T cells that drive in vivo responses in hosts that have donor T cells undergoing immune reconstitution, pharmacologic immunosuppression, and actively developing tolerance to the targeted antigens. Although there is scientific rationale that suggests this is obtainable, we focused our initial studies on first establishing a miHA vaccine regimen in the donor.
These studies describe the first in vivo response to miHA vaccination in a large, outbred animal model without using recipient cells, and justify the need for future experiments. This study focused on the well-established T cell-mediated responses of donor T cells to miHAs. Future studies are needed to evaluate .for humoral responses (38,39), expand our study with a larger numbers of animals, and determine the reason why partial donor conversion occurred in only 1 of 3 transplant recipients. One potential explanation is that the donors were inadequately sensitized and extra rounds of sensitization are needed to drive conversion of chimerism in the recipient following DLI. Consistent with this hypothesis are the weak pooled ELISpot responses in the three vaccinated donors compared to the robust responses to single peptides shown for the initial two test female dogs that each received an extra round of sensitization. Alternatively, DLA restriction may play a role in how efficiently a miHA is presented. Notably, the DLA for the recipient that underwent partial conversion included DRB1 9/22 and DLA-88 03801/50101, while the initial test female H619 that produced the most robust responses also expressed DRB1 9/22 and DLA-88 03801. Thus, one or more of these DLA alleles may be capable of driving a response against a vaccine-encoded epitope with a minimal number of vaccinations. Another possibility is that the effect of a DLI using ubiquitously expressed antigens is blunted as compared to miHAs primarily expressed on hematopoietic cells (13,14). This may explain why R3 experienced only a partial versus a full donor conversion.
Putting aside the ethical limitations, miHA vaccination of human transplant donors followed by adoptive transfer into the recipient would overcome many of the challenges of establishing a miHA vaccine in the transplant recipient. First, miHA vaccination of human transplant donors is likely safe, as miHA sensitization is a naturally occurring phenomenon during pregnancy (40,41) and also occurs following infusion of non-irradiated blood products (42). Second, murine studies have shown that adoptive transfer of CD8+ memory T cells from donors vaccinated against a single miHA (H60) eliminated implanted tumors without GVHD, and was proposed as an alternative approach to ex vivo engineering (13). Third, numerous studies have shown responses to tumor-associated antigens delivered by DCs (43), plasmids (44), or viral vectors (45), and analogous approaches using miHAs are planned or ongoing (46). Fourth, we have shown that the canine model of mixed hematopoietic chimerism provides a large animal model to develop a miHA vaccine (18,20). This study adds to our understanding of this approach, demonstrating that plasmid- and viral-based methods produce miHA-specific responses and in vivo effects following DLI.
The clinical application of a miHA vaccine will likely require targeting hematopoietic-restricted miHAs. In humans, the chance of having at least one mismatch for one of the eight known hematopoietic system-restricted miHAs has been reported to be 21.2% and 33.6% in the Caucasian population for MHC-matched sibling donor-recipients and MHC-matched unrelated donor-recipients, respectively (47). To identify this type of variation in the dog, we plan to cross-reference next generation sequencing data and newly released single nucleotide polymorphism data with tissue expression microarray data (http://www.broad.mit.edu/mammals/dog/snp/) (48). Non-synonymous disparities in genes expressed primarily in hematopoietic cells are ideal candidate vaccine targets. Preliminary expression array analysis has identified several hundred transcripts that are expressed at significant levels in PBMCs, but not in common GVHD target tissues including gut and skin epithelium, and saline-perfused liver (unpublished results). Targeting these variations in a multi-epitope vaccine (49) using our prime/boost regimen will directly evaluate whether targeting hematopoietic-restricted variations will generate a robust GVT-like response with little or no GVHD.
Widespread application of a miHA vaccine is likely to require vaccination of the allogeneic HCT recipients, entailing a therapy that overcomes T regulatory-induced tolerance without affecting the ability of resident donor T cells to respond to the vaccine. One potential approach is suggested by our knowledge of MHC-matched allogeneic HCT. For example, we have shown that 200 cGy TBI overcomes T regulatory cell tolerance for a short period of time, (17,50,51). Also, 200 cGy TBI provided as part of HCT conditioning regimens does not prevent resident T cells from responding to miHAs, as demonstrated by the requirement of post-grafting immunosuppression of recipients in order to prevent graft rejection (16). Collectively, these observations suggest that 2 Gy TBI overcomes T regulatory cell tolerance but not the ability of donor T cells to respond to miHAs, thus offering a window to test a miHA vaccine directly in the recipient. The evolution of novel vaccination techniques (52,53) and manipulation of costimulatory molecules (54) used for cancer immunotherapy may also suggest strategies to induce miHA sensitization in the setting of T regulatory cell tolerance in the recipient.
In conclusion, vaccination with specific miHAs unique to the recipient holds promise as a future therapy to separate GVT from GVHD. However, translating this knowledge into a clinical protocol carries significant risk. We will address these risks by developing a miHA vaccination regimen in a canine model of allogeneic HCT. This platform allows experiments testing a variety of miHA targets, including variations that are expressed primarily on hematopoietic cells. Finally, we plan to explore methods for direct sensitization of the recipient against specific miHAs, thereby eliminating the requirement for donor vaccination and subsequent DLI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Michele Spector, DVM and the technicians at the Fred Hutchinson Cancer Research Center canine facility for caring for the dogs used in our experiments. We want to thank Dr. Nicole Frahm for assistance in developing a canine-specific ELISpot. We also thank Helen Crawford and Bonnie Larson for their help with article preparation.
Funding: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD, grant P01CA078902, P30CA015704, K12CA76930, P30DK56465, support from Gabrielle’s Angel Foundation for Cancer Research (SLR) and by awards from the Joseph Steiner Krebsstiftung, Bern, Switzerland and Lupin Foundation, Metairie, LA (RS).
Abbreviations
- rAd5
replication-deficient human adenovirus type 5
- PMED
particle mediated epidermal delivery device or “gene gun”
Footnotes
Disclosure: The manuscript was neither prepared nor funded in any part by a commercial organization, including educational grants. The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.Goulmy E, Termijtelen A, Bradley BA, van Rood JJ. Y-antigen killing by T cells of women is restricted by HLA. Nature. 1977;266:544. doi: 10.1038/266544a0. [DOI] [PubMed] [Google Scholar]
- 2.Storb R, Rudolph RH, Kolb HJ, et al. Marrow grafts between DL-A-matched canine littermates. Transplantation. 1973;15:92. doi: 10.1097/00007890-197301000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 4.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:832, 895. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood. 1995;86:2041. [PubMed] [Google Scholar]
- 6.Minor Histocompatibility Antigens: From the Laboratory to the Clinic. Landes Bioscience; Georgetown, Texas, USA: 2000. [Google Scholar]
- 7.Goulmy E. Minor histocompatibility antigens: allo target molecules for tumor-specific immunotherapy (Review) Cancer Journal. 2004;10:1. doi: 10.1097/00130404-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Falkenburg JH, Wafelman AR, Joosten P, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94:1201. [PubMed] [Google Scholar]
- 9.Marijt E, Wafelman A, van der Hoorn M, et al. Phase I/II feasibility study evaluating the generation of leukemia-reactive cytotoxic T lymphocyte lines for treatment of patients with relapsed leukemia after allogeneic stem cell transplantation. Haematologica. 2007;92:72. doi: 10.3324/haematol.10433. [DOI] [PubMed] [Google Scholar]
- 10.Meij P, Jedema I, van der Hoorn MA, et al. Generation and administration of HA-1-specific T-cell lines for the treatment of patients with relapsed leukemia after allogeneic stem cell transplantation: a pilot study. Haematologica. 2012;97:1205. doi: 10.3324/haematol.2011.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietiic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Loenen MM, Hagedoorn RS, Kester MG, et al. Kinetic preservation of dual specificity of coprogrammed minor histocompatibility antigen-reactive virus-specific T cells. Cancer Res. 2009;69:2034. doi: 10.1158/0008-5472.CAN-08-2523. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine P, Roy-Proulx G, Knafo L, Baron C, Roy DC, Perreault C. Adoptive transfer of minor histocompatibility antigen-specific T lymphocytes eradicates leukemia cells without causing graft-versus-host disease. Nat Med. 2001;7:789. doi: 10.1038/89907. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Matte-Martone C, Zheng H, et al. Memory T cells from minor histocompatibility antigen-vaccinated and virus-immune donors improve GVL and immune reconstitution. Blood. 2011;118:5965. doi: 10.1182/blood-2011-07-367011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas ED. Nobel Lecture: Bone Marrow Transplantation ? Past, Present and Future. In: Frängsmyr T, Linsten J, editors. Nobel Lectures, Physiology or Medicine 1981-1990. World Scientific Publishing; Singapore: 1993. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1990/thomas-lecture.html-Date Accessed: <enter today’s date>. [Google Scholar]
- 16.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048. [PubMed] [Google Scholar]
- 17.Lesnikova M, Nikitine A, Mason N, Nash RA, Georges GE. Ex vivo expanded T regulatory (Treg) cells block conversion of mixed chimeras to complete donor chimerism. Blood (ASH Annual Meeting Abstracts) 2006;108:5168. (abstract) [Google Scholar]
- 18.Lesnikova M, Nikitine A, Pogosov L, Nash RA, Georges GE. Peripheral CD4+CD25+ regulatory T cells (Treg) block alloreactive host anti-donor T cells in canine mixed hematopoietic chimeras. Blood (ASH Annual Meeting Abstacts) 2005;106:3101. (abstract) [Google Scholar]
- 19.Georges GE, Storb R, Thompson JD, et al. Adoptive immunotherapy in canine mixed chimeras after nonmyeloablative hematopoietic cell transplantation. Blood. 2000;95:3262. [PubMed] [Google Scholar]
- 20.Weiden PL, Storb R, Tsoi M-S, Graham TC, Lerner KG, Thomas ED. Infusion of donor lymphocytes into stable canine radiation chimeras: Implications for mechanism of transplantation tolerance. J Immunol. 1976;116:1212. [PubMed] [Google Scholar]
- 21.Graves SS, Hogan WJ, Kuhr C, et al. Adoptive immunotherapy against allogeneic kidney grafts in dogs with stable hematopoietic trichimerism. Biol Blood Marrow Transplant. 2008;14:1201. doi: 10.1016/j.bbmt.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkataraman GM, Stroup P, Graves SS, Storb R. An improved method for dog leukocyte antigen 88 typing and two new major histocompatibility complex class I alleles, DLA-88*01101 and DLA-88*01201. Tissue Antigens. 2007;70:53. doi: 10.1111/j.1399-0039.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 24.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 25.Sorror ML, Leisenring W, Mielcarek M, et al. Intensified postgrafting immunosuppression failed to assure long-term engraftment of dog leukocyte antigen-identical canine marrow grafts after 1 gray total body irradiation. Transplantation. 2008;85:1023. doi: 10.1097/TP.0b013e318169be24. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701. [PubMed] [Google Scholar]
- 27.Ladiges WC, Storb R, Graham T, Thomas ED. Experimental techniques used to study the immune system of dogs and other large animals. In: Gay WI, Heavener JE, editors. Methods of Animal Experimentation. Academic Press; New York, NY: 1989. p. 103. [Google Scholar]
- 28.Georges GE, Lesnikova M, Storb R, et al. Minor-histocompatibility antigen (miHA)-specific cytotoxic t-lymphocytes (CTL) generated with dendritic cells (DC) between dog leukocyte antigen (DLA)-indentical littermates. Blood. 2000;96(Part 1):770a. doi: 10.1053/bbmt.2003.50023. #3332 (abstract) [DOI] [PubMed] [Google Scholar]
- 29.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 30.Fuller DH, Murphey-Corb M, Clements J, Barnett S, Haynes JR. Induction of immunodeficiency virus-specific immune responses in rhesus monkeys following gene gun-mediated DNA vaccination. Journal of Medical Primatology. 1996;25:236. doi: 10.1111/j.1600-0684.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 31.Sandmaier BM, Storb R, Santos EB, et al. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508. [PubMed] [Google Scholar]
- 32.Lupu M, Gooley T, Zellmer E, Graves SS, Storb R. Principles of peripheral blood mononuclear cell apheresis in a preclinical canine model of hematopoietic cell transplantation. J Vet Intern Med. 2008;22:74. doi: 10.1111/j.1939-1676.2007.0016.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Storb R, Tapscott SJ, Riddell S. Analyzing cellular immunity to AAV in a canine model using ELISPOT assay. In: Kalyuzhny AE, editor. Handbook of ELISPOT: Methods and Protocols. Springer; Clifton, NJ: 2012. p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastwood D, Bird C, Dilger P, et al. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. Br J Clin Pharmacol. 2013;76:299. doi: 10.1111/bcp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 36.Peiperl L, Morgan C, Moodie Z, et al. Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054) PLoS ONE [Electronic Resource] 2010;5:e13579. doi: 10.1371/journal.pone.0013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wick DA, Martin SD, Nelson BH, Webb JR. Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C) Vaccine. 2011;29:984. doi: 10.1016/j.vaccine.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 41.Mommaas B, Stegehuis-Kamp JA, van Halteren AG, et al. Cord blood comprises antigen-experienced T cells specific for maternal minor histocompatibility antigen HA-1. Blood. 2005;105:1823. doi: 10.1182/blood-2004-07-2832. [DOI] [PubMed] [Google Scholar]
- 42.Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. J Immunol. 1970;105:627. [PubMed] [Google Scholar]
- 43.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 44.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer (Review) Nature Reviews. 2008;Cancer. 8:108. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 45.Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines (Review) Cancer Journal. 2011;17:359. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hambach L, Goulmy E. Immunotherapy of cancer through targeting of minor histocompatibility antigens (Review) Curr Opin Immunol. 2005;17:202. doi: 10.1016/j.coi.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Spierings E, Goulmy E. Minor histocompatibility antigen typing by DNA sequencing for clinical practice in hematopoietic stem-cell transplantation. In: Christiansen FT, Tait BD, editors. Immunogenetics: Methods and Applications in Clinical Practice. Springer Science+Business Media; New York: 2012. p. 509. [DOI] [PubMed] [Google Scholar]
- 48.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 49.Suhrbier A. Multi-epitope DNA vaccines (Review) Immunology & Cell Biology. 1997;75:402. doi: 10.1038/icb.1997.63. [DOI] [PubMed] [Google Scholar]
- 50.Graves SS, Mathes DW, Georges GE, et al. Long-term tolerance to kidney allografts after induced rejection of donor hematopoietic chimerism in a preclinical canine model. Transplantation. 2012;94:562. doi: 10.1097/TP.0b013e3182646bf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taranova AG, Georges GE, Yunusov M, Storb RF, Nash RA. Breaking tolerance in stable mixed chimeric dogs with low-dose TBI and donor or recipient lymphocyte infusion. Blood. 2003;102(Part 1):76a. #256 (abstract) [Google Scholar]
- 52.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 53.Murata S, Ladle BH, Kim PS, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 54.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways (Review) Immunol Rev. 2008;224:166. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 55.Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens (Review) Nat Immunol. 2004;5:678. doi: 10.1038/ni1082. [DOI] [PubMed] [Google Scholar]
- 56.Jager L, Hausl MA, Rauschhuber C, Wolf NM, Kay MA, Ehrhardt A. A rapid protocol for construction and production of high-capacity adenoviral vectors. Nature Protocols. 2009;4:547. doi: 10.1038/nprot.2009.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.