Abstract
Nineteen PCBs (chiral or C-PCBs) exist as two stable rotational isomers (atropisomers) that are non-superimposable mirror images of each other. C-PCBs are released into the environment as racemic (i.e., equal) mixtures of both atropisomers and undergo atropisomeric enrichment due to biological, but not abiotic processes. In particular toxicokinetic studies provide important, initial insights into atropselective processes involved in the disposition (i.e., absorption, distribution, biotransformation and excretion) of C-PCBs. The toxicokinetic of C-PCBs is highly congener and species dependent. In particular at lower trophic levels, abiotic processes play a predominant role in C-PCB toxicokinetics. Biotransformation plays an important role in the elimination of C-PCBs in mammals. The elimination of C-PCB follows the approximate order mammals > birds > amphibians > fish, mostly due to a corresponding decrease in metabolic capacity. A few studies have shown differences in the toxicokinetics of C-PCB atropisomers; however, more work in needed to understand the toxicokinetics of C-PCBs and the underlying biological processes. Such studies will not only contribute to our understanding of the fate of C-PCBs in aquatic and terrestrial food webs, but also facilitate our understanding of human exposures to C-PCBs.
Keywords: Chirality, persistent organic pollutant, pharmacokinetics, enantioselective, invertebrate, vertebrate, fish, bird, mammals, humans, disposition, absorption, metabolism, excretion
Introduction
Polychlorinated biphenyls (PCBs) are a group of legacy chemicals that are still relevant for human and environmental health. They were manufactured by batch chlorination of biphenyl, resulting in complex chemical mixtures containing 140 to 150 of the 209 possible PCB congeners (Frame et al. 1996). PCBs are chemically and thermally stable and, therefore, persist in the environment. Like other persistent organic pollutants (POPs), PCBs bioconcentrate in living organisms and biomagnify in terrestrial and aquatic food chains due to their lipophilicity. Their industrial production was banned in the late 1970s due to raising environmental and human health concerns. However, recent studies demonstrate that PCBs are inadvertently generated as part of certain industrial processes, such as the production of paint pigments and adhesives (Anezaki et al. 2014, Anezaki and Nakano 2014, 2015, Hu and Hornbuckle 2010). As a result, PCBs are still present in the environment, resulting in ongoing human exposure through the air (Hu et al. 2010, Thomas et al. 2012) and/or diet (Schecter et al. 2010, Su et al. 2012). Laboratory and epidemiological studies have implicated exposure to PCBs in a range of adverse health effects in humans, including cancer as well as neurological, reproductive, endocrine and other non-cancer effects (Agency for Toxic Substances and Disease Registry 2000, Robertson and Hansen 2001).
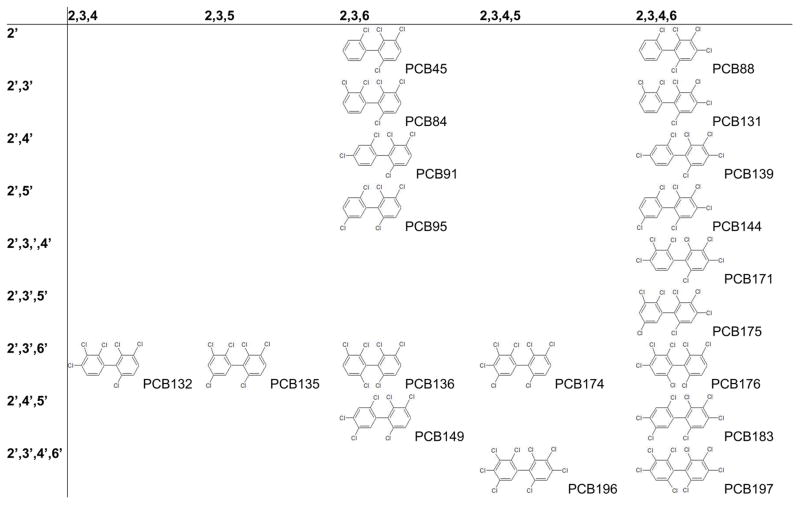
A group of nineteen PCB congeners contain a chiral axis and, due to the presence of three or four bulky ortho chlorine substituents, exist as two stable rotational isomers (or atropisomers) that are non-superimposable mirror images of each other (C-PCBs, Table 1) (Kaiser 1974, Lehmler and Robertson 2001). C-PCBs are major constituents of technical PCB mixtures and, depending on the chlorine content, can represent 6 to 30% by weight of a PCB mixture (Kania-Korwel et al. 2008b). The manufacturing process of PCBs results in the formation of racemic mixtures (Robson and Harrad 2004). Consequently, racemic C-PCBs are released into the environment. Physico-chemical (abiotic) processes, like diffusion, partition or evaporation, do not result in an atropisomeric enrichment of C-PCBs. Indeed, air samples typically do not show any atropisomeric enrichment (Asher et al. 2012, Asher et al. 2007, Jamshidi et al. 2007). On the other hand, biological processes, such as biotransformation, transport processes and protein binding (Muller and Kohler 2004), can result in an atropisomeric enrichment of C-PCBs in soils, sediments and wildlife (Lehmler et al. 2009). In particular cytochrome P450 (P450) enzymes contribute to the congener and species dependent atropisomeric enrichment of C-PCBs in wildlife, laboratory animals and humans (Lehmler et al. 2009).
Table 1.
Structures of all nineteen chiral PCBs that exist as stable atropisomers.
|
Chiral signatures (i.e., the direction and extend of the atropisomeric enrichment of C-PCBs) are a powerful tool to study the movement of PCBs through aquatic and terrestrial food chains (Lehmler et al. 2009). Moreover, a growing number of studies suggest that the atropisomeric enrichment of C-PCBs is toxicologically relevant (Lehmler et al. 2005, Pessah et al. 2009, Yang et al. 2014). However, most environmental and, to some extent, laboratory studies report only a snapshot of chiral signatures (i.e., at the time of sample or specimen collection) in aquatic and terrestrial food webs (Asher et al. 2007, Dang et al. 2010, Lu et al. 2014, Wong et al. 2004). Therefore, many fundamental questions about atropselective processes involved in absorption, distribution, biotransformation and excretion of C-PCB remain unanswered. Inferences about the atropselectivity of biological processes (e.g., biotransformation) involved in C-PCB disposition can be derived from toxicokinetic studies, especially for non-mammalian species where suitable in vitro models (e.g., recombinant enzymes) are not available. The objective of the present review is to summarize our current knowledge of the toxicokinetics of PCB atropisomers in different species and to assess human exposure to atropisomerically enriched C-PCBs. Because of the limited number of studies investigating the toxicokinetics of PCB atropisomers, general toxicokinetics studies that include C-PCBs are also discussed to provide an overall context and identify future research needs.
Species of lower trophic levels (e.g., invertebrate)
Opossum shrimp (Mysis relicta) is an important food source for fish in temperate lakes in North American and Europe (Wong et al. 2004). The total estimated biomass of Mysis relicta in the southern basin of Lake Michigan was approximately 1.16–1.74 × 103 t dry weight in 2000 (Pothoven et al. 2004). As shown in Fig. 1, the elimination of PCBs in mysids (Mysis relicta) depends, at least in part, on the chlorine substitution pattern (Warner and Wong 2006). For example, the uptake and elimination profiles of the pentachlorinated PCBs 91 and 95 are dissimilar, which suggests that factors other than simple physicochemical-processes (i.e., passive diffusion) contribute to their uptake and elimination. A statistically significant atropiomeric enrichment of PCBs 91, 95 and 149, but not PCBs 136 and 183 is observed in Mysis relicta (Fig. 1). It is likely that the atropisomeric enrichment of PCBs 91, 95 and 149 is due to currently uncharacterized biotransformation processes, which provides evidence that even species at lower trophic levels may alter chiral signatures of PCBs in aquatic food webs.
Fig. 1.
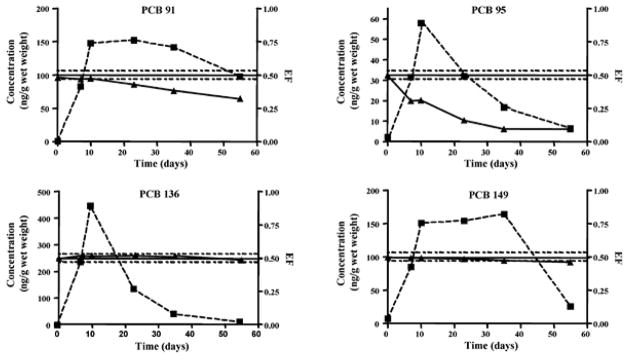
Concentrations (ng/g wet weight) and EFs for PCBs 91, 95, 136 and 149 in Mysis relicta as a function of time. Mysids were maintained in aquaria containing sediment spiked with an organohalogen compound mixture for 10 days, followed by a 50 day depuration period. Dashed line with squares represents PCB concentrations; solid line with triangles represents the enantiomeric fractions (EF); solid and dashed lines around the racemic EF of 0.500 indicate the 95% confidence interval of the EF determination of the racemate. Reprinted with permission from (Warner and Wong 2006). Copyright 2006, American Chemical Society.
The marine worm species Nereis diversicolor and Nereis virens are used as biomonitors for PCB contamination in the sediment compartment of aquatic ecosystems (Bennett et al. 2011, Durou et al. 2007). Nereis diversicolor is capable of metabolizing some PCB congeners, such as PCB 52, to polar biotransformation products (Goerke and Weber 2001). However, it is currently unclear if Nereis diversicolor can metabolize C-PCB congeners, such as PCB 95. In fact, PCB 95 is relatively persistent in this species (elimination half-lives from 6.9 to 11.5 weeks), especially compared to other, lower-to-medium chlorinated PCBs 44, 52, 87 and 101 (elimination half-lives from 0.58 to 5.7 weeks) (Goerke and Weber 1990, Goerke and Weber 2001). This difference in elimination half-lives is potentially due to differences in the biotransformation of the PCB congeners under investigation and suggests that PCB 95 undergoes no or only slow biotransformation in Nereis diverisolor. Another worm species, Nereis virens, exhibits total body elimination rate constants an order of magnitude higher than Nereis diverisolor, effectively eliminating more than 90% of PCB body burden (Bennett et al. 2011). The whole body elimination rate constants for PCBs 95 and 149 are 0.10 and 0.09 d−1, respectively. Similarly rapid C-PCB elimination is observed in Lumbriculus variegatus and Hexagenia spp. (Van Geest et al. 2011). Specifically, PCBs 95 and 149 have elimination rates ranging from 0.301 to 0.367 d−1 in both species. In both studies, the PCB depuration strongly depends on the experimental conditions, including the duration of the uptake and depuration phases, the temperature and the lipid composition of the organism (Bennett et al. 2011, Van Geest et al. 2011), and likely does not involve PCB metabolism.
Bivalves, such as the freshwater mussel, Elliptio complanata, and the green-lipped mussel, Perna viridis, are filter-feeders and consume particle-bound PCBs in aquatic systems. They rapidly reflect changing PCB levels in the water and, therefore, are used to monitor aquatic PCB pollution (O’Rourke et al. 2004, Takabe et al. 2011, Tanabe et al. 1987, Wang et al. 2010a). The rapid change in PCB levels in mussels is due to the large gill surface to volume ratio (Morrison et al. 1995). The comparatively large gill surface area also allows mussels to eliminate PCB faster compared to fish. Typically the elimination rate constants of selected C-PCB congeners range from <0.005 to 0.22 day−1 in different bivalves (Table 2). The elimination rate constants for PCBs inversely depend on the octanol-water partition coefficient KOW (O’Rourke et al. 2004, Wang et al. 2010a) and, thus, are not driven by biological processes. For example, PCBs 91 and 95 have the highest elimination rate constants in Elliptio complanata, whereas PCB 174 has the lowest rate constants (O’Rourke et al. 2004). Similarly, the clearance of higher chlorinated C-PCB, such as PCB 149, is much slower in green-lipped mussels (Perna viridis) (Tanabe et al. 1987). Although bivalves have relatively poor biotransformation capacities (O’Rourke et al. 2004), limited experimental evidence indicates that some C-PCBs undergo atropisomeric enrichment in bivalves (Dang et al. 2010, Hühnerfuss et al. 1995, Morrissey et al. 2007, Wong et al. 2001). It is therefore possible that there are differences in the toxicokinetics of C-PCBs in bivalves due to yet uncharacterized biotransformation processes.
Table 2.
Elimination (depuration) rate constants for selected C-PCBs and the persistent PCB 153 in selected invertebrae species.
| Species | Elimination (depuration) rate constant [10−2 day−1]a
|
Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCB 84 | PCB 91 | PCB 95 | PCB 132 | PCB 136 | PCB 149 | PCB 153 | PCB 174 | ||
| Earthworms (Eisenia foetida) | 8 | 4 | (Wagman et al. 2001) | ||||||
| Freshwater mussel (Elliptio complanata) | 2.1b | 2.8 | 2.9 | 0.9c | 1.8 | 1.2 | 0.9d | < 0.5 | (O’Rourke et al. 2004) |
| Freshwater mussel (Elliptio complanata)e | 3.1 | 2.6 | 2.2 | 1.3 | (Wang et al. 2010a) | ||||
| Green-lipped mussel (Perna viridis) | 8.0 | 12 | 9.7 | 7.9 | (Tanabe et al. 1987) | ||||
| Manila clam (Tapes philippinarum)f | 21 | 22 | 24 | (Raccanelli et al. 2008) | |||||
| Opossum shrimp (Mysis relicta)g | 1.4 | 7.4 | 0.3 | (Warner and Wong 2006) | |||||
| Sandworm (Nereis diversicolor)f | 0.8–1.4 | 0.2–0.8 | (Goerke and Weber 1990, Goerke and Weber 2001) | ||||||
| Earthworms (Eisenia foetida) | 8 | 4 | (Wagman et al. 2001) | ||||||
| Mayfly nymph (Hexagenia spp) | 27 | NS | (Wagman et al. 2001) | ||||||
| Zebra mussel (Dreissena polymorpha) | 8.9h | 4.7 | 5.2 | (Morrison et al. 1995) | |||||
Elimination/depuration rate constants were determined for whole animals if not noted otherwise (please see the respective references for additional details);
co-eluting with PCB101;
co-eluting with PCB 153;
co-eluting with PCB 132;
elimination rate constants were estimated using a quantitative structure-activity relationship;
elimination rate constants were calculated from t1/2 using the equation k = ln 2/t1/2;
minimum elimination rates;
co-eluting with PCB 66. NS – regression analysis not significant.
Fish
Fish represents an important source of human PCB exposure (Darnerud et al. 2006, Kostyniak et al. 2005, Schecter et al. 2010, Voorspoels et al. 2008), thus resulting in significant PCB exposures of certain human subpopulations (see section on C-PCBs levels in human blood and tissue below). Because the atropisomeric enrichment of C-PCBs in fish is highly variable across species and between different studies (Lehmler et al. 2009), human C-PCB exposure via fish likely varies in the direction and extent of the atropisomeric enrichment; however, this hypothesis has not been investigate to date. Fish are exposed to PCBs through the water and their diet (see also Invertebrae section above), which may result in the uptake of non-racemic C-PCBs from their prey (Dang et al. 2010, Lu et al. 2014, Wong et al. 2004) (Table 3). Once exposed, gill ventilation, fecal egestion, biotransformation, growth dilution and mother-to-young transfer are important and well investigated elimination processes in fish. In addition, the duration of the experiment, fish species and fish weights are factors that need to be considered in depuration studies in fish (de Boer et al. 1994, Paterson et al. 2010). Typically, larger fish appear to have longer depuration half-lives (Sijm et al. 1992, Wong et al. 2002a). In some fish species, such has rainbow trout (Oncorhynchus mykiss), the toxicokinetics of PCBs appears to be dose dependent (Brambilla et al. 2007, Buckman et al. 2004), whereas in other species, like Japanese Koi (Cyprinus carpio), the toxicokinetics can be independent of the dose (Paterson et al. 2010). All of these factors need to be taken into account when determining elimination half-lives for PCBs from combined bioconcentration/elimination laboratory studies.
Table 3.
Elimination (depuration) rate constants for selected C-PCBs and the persistent PCB 153 in selected fish species.
| Species | Elimination (depuration) rate constant [10−2 day−1]a
|
Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCB 84 | PCB 91 | PCB 95 | PCB 132 | PCB 136 | PCB 149 | PCB 153 | PCB 174 | ||
| Arctic char (Salvelinus alpinus)b | 6.9 | 7.7 | 8.7 | 7.7 | 7.7 | (Wiberg et al. 2006) | |||
| Eel (Anguilla anguilla) | 0.02 | NA | (de Boer et al. 1994) | ||||||
| Flounder (Platichthys flesus) | 3.8 | 1.0 | (Goerke and Weber 2001) | ||||||
| Guppy (Poecilia reticulata) | 0.34 | 0.41 | (Sijm et al. 1992) | ||||||
| Rainbow trout (Oncorhynchus mykiss) | 0.3c | (−)-PCB 136: 0.14 (±)-PCB 136: 0.19 (+)-PCB 136: 0.27 |
(Wong et al. 2002a) | ||||||
| Rainbow trout (Oncorhynchus mykiss)d | 0.5e | 0.4 | 0.4f | 0.5 | 0.5 | 0.4g | 0.4 | 0.4 | (Buckman et al. 2004) |
| Rainbow trout (Oncorhynchus mykiss)h | 0.5e | 0.3 | 0.3f | 0.5 | 0.5 | 0.3g | 0.3 | 0.4 | (Buckman et al. 2004) |
| Rainbow trout (Oncorhynchus mykiss)b | 0.8 | 0.5c | 0.9 | 0.8 | 0.6d | 0.5 | 0.5 | (Buckman et al. 2006) | |
| Rainbow trout (Oncorhynchus mykiss) | 1.7 | 1.2 | 0.9 | (Konwick et al. 2006) | |||||
| Rainbow trout (Oncorhynchus mykiss)e | 0.4–0.5f | 0.4–0.5 | 0.3–0.4c | 0.4–0.6 | 0.4–0.6 | 0.3–0.5d | 0.3–0.4 | 0.4–0.5 | (Buckman et al. 2007) |
| Rainbow trout (Oncorhynchus mykiss)g | 0.9–1.6 | 1.2–2.0 | 1.3–1.9 | (Brambilla et al. 2007) | |||||
| White prawn (Palaemon longirostris) | 29 | 1.7 | (Goerke and Weber 2001) | ||||||
| Yellow perch (Perca flavescens)h | NA-1.4 | NA-1.4 | NA-1.3 | NA-1.1i | NA-1.0 | NA-1.0 | NA-1.1j | NA-1.0 | (Paterson et al. 2007) |
| Zebrafish (Brachydanio rerio) | 1.6 | 1.0 | (Fox et al. 1994) | ||||||
| Fathead minnow (Pimephales promelas) | 2.2 | NS | (Van Geest et al. 2011) | ||||||
| Japanese koi (Cyprinus carpio)k | 0.06–0.51 | 0.76–1.1 | 0.34–0.66 | 0.36–0.69 | (Paterson et al. 2010) | ||||
Elimination/depuration rate constants were determined for whole animals if not noted otherwise (please see the respective references for additional details);
rainbow trout was fed a diet containing Aroclor 1242, 1254 and 1260 (10 μg/g each) plus PCBs 202 and 209 (0.5 μg/g each);
co-eluting with PCB 66;
co-eluting with PCB 133;
rainbow trout was either treated with a PCB mixture containing Aroclor 1242, 1254 and 1260 (10 μg/g each) plus PCBs 202 and 209 (0.5 μg/g each) or this PCB mixture supplemented with (A) CYP 1A-inducing PCB congeners 77, 126 and 169 (10 ng/g each) or (B) CYP 2B-inducing PCB congeners 87, 99, 101, 153, 180 and 194 (10 ng/g each);
co-eluting with PCB 92;
rainbow trout was fed a diet containing different levels of PCBs (Aroclor 1254) as well as six polychlorinated dibenzodioxins and dibenzofurans;
elimination rate constants summarize the range of elimination rate constants determined during summer, fall, winter, spring and annual temperature cycles;
co-eluting with PCB 153;
co-eluting with PCB 132,
elimination rate constants summarize the range of elimination rate constants determined at three different doses; NA = no apparent elimination. NS – regression analysis not significant.
Water temperature is another, well-known factor influencing elimination rates in fish. In yellow perch (Perca flavescens), the temperature-dependent half-lives of C-PCBs range from 4 to 14 months during the spring, which is comparable to the half-lives reported for rainbow trout (Paterson et al. 2007). In contrast, essentially no PCB elimination is detected during the winter and the highest elimination rate constants are observed during the summer temperature cycle. Since body temperature is not regulated in fish, water temperature can influence elimination rates in fish by altering the activity of P450 enzymes, with the biotransformation rate being inversely proportional to the temperature (Buckman et al. 2004, Buckman et al. 2007, Paterson et al. 2007). While this has not been investigated previously, water temperature may therefore be a determinant of chiral signatures of C-PCBs in fish.
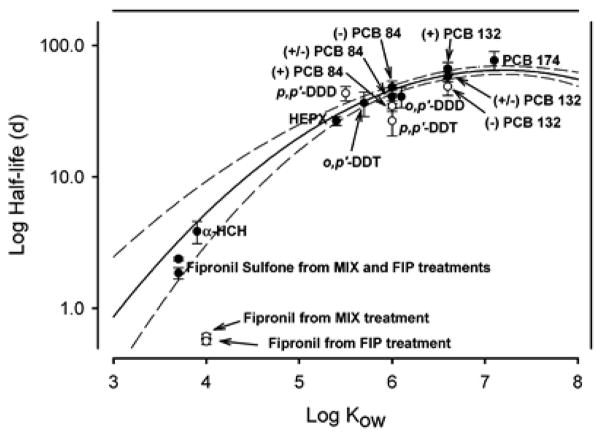
PCB elimination from fish is typically a physicochemical process and, therefore, does not result in differences in the elimination of PCB atropisomers. Briefly, it occurs primarily by partitioning from the gill membranes into the surrounding water, with 80–95% of PCB eliminated by this route in freshwater fish (Paterson et al. 2010). In some fish species, such as Japanese Koi (Cyprinus carpio), fecal egestion is limited and accounts for less than 20 % of the body burden (Paterson et al. 2010). In the absence of biotransformation, PCB elimination is a function of Kow values (Buckman et al. 2004, Buckman et al. 2006, Paterson et al. 2010, Thomann 1989) and the degree of chlorination (Niimi and Oliver 1988). Specifically, the half-life of persistent PCB congeners is expected to increase linearly with Kow. Interestingly, regression of the log of the PCB half-life and log Kow shows a curvilinear relationship, with a shorter half-life for PCBs with a log Kow > 7 (Fisk et al. 1998, Konwick et al. 2006) <ref.>. Possible explanation for this trend are that: Kow is not a good surrogate of the biological lipids; Kow’s for super hydrophobic compounds are inaccurate; and an increasing resistance of PCBs with a log Kow > 7 to mass transfer to storage compartments, thus resulting in an apparently faster clearance of super-hydrophobic PCBs and other persistent organic pollutants. Deviations from this curvilinear relationship between half-life and the Kow are used to indirectly assess if a compound, such as PCB 84 atropisomers, is subject to biotransformation processes (Konwick et al. 2006).
Half-lives of C-PCBs have been extensively investigated in rainbow trout (Brambilla et al. 2007, Buckman et al. 2004, Buckman et al. 2007, Buckman et al. 2006) (Table 3). The depletion half-lives of PCBs 95 and 149 were determined in rainbow trout fed a diet containing different levels of Aroclor 1254 as well as six polychlorinated dibenzodioxins and dibenzofurans (Brambilla et al. 2007). Estimated half-lives, adjusted for clearance and growth dilution, range from 2.5–5.2 months for PCB 95 and from 1.7–3.0 months for PCB 149. Similarly, depuration half-lives of eight C-PCBs in juvenile (Buckman et al. 2004) and immature (Buckman et al. 2007, Buckman et al. 2006) rainbow trout range from 2 to 8 months after administration of a mixture containing Aroclors 1242, 1254 and 1260, with the smaller fish displaying overall longer half-lives. In comparison, the elimination half-life of PCB 136 in guppy is 3.5 months (Sijm et al. 1992), but approximately 10 years for PCB 149 in eel (Anguilla anguilla) (de Boer et al. 1994).
Fish in general have a limited biotransformation capacity (Paterson et al. 2010). However, PCB biotransformation can make a contribution to PCB elimination in some fish species (Buckman et al. 2007, Buckman et al. 2006, White et al. 1997) and represent a dominant elimination process for these highly hydrophobic compounds (de Boer et al. 1994, Sijm et al. 1992). For example, PCBs 91, 132, 136 and 174 are subject to biotransformation in rainbow trout in some studies (Buckman et al. 2007, Buckman et al. 2006), with the biotransformation rate being higher in trout treated with a mixture of Aroclors fortified with PCB congeners known to induce the expression of CYP2B enzymes in rodents (Buckman et al. 2007). In contrast, zebrafish (Brachydanio rerio) does not appear to metabolize PCBs and, in the case of PCB 136, biotransformation does not contribute to the clearance rate constant of 0.0161 day−1 in zebrafish (Fox et al. 1994).
Biotransformation requires the interaction of PCB congeners with chiral biomacromolecules and, unlike physicochemical processes (e.g. passive diffusion), can result in an atropisomeric enrichment. In addition to a deviation from the above mentioned curvilinear relationship between half-life and the Kow, such an atropisomeric enrichment provides evidence of biotransformation processes in an aquatic ecosystem. In particular rainbow trout has been used as a model system to investigate atropselective biotransformation processes. As shown by Wong and co-authors, (+)-PCB 136 has a shorter half-life compared to (−)-PCB 136 in rainbow trout (8.7 vs. 16.9 months, respectively) (Wong et al. 2002a). In contrast, PCB 95 is not eliminated atropselectively and has a half-life of 8.7 months.
Several other studies also show an atropisomeric enrichment of PCBs in rainbow trout. Treatment of rainbow trout with a mixture containing PCBs 84, 132 and 174 plus several current use and legacy pesticides results in an atropisomeric enrichment of (−)-PCB 84 and (+)-PCB 132, whereas no enrichment is observed for PCB 174 (Konwick et al. 2006). Buckman et al. report no atropisomeric enrichment for PCBs 95, 132, 149, 174 and 183 after administration of a complex PCB mixture containing Aroclors 1242, 1254 and 1260 to rainbow trout (Buckman et al. 2006). However, PCB 136 and PCB 91 display atropisomeric enrichment in rainbow trout.
Although atropselective biotransformation is a logical explanation for the atropisomeric enrichment of C-PCBs observed in various fish species, atropselective uptake processes may also contribute to the atropisomeric enrichment. However, studies by Wong et al. (Wong et al. 2002b) (PCB 136) and Konwick et al. (Konwick et al. 2006) (PCBs 84 and 132) report an atropisomeric enrichment only during the depuration but not the uptake phase (Figs. 2 and 3). These findings provide a good indication that biotransformation is the cause of the atropisomeric enrichment in rainbow trout because PCB uptake from the gastrointestinal tract occurs by passive diffusion (Gobas et al. 1993) and, therefore, is not enantioselective. This interpretation is further supported by the fact that (+)-PCB 84 and (−)-PCB 132 fall slightly below the curvilinear relationship of the half-life and Kow (Fig. 2), which also is thought to indicate biotransformation of a compound (i.e., PCB atropisomer). Consistent with this explanation, (−)-PCB 84 and (+)-PCB 132 are enriched in juvenile rainbow trout in the study by Konwick et al. (2006).
Fig. 2.
Regression of the log of the half-life of 16 persistent PCBs and log Kow in juvenile rainbow trout displays a curvilinear relationship. Compounds falling below this relationship (open circles), for example (+)-PCB 84 and (−)-PCB 132, are thought to be biotransformed. The solid line represents the quadratic regression line and the dashed lines represent the 95% confidence intervals. Reprinted with permission from (Konwick et al. 2006). Copyright 2006, American Chemical Society.
Fig. 3.
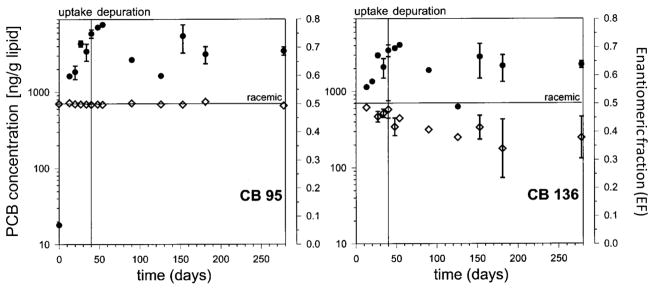
Time-dependent changes in concentration (●) and enantiomeric fractions (◇) of PCB 95 and 136 in rainbow trout carcasses. PCB concentrations are lipid and growth normalized. Each time point represents the mean ± one standard deviation. Reprinted with permission from (Wong et al. 2002a). Copyright 2002, American Chemical Society.
An atropisomeric enrichment of PCBs was also observed in arctic char (Salvelinus alpinus), where (+)-PCB 136 is slightly enriched after intraperitoneal administration of a mixture of containing PCBs 95, 132, 136, 149 and 174 plus -hexachlorocyclohexane, cis-chlordane and heptachlor (Wiberg et al. 2006). (−)-PCB 132 is slightly enriched in the muscle, but not the liver, whereas (+)-PCB 136 is enriched in the liver and muscle (5 week time point only). All other PCB congeners do not display any significant atropisomeric enrichment. Similarly intraperitoneal administration of racemic PCB 136 results in near-racemic chiral signatures of PCB 136 in different mouse tissues (Kania-Korwel et al. 2007). Consequently, the lack of atropisomeric enrichment in arctic char in the study by Wiberg et al. may be the result of the route of administration. The elimination half-lives of C-PCB in arctic char are approximately 10 days, which is significantly shorter than the half-lives in rainbow trout and yellow perch. Unfortunately, the half-lives of individual PCB atropisomers are not reported in the study by Wiberg et al. (2006).
The fact that C-PCBs undergo atropisomeric enrichment due to atropselective biotransformation can be used to assess the movement of PCBs through food webs and to identify species with biotransformation capacity. Wong and co-workers report near racemic PCB signatures at most low trophic level organisms in the aquatic food web of Lake Superior, indicative of a negligible capability of PCB biotransformation by these organisms (Wong et al. 2004). In contrast, some forage fish, such as slimy sculpins (Cottus cognatus) and lake herring (Coregonus artedii), display a change in atropisomeric enrichment relative to its prey, suggesting some ability of these fish species to biotransform C-PCBs. The estimated half-lives of these congeners range from 2 to 7 years. Lake trout (Salvelinus namaycush), a salmonoid closely related to the rainbow trout, displays nonracemic signatures of PCBs 91, 95, 149 and 179. These chiral signatures are not significantly different from the chiral signatures in the various major prey species, which suggests that these congeners do not undergo atropselective biotransformation in lake trout. However, as in rainbow trout, a significant enrichment of (−)-PCB 136 is observed, suggesting the preferential metabolism of (+)-PCB 136 in lake trout. The estimated half-live of PCB 136 in lake trout is comparatively long with 8 year.
Overall, growing evidence suggests that C-PCBs can undergo atropselective biotransformation in fish (reviewed in (Lehmler et al. 2009). However, the enzymes involved in C-PCB biotransformation in fish are largely unknown. Some P450 enzyme isoforms are likely involved in the metabolism of PCB in fish analogous to higher organisms. A number of studies demonstrate the presence of CYP2B-like enzymes in fish using pentoxyresorufin O-depentylase (PROD) activity (Koenig et al. 2012, Machala et al. 1997, Stegeman et al. 1997) and/or mammalian antibodies for CYP2B enzymes (Stegeman et al. 1997). Moreover, functional CYP2B-like enzymes have been isolate from fish liver microsomes (Bozcaarmutlu and Arinc 2008). However, these CYP2B-like enzymes are not inhibited by mammalian CYP2B inhibitors (Koenig et al. 2012, Machala et al. 1997) and their gene expression is not induced by classical, mammalian CYP2B-inducers, such as phenobarbital. Moreover, no genes encoding for CYP2B enzymes have been identified in fish (Goldstone et al. 2010, Uno et al. 2012). Instead, other members of the fish CYP2 family fish are immunoreactive with rat CYP2B antibodies (Yang et al. 1998) and, therefore, may account for CYP2B-like metabolism of PCBs in fish. Thus, additional work is needed to identify which P450 isoform(s) in fish are involved in the metabolism of PCBs in general and C-PCBs in particular.
Amphibians
Although amphibians represent a large proportion of the biomass in some ecosystem and are an important food source for fish, birds, and mammals from higher trophic levels, surprisingly little is known about the toxicokinetics of PCBs in general and C-PCBs in particular. Elimination by passive diffusion via the feces plays a major role in the elimination and, thus, the persistence of PCBs in amphibians. In addition, recent evidence suggests that at least some amphibians, such as green frogs (Rana clamitans) and leopard frogs (Rana pipiens), have limited biotransformation capacity for PCB congeners with vicinal H-atoms in meta and para position and, to a lesser extent, ortho and meta position (Leney et al. 2006a).
The elimination rate constants of C-PCBs 84, 91, 95, 132, 136, 149, 132, 174, 176 and 183 range from 0.0036 to 0.37 d−1 in frogs. They are highly dependent on the season, with elimination rate constants typically lowest in hibernating frogs in winter, and increases by approximaletly an order of magnitude in adults in warmer seasons. Also, the life stage is an important factor, with the elimination rate constants lower in tadpoles compared to metamorphs (Angell and Haffner 2010, Leney et al. 2006c, b). More persistent PCB congeners can have elimination rate constants in amphibians that are at least one order of magnitude smaller compared to C-PCBs. It is currently unclear which specific enzymes are responsible for the biotransformation of PCBs in frogs and other amphibians; if the underlying biotransformation processes are atropselective; and which metabolites are formed. Therefore, amphibians may represent a currently unrecognized source of atropisomerically enriched PCBs and PCB metabolites in the environment.
Birds
Eggs, in particular free-range eggs, may represent a source of human exposure to C-PCBs (Kijlstra et al. 2007, Van Overmeire et al. 2009, Voorspoels et al. 2008, Windal et al. 2009). For example, PCB levels in eggs are the second highest contributor after fish to PCB intake based on a food market-basket study representative for the general Belgian population (Voorspoels et al. 2008). Despite the importance of eggs as a potential dietary source of PCBs in some populations, neither the toxicokinetics of C-PCBs nor the extent of their atropisomeric enrichment has been reported in chicken and, ultimately, eggs. In fact, little is known about the toxicokinetics of C-PCBs in birds in general and systematic toxicokinetic studies have only been reported ring doves (Streptotpelia risoria) (Drouillard and Norstrom 2000, Drouillard and Norstrom 2003) and American kestrels (Falco sparverius) (Drouillard et al. 2007, Drouillard et al. 2001). In contrast to fish and amphibians, birds readily metabolize PCBs to hydroxylated (Borlakoglu and Wilkins 1993) and methylsulfonylated metabolites (Jorundsdottir et al. 2010, Karasek et al. 2007). In the case of methylsulfonyl PCBs, this metabolism appears to be atropselective (Karasek et al. 2007).
Oral administration of a mixture containing Aroclor 1242 : 1254 : 1260 (1:1:1) to ring doves results in rapid PCB absorption. The maximum concentrations (Cmax) for twelve PCB congeners, including C-PCBs 95 and 149, occurs within 5.3 to 7.6 h after feeding (Fig. 4) (Drouillard and Norstrom 2000). Plasma lipids achieve Cmax in the same time period, which suggests PCBs uptake through the same mechanisms as dietary lipids. The plasma clearance rate constants for PCBs 95 and 149 are 0.19 h−1 and 0.24 h−1, respectively. For comparison, the persistent congener PCB 153 has a slower clearance rate of 0.14 h−1in ring doves. A similar study from the same group also reports a rapid elimination of C-PCB 149, with a whole body elimination rate constant of 0.021 to 0.025 day−1 (Drouillard and Norstrom 2003). The clearance of this PCB congener appears to be independent of the dietary fat content. Overall, clearance of PCB congeners with open meta-para position is faster compared to congeners with a 4,4′-dichloro substitution pattern, which indicates that PCB undergo biotransformation in ring doves.
Fig. 4.
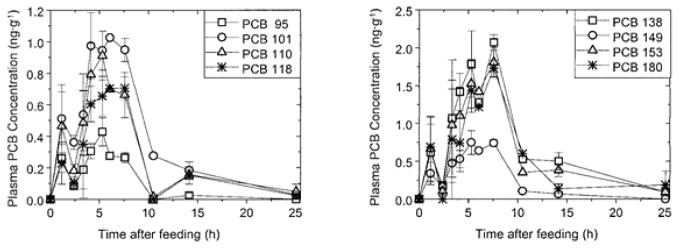
Toxicokinetics of selected PCB congeners in blood plasma of ring doves after dietary administration of a PCB mixture containing Aroclor 1242: 1254: 1260 (1:1:1). The graphics show the trends in PCB concentrations of penta- (left) and hexachlorobiphenyls (right) in replicate birds over a 25 hour blood sampling period. Maximum plasma concentrations of C-PCBs 95 and 149 are lower compared to the plasma concentrations of more persistent PCB congeners but occur in a similar time period. Error bars indicate the standard mean of error. Reprinted with permission from (Drouillard and Norstrom 2000). Copyright 2000, SETAC.
Toxicokinetic parameters have also been determined in juvenile and adult male American kestrels (Falco sparverius) receiving a diet containing Aroclor 1242 : 1254 : 1260 in a 1:1:1 ratio (Drouillard et al. 2007, Drouillard et al. 2001). PCBs 95 and 149 are completely cleared from the whole body of adult birds after 348 days, with plasma clearance constants of 0.41 and 0.19, respectively. In contrast, only 65 % of the persistent congener PCB 183 is cleared from the whole body, with a clearance rate of only 0.04. The whole body elimination is more than 10 fold faster in the young than in adult kestrels. The extent of the biomagnifications in American kestrels is controlled by the elimination kinetics and not the uptake rate constants (Drouillard et al. 2001). Based on this study, metabolic biotransformation and fecal egestion of the parent PCB are likely major factors controlling the elimination of PCBs, including C-PCBs 95 and 149.
Toxicokinetics and disposition of C-PCBs in mammalian animal models
In mammals, such as rats, dogs and monkeys, the liver and muscle are the most important tissues for the initial distribution of PCBs because they are highly perfused, have a large mass and a high intrinsic ability to extract xenobiotics from blood (Kania-Korwel et al. 2010, Matthews and Anderson 1975, Sipes et al. 1982). This results in a rapid initial clearance of PCBs from the blood (Kania-Korwel et al. 2010, Matthews and Anderson 1975, Matthews and Tuey 1980, Sipes et al. 1982). After the initial clearance phase, PCBs are redistributed from highly into poorly perfused tissues with high affinity for PCBs (such as adipose tissue), metabolized and/or eliminated into the feces. Differences in the initial clearance phase are small for different PCB congeners, whereas the half-lives differ drastically depending on the degree of chlorination and the substitution pattern of the PCB congeners (Matthews and Tuey 1980). For example, the half-life of PCB 136 is 10-times smaller compared to PCB 153 in rats (3.6 days versus 37.2 days, respectively) (Matthews and Tuey 1980). In addition to liver and muscle, the fat compartment is another important factor in the distribution of PCBs (e.g., see: (Hansen and Welborn 1977, Kania-Korwel et al. 2010, Schnellmann et al. 1983, Wyss et al. 1982). In all species, the fat compartment is the storage tissue for PCBs and always has the highest PCB tissue-to-blood partition coefficient due to the high lipophilicity of PCBs (Schnellmann et al. 1983). For example, the human adipose tissue-to-blood partition coefficient for PCB 95 ranges from 45 to 62 (Parham et al. 1997, Wolff et al. 1982).
The estimated half-lives of C-PCBs in different mammalian species range from 0.13 to 7.9 years (Table 4). Ultimately, the rate of metabolism determines whether a PCB congener will accumulate in the body tissue of a particular species (Lutz et al. 1984, Matthews and Tuey 1980, Sipes et al. 1982). In particular rats (Luotamo et al. 1991a, Lutz et al. 1984) and dogs (Lutz et al. 1984, Zimmer et al. 1980) metabolize PCB rapidly compared to other mammalian species. For example, PCB 136 is more rapidly eliminated from the body in dogs and rats (Lutz et al. 1984, Matthews and Tuey 1980, Sipes et al. 1982) compared to monkeys (Sipes et al. 1982). As a result of the slower metabolism, monkeys have higher PCB 136-to-PCB 136 metabolite ratios and an overall higher percentage of the total PCB 136 dose in the adipose tissue compare to dogs (Sipes et al. 1982). In rats, PCB 136 is primarily eliminated as metabolites, independent of the age (Birnbaum 1983).
Table 4.
Half-life of C-PCBs in laboratory animals and humans.
| PCB congener | Species | Gender | Route of administration | Half-life
|
Reference | |
|---|---|---|---|---|---|---|
| t1 [days] | t2 [days] | |||||
| E1-PCB 911 | Mice | Female | Oral | 1.9 | (Kania-Korwel et al. 2010) | |
| E1-PCB 911 | Mice | Female | Oral | 2.7 | (Kania-Korwel et al. 2010) | |
| E1-PCB 951 | Mice | Female | Oral | 12.9 | (Kania-Korwel et al. 2010) | |
| E1-PCB 951 | Mice | Female | Oral | 6.9 | (Kania-Korwel et al. 2010) | |
| PCB 952 | Wister rats | Male | Oral | 1.43 | 163 | (Tanabe S. 1981) |
| PCB 95 | Humans | 0.4 years4 | (Agency for Toxic Substances and Disease Registry 2000, Wolff and Schecter 1991) | |||
| PCB 95 | Humans | 3 years4 | (Agency for Toxic Substances and Disease Registry 2000, Wolff et al. 1992) | |||
| (−)-PCB 1321 | Mice | Female | Oral | 15.8 | (Kania-Korwel et al. 2010) | |
| (+)-PCB 1321 | Mice | Female | Oral | 5.1 | (Kania-Korwel et al. 2010) | |
| PCB 136 | Mice | Female | Oral with diet | 3.2 (2.1–6.9) | (Mizutani et al. 1980) | |
| (−)-PCB 1361 | Mice | Female | Oral | 8.3 | (Kania-Korwel et al. 2010) | |
| (+)-PCB 1361 | Mice | Female | Oral | 7.1 | (Kania-Korwel et al. 2010) | |
| PCB 1362 | Wister rats | Male | Oral | 2.63 | (Tanabe S. 1981) | |
| PCB 136 | Beagles (dog) | Male | i.v. | 0.05 | 3.05 | (Sipes et al. 1982) |
| PCB 136 | Sprague-Dawley rats | i.v. | 0.24 | 3.6 | (Matthews and Tuey 1980) | |
| PCB 136 | Sprague- Dawley rats | Male | i.v. | 0.44 | (Birnbaum 1983) | |
| PCB 136 | Cynomolgus monkey | Male | i.v. | 0.16 | 4.57 | (Sipes et al. 1982) |
| (−)-PCB 1491 | Mice | Female | Oral | 11.7 | (Kania-Korwel et al. 2010) | |
| (+)-PCB 1491 | Mice | Female | Oral | 7.5 | (Kania-Korwel et al. 2010) | |
| (−)-PCB 1741 | Mice | Female | Oral | 11.2 | (Kania-Korwel et al. 2010) | |
| (+)-PCB 1741 | Mice | Female | Oral | 11.4 | (Kania-Korwel et al. 2010) | |
| (−)-PCB 1761 | Mice | Female | Oral | 0.54 | (Kania-Korwel et al. 2010) | |
| (+)-PCB 1761 | Mice | Female | Oral | 1.2 | (Kania-Korwel et al. 2010) | |
| PCB 1762 | Wister rats | Male | Oral | 3.13 | >903 | (Tanabe S. 1981) |
| PCB 1831 | Mice | Female | Oral | 6.9 | (Kania-Korwel et al. 2010) | |
| PCB 1832 | Wister rats | Male | Oral | 9.33,5 | >903,5 | (Tanabe S. 1981) |
| PCB 1836 | Rhesus monkey (Macaca mulatta) | Female | Oral | 0.63 years (0.33–1.00 years) | (Mes et al. 1995) | |
| PCB 183 | Humans | 0.13 years7 | (Agency for Toxic Substances and Disease Registry 2000, Luotamo et al. 1991b) | |||
| PCB 183 | Humans | 7.9 years8 | (Agency for Toxic Substances and Disease Registry 2000, Wolff et al. 1992) | |||
Female C57Bl/6 mice received 50 mg/kg body weight of a PCB mixture containing racemic PCB 91 (6.1% by weight), PCB 95 (24.5% by weight), PCB 132 (12.7% by weight), PCB 136 (4.7% by weight), PCB 149 (30.5% by weight), PCB 174 (13.4% by weight), PCB 176 (1.6% by weight) and PCB 183 (6.5% by weight) in corn oil (5.0 mg PCB/ml corn oil) by oral gavage (Kania-Korwel et al. 2010).
A equivalent mixture of Kanechlor-300, -400, -500 and -600 (3 mg in 1 mL corn oil) was orally administered each day for 5 days (Tanabe S. 1981);
half-life of the total PCB content in the animals;
co-eluting PCBs 95, 56 and 60;
co-eluting PCBs 174 and 183;
Aroclor 1254 was administered daily for six years, followed by a deplete period of three years. For additional experimental details see (Mes et al. 1995);
adipose tissue;
co-eluting PCBs 128 and 183; E1 = first eluting PCB atropisomer; E2 = second eluting PCB atropisomer.
The atropselective disposition of PCB 136 has been investigated in C56Bl/6 mice. In all studies, (+)-PCB 136 is enriched in all tissues and excreta, which is consistent with an atropselective metabolism of PCB 136 by P450 enzymes. The disposition of PCB 136 atropisomers is independent of gender (Kania-Korwel et al. 2007) and dietary fat content (Kania-Korwel et al. 2008a), but depends on the dose (Kania-Korwel et al. 2008b) and route of exposure (Kania-Korwel et al. 2007). Induction of P450 enzymes with classical inducers, such as phenobarbital, dexamethasone and -naphthoflavone, prior to PCB administration consistently resulted in a slight increase in the atropisomeric enrichment (Kania-Korwel et al. 2008d). This effect is not a result of the induction of certain P450 enzymes, but most likely due to a dilution of the dose in the liver resulting from the increased liver weight after treatment with any of the above mentioned inducers. The atropisomeric enrichment of PCB 136 also depends on the mammalian species. For example, (−)-PCB 136 undergoes a slight atropisomeric enrichment in male and female Sprague–Dawley rats following intraperitoneal administration of PCB 136 (Kania-Korwel et al. 2008c). In agreement with this observation, a number of in vitro studies also reports an enrichment of (−)-PCB 136 due to a slower, P450 enzyme mediated metabolism (Kania-Korwel et al. 2011, Kania-Korwel and Lehmler 2013, Wu et al. 2013b, Wu et al. 2011).
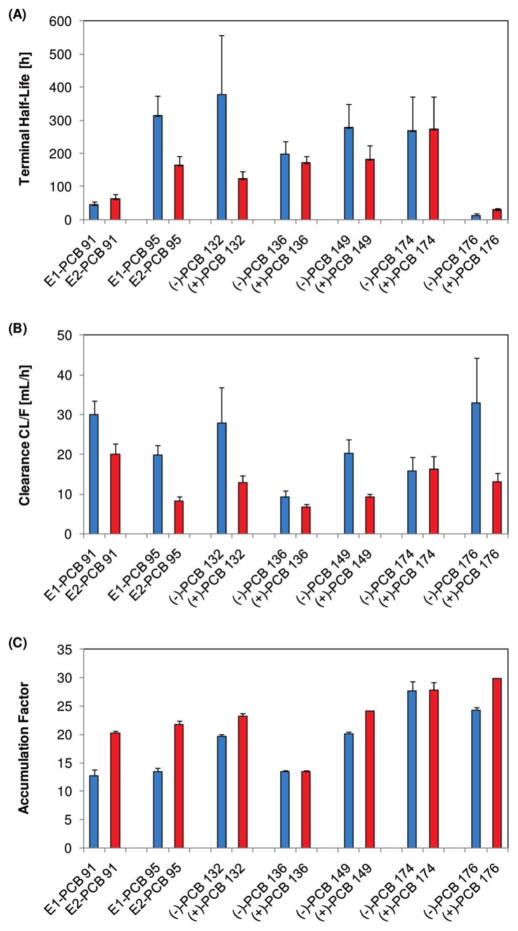
A likely explanation for the atropisomeric enrichment of C-PCBs in mice is a difference in the toxicokinetics of the two PCB atropisomers. A recent study by Kania-Korwel et al. investigated toxicokinetic parameters of PCB atropisomers after oral administration of a mixture containing several C-PCBs (Table 4; Fig. 5) (Kania-Korwel et al. 2010). In this study, the maximum PCB concentration in blood and selected tissues is typically higher for the second compared to the first eluting atropisomer. No clear rank order is observed for the terminal half-lives of the atropisomers. The longest and shortest terminal half-lives are observed for (−)-PCB 132 and (−)-PCB 176, respectively. Only the half-lives of the atropisomers of PCBs 95, 132 and 149 are distinctively different. For these three congeners, the half-lives of the first eluting, (−)-atropisomer are shorter compared to the second eluting (+)-atropisomer. For example, the (−)- and (+)-PCB 132 half-lives differ by a factor of three.
Fig. 5.
The first eluting atropisomer (blue) is typically cleared faster and accumulates less than the second atropisomer (red) in female mice as indicated by (A) terminal half-live, (B) bioavailability normalized clearance and (C) accumulation factors. Reprinted with permission from (Kania-Korwel et al. 2010). Copyright 2010, American Chemical Society.
The bioavailability adjusted clearances (CL/F) in the study by Kania-Korwel et al. shows a clear difference between the first and second eluting atropisomer, with the CL/F of the second eluting atropisomer being smaller for all PCB congeners (Fig. 5) (Kania-Korwel et al. 2010). This observation is consistent with the direction of the atropisomeric enrichment of C-PCBs reported in in vitro metabolism studies in mouse liver microsomes or tissue slices (Wu et al. 2013a, Wu et al. 2014). The accumulation factors, a measure of the relative persistence of a xenobiotic in an organism (Fig. 5), suggest a generally higher tendency of the second eluting PCB atropisomer to accumulate in mice, which is in agreement with the slower clearance of the second eluting atropisomer. The estimated blood-to-tissue distribution coefficients are the highest for adipose tissue and decreased in the order adipose tissue > liver > brain. Overall, no significant differences in the blood-to-tissue distribution coefficients are observed for the first versus the second atropisomers, which indicates that the blood-to-tissue partitioning is primarily a result of passive diffusion in mice.
Monkeys appear to have relatively long half-lives compared to other mammalian species (Table 4). This is consistent with the relatively slow metabolism of PCB 136 by liver microsomes (Wu et al. 2014). The half-life of several PCB congeners, including chiral PCB 183, was determined in the blood of female Rhesus monkeys (Macaca mulatta) treated for six years with Aroclor 1254, followed by a depletion period of three years (Mes et al. 1995). The half-lives of selected PCB congeners are independent of the Aroclor 1254 doses employed (5 to 80 μg/kg body weight/day). The half-life of PCB 183 is 0.63 years (0.33 to 1.00 years, n = 8). In comparison, the highly persistent PCB 153 has a comparable half-life of 0.67 years (0.27 to 0.96 years) in this study, which suggests that PCB 183 is not subject to detectable levels of metabolism in monkeys. This observation is not surprising because PCB 183 does not have any vicinal hydrogen atoms and, based on established structure-activity relationships for PCB metabolism, is poorly metabolized by P450 enzymes (Kania-Korwel and Lehmler 2013).
C-PCBs in humans: Toxicokinetics, levels and chiral signatures
Toxicokinetics of C-PCBs in humans
Understanding the toxicokinetics of PCBs in humans is important for assessing the risk of PCB exposed populations (Lipscomb and Ohanian 2006). Unfortunately, only few studies have reported the half-lives of PCB congeners in humans (Table 4), and published half-life estimates are limited to relatively few congeners (Broding et al. 2007, Grandjean et al. 2008, Ritter et al. 2011, Seegal et al. 2011, Shirai and Kissel 1996) or are Aroclor-based (Hopf et al. 2013). The reported half-lives display great disparities and range from 0.02 years to infinity, with 2–6 years being the most reliable estimates to date (Agency for Toxic Substances and Disease Registry 2000, Shirai and Kissel 1996). The human metabolic elimination rates for C-PCBs are 1.4 (PCBs 149/139), 1.5 (PCBs 84/92), 2 (PCBs 132/161), 2.4 (PCBs 174/181), 6 (PCB 91 and PCBs 98/95/93/102) and 7 years (PCB 136), respectively (Brown 1994). These human half-lives are relatively long and, overall, consistent with metabolism studies using human liver microsomes (Schnellmann et al. 1983, Wu et al. 2014) as well as animal (Matthews and Anderson 1975, Matthews and Tuey 1980, Tanabe S. 1981) and human studies of the elimination of structurally related, but non-chiral PCB congeners (Brown 1994, Chen et al. 1982). These half-life estimates need to be interpreted carefully because they are based on body burden measurements at only a few time points in the same individual. Moreover, half-life determinations assume negligible PCB intake between sample collections and first order elimination kinetics. Additional limitations of any study used for the estimation of human half-lives include analytical issues (e.g., co-elution of congener, lack of authentic PCB standards, and quantification using non-specific electron capture detection; small sample size; short sampling intervals; and low initial body burdens).
C-PCBs levels in human blood and tissues
The lack of toxicokinetic data for C-PCBs in humans raises the questions whether C-PCBs are detected in the human population. Most studies measure blood PCB levels in humans, with the underlying assumption that the serum PCB levels are in an equilibrium with tissue PCB levels (Brown and Lawton 1984). This assumption is problematic for several reasons. Serum lipids differ between males and females (Phillips et al. 1989) and fluctuate with time, for example between fasting to non-fasting states (Phillips et al. 1989, Schisterman et al. 2005). Moreover, not all studies analyze PCBs in serum, but determine PCB levels in whole blood or plasma, which makes it difficult to translate blood PCB levels into body burden and compare PCB levels from different studies. A summary of representative studies reporting C-PCB levels in human blood (depending on the study, whole blood, serum or plasma) is presented in Table 5A (see Table S1, Supplementary Material for a comprehensive summary). To facilitate a comparison between studies, all blood levels are expressed in ng/g wet weight, if necessary by applying appropriate conversions.
Table 5A.
Levels of C-PCBs in human blood samples. All units were adjusted to ng/g, see footnotes.
| Matrix | Country/population | Number of samples | PCB 91 | PCB 95 | PCB 132 | PCB 136 | PCB 149 | PCB 153 | PCB 183 | Reference Year of sampling |
|---|---|---|---|---|---|---|---|---|---|---|
| Seruma | US/gen. pop. (NHANES) | 1873 | 0.004d | 0.14 | 0.01d | (Patterson et al. 2009) 2009 |
||||
| Plasmab | Canada/gen. pop. and non-H lymp | 422 | 4.4 | nd - 0.5 | (Spinelli et al. 2007) 2007 |
|||||
| Serumb | US/natives | 753 | nd-0.19 | nd-0.32 | nd-0.23 | nd-0.16 | nd-0.25c | 6.7 | nd-0.75 | (DeCaprio et al. 2005) 2005 |
| Seruma | US/natives | 314 | 0.8–8.6 | 26–32 | 1.8–4.2 | (Schaeffer et al. 2006) 2006 |
||||
| Serumb | US/sport fishermen | 99 | 0.8c | 0.06d | (Turyk et al. 2006) 2006 |
|||||
| Serumb | US/occup. exp. | 165 | 0.4–93c | 0.2–90c | 0.06–26c | (Wolff et al. 1992) 1992 |
||||
| Seruma,b | US/occup. exp. | 52 | 0.05–4.2 | 0.16–11 | 0.32–23 | 0.14–6.9c | (Fait et al. 1989) 1989 |
|||
| US/gen. pop. | 56 | 0.07–7.8 | 0.15–5.5 | 0.17–6.1 | 0.05–2.4c | |||||
| Seruma | Slovakia/gen. pop. | 315 | nd-0.3 | 0.0003–0.5 | nd-0.9c | nd-1.2 | 0.4–65 | 0.3–5.3 | (Jursa et al. 2006) 2006 |
|
| Seruma,b | Romania/gen. pop. | 2 | 0.13–2.9 | 0.7–3.8 | 0.02–0.6 | (Covaci et al. 2001) 2001 |
||||
| Serumb | US/women gen. pop. | 15 | nd-0.06 | 0.004–0.2 | 0.01–0.05 | (Whitcomb et al. 2005) 2005 |
Analytical techniques:
GC/MS or HRMS,
GC-ECD,
co-eluting with another congener(s),
geometric mean;
Abbreviations: US – United States, nd - MDL or otherwise defined detection limit, where available; non-H. Lymph. – non-Hodginks lymphoma; gen. pop. – general population; occup. exp. – occupational exposure; data reexpression for the table: a) according to published data (Ward et al. 2000), the density of serum used was 1.026 g/ml, and the following units were assumed to be equivalent: ppb = ng/g, ng/ml = μg/L. The same assumption was made for blood plasma. b) The serum lipids content was calculated as 7 mg/g according to (Phillips et al. 1989) (factor x 0.007), and plasma lipids were calculated as 6 mg/ml according to (Michaels et al. 1960). The whole blood lipids were assumed to be same as plasma.
Unfortunately, many large human biomonitoring studies focus on highly persistent and/or dioxin-like PCB congeners and do not assess levels of C-PCBs. For example, Japanese (Ueda et al. 1999) and Australian (Harden et al. 2004) biomonitoring studies focus exclusively on dioxin-like PCBs. These congeners are frequently measured in human studies to facilitate risk assessment using Toxic Equivalency Factors (Ahlborg et al. 1994, Van den Berg et al. 2006). Analogous Neurotoxic Equivalents have been proposed by Simon for PCBs, including several C-PCBs (Simon, 2007); however, there is still limited need to measure C-PCB levels from a regulatory perspective because Neurotoxic Equivalency Factors are not widely accepted for risk assessment. Several large European biomonitoring studies, such as the German Environmental Survey (Becker et al. 1998) and the two Belgian studies (Colles et al. 2008, Verhulst et al. 2009), analyzed only a handful of “marker” congeners (PCBs 118, 138, 153, 170 and 180), but no C-PCBs. One notable exception is the National Health and Nutrition Evaluation Survey (NHANES) in the United States, which includes PCB 149 and 183 in its analyses of environmental contaminants in the general US population. Blood concentrations of PCB 149 and 183 of up to 0.004 and 0.01 ng/g wet weight, respectively, are reported by NHANES (Table 5) (Patterson et al. 2009). Several C-PCB congers, in particular PCB 183, are measured by a few biomonitoring studies, such as New Zealand’s Organochlorine Programme (Buckland et al. 2001) and the Norwegian Arctic Monitoring and Assessment Programme (AMAP; www.amap.no). PCB 196 and PCB 183 (measured by AMAP only) are below the detection limit in human serum samples in both studies.
A number of smaller studies report C-PCB levels in human blood. PCB 183 is a persistent PCB congener (Hansen 2001) and, unlike other C-PCBs, is commonly found in human blood. High levels of PCB 183 are present in blood from non-Hodgkings lymphoma patients in British Columbia, Canada, with concentrations up to 0.5 ng/g plasma (Spinelli et al. 2007) (Table 5A). However, there is no apparent correlation between PCB 183 levels and cancer outcomes (Aronson et al. 2010, Spinelli et al. 2007). Populations eating wild-caught fish, including native tribes consuming a traditional diet, also display increased C-PCB levels. For example, PCB 91 levels as high as 0.19 ng/g are found in Mohawks from Akwesasne Reserve at the United States/Canadian border (Table 5A) (DeCaprio et al. 2005). PCB 95, 132, 136, 149 and 183 levels are also reported by this study, with maximum levels of 0.29, 0.17, 0.16, 00.24 and 0.55 ng/g. PCB 149 and 183 levels as high as 8.6 ng/g and 5.0 ng/g serum, respectively, are detected in Native Americans from the Great Lakes (Schaeffer et al. 2006). The highest concentration of PCB 183 is reported in consumers of Great Lakes fish, where the mean is 8.5 ng/g serum (Turyk et al. 2006).
Occupation and accidental PCB exposures can result in C-PCB levels that are higher than levels in the general population; however, the C-PCB levels in these populations are lower than levels in populations eating wild-caught fish. For example, the highest PCB 95 levels reported in the literature are found in capacitor manufacturing workers, with a maximum of 93 ng/g in 1976 and 19 ng/g in 1979 (Wolff et al. 1992) (Table 5A). Similarly, PCBs 149 and 183 are reported in concentrations of up to 11 and 7 ng/g, respectively, in transformer repair workers (Fait et al. 1989). Levels of both C-PCB congeners are at least 2-times lower in the control population from the same study.
Elevated levels of C-PCBs can sometimes be found in general populations. Oftentimes, such elevated levels can be linked to known manufacturing site, like in Slovakia (reported PCBs 95, 132, 136, 149 and 183 at maximum 0.31, 0.5, 0.32, 1.2 and 5.3 ng/g, respectively (Jursa et al. 2006). Occasionally, elevated levels of C-PCBs are detected in populations without identifying the source(s) of PCB exposure. For example, PCB 149 levels in the Timisoara province of Romania reach 2.9 ng/g. In the same study, PCB 183 levels are on the low side with 0.64 ng/g (Covaci et al. 2001). Also, a study of young American women reports maximum concentrations of PCBs 132, 136 and 183 of 0.06, 0.2 and 0.05 ng/g, respectively (Whitcomb et al. 2005).
Only a limited number of C-PCB levels in postmortem tissue samples are reported in the literature (Table 5B). Different units (e.g., lipid vs. wet weight adjusted PCB levels) and large variability between individuals make comparisons of these data challenging. In some studies, C-PCB tissue levels are one or two orders of magnitude lower compared to PCB 153 levels, a PCB congeners frequently detected in human samples. In other studies, levels of C-PCBs are comparable to levels of PCB 153. For example, Mitchell et al. (2012) report PCB 95 levels ranging from not detectable to 67 ng/g lipid in postmortem brain samples. PCB 153 levels appear to be only slightly higher and ranged from 1.9 to 67 ng/g lipid. Interestingly, this study also observes higher PCB 95 levels in brain samples from individuals with a genetic neurodevelopmental disorder compared to neurotypical controls, which suggest a possible link between PCB 95 exposure and some autism spectrum disorders.
Table 5B.
Levels of C-PCBs in postmortem human tissue samples. Blank fields indicate that the respective PCB congener was not quantified.
| Tissue | Country | Subjects | PCB 95 | PCB 132 | PCB 149 | PCB 153 | PCB 183 | Reference |
|---|---|---|---|---|---|---|---|---|
| [ng/g tissue] | ||||||||
|
| ||||||||
| Brain | USa | 72 | 0.52–0.64 | 1.2–2.6 | (Hatcher-Martin et al. 2012) | |||
| Adipose | Chinaa | 303 | nd | nd-31 | (Wang et al. 2010b) | |||
| Adipose | Polandc | 5 | 0.27–5.2 | 27–215 | (Szafran-Urbaniak 2008) | |||
| Brain | nd | 0.25–1.8 | ||||||
| Liver | nd-2.4 | 2.5–22 | ||||||
| Kidney | nd | 0.64–2.0 | ||||||
| Brain | Belgiuma,c | 1 | nd | 0.36 | nd | 3.1 | 0.19 | (Chu et al. 2003) |
| Liver | 11 | nd | 0.54–1.6 | nd-0.15 | 0.69–23 | 0.11–2.0 | ||
| Kidney | 3 | nd | nd-0.78 | nd | 0.62–6.3 | nd-0.36 | ||
| Muscle | 3 | nd | nd-0.79 | nd | 1.7–14 | 0.14–1.1 | ||
|
| ||||||||
| [ng/g lipid] | ||||||||
|
| ||||||||
| Brain | USc | 107 | nd-67 | 1.9–86 | (Mitchell et al. 2012) | |||
| Adipose | US | 3 | 10–227 | 0.4–27 | (Schecter et al. 1989) | |||
| Liver | 20–34 | 0.5–22 | ||||||
| Kidney | 6–117 | 0.5–17 | ||||||
| Muscle | 23–30 | 0.3–39 | ||||||
| Adipose | Belgiuma | 25 | 131 | 11 | (Covaci et al. 2008) | |||
| Liver | 100 | 8 | ||||||
| Adipose | Netherlands | 245 | 22–25d | 280–310d | (Bräuner et al. 2010) | |||
| Adipose | Finland | 420 | 17–958 | 1.4–65 | (Kiviranta et al. 2005) | |||
| Brain | Germany | 25 | 135 | 5.3 | (Bachour et al. 1998) | |||
| Liver | 703 | 39 | ||||||
| Lung | 1027 | 50 | ||||||
| Muscle | 405 | 29 | ||||||
| Adipose | Greenlandc | 67 | 280–5580 | 14–413 | (Dewailly et al. 1999) | |||
| Brain | 17 | 53–397 | 0.5–29 | |||||
| Liver | 26 | 242–3770 | 11–241 | |||||
GC-MS or GC-HRMS;
range of means,
GC-ECD,
range of medians; nd – not detected/below detection limit.
Chiral signatures in humans
A non-racemic composition of C-PCBs is likely an indicator of differences in the toxicokinetics of PCB atropisomers; however, only a handful of studies report the atropisomeric enrichment of C-PCBs in humans (Table 7). Typically, these studies report the atropisomeric enrichment as an enantiomeric fraction (EF), i.e. the concentration of one PCB atropisomer divided by the sum of the concentration of both atropisomers [i.e., EF = C1/(C1+C2). EF values can range from 0 to 1 for pure atropisomers, with 0.5 representing a racemic mixture (De Geus et al. 2000, Harner et al. 2000). Similar to environmental and laboratory studies (Lehmler et al. 2009) atropselective metabolism by P450 enzymes is the most likely explanation for an atropisomeric enrichment of C-PCBs in humans. However, surprisingly little is known about the atropselective metabolism of C-PCBs in humans. Atropselective formation of hydroxylated C-PCB metabolites occurs in in vitro studies with pooled human microsomes (Wu et al. 2014); unfortunately, the cytochrome P450 isoforms responsible for the atropselective formation of hydroxylated C-PCB metabolites remain unknown. There is some evidence that human CYP2B6 is involved in formation of hydroxylated metabolites from ortho-substituted PCBs, like PCB 153 (Ariyoshi et al. 1995). Also, C-PCBs 45, 91 and 132 are atropselectively biotransformed by CYP2B6; however, the metabolism is not as extensive as by rat CY2B1 (Warner et al. 2009).
Table 6.
Atropisomeric enrichment of C-PCBs in human tissues
| Tissue n of samples |
PCB 91 | PCB 95 | PCB 132 | PCB 149 | PCB 174 | PCB 176 | PCB 183 | Reference |
|---|---|---|---|---|---|---|---|---|
| Milk/Spain n=11 |
0.53–0.65 | 0.45–0.60 | 0.52–0.65 | 0.50–0.63 | 0.50–066 | 0.54–0.65 | 0.26–0.40 | (Bordajandi et al. 2008) |
| Milk/Swiss n=4 |
na | 0.64–0.76 | 0.67–0.82 | 0.52–055 | 0.59–0.62 (n=2) | na | na | (Bucheli and Brandli 2006) |
| Milk/Germany n=10 |
na | 0.51–0.54 | 0.54–0.71 | 0.49–0.51 | na | na | na | (Blanch et al. 1999, Glausch et al. 1995) |
| Feces/UK n=2 |
na | 0.42–0.50 | na | 0.49–0.55 | na | na | na | (Harrad et al. 2006) |
| Liver/Belgium n=11 |
na | 0.51–0.75 | 0.51–0.68 | 0.42–0.50 | na | na | na | (Chu et al. 2003) |
| Brain/Belgium n=1 |
na | 0.50 | 0.48 | 0.49 | na | na | na | (Chu et al. 2003) |
| Kidney/Belgium n=3 |
na | 0.50–0.57 | 0.48–0.50 | 0.46–0.50 | na | na | na | (Chu et al. 2003) |
| Muscle/Belgium n=3 |
na | 0.51–0.53 | 0.50–0.53 | 0.49–0.50 | na | na | na | (Chu et al. 2003) |
| Hair/China n=97 |
na | 0.50a | 0.48a | na | na | na | nd | (Zheng et al. 2013) |
na – not analyzed, nd – not detected.
mean.
The atropisomeric enrichment of C-PCBs has been studies in a number of human breast milk samples from Europe (Ariyoshi et al. 1995, Blanch et al. 1999, Bordajandi et al. 2008, Bucheli and Brandli 2006, Glausch et al. 1995). (+)-PCB 132 is enriched in most breast milk samples (EF = 0.52 to 0.82), whereas PCB 149 is typically near racemic (EF = 0.49 to 0.63). PCB 95 is atropisomerically enriched in breast milk from Switzerland (EF = 0.64–0.76), but near racemic in breast milk from Germany and Spain (EF = 0.45–0.60). Several other C-PCBs also show some atropisomeric enrichment in breast milk from Spain (Bordajandi et al. 2008). Additionally, EF values of PCBs 95, 132 and 149 are published for a small number of human tissue and feces samples. A significant atropisomeric enrichment of PCBs 95, 132 and 149 is observed in livers samples from Belgium (Chu et al. 2003). Enrichment of (+)-PCB 183 is observed in a single human adipose tissue sample from Japan (Toda et al. 2012). In contrast, chiral signatures in muscle, brain and kidney are essentially racemic (Chu et al. 2003). Moreover, PCBs 95 and 149 in the diet and PCB 95 in the feces of healthy volunteers from the UK are near racemic, while PCB 149 is only detected in two feces samples with a slight atropisomeric enrichment (Harrad et al. 2006). PCBs 95 and 132 are found to be near racemic in human hair in China (Zheng et al. 2013); unfortunately, it is unclear if PCBs present in the hair samples represent an internal PCB exposure.
Conclusion
C-PCBs are an important group of environmentally and toxicologically relevant PCBs congeners. Because they atropselectively interact with biological but not abiotic systems, C-PCBs represent a powerful, but underutilized tool to study absorption, distribution, biotransformation and excretion processes in wildlife and, ultimately, humans. In general, toxicokinetics studies provide some evidence that C-PCBs undergo biotransformation in species at different trophic levels; however, further studies as needed to determine if these biotransformation processes are atropselective and, thus, make a contribution to non-racemic signatures observed in wildlife. Moreover, the enzymes involved in atropselective PCB biotransformation in species at all trophic levels, including humans, need to be further characterized. A better understanding of the biological processes responsible for the atropisomeric enrichment of C-PCBs will ultimately enhance our understanding of the environmental fate of PCBs and their adverse health effects.
Supplementary Material
Acknowledgments
Funding: The authors would like to acknowledge support through grants from the National Institute for Environmental Health Sciences/National Institutes of Health (ES05605, ES012475, ES013661 and ES017425) where their own work is cited.
Footnotes
Compliance with Ethical Standards: This manuscript does not involve research with humans or animals.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Agency for Toxic Substances and Disease Registry. Toxicological profile for polychlorinated biphenyls (PCBs) Department of Health and Human Services, Public Health Service; Atlanta, GA: 2000. [PubMed] [Google Scholar]
- Ahlborg UG, Becking GC, Birnbaum LS, Brouwer A, Derks HJGM, Feeley M, Golor G, Hanberg A, Larsen JC, Liem AKD, Safe SH, Schlatter C, Waern F, Younes M, Yrjanheikki E. Toxic Equivalency Factors for dioxin-like PCBs. Chemosphere. 1994;28:1049–1067. [Google Scholar]
- Anezaki K, Kannan N, Nakano T. Polychlorinated biphenyl contamination of paints containing polycyclic- and Naphthol AS-type pigments. Environ Sci Pollut Res. 2014:1–11. doi: 10.1007/s11356-014-2985-6. [DOI] [PubMed] [Google Scholar]
- Anezaki K, Nakano T. Concentration levels and congener profiles of polychlorinated biphenyls, pentachlorobenzene, and hexachlorobenzene in commercial pigments. Environ Sci Pollut Res. 2014;21:998–1009. doi: 10.1007/s11356-013-1977-2. [DOI] [PubMed] [Google Scholar]
- Anezaki K, Nakano T. Unintentional PCB in chlorophenylsilanes as a source of contamination in environmental samples. J Hazard Mater. 2015;287:111–117. doi: 10.1016/j.jhazmat.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Angell RA, Haffner GD. Polychlorinated biphenyl elimination rates and changes in chemical activity in hibernating amphibians. Environ Toxicol Chem. 2010;29:700–707. doi: 10.1002/etc.90. [DOI] [PubMed] [Google Scholar]
- Ariyoshi N, Oguri K, Koga N, Yoshimura H, Funae Y. Metabolism of highly persistent PCB congener, 2,4,5,2′,4′,5′-hexachlorobiphenyl, by human CYP2B6. Biochem Biophys Res Commun. 1995;212:455–60. doi: 10.1006/bbrc.1995.1991. [DOI] [PubMed] [Google Scholar]
- Aronson KJ, Wilson JWL, Hamel M, Diarsvitri W, Fan W, Woolcott C, Heaton JPW, Nickel JC, Macneily A, Morales A. Plasma organochlorine levels and prostate cancer risk. J Expo Sci Environ Epidemiol. 2010;20:434–45. doi: 10.1038/jes.2009.33. [DOI] [PubMed] [Google Scholar]
- Asher BJ, Wong CS, Rodenburg LA. Chiral source apportionment of polychlorinated biphenyls to the Hudson River estuary atmosphere and food web. Environ Sci Technol. 2007;41:6163–6169. doi: 10.1021/es070763n. [DOI] [PubMed] [Google Scholar]
- Asher BJ, Ross MS, Wong CS. Tracking chiral polychlorinated biphenyl sources near a hazardous waste incinerator: Fresh emissions or weathered revolatilization? Environ Toxicol Chem. 2012;31:1453–1460. doi: 10.1002/etc.1852. [DOI] [PubMed] [Google Scholar]
- Bachour G, Failing K, Georgii S, Elmadfa I, Brunn H. Species and organ dependence of PCB contamination in fish, foxes, roe deer, and humans. Arch Environ Contam Toxicol. 1998;35:666–73. doi: 10.1007/s002449900429. [DOI] [PubMed] [Google Scholar]
- Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, Seifert B. German Environmental Survey 1998, Vol. III: Human biomonitoring. Pollutants in blood and urine of the German population. 1998 doi: 10.1078/1438-4639-00155. [DOI] [PubMed] [Google Scholar]
- Bennett ER, Steevens JA, Lotufo GR, Paterson G, Drouillard KG. Novel control and steady-state correction method for standard 28-day bioaccumulation tests using Nereis virens. Environ Toxicol Chem. 2011;30:1366–1375. doi: 10.1002/etc.520. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. Distribution and excretion of 2,3,6,2′,3′,6′- and 2,4,5,2′,4′,5′-hexachlorobiphenyl in senescent rats. Toxicol Appl Pharmacol. 1983;70:262–272. doi: 10.1016/0041-008x(83)90102-3. [DOI] [PubMed] [Google Scholar]
- Blanch GP, Glausch A, Schurig V. Determination of the enantiomeric ratios of chiral PCB 95 and 149 in human milk samples by multidimensional gas chromatography with ECD and MS(SIM) detection. Eur Food Res Technol. 1999;209:294–296. [Google Scholar]
- Bordajandi LR, Abad E, Gonzalez MJ. Occurrence of PCBs, PCDD/Fs, PBDEs and DDTs in Spanish breast milk: Enantiomeric fraction of chiral PCBs. Chemosphere. 2008;70:567–575. doi: 10.1016/j.chemosphere.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Borlakoglu JT, Wilkins JPG. Metabolism of di, tri-, tetra-, penta- and hexachlorobiphenyls by hepatic microsomes isolated from control animals and animals treated with Aroclor 1254, a commercial mixture of polychlorinated biphenyls (PCBs) Comp Biochem Physiol., C: Comp Pharmacol Toxicol. 1993;105C:95–106. doi: 10.1016/0742-8413(93)90064-r. [DOI] [PubMed] [Google Scholar]
- Bozcaarmutlu A, Arinc E. Purification of CYP2B-like protein from feral leaping mullet (Liza saliens) liver microsomes and its biocatalytic, molecular, and immunological characterization. J Biochem Mol Toxicol. 2008;22:284–298. doi: 10.1002/jbt.20239. [DOI] [PubMed] [Google Scholar]
- Brambilla G, Dellatte E, Fochi I, Iacovella N, Miniero R, di Domenico A. Depletion of selected polychlorinated biphenyl, dibenzodioxin, and dibenzofuran congeners in farmed rainbow trout (Oncorhynchus mykiss): A hint for safer fish farming. Chemosphere. 2007;66:1019–1030. doi: 10.1016/j.chemosphere.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Bräuner EV, Raaschou-Nielsen O, Gaudreau E, LeBlanc A, Tjønneland A, Overvad K, Sørensen M. Predictors of polychlorinated biphenyl boncentrations in adipose tissue in a general Danish population. Environ Sci Technol. 2010;45:679–685. doi: 10.1021/es102489c. [DOI] [PubMed] [Google Scholar]
- Broding HC, Schettgen T, Göen T, Angerer J, Drexler H. Development and verification of a toxicokinetic model of polychlorinated biphenyl elimination in persons working in a contaminated building. Chemosphere. 2007;68:1427–1434. doi: 10.1016/j.chemosphere.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Brown J, Jr, Lawton RW. Polychlorinated biphenyl (PCB) partitioning between adipose tissue and serum. Bull Environ Contam Toxicol. 1984;33:277–280. doi: 10.1007/BF01625543. [DOI] [PubMed] [Google Scholar]
- Brown JF. Determination of PCB metabolic, excretion, and accumulation rates for use as indicators of biological response and relative risk. Environ Sci Technol. 1994;28:2295–2305. doi: 10.1021/es00062a013. [DOI] [PubMed] [Google Scholar]
- Bucheli TD, Brandli RC. Two-dimensional gas chromatography coupled to triple quadrupole mass spectrometry for the unambiguous determination of atropisomeric polychlorinated biphenyls in environmental samples. J Chromatogr A. 2006;1110:156–164. doi: 10.1016/j.chroma.2006.01.069. [DOI] [PubMed] [Google Scholar]
- Buckland SMN, Garrett B, Ellis N, van Maanen HKT. Concentrations of selected organochlorines in the serum of the non-occupationally exposed New Zealand population, Organochlorines Programme. Ministry for the Environment; 2001. [Google Scholar]
- Buckman AH, Brown SB, Hoekstra PF, Solomon KR, Fisk AT. Toxicokinetics of three polychlorinated biphenyl technical mixtures in rainbow trout (Oncorhynchus mykiss) Environ Toxicol Chem. 2004;23:1725–1736. doi: 10.1897/03-336. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat Toxicol. 2006;78:176–185. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Buckman AH, Brown SB, Small J, Muir DCG, Parrott J, Solomon KR, Fisk AT. Role of temperature and enzyme induction in the biotransformation of polychlorinated biphenyls and bioformation of hydroxylated polychlorinated biphenyls by rainbow trout (Oncorhynchus mykiss) Environ Sci Technol. 2007;41:3856–3863. doi: 10.1021/es062437y. [DOI] [PubMed] [Google Scholar]
- Chen PH, Luo ML, Wong CK, Chen CJ. Comparative rates of elimination of some individual polychlorinated biphenyls from the blood of PCB-poisoned patients in Taiwan. Food Chem Toxicol. 1982;20:417–425. doi: 10.1016/s0278-6915(82)80107-5. [DOI] [PubMed] [Google Scholar]
- Chu S, Covaci A, Schepens P. Levels and chiral signatures of persistent organochlorine pollutants in human tissues from Belgium. Environ Res. 2003;93:167–176. doi: 10.1016/s0013-9351(03)00016-1. [DOI] [PubMed] [Google Scholar]
- Colles A, Koppen G, Hanot V, Nelen V, Dewolf MC, Noel E, Malisch R, Kotz A, Kypke K, Biot P, Vinkx C, Schoeters G. Fourth WHO-coordinated survey of human milk for persistent organic pollutants (POPs): Belgian results. Chemosphere. 2008;73:907–14. doi: 10.1016/j.chemosphere.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Covaci A, Hura C, Schepens P. Selected persistent organochlorine pollutants in Romania. Sci Total Environ. 2001;280:143–152. doi: 10.1016/s0048-9697(01)00820-8. [DOI] [PubMed] [Google Scholar]
- Covaci A, Voorspoels S, Roosens L, Jacobs W, Blust R, Neels H. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere. 2008;73:170–175. doi: 10.1016/j.chemosphere.2008.02.059. [DOI] [PubMed] [Google Scholar]
- Dang VD, Walters DM, Lee CM. Transformation of chiral polychlorinated biphenyls (PCBs) in a stream food web. Environ Sci Technol. 2010;44:2836–41. doi: 10.1021/es902227a. [DOI] [PubMed] [Google Scholar]
- Darnerud P, Atuma S, Aune M, Bjerselius R, Glynn A, Grawé KP, Becker W. Dietary intake estimations of organohalogen contaminants (dioxins, PCB, PBDE and chlorinated pesticides, eg DDT) based on Swedish market basket data. Food Chem Toxicol. 2006;44:1597–1606. doi: 10.1016/j.fct.2006.03.011. [DOI] [PubMed] [Google Scholar]
- de Boer J, van der Valk F, Kerkhoff MAT, Hagel P, Brinkman UAT. 8-Year study on the elimination of PCBs and other organochlorine compounds from eel (Anguilla anguilla) under natural conditions. Environ Sci Technol. 1994;28:2242–2248. doi: 10.1021/es00062a007. [DOI] [PubMed] [Google Scholar]
- De Geus H, Wester P, de Boer J, Brinkman U. Enantiomer fractions instead of enantiomer ratios. Chemosphere. 2000;41:725–727. doi: 10.1016/s0045-6535(99)00431-2. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ Health Perspect. 1999;107:823–8. doi: 10.1289/ehp.99107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouillard KG, Norstrom RJ. Dietary absorption efficiencies and toxicokinetics of polychlorinated biphenyls in ring doves following exposure to Aroclor mixtures. Environ Toxicol Chem. 2000;19:2707–2714. [Google Scholar]
- Drouillard KG, Fernie KJ, Smits JE, Bortolotti GR, Bird DM, Norstrom RJ. Bioaccumulation and toxicokinetics of 42 polychlorinated biphenyl congeners in American kestrels (Falco sparverius) Environ Toxicol Chem. 2001;20:2514–2522. [PubMed] [Google Scholar]
- Drouillard KG, Norstrom RJ. The influence of diet properties and feeding rates on PCB toxicokinetics in the ring dove. Arch Environ Contam Toxicol. 2003;44:97–106. doi: 10.1007/s00244-002-1199-y. [DOI] [PubMed] [Google Scholar]
- Drouillard KG, Fernie KJ, Letcher RJ, Shutt LJ, Whitehead M, Gebink W, Bird DM. Bioaccumulation and biotransformation of 61 polychlorinated biphenyl and four polybrominated diphenyl ether congeners in juvenile American kestrels (Falco sparverius) Environ Toxicol Chem. 2007;26:313–24. doi: 10.1897/06-052r.1. [DOI] [PubMed] [Google Scholar]
- Durou C, Poirier L, Amiard J-C, Budzinski H, Gnassia-Barelli M, Lemenach K, Peluhet L, Mouneyrac C, Romeo M, Amiard-Triquet C. Biomonitoring in a clean and a multi-contaminated estuary based on biomarkers and chemical analyses in the endobenthic worm Nereis diversicolor. Environ Pollut. 2007;148:445–458. doi: 10.1016/j.envpol.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Fait A, Grossman E, Self S, Jeffries J, Pellizzari E, Emmett E. Polychlorinated biphenyl congeners in adipose tissue lipid and serum of past and present transformer repair workers and a comparison group. Fundam Appl Toxicol. 1989;12:42–55. doi: 10.1016/0272-0590(89)90060-2. [DOI] [PubMed] [Google Scholar]
- Fisk AT, Norstrom RJ, Cymbalisty CD, Muir DCG. Dietary accumulation and depuration of hydrophobic organochlorines: Bioaccumulation parameters and their relationship with the octanol/water partition coefficient. Environmental Toxicology and Chemistry. 1998;17:951–961. [Google Scholar]
- Fox K, Zauke GP, Butte W. Kinetics of bioconcentration and clearance of 28 polychlorinated biphenyl congeners in zebrafish (Brachydanio rerio) Ecotoxicol Environ Saf. 1994;28:99–109. doi: 10.1006/eesa.1994.1038. [DOI] [PubMed] [Google Scholar]
- Frame GM, Cochran JW, Bowadt SS. Complete PCB congener distribution for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resolut Chromatogr. 1996;19:657–668. [Google Scholar]
- Glausch A, Hahn J, Schurig V. Enantioselective determination of chiral 2,2′,3,3′,4,6′-hexachlorobiphenyl (PCB 132) in human milk samples by multidimensional gas chromatography/electron capture detection and by mass spectrometry. Chemosphere. 1995;30:2079–2085. doi: 10.1016/0045-6535(95)00085-m. [DOI] [PubMed] [Google Scholar]
- Gobas FAPC, Zhang X, Wells R. Gastrointestinal magnification: the mechanism of biomagnification and food chain accumulation of organic chemicals. Environ Sci Technol. 1993;27:2855–2863. [Google Scholar]
- Goerke H, Weber K. Population-dependent elimination of various polychlorinated biphenyls in Nereis diversicolor (Polychaeta) Mar Environ Res. 1990;29:205–26. [Google Scholar]
- Goerke H, Weber K. Species-specific elimination of polychlorinated biphenyls in estuarine animals and its impact on residue patterns. Mar Environ Res. 2001;51:131–149. doi: 10.1016/s0141-1136(00)00036-2. [DOI] [PubMed] [Google Scholar]
- Goldstone J, McArthur A, Kubota A, Zanette J, Parente T, Jonsson M, Nelson D, Stegeman J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, Barr DB, Needham LL, Weihe P, Heinzow B. Elimination half-lives of polychlorinated biphenyl congeners in children. Environ Sci Technol. 2008;42:6991–6. doi: 10.1021/es800778q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LG, Welborn ME. Distribution, dilution, and elimination of polychlorinated biphenyl analogs in growing swine. J Pharm Sci. 1977;66:497–501. doi: 10.1002/jps.2600660410. [DOI] [PubMed] [Google Scholar]
- Hansen LG. Identification of steady-state and episodic PCB congeners from multiple pathway exposures. In: Robertson LW, HLG, editors. PCBs. Recent advances in environmental toxicology and heath effects. University Press of Kentucky; Lexington: 2001. p. 47. [Google Scholar]
- Harden F, Muller J, Toms L. National Dioxins Program, Technical Report No. 9 Dioxins in the Australian population: Levels in blood. Australian Government, Department of the Environment and Heritage; 2004. [Google Scholar]
- Harner T, Wiberg K, Norstrom R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ Sci Technol. 2000;34:218–220. [Google Scholar]
- Harrad S, Hazrati S, Ibarra C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: Implications for human exposure. Environ Sci Technol. 2006;40:4633–4638. doi: 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW, Pennell KD. Association between polychlorinated biphenyls and Parkinson’s disease neuropathology. NeuroToxicology. 2012;33:1298–1304. doi: 10.1016/j.neuro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Waters MA, Succop P. Concentration-dependent half-lives of polychlorinated biphenyl in sera from an occupational cohort. Chemosphere. 2013;91:172–8. doi: 10.1016/j.chemosphere.2012.12.039. [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol. 2010;44:2822–7. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Lehmler HJ, Martinez A, Wang K, Hornbuckle KC. Atmospheric PCB congeners across Chicago. Atmos Environ. 2010;44:1550–1557. doi: 10.1016/j.atmosenv.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hühnerfuss H, Pfaffenberger B, Gehrecke B, Karbe L, König WA, Landgraff O. Stereochemical effects of PCBs in the marine environement: seasonal variation of coplanar and atropisomeric PCBs in blue mussles (Mytilus edulis L.) of the German Bight. Mar Poll Bull. 1995;30:332–340. [Google Scholar]
- Jamshidi A, Hunter S, Hazrati S, Harrad S. Concentrations and chiral signatures of polychlorinated biphenyls in indoor and outdoor air and soil in a major UK conurbation. Environ Sci Technol. 2007;41:2153–2158. doi: 10.1021/es062218c. [DOI] [PubMed] [Google Scholar]
- Jorundsdottir H, Lofstrand K, Svavarsson J, Bignert A, Bergman A. Organochlorine compounds and their metabolites in seven Icelandic seabird species - a comparative study. Environ Sci Technol. 2010;44:3252–9. doi: 10.1021/es902812x. [DOI] [PubMed] [Google Scholar]
- Jursa S, Chocanowa J, Petrik J, Loksa J. Dioxin-like and non-dixin like PCBs in human serum of Slovak population. Chemosphere. 2006;64:686–691. doi: 10.1016/j.chemosphere.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Kaiser K. On the optical activity of polychlorinated biphenyls. Environ Poll. 1974;7:93–101. [Google Scholar]
- Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler H-J. Influence of dietary fat on the enantioselective disposition of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) in female mice. Food Chem Toxicol. 2008a;46:637–644. doi: 10.1016/j.fct.2007.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler HJ. Dose-dependent enantiomeric enrichment of 2,2′,3,3′,6,6′-hexachlorobiphenyl in female mice. Environ Toxicol Chem. 2008b;27:299–305. doi: 10.1897/07-359R.1. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Vyas SM, Song Y, Lehmler H-J. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomers. J Chromatogr A. 2008c;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Xie W, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantiomeric enrichment of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) in mice after induction of CYP enzymes. Arch Environ Contam Toxicol. 2008d;55:510–517. doi: 10.1007/s00244-007-9111-4. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, El-Komy MHME, Veng-Pedersen P, Lehmler HJ. Clearance of polychlorinated biphenyl atropisomers is enantioselective in female C57Bl/6 mice. Environ Sci Technol. 2010;44:2828–2835. doi: 10.1021/es901781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Duffel MW, Lehmler HJ. Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ Sci Technol. 2011;45:9590–9596. doi: 10.1021/es2014727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Lehmler HJ. Assigning atropisomer elution orders using atropisomerically enriched polychlorinated biphenyl fractions generated by microsomal metabolism. J Chromatogr A. 2013;1278:133–144. doi: 10.1016/j.chroma.2012.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasek L, Hajslova J, Rosmus J, Huehnerfuss H. Methylsulfonyl PCB and DDE metabolites and their enantioselective gas chromatographic separation in human adipose tissues, seal blubber and pelican muscle. Chemosphere. 2007;67:S22–S27. doi: 10.1016/j.chemosphere.2006.05.081. [DOI] [PubMed] [Google Scholar]
- Kijlstra A, Traag WA, Hoogenboom LAP. Effect of flock size on dioxin levels in eggs from chickens kept outside. Poult Sci. 2007;86:2042–2048. doi: 10.1093/ps/86.9.2042. [DOI] [PubMed] [Google Scholar]
- Kiviranta H, Tuomisto JT, Tuomisto J, Tukiainen E, Vartiainen T. Polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in the general population in Finland. Chemosphere. 2005;60:854–869. doi: 10.1016/j.chemosphere.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Koenig S, Fernandez P, Sole M. Differences in cytochrome P450 enzyme activities between fish and crustacea: Relationship with the bioaccumulation patterns of polychlorobiphenyls (PCBs) Aquat Toxicol. 2012;108:11–17. doi: 10.1016/j.aquatox.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Konwick BJ, Garrison AW, Blanck MC, Avants JK, Fisk AT. Bioaccumulation, biotransformation, and metabolite formation of Fipronil and chiral legacy pesticides in rainbow trout. Environ Sci Technol. 2006;40:2930–2936. doi: 10.1021/es0600678. [DOI] [PubMed] [Google Scholar]
- Kostyniak P, Hansen L, Widholm J, Fitzpatrick R, Olson J, Helferich J, Kim K, Sable H, Seegal R, Pessah I, Schantz S. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Robertson LW. Atropisomers of PCBs. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington: 2001. pp. 61–65. [Google Scholar]
- Lehmler H-J, Robertson LW, Garrison AW, Kodavanti PRS. Effects of PCB 84 enantiomers on [3H] phorbol ester binding in rat cerebellar granule cells and 45Ca2+-uptake in rat cerebellum. Toxicol Lett. 2005;156:391–400. doi: 10.1016/j.toxlet.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Harrad SJ, Hühnerfuss H, Kania-Korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism and distribution: A review. Environ Sci Technol. 2009;44:2757–2766. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leney JL, Balkwill KC, Drouillard KG, Haffner GD. Determination of polychlorinated biphenyl and polycyclic aromatic hydrocarbon elimination rates in adult green and leopard frogs. Environ Toxicol Chem. 2006a;25:1627–1634. doi: 10.1002/etc.5620250623. [DOI] [PubMed] [Google Scholar]
- Leney JL, Drouillard KG, Haffner GD. Metamorphosis increases biotransformation of polychlorinated biphenyls: A comparative study of polychlorinated biphenyl metabolism in green frogs (Rana clamitans) and leopard frogs (Rana pipiens) at various life stages. Environ Toxicol Chem. 2006b;25:2971–2980. doi: 10.1897/05-561r1.1. [DOI] [PubMed] [Google Scholar]
- Leney JL, Drouillard KG, Haffner GD. Does metamorphosis increase the susceptibility of frogs to highly hydrophobic contaminants? Environ Sci Technol. 2006c;40:1491–1496. doi: 10.1021/es0515506. [DOI] [PubMed] [Google Scholar]
- Lipscomb JC, Ohanian EV. Toxicokinetics and risk assessment. Informa Health Care; 2006. [Google Scholar]
- Lu Z, Fisk AT, Kovacs KM, Lydersen C, McKinney MA, Tomy GT, Rosenburg B, McMeans BC, Muir DCG, Wong CS. Temporal and spatial variation in polychlorinated biphenyl chiral signatures of the Greenland shark (Somniosus microcephalus) and its arctic marine food web. Environ Pollut. 2014;186:216–225. doi: 10.1016/j.envpol.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Luotamo M, Elovaara E, Raunio H, Pelkonen O, Riihimäki V, Vainio H. Distribution and effects on cytochrome P450 system of two hexachlorobiphenyl isomers in the rat. Arch Toxicol. 1991a;65:661–665. doi: 10.1007/BF02098033. [DOI] [PubMed] [Google Scholar]
- Luotamo M, Järvisalo J, Aitio A. Assessment of exposure to polychlorinated biphenyls: analysis of selected isomers in blood and adipose tissue. Environ Res. 1991b;54:121–134. doi: 10.1016/s0013-9351(05)80095-7. [DOI] [PubMed] [Google Scholar]
- Lutz RJ, Dederick RL, Tuey D, Sipes IG, Anderson MW, Matthews HB. Comparison of the pharmacokinetics of several polychlorinated biphenyls in mouse, rat, dog and monkey by means of a physiological pharmacokinetic model. Drug Metab Dispos. 1984;12:527–535. [PubMed] [Google Scholar]
- Machala M, Nezveda K, Petrivalsky M, Jarosova AB, Piacka V, Svobodova Z. Monooxygenase activities in carp as biochemical markers of pollution by polycyclic and polyhalogenated aromatic hydrocarbons: Choice of substrates and effects of temperature, gender and capture stress. Aquat Toxicol. 1997;37:113–123. [Google Scholar]
- Matthews HB, Anderson MW. Effect of chlorination on the distribution and excretion of polychlorinated biphenyls. Drug Metab Disp. 1975;3:371–380. [PubMed] [Google Scholar]
- Matthews HB, Tuey DB. The effect of chlorine position on the distribution and excretion of four hexachlorobiphenyl isomers. Toxicol Appl Pharmacol. 1980;53:377–388. doi: 10.1016/0041-008x(80)90351-8. [DOI] [PubMed] [Google Scholar]
- Mes J, Arnold DL, Bryce F. The elimination and estimated half-lives of specific polychlorinated biphenyl congeners from the blood of female monkeys after discontinuation of daily dosing with Aroclor 1254. Chemosphere. 1995;30:789–800. doi: 10.1016/0045-6535(94)00408-m. [DOI] [PubMed] [Google Scholar]
- Michaels G, Wheeler P, Barcellini A, Kinsell L. Freely extractable lipid of human blood plasma: I. Methodology and observations in normal and abnormal subjects. Am J Clin Nutr. 1960;38:38–43. [Google Scholar]
- Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53:589–98. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Hidaka K, Ohe T, Matsumoto M, Yamamoto K, Tajima K. Comparative study on accumulation and elimination of hexachlorobiphenyls and decachlorobiphenyl in mice. Bull Environ Contam Toxicol. 1980;25:181–187. doi: 10.1007/BF01985508. [DOI] [PubMed] [Google Scholar]
- Morrison H, Yankovich T, Lazar R, Haffner GD. Elimination rate constants of 36 PCBs in zebra mussels (Dreissena polymorpha) and exposure dynamics in the Lake St. Clair - Lake Erie corridor. Can J Fish Aquat Sci. 1995;52:2574–2582. [Google Scholar]
- Morrissey JA, Bleackley DS, Warner NA, Wong CS. Enantiomer fractions of polychlorinated biphenyls in three selected Standard Reference Materials. Chemosphere. 2007;66:326–331. doi: 10.1016/j.chemosphere.2006.04.085. [DOI] [PubMed] [Google Scholar]
- Muller TA, Kohler H-PE. Chirality of pollutants - effects on metabolism and fate. Appl Microbiol Biotechnol. 2004;64:300–316. doi: 10.1007/s00253-003-1511-4. [DOI] [PubMed] [Google Scholar]
- Niimi AJ, Oliver BG. Influence of molecular weight and molecular volume on dietary absorption efficiency of chemicals by fishes. Can J Fish Aquat Sci. 1988;45:222–227. [Google Scholar]
- O’Rourke S, Drouillard KG, Haffner GD. Determination of laboratory and field elimination rates of polychlorinated biphenyls (Pcbs) in the freshwater mussel, Elliptio complanata. Arch Environ Contam Toxicol. 2004;47:74–83. doi: 10.1007/s00244-004-2295-y. [DOI] [PubMed] [Google Scholar]
- Parham FM, Kohn MC, Matthews HB, DeRosa C, Portier CJ. Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. I Tissue:blood partition coefficients. Toxicol Appl Pharmacol. 1997;144:340–347. doi: 10.1006/taap.1997.8139. [DOI] [PubMed] [Google Scholar]
- Paterson G, Drouillard KG, Haffner GD. PCB elimination by Yellow Perch (Perca flavescens) during an annual temperature cycle. Environ Sci Technol. 2007;41:824–829. doi: 10.1021/es060266r. [DOI] [PubMed] [Google Scholar]
- Paterson G, Liu J, Haffner GD, Drouillard KG. Contribution of fecal egestion to the whole body elimination of polychlorinated biphenyls by Japanese Koi (Cyprinus carpio) Environ Sci Technol. 2010;44:5769–5774. doi: 10.1021/es903348z. [DOI] [PubMed] [Google Scholar]
- Patterson JDG, Wong L-Y, Turner WE, Caudill SP, DiPietro ES, McClure PC, Cash TP, Osterloh JD, Pirkle JL, Sampson EJ, Needham LL. Levels in the U.S. population of those Persistent Organic Pollutants (2003–2004) included in the Stockholm Convention or in other long-range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Lehmler HJ, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (−)-2,2′,3,3′,6,6′-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol. 2009;22:201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Pothoven SA, Fahnenstiel GL, Vanderploeg HA. Spatial distribution, biomass and population dynamics of Mysis relicta in Lake Michigan. Hydrobiologia. 2004;522:291–299. [Google Scholar]
- Raccanelli S, Libralato S, Favotto M. On the detoxification of benthic bivalves contaminated by POPs: insights from experimental and modelling approaches. Environ Chem Lett. 2008;6:251–258. [Google Scholar]
- Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, Hungerbuhler K. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ Health Perspect. 2011;119:225–31. doi: 10.1289/ehp.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington, KY: 2001. p. 461. [Google Scholar]
- Robson M, Harrad S. Chiral PCB signatures in air and soil: implications for atmospheric source apportionment. Environ Sci Technol. 2004;38:1662–1666. doi: 10.1021/es0349002. [DOI] [PubMed] [Google Scholar]
- Schaeffer D, Dellinger J, Needham L, Hansen L. Serum PCB profiles in Native Americans from Wisconsin based on region, diet, age, and gender: Implications for epidemiology studies. Sci Total Environ. 2006;357:74–87. doi: 10.1016/j.scitotenv.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Schecter A, Mes J, Davies D. Polychlorinated biphenyl (PCB), DDT, DDE and hexachlorobenzene (HCB) and PCDD/F isomer levels in various organs in autopsy tissue from North American patients. Chemosphere. 1989;18:811–818. [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated compounds, polychlorinated biphenyl, and organochlorine pesticide contamination in composite food samples from Dallas, Texas. Environ Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GMB, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellmann R, Putnam C, Sipes I. Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl and 2,2′,4,4′,5,5′-hexachlorobiphenyl by human hepatic microsomes. Biochem Pharmacol. 1983;32:3233–3239. doi: 10.1016/0006-2952(83)90209-5. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Factor SA. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J Expo Sci Environ Epidemiol. 2011;21:234–246. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- Shirai JH, Kissel JC. Uncertainty in estimated half-lives of PCBS in humans: impact on exposure assessment. Sci Total Environ. 1996;187:199–210. doi: 10.1016/0048-9697(96)05142-x. [DOI] [PubMed] [Google Scholar]
- Sijm DTHM, Seinen W, Opperhuizen A. Life-cycle biomagnification study in fish. Environ Sci Technol. 1992;26:2162–74. [Google Scholar]
- Sipes IG, Slocumb ML, Chen HS, Carter DE. 2,3,6,2′,3′,6′-Hexachlorobiphenyl: distribution, metabolism, and excretion in the dog and the monkey. Toxicol Appl Pharmacol. 1982;62:317–24. doi: 10.1016/0041-008x(82)90130-2. [DOI] [PubMed] [Google Scholar]
- Spinelli J, Ng C, Weber J, Connors J, Gascoyne R, Lai A, Brooks-Wilson A, Le N, Berry B, Gallagher R. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007;121:2767–2775. doi: 10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, Woodin BR, Singh H, Oleksiak MF, Celander M. Cytochromes p450 (CYP) in tropical fishes: Catalytic activities, expression of multiple CYP proteins and high levels of microsomal p450 in liver of fishes from Bermuda. Comp Biochem Phys C. 1997;116:61–75. doi: 10.1016/s0742-8413(96)00128-4. [DOI] [PubMed] [Google Scholar]
- Su G, Liu X, Gao Z, Xian Q, Feng J, Zhang X, Giesy JP, Wei S, Liu H, Yu H. Dietary intake of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) from fish and meat by residents of Nanjing, China. Environ Int. 2012;42:138–143. doi: 10.1016/j.envint.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Szafran-Urbaniak B. Application of validated method for determination of selected polychlorinated biphenyls in human adipose tissue samples. Environ Toxicol Pharmacol. 2008;25:131–5. doi: 10.1016/j.etap.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Takabe Y, Tsuno H, Nishimura F, Guan Y, Mizuno T, Matsumura C, Nakano T. Applicability of Corbicula as a bioindicator for monitoring organochlorine pesticides in fresh and brackish waters. Environ Monit Assess. 2011;179:47–63. doi: 10.1007/s10661-010-1718-7. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Tatsukawa R, Phillips DJH. Mussels as bioindicators of PCB pollution: A case study on uptake and release of PCB isomers and congeners in green-lipped mussels (Perna viridis) in Hong Kong waters. Environ Pollut. 1987;47:41–62. doi: 10.1016/0269-7491(87)90120-5. [DOI] [PubMed] [Google Scholar]
- Tanabe SNY, Tatsukawa R. Absorption efficiency and biological half-life of individual chlorobiphenyls in rats treated with Kanechlor products. Agric Biol Chem. 1981;45:717–716. [Google Scholar]
- Thomann RV. Bioaccumulation model of organic chemical distribution in aquatic food chains. Environ Sci Technol. 1989;23:699–707. [Google Scholar]
- Thomas K, Xue J, Williams R, Jones P, Whitaker D. Polychlorinated biphenyls (PCBs) in school buildings: Sources, environmental levels, and exposures. United States Environmental Protection Agency, Office of Research and Development, National Exposure Research Laboratory; 2012. [Google Scholar]
- Toda M, Matsumura C, Tsurukawa M, Okuno T, Nakano T, Inoue Y, Mori T. Absolute configuration of atropisomeric polychlorinated biphenyl 183 enantiomerically enriched in human samples. J Phys Chem A. 2012:9340–9346. doi: 10.1021/jp306363n. [DOI] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Hanrahan L, Falk C, Steenport D, Needham L, Patterson DJ, Freels S, Persky V Great Lakes Consortium. Relationship of serum levels of individual PCB, dioxin, and furan congeners and DDE with Great Lakes sport-caught fish consumption. Environ Res. 2006;100:173–183. doi: 10.1016/j.envres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Ueda H, Nakayama T, Kanai M, Araki S. The state of dioxin accumulation in the human body, blood, wildlife, and food: findings of the fiscal 1998 survey. Environmental Health and Safety Division, Environmental Health Department, Environment Agency of Japan; 1999. http://www.env.go.jp/en/chemi/dioxins/accumulation.pdf. [Google Scholar]
- Uno T, Ishizuka M, Itakura T. Cytochrome P450 (CYP) in fish. Environ Toxicol Pharmacol. 2012;34:1–13. doi: 10.1016/j.etap.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Geest JL, Mackay D, Poirier DG, Sibley PK, Solomon KR. Accumulation and depuration of polychlorinated biphenyls from field-collected sediment in three freshwater organisms. Environ Sci Technol. 2011;45:7011–7018. doi: 10.1021/es1043744. [DOI] [PubMed] [Google Scholar]
- Van Overmeire I, Pussemier L, Waegeneers N, Hanot V, Windal I, Boxus L, Covaci A, Eppe G, Scippo ML, Sioen I, Bilau M, Gellynck X, De Steur H, Tangni EK, Goeyens L. Assessment of the chemical contamination in home-produced eggs in Belgium: General overview of the CONTEGG study. Sci Total Environ. 2009;407:4403–4410. doi: 10.1016/j.scitotenv.2008.10.066. [DOI] [PubMed] [Google Scholar]
- Verhulst SL, Nelen V, Hond ED, Koppen G, Beunckens C, Vael C, Schoeters G, Desager K. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117:122–6. doi: 10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorspoels S, Covaci A, Neels H. Dietary PCB intake in Belgium. Environ Toxicol Pharmacol. 2008;25:179–182. doi: 10.1016/j.etap.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Wagman N, Strandberg B, Tysklind M. Dietary uptake and elimination of selected polychlorinated biphenyl congeners and hexachlorobenzene in earthworms. Environ Toxicol Chem. 2001;20:1778–1784. doi: 10.1897/1551-5028(2001)020<1778:duaeos>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu X, Shan Z, Shi L. Using electrotopological state indices to model the depuration rates of polychlorinated biphenyls in mussels of Elliptio complanata. J Environ Sci. 2010a;22:1544–1550. doi: 10.1016/s1001-0742(09)60287-4. [DOI] [PubMed] [Google Scholar]
- Wang N, Kong D, Cai D, Shi L, Cao Y, Pang G, Yu R. Levels of polychlorinated biphenyls in human adipose tissue samples from Southeast China. Environ Sci Technol. 2010b;44:4334–4340. doi: 10.1021/es9038775. [DOI] [PubMed] [Google Scholar]
- Ward E, Schulte P, Grajewski B, Andersen A, Patterson DJ, Turner W, Jellum E, Deddens J, Friedland J, Roeleveld N, Waters M, Butler M, DiPietro E, Needham L. Serum organochlorine levels and breast cancer: a nested case-control study of Norwegian women. Cancer Epidemiol Biomarkers Prev. 2000;9:1357–1367. [PubMed] [Google Scholar]
- Warner NA, Wong CS. The freshwater invertebrate Mysis relicta can eliminate chiral organochlorine compounds enantioselectively. Environ Sci Technol. 2006;40:4158–4164. doi: 10.1021/es052166b. [DOI] [PubMed] [Google Scholar]
- Warner NA, Martin JW, Wong CS. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ Sci Technol. 2009;43:114–121. doi: 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- Whitcomb B, Schisterman E, Buck G, Weiner J, Greizerstein H, Kostyniak P. Relative concentrations of organochlorines in adipose tissue and serum among reproductive age women. Environ Toxicol Pharma. 2005;19:203–213. doi: 10.1016/j.etap.2004.04.009. [DOI] [PubMed] [Google Scholar]
- White RD, Shea D, Stegeman JJ. Metabolism of the aryl hydrocarbon receptor agonist 3,3′,4,4′-tetrachlorobiphenyl by the marine fish scup (Stenotomus Chrysops) in vivo and in vitro. Drug Metab Disp. 1997;25:564–572. [PubMed] [Google Scholar]
- Wiberg K, Andersson PL, Berg H, Olsson P-E, Haglund P. The fate of chiral organochlorine compounds and selected metabolites in intraperitoneally exposed Arctic char (Salvelinus alpinus) Environ Toxicol Chem. 2006;25:1465–1473. doi: 10.1897/05-329r.1. [DOI] [PubMed] [Google Scholar]
- Windal I, Hanot V, Marchi J, Huysmans G, Van Overmeire I, Waegeneers N, Goeyens L. PCB and organochlorine pesticides in home-produced eggs in Belgium. Sci Total Environ. 2009;407:4430–4437. doi: 10.1016/j.scitotenv.2008.11.063. [DOI] [PubMed] [Google Scholar]
- Wolff M, Schecter A. Accidental exposure of children to polychlorinated biphenyls. Arch Environ Contam Toxicol. 1991;20:449–453. doi: 10.1007/BF01065832. [DOI] [PubMed] [Google Scholar]
- Wolff M, Fischbein A, Selikoff I. Changes in PCB serum concentrations among capacitor manufacturing workers. Environ Res. 1992;59:202–216. doi: 10.1016/s0013-9351(05)80240-3. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Thornton J, Fischbein A, Lilis R, Selikoff IJ. Disposition of polychlorinated biphenyl congeners in occupationally exposed persons. Toxicol Appl Pharmacol. 1982;62:294–306. doi: 10.1016/0041-008x(82)90128-4. [DOI] [PubMed] [Google Scholar]
- Wong CS, Garrison AW, Smith PD, Foreman WT. Enantiomeric composition of chiral polychlorinated biphenyl atropisomers in aquatic and riparian biota. Environ Sci Technol. 2001;35:2448–2454. doi: 10.1021/es0018872. [DOI] [PubMed] [Google Scholar]
- Wong CS, Lau F, Clark M, Mabury SA, Muir DCG. Rainbow trout (Oncorhynchus mykiss) can eliminate chiral organochlorine compounds enantioselectively. Environ Sci Technol. 2002a;36:1257–1262. doi: 10.1021/es0156791. [DOI] [PubMed] [Google Scholar]
- Wong CS, Lau F, Clark M, Mabury SA, Muir DCG. Rainbow Trout (Oncorhynchus mykiss) can eliminate chiral organochlorine compound enantioselectively. Environ Sci Technol. 2002b;36:1257–1262. doi: 10.1021/es0156791. [DOI] [PubMed] [Google Scholar]
- Wong CS, Mabury SA, Whittle DM, Backus SM, Teixeira C, Devault DS, Bronte CR, Muir DCG. Organochlorine compounds in Lake Superior: Chiral polychlorinated biphenyls and biotransformation in the aquatic food web. Environ Sci Technol. 2004;38:84–92. doi: 10.1021/es0346983. [DOI] [PubMed] [Google Scholar]
- Wu X, Pramanik A, Duffel MW, Hrycay EG, Bandiera SM, Lehmler HJ, Kania-Korwel I. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem Res Toxicol. 2011;24:2249–2257. doi: 10.1021/tx200360m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Duffel M, Lehmler H-J. Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-preatreated mice is congener-specific and atropselective. Chem Res Toxicol. 2013a;26:1642–1651. doi: 10.1021/tx400229e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kania-Korwel I, Chen H, Stamou M, Dammanahalli KJ, Duffel M, Lein PJ, Lehmler HJ. Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica. 2013b;43:933–947. doi: 10.3109/00498254.2013.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kammerer A, Lehmler H-J. Microsomal oxidation of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) results in species-dependent chiral signatures of the hydroxylated metabolites. Environ Sci Technol. 2014;48:2436–2444. doi: 10.1021/es405433t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss P, Mühlebach S, Bickel M. Pharmacokinetics of 2,2′,4,4′,5,5′-hexachlorobiphenyl (6-CB) in rats with decreasing adipose tissue mass. I Effects of restricting food intake two weeks after administration of 6-CB. Drug Metab Disp. 1982;10:657–661. [PubMed] [Google Scholar]
- Yang D, Kania-Korwel I, Ghogha A, Chen H, Stamou M, Bose DD, Pessah IN, Lehmler HJ, Lein PJ. PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci. 2014:379–92. doi: 10.1093/toxsci/kft334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Wang JL, Miranda CL, Buhler DR. CYP2M1: Cloning, sequencing, and expression of a new cytochrome P450 from rainbow trout liver with fatty acid (omega-6)-hydroxylation activity. Arch Biochem Biophys. 1998;352:271–280. doi: 10.1006/abbi.1998.0607. [DOI] [PubMed] [Google Scholar]
- Zheng J, Yan X, Chen S-J, Peng X-W, Hu G-C, Chen K-H, Luo X-J, Mai B-X, Yang Z-Y. Polychlorinated biphenyls in human hair at an e-waste site in China: Composition profiles and chiral signatures in comparison to dust. Environ Int. 2013;54:128–133. doi: 10.1016/j.envint.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Balker C, Sipes IG. Correlation of microsomal metabolism of PCBs to their rates of excretion by dogs and monkeys. Fed Proc. 1980;39:998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.