Abstract
The susceptibility of the reproductive system to early exposure to steroid hormones has become a major concern in our modern societies. Human fetuses are at risk of abnormal programming via exposure to endocrine disrupting chemicals, inadvertent use of contraceptive pills during pregnancy, as well as from excess exposure to steroids through disease states. Animal models provide an unparalleled resource to understand the developmental origin of diseases. In female sheep, prenatal exposure to testosterone (T) excess results in an array of adult reproductive disorders that recapitulate those seen in women with polycystic ovary syndrome (PCOS), including disrupted neuroendocrine feedback mechanisms, increased pituitary sensitivity to gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) excess, functional hyperandrogenism, and multifollicular ovarian morphology culminating in early reproductive failure. Prenatal T-treatment also leads to fetal growth retardation, insulin resistance, and hypertension. Mounting evidence suggests that developmental exposure to improper steroidal as well as metabolic environment may mediate the programming of adult disorders in prenatal T-treated females and these defects are maintained or amplified by postnatal sex steroid and metabolic milieu. This review addresses the steroidal and metabolic contributions to the development and maintenance of PCOS phenotype in the prenatal T-treated sheep model, centering specifically on the effects of prenatal and postnatal treatment with androgen antagonist or insulin sensitizer as potential strategies to prevent/ameliorate these dysfunctions. Insights obtained from these intervention strategies on the mechanisms underlying these defects are likely to have translational relevance to human PCOS.
Keywords: androgens, estrogens, insulin, fertility, PCOS
Introduction
A developing fetus is extremely susceptible to even subtle changes in the intrauterine environment. Some of the changes that the fetus is exposed to may be beneficial for healthy development and survival of the fetus while others may prove to be detrimental. Existing evidence indicates that exposure of pregnant mothers to adverse conditions via nutritional deficits/excess, stress, drugs, disease states, environmental endocrine disrupting chemicals, and/or infectious agents can have an impact on the maternal milieu culminating in adult pathologies [1–6]. Lifestyle choices made by the mother such as diet, smoking, drinking and drugs, medical interventions for treatment for pathologies, and intentional or unintentional exposure to environmental endocrine disrupting chemicals therefore pose a threat to the normal developmental trajectory of the fetus.
The concept of developmental programming is not new. Steroid hormones play a critical role during development influencing cell differentiation into organ systems [6, 7]. The reproductive and metabolic systems are especially susceptible to improper steroid exposure during development culminating in pathologies during adulthood. The developing fetus/offspring can be exposed to steroids through disease states, failed contraception and continued exposure to contraceptive steroids, maternal use of anabolic steroids, and inadvertent exposure to environmental compounds with steroidogenic potential [8]. Epidemiological studies point to developmental effects of such environmental disruptors, which act as steroid mimics, in humans. Recent increases in estrogen sensitive cancers (breast, prostate, and testis), endometriosis, male genital abnormalities, decline in semen quality, and early onset of puberty in girls all point to the looming problem [9].
It has been long known that exposure to excess testosterone (T) during fetal life induces phenotypic virilization and behavioral masculinization in the female offspring [10, 11]. In the last decade, the concept has gained momentum in the context of development of adult pathologies [1]. For instance, studies in several animal models have shown that exposure to T excess during fetal life induces reproductive neuroendocrine, ovarian, and metabolic defects in the female offspring, characteristics also seen in women with polycystic ovary syndrome (PCOS) [8]. Among the various models comprising different species (monkeys, rats, mice, and sheep), sheep is one model in which longitudinal studies focusing on multiple developmental time points in the reproductive life span have been carried out. This review focuses mainly on the prenatal T-treated sheep model with emphasis on the mediators of the reproductive neuroendocrine, ovarian, and metabolic disruptions reported in these animals drawing information from other animal models where necessary.
Disruptions in reproductive cyclicity and fertility
Studies using different breeds of sheep (Finish-Landrace × Dorset Horn, Poll Dorset, and Suffolk) found progressive deterioration of the reproductive axis in prenatally T-treated females, though with considerable variability in the degree of severity [12–15]. These differences between distinct breeds are supportive of contribution from genetic predisposition relative to how they respond to insults. The fact that most of the gestational day (GD) 60–90-treated females cycled during the second breeding season as opposed to GD30–90-treated animals becoming anovulatory [15] is supportive of the existence of critical windows of susceptibility and may be a function of exposure relative to when the various organ systems are differentiating.
Mating trials found that rams ignored prenatal T-treated females (GD60–90) when control females were around; however, mating success was 100% when T animals were separated from controls and bred [16]. Nevertheless, pregnancy rate in GD60–90 females was only 40% compared with controls that presented a 90% pregnancy rate [16]. Fertility testing is not possible in GD30–90 females, which are phenotypically virilized [17]. Studies carried out to determine the impact of metabolic status and adiposity found that postnatal overfeeding amplifies the severity of the reproductive phenotype in GD30–90 females with majority becoming anovulatory in the first breeding season [18]. These findings are supportive of a two-step process proposed earlier [19]: the first insult (e.g., gestational T exposure) leading to organizational changes (programming) and the second (e.g., obesity) amplifying the severity of the phenotype.
Steroidal vs. metabolic contributions to deficits seen in prenatal testosterone-treated sheep
Gestational T excess increases not only maternal T, but also fetal T and estradiol (E2) concentrations in sheep [20]. Androgens and estrogens play an important role in the development of the brain [21, 22] and establishment of neuroendocrine feedback mechanisms controlling gonadotropin-releasing hormone (GnRH) and gonadotropin release [17]. Steroid hormones also act directly at the pituitary levels regulating synthesis and secretion of gonadotropins [23, 24], and at the ovarian level controlling folliculogenesis and steroidogenesis [25, 26]. Thus, improper developmental steroid exposure mediates the programming of adult reproductive disorders in prenatal T-treated females.
A second possibility is that adult defects in prenatal T-treated sheep are also facilitated by altered maternal and/or fetal metabolic milieu. Gestational T excess leads to maternal hyperinsulinemia [27] and disrupts insulin signaling in classical insulin target tissues (Lu and Padmanabhan, unpublished observations) during a period of fetal development that encompasses the time of organization of the GnRH neuronal network [28], as well as pituitary [29] and ovarian differentiation [30, 31]. Because insulin is an essential contributor of brain [32, 33] and pituitary [34] development, and establishment of ovarian reserve [35, 36], reproductive alterations may be mediated, in part, by altered insulin sensitivity. Interestingly, women with PCOS, whose characteristics prenatal T-treated sheep recapitulate, also present elevated concentrations of T and insulin during pregnancy [37].
In addition to programming during fetal development, reproductive and metabolic defects seen in prenatal T-treated sheep may be maintained or amplified by postnatal alterations in sex steroid (functional hyperandrogenism) and metabolic (hyperinsulinemia) profile. Indeed, gestational T-treatment increases androgen receptor (AR) expression in the hypothalamus [38], pituitary (Nada and Padmanabhan, unpublished observations), and granulosa cells of antral follicles [39] during adult life. Furthermore, gestational T-treatment also impairs insulin signaling in a tissue-specific manner [40] leading to hyperinsulinemia [41] in the adult offspring.
Over the last years, our group has been investigating the effects of prenatal and postnatal treatment with androgen antagonist or insulin sensitizer as potential strategies to prevent/ameliorate dysfunctions seen in T-treated females and gain insights on the mechanisms underlying these defects. These studies found that prenatal and postnatal treatment with both androgen antagonist and insulin sensitizer prevented the advancement in onset of puberty seen in these females [42]. In addition, postnatal administration of rosiglitazone, an insulin sensitizer, was found to increase the insulin sensitivity index, decrease the number of aberrant estrous cycles during the second breeding season, and prevent further deterioration of the reproductive axis in T females [43]. As discussed below, this amelioration in reproductive function may result from improvements in 1) feedback mechanisms controlling secretion of GnRH and gonadotropins; 2) follicular development and steroidogenesis; and/or 3) general metabolic status and insulin sensitivity.
Neuroendocrine disruptions
Progressive reproductive deterioration seen in female sheep prenatally exposed to T excess may stem, at least in part, from tonic activation of the reproductive neuroendocrine axis [24, 44, 45]. Prenatal T-treated sheep present defects in all three steroid feedback mechanisms controlling GnRH and gonadotropin secretion (Figure 1), namely E2 negative [17, 44], E2 positive [17, 47, 48], and progesterone (P4) negative feedback [45, 49]. Moreover, pituitary sensitivity to GnRH is remarkably increased in prenatal T-treated sheep [24]. The defects in steroid negative feedback and augmented pituitary responsiveness to GnRH together contribute to the luteinizing hormone (LH) excess and consequent functional hyperandrogenism seen in prenatal T-treated females (Figure 2). Further studies investigating the effects of prenatal treatment with T, dihydrotestosterone (DHT), a non-aromatizable androgen, or co-administration of the androgen antagonist, flutamide, with T have pointed to disruptions of E2 negative feedback being programmed by androgenic action of T, with both T and DHT and not T + flutamide reducing sensitivity to E2 [17, 45, 50]. On the other hand, disruptions in E2 positive feedback were found in T- but not DHT-treated females suggesting that this defect is likely programmed via estrogenic actions of prenatal T [17, 45]. The observation that co-treatment with prenatal T and androgen antagonist failed to reverse the defects in E2 positive feedback is supportive of this premise [51].
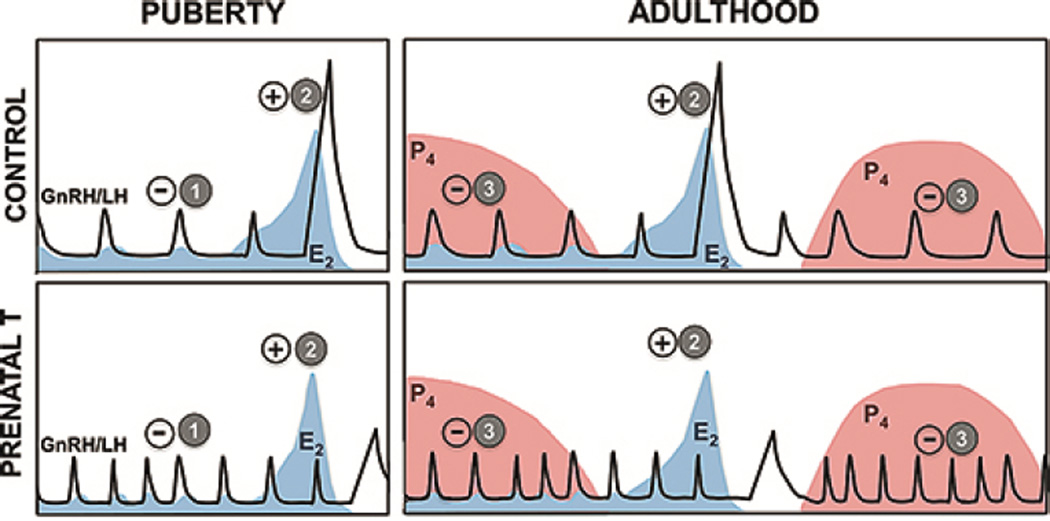
Fig. 1.
Schematic of neuroendocrine feedback systems involved in the control of GnRH/LH secretion that are reprogrammed by prenatal T excess. Upper panel: Pattern of secretion of GnRH/LH in control female sheep; lower panel: Pattern of secretion of GnRH/LH in prenatal T-treated female sheep. (1) E2 negative feedback: GnRH/LH release is under the control of negative feedback action of E2, which is predominant during the prepubertal and anestrus period. Prenatal T-treatment decreases the sensitivity of the neuroendocrine axis to E2, resulting in increased LH pulse frequency. (2) E2 positive feedback: Positive feedback actions of E2 responsible for generation of the preovulatory GnRH/LH surge and onset of cyclicity. Prenatal T-treated females present delayed and dampened LH surge. (3) P4 negative feedback: After puberty (right panels), elevated concentrations of P4 reduce secretion of GnRH/LH pulses preventing ovulation to occur during the luteal phase. Prenatal T-treatment decreases the sensitivity of the neuroendocrine axis to P4, leading to increased LH pulsatile release. Panels illustrating the hormonal profile in control females have been modified from [46].
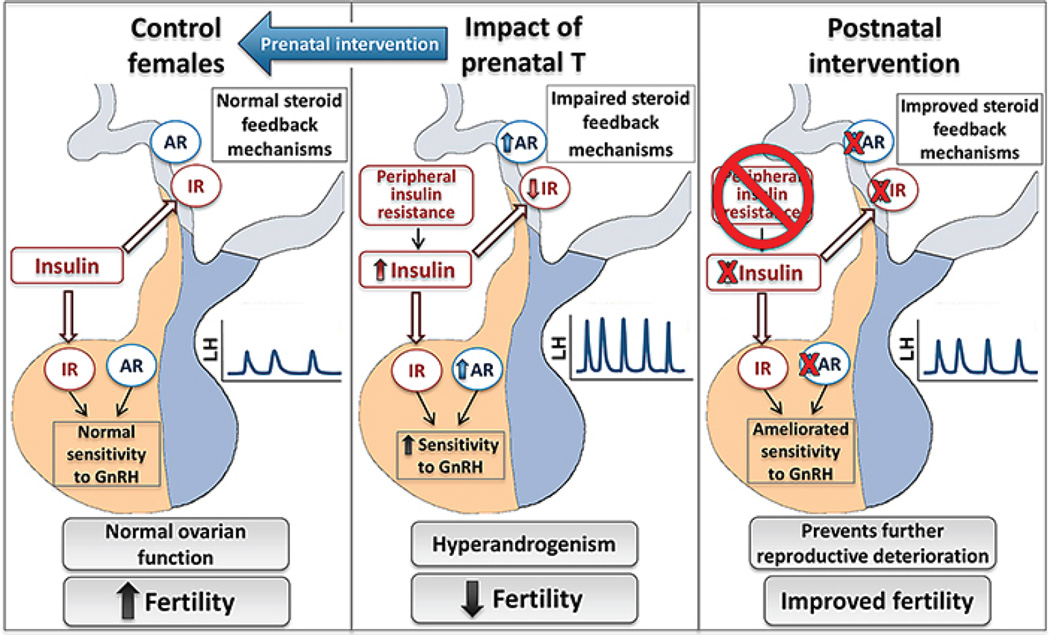
Fig. 2.
Impact of prenatal exposure to T excess on the reproductive neuroendocrine axis in female sheep. Prenatal T-excess (middle panel) results in impaired steroid feedback mechanisms and increased pituitary responsiveness to GnRH, leading to increased frequency and amplitude of LH pulses (LH hypersecretion). Prenatal intervention with androgen antagonist (left panel) prevents the organizational alterations programmed by T excess during fetal life. Postnatal interventions with androgen antagonist and insulin sensitizer agents (right panel) improve neuroendocrine functions and prevent further deterioration of the reproductive system. These interventions are believed to revert/ameliorate (red X) organizational modifications programmed prenatally by T excess.
At the hypothalamic level, neurons colocalizing the neuropeptides kisspeptin, neurokinin B (NKB), and dynorphin (KNDy neurons) in the arcuate nucleus are thought to play a key role in mediating the negative feedback effects of E2 and P4 upon GnRH [52]. Prenatal T-treatment results in marked reduction in NKB and dynorphin in KNDy neurons with kisspeptin remaining unaltered [53]. This peptide imbalance within a single neuronal population has been proposed to underlie some of the defects in responsiveness of the GnRH system to E2 and P4 seen in prenatal T-treated sheep [54]. Recent findings demonstrate that exposure to T excess during fetal development decreases the number of KNDy neurons colocalizing NKB receptors (NK3R) in adult females [55]. Because NKB may act as an autoregulatory transmitter in KNDy neurons, a combined decrease in both ligand and receptor may contribute to defects in the control of GnRH/LH secretion.
At the positive feedback level, postnatal treatment with androgen antagonist or insulin sensitizer has been shown to partially improve the neuroendocrine response, increasing the magnitude but failing to prevent the delay in LH surge response to the E2 positive feedback challenge [51]. These results indicate that timing and magnitude of the LH surge are programmed by different neuroendocrine mechanisms with postnatal androgens and insulin determining the magnitude and estrogens likely the timing of the LH surge. The observation that prenatal T-treated females subjected to neonatal ovariectomy present improvements in E2 positive feedback [56] further supports the hypothesis that postnatal exposure of the neuroendocrine axis to sex steroids, or other ovarian factors, is required to fully defeminize the GnRH/LH surge mechanism in T females.
The potential mediators of the alterations in hypothalamic neuropeptide (NKB and dynorphin) and receptor (NK3R) abundance are not completely elucidated but may involve androgens, estrogens, and insulin. Prenatal treatment with T and DHT, but not E2, results in an increase in AR immunoreactivity in the arcuate nucleus and specifically in KNDy neurons in adult females and co-treatment with flutamide reverts this effect, suggesting that prenatal organization of AR distribution and expression is mediated by androgenic actions of T [38]. In contrast, prenatal T-treatment decreases the percentage of KNDy neurons that colocalize the beta subunit of insulin receptor (IRβ) and co-administration of flutamide fails to prevent this change in female sheep, indicating that this alteration is programmed likely by estrogenic actions of T [57]. Because KNDy neurons are believed to mediate in part the stimulatory effects of insulin on GnRH and LH release [58, 59], alterations in IRβ expression in this cell population may contribute to defects in reproductive functions seen in this animal model.
At the pituitary level, the increased sensitivity to GnRH seen in T females appears to be programmed via androgenic actions of T, since prenatal treatment with DHT also results in increased amplitude of LH pulses after intermittent administration of GnRH boluses under conditions in which endogenous GnRH secretion is suppressed [24]. Consistent with a pituitary effect, prenatal T-treatment increased expression of GnRH receptor (GnRHR) and decreased abundance of estrogen receptor alpha (ESR1) mRNA in the fetal pituitary [24]. These findings combined with the observation that prenatal T-excess decreases the percentage of gonadotropes colocalizing ESR1 in adult ovariectomized females [60] suggest that developmental changes in regulators of gonadotropin synthesis/secretion, including GnRHR and ESR1, may be involved in the increased pituitary responsiveness to GnRH and decreased sensitivity to E2 seen in these animals. Other mediators involved in the increased pituitary sensitivity to GnRH are unclear, however, observations that insulin augments the effects of GnRH on LH synthesis and secretion [61, 62] suggest that insulin may play a role.
From a mechanistic perspective, future studies investigating the effects of androgen antagonist and insulin sensitizer on the neuroendocrine response to E2 and P4 negative feedback mechanisms may shed light on the mediators leading to these defects.
Ovarian disruptions
In addition to reproductive neuroendocrine disruptions, prenatal T-treatment results in multifollicular ovary [63, 64]. This mutifollicular phenotype may stem from aberrant increase in follicular recruitment coupled with arrest in antral follicular development causing persistence.
Follicular activation/recruitment
Ovaries have a finite pool of primordial follicles from which the folliculogenesis starts through a process of activation/recruitment. Majority of the primordial follicles remain in quiescent state and only a few follicles are activated during each reproductive cycle. In addition to activation, the depletion of this pool occurs through the process called follicular atresia [65]. Follicular activation is gonadotropin-independent and is regulated by locally produced growth factors and cytokines [66] and include kit ligand (KITL), fibroblast growth factor (FGF), transforming growth factor (TGF) α, leukemia inhibitory factor (LIF), bone morphogenetic protein 4 (BMP4), anti-Mullerian hormone (AMH), and transforming growth factor (TGF) β [67, 68]. The exact mechanism of activation is not known but it is believed that the balance between activators (KITL, FGF, TGFα, LIF, and BMP4) and inhibitors (AMH and TGFβ) drives the initiation process [69]. These factors activate intracellular signaling pathways such as phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and mammalian target of rapamycin (mTOR) that promote follicle survival and primordial follicle activation [70]. Gene knockout studies suggest that PI3K/AKT-dependent phosphorylation of the transcription factor forkhead box O3a (FOXO3A) leads to premature follicular activation [71]. Gene deletion of the AKT inhibitor, phosphatase and tensin homolog (PTEN), also increases activation of follicles as a result of FOXO3A phosphorylation [72]. Similarly, gene deletion of the inhibitor of follicular activation, AMH, induces premature depletion of primordial follicles [73]. Androgen signaling is also implicated in this process; androgen through either its nuclear receptor or non-genomic mechanisms can activate the PI3K-AKT-FOXO3 signaling in oocyte [74, 75]. On the other hand, follicular atresia underlying follicular depletion is believed to be controlled by the balance between pro- and anti-apoptotic factors. These belong to the B-cell lymphoma-2 (BCL2) family that comprises anti-apoptotic BCL2 and myeloid cell leukemia-1 (MCL1), as well as pro-apoptotic BCL2-associated X protein (BAX) and BCL2-related ovarian killer (BOK) [76]. Growth factors such as KITL are known to increase BCL2 gene expression and promote early follicular survival [65]. A player receiving attention recently in the follicular transition from primordial to primary is the extracellular matrix protein fibrillin 3 (FBN3), which reduces bioavailability of TGF family members [77–79].
In terms of ovarian disruptions, prenatal T-treatment decreases AMH levels in early growing follicles, thereby reducing its inhibitory actions on early follicular growth allowing increased recruitment [80]. Moreover, prenatal T reduces BAX without changing BCL2 in early growing follicles making them resistant to atresia [81]. Prenatal T excess also increases AR protein in primordial and primary follicles [39]. All these changes are consistent with increased follicular recruitment (Figure 3A). Absence of changes in growth differentiation factor (GDF) 9 [80] in prenatal T-treated sheep ovaries and reduced GDF9 mRNA in oocytes of PCOS women [82] are inconsistent with the increased number of growing follicles, considering GDF9 knockout mice show an arrest in follicular development at the primary follicular stage [83].
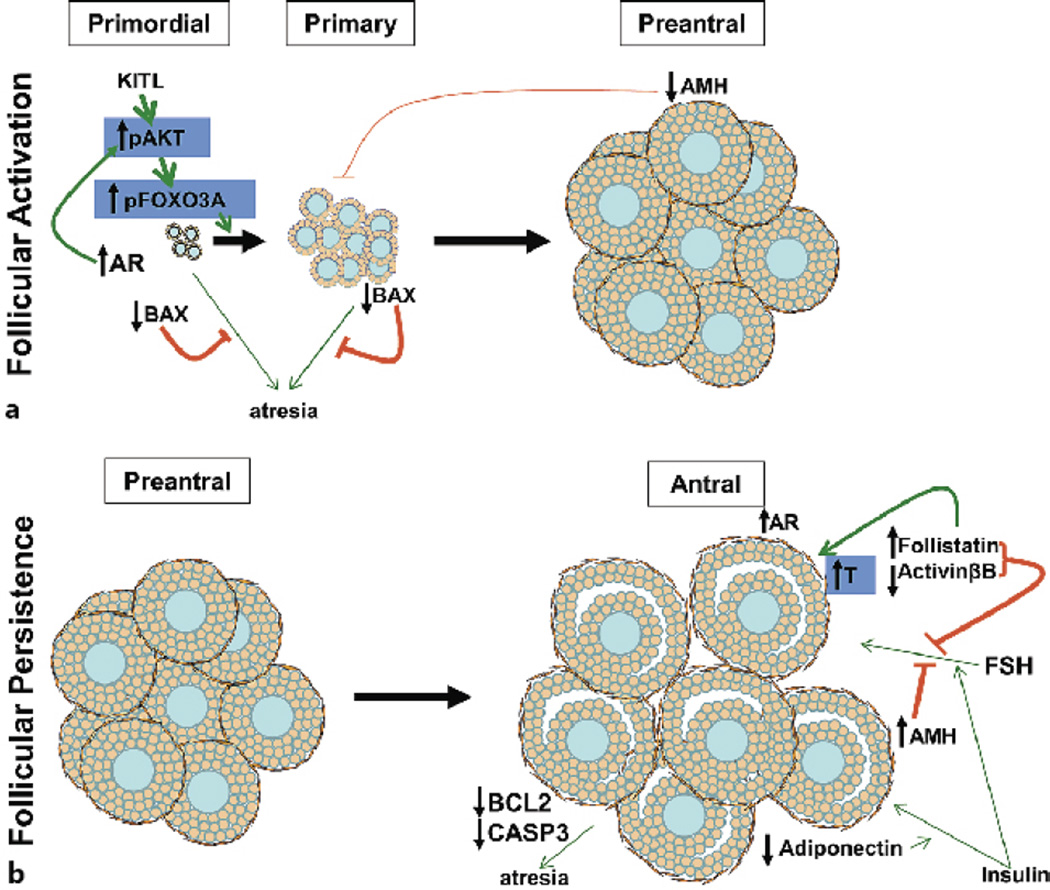
Fig. 3.
Schematic showing the impact of prenatal T-treatment on the ovary. Prenatal T-treatment causes multifollicular ovarian phenotype by increasing primordial follicular activation/recruitment (A) and follicular arrest/persistence (B). Increased follicular activation may result from increased phosphorylation of FOXO transcription factors, decrease in inhibitory AMH levels, or follicular atresia. Follicular arrest may stem from reduced gonadotropin sensitivity through increased follistatin and AMH, reduced insulin sensitivity due to decreased adiponectin, or lack of follicular atresia. Green lines indicate activation, red lines indicate inhibition, and the thickness of the lines represents the intensity of activation or inhibition. The factors in blue blocks have not been investigated in prenatal T-treated sheep but have been reported in other models of T treatment.
To better understand the mechanism of increased follicular recruitment in prenatal T-treated females, PI3K/AKT and FOXO family of transcription factors together with FBN3 (dinucleotide repeat marker in intron 55 of this gene has been linked to PCOS [84]) and other apoptotic genes that seem to be central players need to be investigated.
Follicular arrest and persistence
With progression of follicular development, pre-antral/secondary follicles with acquisition of multiple layers of granulosa cells are formed. The somatic cells of these follicles become less sensitive to oocyte-secreted growth factors changing from heretofore gonadotropin-independent to gonadotropin-dependent. Follicle-stimulating hormone (FSH) is the main gonadotropin required by these follicles and its actions at the gonadal level are controlled by activin, which promotes FSH actions, as well as inhibins and follistatin, which inhibit [85]. FOXO1 has also been shown to interact with activin to promote follicular growth [86]. FSH actions are also mediated by insulin-like growth factor (IGF) I or II [87]. As follicles grow, the amount of AMH produced by these follicles reduces, thus increasing sensitivity of granulosa cells to FSH [88].
As follicles develop further transitioning into antral follicles the following factors promote preovulatory follicle formation. In granulosa cells, FSH and activins stimulate aromatase (CYP19A1) expression while LH and inhibins stimulate thecal CYP17A1. Activins produced by granulosa cells suppress CYP17A1 expression and are blocked by follistatin binding of activin [89]. Insulin can act through either its receptor or through IGF receptors and insulin action is greatly enhanced by the adipocytokine, adiponectin [90]. Insulin together with adiponectin promotes gonadotropin action and granulosa cell steroidogenesis [91]. Non-dominant follicles that do not develop into preovulatory follicles undergo atresia and pro-apoptotic factors such as tumor necrosis factor (TNF) α, prohibitin, Fas, p53 and others are implicated [65]. In addition to secreting growth factors such as GDF9, oocytes also acquire developmental competence with the ability to complete meiosis and undergo fertilization [92]. Other factors such as WNT/Frizzled signaling pathway, IGF and IGF binding proteins (IGFBP), and vascular endothelial growth factor (VEGF) can also influence follicular growth and maturation [93–95].
In the context of ovarian defects, prenatal T-treatment alters the ratio of FSH regulatory proteins with increased mRNA levels of follistatin and reduced activin βB [64]. In addition, antral follicles have greater AMH protein expression, which reduces sensitivity to FSH [80]. These likely create a net negative FSH milieu in the follicle and contribute to follicular arrest in development (Figure 3B). The increased level of follistatin observed in prenatal T-treated females [64] could increase ovarian androgen production by negating activin action. The thecal androgen production could also be influenced by LH hypersecretion [44, 45, 96]. Although the reduced expression of the enzyme CYP17A1 in the theca interna of antral follicles in prenatal T-treated sheep [97] is inconsistent with hyperandrogenism, this may be offset by an increase in the activity of this enzyme, compensatory induction of another isoform, or autocrine/paracrine feedback inhibition [97, 98]. However, gestational T increases AR in these follicles supporting functional hyperandrogenism [39]. The reduced adiponectin in granulosa cells of prenatal T sheep may affect follicular insulin sensitivity and compromise growth [99]. Findings that prenatal T-treatment reduces anti-apoptotic protein BCL2 and the apoptosis effector protein caspase-3 in granulosa cells of antral follicles [81] support a shift in the balance of the pro- versus anti-apoptotic genes that likely arrest the follicle from undergoing atresia or developing further. While oocyte fertilization has not been examined directly in prenatal T-treated sheep, mating studies showed a reduced pregnancy rate [16]. This is consistent with impaired oocyte fertilization observed in PCOS women and prenatal T-treated macaques [96, 100, 101].
Together, these findings suggest that follicular arrest in prenatal T-treated models results from failure at multiple levels involving coordinated effect of several factors. Analysis of other factors such as IGFs, VEGFs, WNTs and other pro-apoptotic proteins and interventional studies negating actions of these mediators are required to completely elucidate how follicular arrest and persistence develop in prenatal T-treated models.
Metabolic dysfunctions
In addition to reproductive disruptions, prenatal T-treatment leads to intrauterine growth restriction (IUGR), low birth weight and postnatal catch-up growth [102], risk factors for adult well-being [103, 104]. Developmental changes in the IGF/IGFBP system in prenatal T-treated sheep are consistent with changes in growth trajectory with a reduction in IGF bioavailability evident during IUGR and an increase during postnatal catch-up growth [102, 105]. Radiotelemetric studies found gestational T excess to also increase arterial and diastolic blood pressure [106]. Metabolic disruptions have also been reported in other prenatal T-treated animal models [107, 108].
Gestational T also reduces peripheral insulin sensitivity leading to hyperinsulinemic status indicative of insulin resistance in sheep [41, 109–111]. Comparative studies with T- and DHT-treated females indicate that the programming of insulin sensitivity defects occurs via androgenic actions of T [41]. Importantly, the window of susceptibility for developing insulin resistance was found to be confined to a shorter programming window, namely 60–90 days of gestation [41]. Interestingly, at postpubertal time points (~16 mo of age), insulin sensitivity is increased, visceral adiposity and adipocyte size are reduced, and circulating palmitic acid is increased in prenatal T females [112]. Relative to earlier observations of reduced insulin sensitivity during early life and adulthood, these findings of increased insulin sensitivity and reduced adiposity postpubertally are suggestive of a period of developmental adaptation.
Investigation of the expression of IR and members of its signaling pathway in adult females revealed that T excess leads to a general down-regulation of many members of the insulin signaling cascade in liver and muscle consistent with them being insulin resistant [40]. In contrast, prenatal T excess upregulated many members of the insulin signaling cascade in the adipose tissue, supportive of increased insulin sensitivity [40]. These findings parallel changes reported in women with PCOS [113–117].
In addition to programming by steroids and insulin, dysfunctions may also be facilitated by deficits in nutrient transfer to the fetus. Gestational T-treatment advances placental differentiation, evident as early as day 65 of gestation [118]. Advanced placental differentiation was sufficient to maintain placental efficiency during early stages of gestation, but not at later stages, culminating in low birth weight [118]. The observations that DHT also advanced placental differentiation, but not T + androgen antagonist, support programming via androgenic actions of T [118]. Because alterations in fetal nutrition result in developmental adaptations that affect adult health [119], compromised placental differentiation and function likely play a role in the phenotype seen in this sheep model.
It is well-established that obesity, a condition associated with systemic inflammation, is a risk factor for insulin resistance and diabetes [120, 121]. Adipokines, oxidative stress, and pro-inflammatory cytokines, such as interleukin 4 (IL4), IL6, and TNFα have been identified as important factors linking obesity and insulin resistance [121–123]. Whether these factors also mediate insulin resistance in females prenatally exposed to T excess remains to be determined. Nevertheless, it is known that postnatal overfeeding leading to increased body weight gain exacerbates the insulin resistance in prenatal T females [41].
Human translation
The phenotypic attributes of prenatal T sheep are similar to features seen in women with PCOS, a major reproductive disorder affecting women of reproductive age [124]; as such, prenatal T sheep may serve as a valuable model for understanding the developmental origin of the PCOS phenotype. This is especially so because the organization of GnRH neuronal network, completion of ovarian differentiation and pancreatic islet differentiation in sheep occurs in utero as is the case in humans [125]. Other benefits of sheep for studying developmental origin of reproductive and metabolic disorders include the wealth of available normative information, feasibility of performing studies in natural environment, availability of hypophyseal-portal approaches to gain an understanding of neural secretory dynamics, ability to non-invasively monitor follicular dynamics longitudinally, feasibility to tap into fetal circulation and the relatively short time line from birth to adulthood (28 wk to puberty).
The attributes of prenatal T treated sheep recapitulate both the reproductive and metabolic phenotype of PCOS women (oligo/anovulation, functional hyperandrogenism, multifollicular ovarian morphology, and insulin resistance) and meet the NIH, Rotterdam, and AE-PCOSS criteria (125). One limitation of the sheep model is that their longer life span compared to rats and mice makes it challenging to study transgenerational effects of prenatal steroid excess. The benefit of knocking in/out genes, which provide a powerful tool for addressing functionality of specific genes in mice, are also difficult to perform in sheep. On the other hand, rats and mice are polyovular and altricial, thus making it difficult to translate some findings to humans. The long developmental timeline and elevated costs limit extensive use in research of prenatal T-treated rhesus monkeys that bear developmental and genealogical similarity to humans. Therefore, it is important to take into account the developmental time line of organ systems of each model while probing mechanisms to enable human translation.
Conclusions
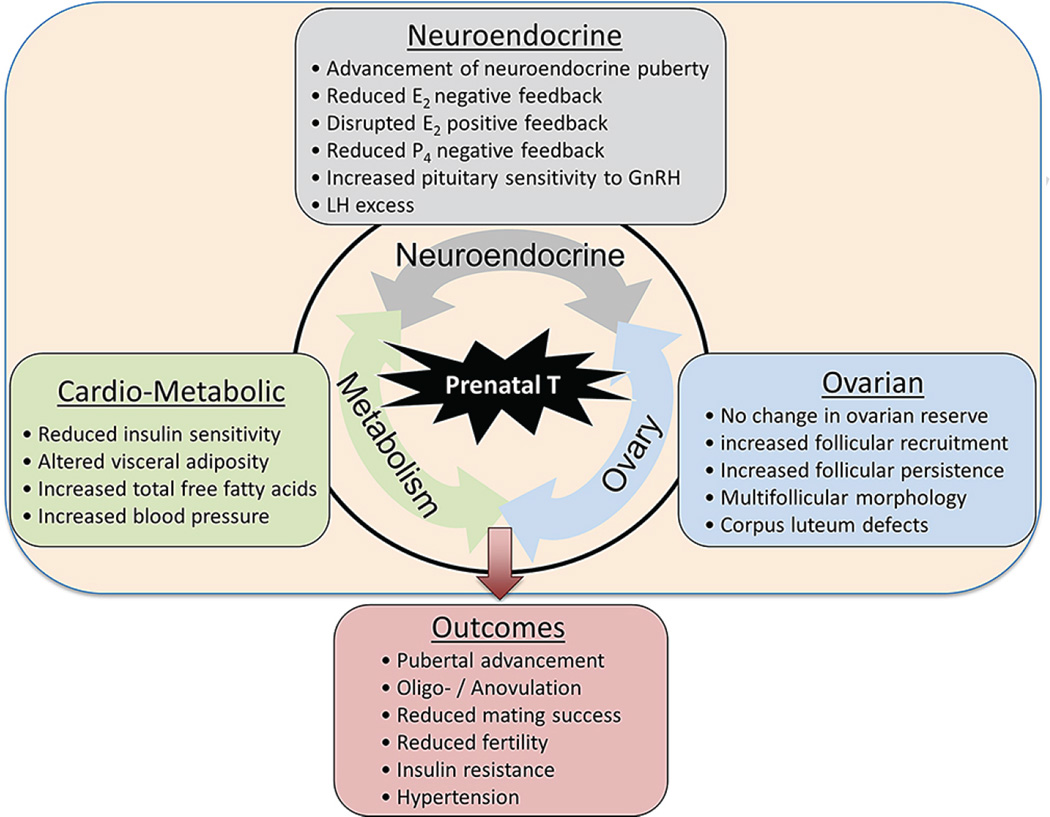
Studies discussed in this review centering on sheep as a model system highlight the concerns inappropriate exposure to steroid hormones/steroid mimics pose to the well-being of the developing offspring. Importantly, these studies point to the coordinated impact of several systems (Figure 4) in establishing or disrupting the final phenotype and thereby emphasizing the need for developing integrative approaches to overcome pathology. Our studies in the prenatal T model clearly point to disruptions at the neuroendocrine, ovarian and metabolic level each impinging on the other. This highlights the need for interventions targeting at multiple levels for achieving optimal success.
Fig. 4.
Self-perpetuating vicious cycle involving the three systems (neuroendocrine, ovarian, and metabolic) and main alterations observed in females exposed to prenatal T excess.
From a mechanistic perspective, future studies should focus on further elucidating the mediators involved in programming and maintaining the dysfunctions seen in this animal model. Moreover, because of the potential for these alterations to be carried forward to subsequent generations, transgenerational studies are needed to help elucidate potential epigenetic mechanisms implicated in the reprogramming of reproductive and metabolic systems.
Footnotes
Disclaimer: Accepted, unedited article not yet assigned to an issue. The statements, opinions and data contained in this publication are solely those of the individual authors and contributors and not of the publisher and the editor(s). The publisher and the editor(s) disclaim responsibility for any injury to persons or property resulting from any ideas, methods, instructions or products referred to in the content. Copyright: All rights reserved. No part of this publication may be translated into other languages, reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, microcopying, or by any information storage and retrieval system, without permission in writing from the publisher or, in the case of photocopying, direct payment of a specified fee to the Copyright Clearance Center.
References
- 1.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588–595. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 2.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology. 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 3.Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, Hyde MJ, Modi N. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;55:3114–3127. doi: 10.1007/s00125-012-2689-8. [DOI] [PubMed] [Google Scholar]
- 4.Poston L. Developmental programming and diabetes - The human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24:541–552. doi: 10.1016/j.beem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clin Perinatol. 2007;34:515–526. doi: 10.1016/j.clp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 7.McEwen B. Steroid hormones: effect on brain development and function. Horm Res Paediatr. 1992;37:1–10. doi: 10.1159/000182393. [DOI] [PubMed] [Google Scholar]
- 8.Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78:734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- 11.Gorski RA. Sexual differentiation of the brain: a model for drug-induced alterations of the reproductive system. Environ Health Perspect. 1986;70:163–175. doi: 10.1289/ehp.8670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Short R. Sexual differentiation of the brain of the sheep. J Endocrinol. 1975;66:5P–5P. [PubMed] [Google Scholar]
- 13.Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- 14.Clarke I, Scaramuzzi R, Short R. Ovulation in prenatally androgenized ewes. J Endocrinol. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- 15.Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- 16.Steckler T, Roberts E, Doop D, Lee T, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
- 18.Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity—implication for polycystic ovary syndrome. Endocrinology. 2009;150:1456–1465. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent Hypomethylation in the Promoter of Nucleosomal Binding Protein 1 (Nsbp 1) Correlates with Overexpression of Nsbp 1 in Mouse Uteri Neonatally Exposed to Diethylstilbestrol or Genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 22.Toran-Allerand CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res. 1976;106:407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- 23.Brooks AN, Thomas GB. Ontogeny and function of the pituitary-gonodal axis during fetal development in sheep. Reprod Domest Anim. 1995;30:158–162. [Google Scholar]
- 24.Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod. 2008;78:648–660. doi: 10.1095/biolreprod.107.063347. [DOI] [PubMed] [Google Scholar]
- 25.Pepe GJ, Billiar RB, Albrecht ED. Regulation of baboon fetal ovarian folliculogenesis by estrogen. Mol Cell Endocrinol. 2006;247:41–46. doi: 10.1016/j.mce.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 26.Abbott DH, Padmanabhan V, Dumesic DA. Contribution of estrogen and androgen to fetal programming of ovarian dysfunction. Reprod Biol Endocrinol. 2006;4:17. doi: 10.1186/1477-7827-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abi-Salloum BA, Veiga-Lopez A, Abbott DH, Padmanabhan V. Developmental Programming: Gestational Exposure to Excess Testosterone, by Its Androgenic Action, Disrupts Maternal Steroidal and Metabolic Environment in Sheep. Endocr Rev; 94th Annual Meeting of the Endocrine Society; Houston, TX. 2012. MON-15 (Abstr). [Google Scholar]
- 28.Wood R, Newman S, Lehman M, Foster D. GnRH neurons in the fetal lamb hypothalamus are similar in males and females. Neuroendocrinology. 1992;55:427–433. doi: 10.1159/000126154. [DOI] [PubMed] [Google Scholar]
- 29.Brooks A, Hagan D, Sheng C, McNeilly A, Sweeney T. Prenatal gonadotrophins in the sheep. Anim Reprod Sci. 1996;42:471–481. [Google Scholar]
- 30.Sawyer HR, Smith P, Heath DA, Juengel JL, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- 31.McNatty K, Fidler A, Juengel J, Quirke L, Smith P, Heath D, Lundy T, O’Connell A, Tisdall D. Growth and paracrine factors regulating follicular formation and cellular function. Mol Cell Endocrinol. 2000;163:11–20. doi: 10.1016/s0303-7207(99)00235-x. [DOI] [PubMed] [Google Scholar]
- 32.Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai M, Li T, Ross MG. Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology. 2011;152:3192–3201. doi: 10.1210/en.2010-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubois PM. Ontogenesis of gonadotropic cells. Ann Endocrinol. 1990;51:47–53. [PubMed] [Google Scholar]
- 35.Isik S, Ozcan HN, Ozuguz U, Tutuncu YA, Berker D, Alimli AG, Akbaba G, Karademir MA, Guler S. Evaluation of ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with type 2 diabetes mellitus. J Clin Endocrin Metab. 2011;97:261–269. doi: 10.1210/jc.2011-1923. [DOI] [PubMed] [Google Scholar]
- 36.Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers: postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835. doi: 10.1001/archpedi.1960.04020040820004. [DOI] [PubMed] [Google Scholar]
- 37.Sir-Petermann T, Maliqueo M, Angel B, Lara H, Perez-Bravo F, Recabarren S. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 38.Cernea M, Lee T, Cheng G, Padmanabhan V, Lehman MN, Coolen LM. Excess prenatal testosterone increases androgen receptor expression in hypothalamic areas of the female sheep brain. 41st Annual Meeting of the Society for Neuroscience; Washington DC. 2011. (Abstr). [Google Scholar]
- 39.Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877. doi: 10.1530/REP-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nada SE, Thompson RC, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology. 2010;151:5165–5173. doi: 10.1210/en.2010-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padmanabhan V, Veiga-Lopez A, Abbott D, Recabarren S, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veiga-Lopez A, Salloum BA, Williams A, Herkimer C, Beckett E, Padmanabhan V. Developmental Programming: Prenatal and Postnatal Contribution of Androgen and Insulin in the Reprogramming of Pubertal Onset in Prenatal Testosterone-Treated Sheep. Endocr Rev; 94th Annual Meeting of the Endocrine Society; Houston, TX. 2012. SUN-31 (Abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veiga-Lopez A, Lee JS, Padmanabhan V. Developmental programming: insulin sensitizer treatment improves reproductive function in prenatal testosterone-treated female sheep. Endocrinology. 2010;151:4007–4017. doi: 10.1210/en.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- 45.Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunctions: The sheep model. Soc Reprod Fertil Suppl. 2007;64:83–107. doi: 10.5661/rdr-vi-83. [DOI] [PubMed] [Google Scholar]
- 47.Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- 48.Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- 49.Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- 50.Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abi Salloum B, Herkimer C, Lee JS, Veiga-Lopez A, Padmanabhan V. Developmental programming: prenatal and postnatal contribution of androgens and insulin in the reprogramming of estradiol positive feedback disruptions in prenatal testosterone-treated sheep. Endocrinology. 2012;153:2813–2822. doi: 10.1210/en.2011-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153:5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151:301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn T, Fergani C, Coolen L, Padmanabhan V, Lehman M. Prenatal Testosterone Excess Decreases Neurokinin 3 Receptor Immunoreactivity within the Arcuate Nucleus KNDy cell population. J Neuroendocrin. 2014 doi: 10.1111/jne.12244. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson LM, Mytinger A, Roberts EK, Lee TM, Foster DL, Padmanabhan V, Jansen HT. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154:1612–1623. doi: 10.1210/en.2012-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cernea M, Phillips R, Padmanabhan V, Coolen LM, Lehman MN. Prenatal testosterone decreases co-expression of insulin receptors in KNDy neurons in adult ewes. 2nd World Conference on Kisspeptin Signaling in the Brain; Tokyo, Japan. 2012. (Abstr). [Google Scholar]
- 58.Sliwowska JH, Fergani C, Gawałek M, Skowronska B, Fichna P, Lehman MN. Insulin: Its Role in the Central Control of Reproduction. Physiol Behav. 2014;133:197–206. doi: 10.1016/j.physbeh.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu X, Dowling AR, Marino JS, Faulkner LD, Bryant B, Bruning JC, Elias CF, Hill JW. Delayed puberty but normal fertility in mice with selective deletion of insulin receptors from Kiss1 cells. Endocrinology. 2013;154:1337–1348. doi: 10.1210/en.2012-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson JE, Hastie PM, Shah A, Smith A, Evans NP. Developmental programming: prenatal androgen exposure alters the gonadotroph population of the ovine pituitary gland. J Neuroendocrinol. 2012;24:434–442. doi: 10.1111/j.1365-2826.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 61.Buggs C, Weinberg F, Kim E, Wolfe A, Radovick S, Wondisford F. Insulin augments GnRH-stimulated LHbeta gene expression by Egr-1. Mol Cell Endocrinol. 2006;249:99–106. doi: 10.1016/j.mce.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur J Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- 63.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 64.West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrin. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 65.Escobar M, Vázquez-Nin G, Echeverría O. Cell Death in Mammalian Ovary. Netherlands: Springer; 2011. Follicular Cells; pp. 185–200. [Google Scholar]
- 66.McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- 67.Fortune J. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 68.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 69.Gougeon A. The ovary. Vol. 2. Elsevier Academic Press; 2004. Dynamics of human follicular growth: morphologic, dynamic, and functional aspects; pp. 25–43. [Google Scholar]
- 70.Sobinoff AP, Sutherland JM, McLaughlin EA. Intracellular signalling during female gametogenesis. Mol Hum Reprod. 2013;19:265–278. doi: 10.1093/molehr/gas065. [DOI] [PubMed] [Google Scholar]
- 71.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 72.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 73.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 74.Yang J-L, Zhang C-P, Li L, Huang L, Ji S-Y, Lu C-L, Fan C-H, Cai H, Ren Y, Hu Z-Y. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 2010;151:774–782. doi: 10.1210/en.2009-0751. [DOI] [PubMed] [Google Scholar]
- 75.Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19:828–837. doi: 10.1093/molehr/gat065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pru JK, Tilly JL. Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol Endocrinol. 2001;15:845–853. doi: 10.1210/mend.15.6.0646. [DOI] [PubMed] [Google Scholar]
- 77.Hubmacher D, Tiedemann K, Reinhardt DP. Fibrillins: from biogenesis of microfibrils to signaling functions. Curr Top Dev Biol. 2006;75:93–123. doi: 10.1016/S0070-2153(06)75004-9. [DOI] [PubMed] [Google Scholar]
- 78.Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010;58:903–915. doi: 10.1369/jhc.2010.956615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hatzirodos N, Bayne RA, Irving-Rodgers HF, Hummitzsch K, Sabatier L, Lee S, Bonner W, Gibson MA, Rainey WE, Carr BR. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertil Steril. 2012;97:748–756. doi: 10.1016/j.fertnstert.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol Reprod. 2012;87:22. doi: 10.1095/biolreprod.112.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, Shimasaki S, Erickson GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrin Met. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- 83.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 84.Raja-Khan N, Urbanek M, Rodgers RJ, Legro RS. The role of TGF-β in polycystic ovary syndrome. Reprod Sci. 2014;21:20–31. doi: 10.1177/1933719113485294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knight PG, Satchell L, Glister C. Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol. 2012;359:53–65. doi: 10.1016/j.mce.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 86.Liu Z, Castrillon DH, Zhou W, Richards JS. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol Endocrinol. 2013;27:238–252. doi: 10.1210/me.2012-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazerbourg S, Bondy CA, Zhou J, Monget P. The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles? a comparative species study. Reprod Domest Anim. 2003;38:247–258. doi: 10.1046/j.1439-0531.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 88.Visser JA, Themmen AP. Role of anti-Mullerian hormone and bone morphogenetic proteins in the regulation of FSH sensitivity. Mol Cell Endocrinol. 2014;382:460–465. doi: 10.1016/j.mce.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 89.Mather JP, Moore A, Li RH. Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Exp Biol Med. 1997;215:209–222. doi: 10.3181/00379727-215-44130. [DOI] [PubMed] [Google Scholar]
- 90.Palin M-F, Bordignon VV, Murphy BD. Adiponectin and the control of female reproductive functions. Vitam Horm. 2012;90:239–287. doi: 10.1016/B978-0-12-398313-8.00010-5. [DOI] [PubMed] [Google Scholar]
- 91.Maillard V, Uzbekova S, Guignot F, Perreau C, Ramé C, Coyral-Castel S, Dupont J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod Biol Endocrinol. 2010;8 doi: 10.1186/1477-7827-8-23. 10.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dumesic DA, Padmanabhan V, Abbott DH. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63:39–48. doi: 10.1097/OGX.0b013e31815e85fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100:23–38. doi: 10.1016/j.fertnstert.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kwintkiewicz J, Giudice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- 95.McFee RM, Rozell TG, Crupp AS. The balance between proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res. 2012;349:635–647. doi: 10.1007/s00441-012-1330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Padmanabhan V, Salvetti NR, Matiller V, Ortega HH. Developmental Programming: Prenatal Steroid Excess Disrupts Key Members of Intraovarian Steroidogenic Pathway in Sheep. Endocrinology. 2014;155:3649–3660. doi: 10.1210/en.2014-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hough D, Cloete S, Storbeck K, Swart A, Swart P. Cortisol production in sheep is influenced by the functional expression of two cytochrome P450 17α-hydroxylase/17, 20-lyase (CYP17) isoforms. J Anim Sci. 2013;91:1193–1206. doi: 10.2527/jas.2012-5800. [DOI] [PubMed] [Google Scholar]
- 99.Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol Reprod. 2010;82:1065–1075. doi: 10.1095/biolreprod.109.082719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocr Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 101.Dumesic DA, Abbott DH. Implications of polycystic ovary syndrome on oocyte development. Semin Reprod Med. 2008;26:53–61. doi: 10.1055/s-2007-992925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 103.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 104.Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab. 2008;22:155–171. doi: 10.1016/j.beem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Crespi EJ, Steckler TL, MohanKumar PS, Padmanabhan V. Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep. J Physiol. 2006;572:119–130. doi: 10.1113/jphysiol.2005.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.King AJ, Olivier NB, MohanKumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab. 2007;292:e1837–e1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- 107.Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295:e262–e268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.DeHaan K, Berger L, Bechtel P, Kesler D, McKeith F, Thomas D. Effect of prenatal testosterone treatment on nitrogen utilization and endocrine status of ewe lambs. J Anim Sci. 1990;68:4100–4108. doi: 10.2527/1990.68124100x. [DOI] [PubMed] [Google Scholar]
- 110.Hansen L, Drackley J, Berger L, Grum D. Prenatal androgenization of lambs: I. Alterations of growth, carcass characteristics, and metabolites in blood. J Anim Sci. 1995;73:1694–1700. doi: 10.2527/1995.7361694x. [DOI] [PubMed] [Google Scholar]
- 111.Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289:e801–e806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- 112.Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology. 2013;154:1731–1742. doi: 10.1210/en.2012-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corbould A, Kim Y-B, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:e1047–e1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 114.Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS) Am J Physiol Endocrinol Metab. 2001;281:e392–e399. doi: 10.1152/ajpendo.2001.281.2.E392. [DOI] [PubMed] [Google Scholar]
- 115.Glintborg D, Højlund K, Andersen NR, Hansen BF, Beck-Nielsen H, Wojtaszewski JF. Impaired insulin activation and dephosphorylation of glycogen synthase in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. J Clin Endocrinol Metab. 2008;93:3618–3626. doi: 10.1210/jc.2008-0760. [DOI] [PubMed] [Google Scholar]
- 116.Cortón M, Botella-Carretero JI, Benguria A, Villuendas G, Zaballos A, San Millán JL, Escobar-Morreale HF, Peral B. Differential gene expression profile in omental adipose tissue in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:328–337. doi: 10.1210/jc.2006-1665. [DOI] [PubMed] [Google Scholar]
- 117.Seow K-M, Juan C-C, Hsu Y-P, Hwang J-L, Huang L-W, Ho L-T. Amelioration of insulin resistance in women with PCOS via reduced insulin receptor substrate-1 Ser312 phosphorylation following laparoscopic ovarian electrocautery. Hum Reprod. 2007;22:1003–1010. doi: 10.1093/humrep/del466. [DOI] [PubMed] [Google Scholar]
- 118.Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of testosterone on placental differentiation. Reproduction. 2014 doi: 10.1530/REP-14-0055. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutri. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 120.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Antuna-Puente B, Feve B, Fellahi S, Bastard J-P. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 122.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yudkin JS, Stehouwer C, Emeis J, Coppack S. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction a potential role for cytokines originating from adipose tissue? Arterioscl Throm Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 124.Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JSE, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 125.Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]