Abstract
Collagen crosslinking enhances many beneficial properties of articular cartilage, including resistance to chemical degradation and mechanical wear, but many crosslinking agents are cytotoxic. The purpose of this study was to evaluate the effectiveness of genipin, a crosslinking agent with favorable biocompatibility and cytotoxicity, as a potential treatment to prevent the degradation and wear of articular cartilage. First, the impact of genipin concentration and treatment duration on the viscoelastic properties of bovine articular cartilage was quantified. Next, two short-term (15 minute) genipin crosslinking treatments were chosen, and the change in collagenase digestion, cartilage wear, and the friction coefficient of the tissue with these treatments was measured. Finally, chondrocyte viability after exposure to these genipin treatments was assessed. Genipin treatment increased the stiffness of healthy, intact cartilage in a dose-dependent manner. The 15-minute crosslinking treatments improved cartilage's resistance to both chemical degradation, particularly at the articular surface, and to damage due to mechanical wear. These enhancements were achieved without sacrificing the low coefficient of friction of the tissue and at a genipin dose that preserved chondrocyte viability. The results of this study suggest that collagen crosslinking via genipin may be a promising preventative treatment to slow the degradation of cartilage.
Keywords: biotribology, collagenase digestion, wear, friction, articular cartilage, collagen, crosslinking, genipin, cytotoxicity
INTRODUCTION
Osteoarthritis (OA) is a leading cause of disability in developed countries,1 affecting an estimated 15% of the United States population over the age of 25,2 and costing the U.S. economy just under $186 billion per year in lost wages and productivity.3 Despite the widespread prevalence of OA, few treatments are available to prevent its occurrence or even slow the advancement of the disease. The progression of OA involves a complex interplay of mechanical, cellular, and biochemical processes. In OA, the expression of degradative enzymes by the chondrocytes and the inflamed synovium are greatly enhanced,4 resulting in a reduction of the mechanical properties of the cartilage.5 Over time, the weakened tissue is less able to withstand mechanical loading, to the point that even normal activities produce abnormal wear and damage.
One method to decrease the susceptibility of cartilage to chemical degradation and mechanical wear is to enhance the collagen crosslinking in the tissue. Collagen fibers in biological tissue are strengthened by the formation of native crosslinks, and can be further stabilized through exogenous crosslinkinking.6-8 Crosslinks formed from advanced glycation end products (AGEs) resulted in increased tissue stiffness and strength9, 10 and decreased cartilage degradation by matrix metalloproteinases (MMPs).11 Additionally, collagen crosslinking via glutaraldehyde and formaldehyde fixation has been shown to increase the wear resistance of cartilage in in vitro testing.6-8 Glutaraldehyde fixation of healthy bovine cartilage resulted in an increase in stiffness of 28%, and a decrease in wear of 20%.7 Formaldehyde fixation of cartilage that was previously digested in trypsin showed reduced wear rates by 3 to 4 fold compared to digested cartilage, and 2 fold compared to healthy cartilage.6 While these crosslinking techniques demonstrated the promise of crosslinking to decrease biochemical degradation and the wear of articular cartilage, the chemicals are highly cytotoxic or cause an adverse biological response. A crosslinking agent that is both of low cytotoxicity and produces stable, biocompatible crosslinks is needed if a viable treatment to prevent native cartilage degradation and wear is to be developed.
One potential crosslinking agent that has received recent interest is genipin, a natural plant extract. Genipin is considered to be cell-tolerated12-14 and has been used to crosslink cellular and acellular tissues,16-19 as well as biomaterials such as chitosan and chitosan composites,20, 21 gelatin22-24 and polyethylene glycol hydrogels.25, 26 Genipin has been found to have relatively good biocompatibility in numerous studies on the viability of cells that have been seeded onto previously crosslinked scaffolds.27-29 Other studies have investigated the effect of genipin on cellular tissues and found the maximum nontoxic concentrations of genipin to range from 0.22 to 1 mM.30-32 However, these studies investigated the cellular response to genipin incubation periods of 1 to 7 days. A clinically relevant genipin treatment that could be applied during a surgical procedure would have incubation times on the order of minutes as opposed to days. As yet, the toxicity of short durations of genipin exposure has not been reported.
Genipin has been shown to increase the strength and modulus of a number of collagenous tissues, but its effect on native cartilage is unknown. Many of the previous genipin studies investigated collagen crosslinking in tissues composed of type I collagen. In contrast, the collagen network of cartilage is composed primarily of type II collagen, and genipin crosslinking of type II collagen has, to our knowledge, not been studied. Therefore, the change in the mechanical behavior of bovine articular cartilage with the concentration and duration of genipin crosslinking treatments was first quantified via indentation. From these results, two short-term (15 minute) crosslinking treatments were chosen, and their effect on collagenase digestion of the tissue, the friction coefficient and cartilage wear was investigated. Finally, chondrocyte viability after exposure to these treatments was assessed.
METHODS
VISCOELASTIC PROPERTIES FROM INDENTATION
Bovine stifles from approximately one year old animals were obtained from a local abattoir (Martins Meats; Wakarusa, IN) and were stored frozen at -23°C until use. Osteochondral specimens approximately 0.75 cm square and 1 cm high were taken from the condyles such that the specimen surface was perpendicular to the testing axis.33 Cartilage surfaces were coated with Tissue-Tek® Optimal Cutting Temperature (OCT) compound (Ted Pella, Inc.; Redding, CA) to preserve the surface, vacuum sealed and again stored at -23°C until the day of testing. Specimens were thawed and the surface coating was washed away with abundant phosphate buffered saline (PBS).
Stress-relaxation tests were performed with a Hysitron TI950 TriboIndenter (Minneapolis, MN) equipped with a 3-D OmniProbe® transducer and a 750 μm diameter flat punch probe. The load function consisted of a 20-second ramp to peak displacement, followed by a 50-second hold at peak displacement and a 1-second unloading segment. Testing was carried out with a 0.5 mN preload followed by a 67 μm peak indentation depth. During testing, specimens were immersed in PBS inside a fluid chamber. This protocol was previously found to produce repeatable measurements of the unloading stiffness that were sensitive to changes due to crosslinking.33 Indentations were performed in quadruplicate at three locations selected at random on each specimen. Immediately following indentation testing, specimens were crosslinked in genipin, as described below. Following crosslinking, samples equilibrated in PBS at room temperature for 2 h and then indentation testing was repeated at the same locations on four specimens per condition (n = 12).
To analyze the indentation data, the first 10% of the unloading curve was fit with a power law relation,34
| (1) |
where P is the load, h is the displacement, hf is the final displacement after complete unloading and a and m are power-law parameters. Values of a, m and hf were determined from regression analysis of the experimental data. The unloading stiffness, S, of the cartilage samples was
| (2) |
and was calculated at the maximum load. The unloading stiffness is directly proportional to the elastic modulus of the tissue for a flat punch indenter.35
For the 15 minute crosslinking protocols, the holding portion of the indentation stress relaxation data was further analyzed with a standard linear solid model (SLS) of a free spring in series with a parallel spring and dashpot. A linear viscoelastic Boltzmann integral defines the load as a function of time, F(t), for an arbitrary displacement
| (3) |
where h is the displacement of the indenter, u is a dummy time variable, and G(t)) is the relaxation function for the material.36, 37 The relaxation function for the SLS model is
| (4) |
in which k1 is the stiffness of the free spring, k2 is the stiffness of the spring in parallel with the dashpot, and η is the dashpot viscosity. The ramp displacement and holding conditions can be described by
| (5) |
where r is the slope of the ramp displacement, tR is the time to peak displacement, and h0 is the peak displacement.
Equation (3) was solved using equation (4) and the conditions from equation (5) to define a load-displacement expression for holding following the ramp displacement. The parameters k1, k2, and η were fit to the holding portion of the indentation stress relaxation data using MATLAB's optimization toolbox (Mathworks, Natick, MA) from one location per specimen initially and after the crosslinking treatment (n=4), and the changes in instantaneous stiffness k0, equilibrium stiffness k∞, and relaxation time constant τ were reported.
GENIPIN CROSSLINKING
Cartilage specimens were incubated in genipin (Challenge Bioproducts, Taiwan) solutions in phosphate-buffered saline (PBS) at 37°C for up to 6 h in a shaking waterbath. After incubation in the genipin, specimens were transferred to PBS and incubated at 37°C to bring the total incubation time to 24 h. For time-dependent studies, specimens were incubated in 1 mM genipin in PBS for 0.5, 1 or 6 h then transferred to PBS and incubated an additional 23.5, 23 or 18 h, respectively. For dose dependent studies, specimens were incubated in genipin in PBS for 15 or 30 min, and then transferred to PBS until the total incubation time was 24 h. For the 15 min study, specimens were incubated in 0, 2, 5, 10 or 20 mM genipin in PBS and for the 30 min study specimens were incubated in 0, 1, 2, 5, or 10 mM genipin. From the above studies, 15 min incubations in 2 and 10 mM genipin solutions were chosen for the following experiments.
COLLAGENASE DIGESTION
Osteochondral specimens 5.89 mm in diameter were cored from femoral condyles such that the articular surface was perpendicular to the coring axis. Specimens were incubated in 0, 2, and 10 mM solutions of genipin in PBS for 15 minutes and then in genipin-free PBS for the remaining 24 hours, as above (n=6). Using a sledge microtome (HM 450 Richard Allan, Kalamazoo, MI) equipped with a freezing stage (Physitemp, Clifton, NJ) set at -25°C, 150 μm sections were taken through the depth of the articular cartilage. To facilitate handling of the sections, a thin polyester membrane was coated with OCT freezing compound and placed on the exposed surface of the cartilage before each section was taken.38 Excess OCT was washed away with abundant PBS. Individual sections were incubated for 45 minutes at 37 °C in 0.5 mL of a 2 mg/mL solution of type I collagenase from Clostridium histolyticum in 50 mM Trizma® buffer at pH 7.42 containing 10 mM CaCl2 (all from Sigma). Collagenase digestion was stopped by removing the cartilage section from the solution. To quantify the collagen that had been digested, HPLC was used to measure the hydroxyproline content in the hydrating solution in triplicate as previously described.39, 40 Hydroxyproline is an amino acid constituent found almost exclusively in collagen which can be detected down to the femtomole via HPLC. Linear regressions were conducted for each specimen in order to relate the amount of digested hydroxyproline to the depth of the tissue for the different crosslinking protocols.
FRICTION AND WEAR TESTING
Osteochondral specimens 9.52 mm in diameter were cored from bovine femoral condyles such that the articular surface was perpendicular to the coring axis, with no more than 2 specimens taken per condyle.40 The specimens were immediately incubated in either 0, 2 or 10 mM genipin solution in PBS (n = 4 per condition) as described above. The coefficient of friction (COF) between cartilage and stainless steel (T316; Ra = 0.016 ± 0.004 μm) was measured with a hydrating solution consisting of 0.15 M NaCl with protease inhibitors (1mM ethylenediaminetetraacetic acid, 5 mM benzamadine and 10 mM n-ethylmaleimide). To provide insight into the subsequent wear test, reciprocal sliding motion was carried out using a Universal Micro-Tribometer (Center For Tribology, Inc., Campbell, CA) under a constant normal load of 53 N for 30 min at a sliding speed of 4 mm/s, with each back and forth portion being 18 mm long. The friction and normal forces were averaged over each cycle of reciprocal motion and the COF was obtained from the ratio of these values. The time-dependent COF was calculated from the ratio of the friction force to the normal force, and the initial value at time 0 and final value at 30 minutes was reported. Between tests, the stainless steel was thoroughly cleaned with 70% ethanol, followed by a distilled water rinse.
To perform wear testing, cartilage was worn against 316L stainless steel discs (Ra = 0.015 ± 0.002 μm) as previously described.40 Briefly, specimens were loaded into a pin-on-disk tribometer (OrthoPOD from AMTI; Watertown, MA) with the hydrating fluid and tested in cycles consisting of four 18 mm strokes in a square path with a sliding velocity of 4 mm/s.41 A load of 53 N, corresponding to an average pressure of 1.4 MPa, was applied at a rate of 150 N/s and removed for the final 45% (~8 mm) of each stroke to permit specimen rehydration.41 Testing was conducted at room temperature for a total of 4800 cycles, for a wear distance of 192 m.
To quantify the cartilage wear released to the hydrating saline solution, HPLC was used to measure the hydroxyproline content in the hydrating solution.39, 40 Verification of wear was performed with india ink; the areas that were stained after wiping the surface with a damp cloth indicated damage based on the adherence of india ink to fibrillated cartilage.40
Additional specimens treated with 0 mM genipin served as controls. The control specimens were placed in the hydrating bath and were run through the wear test protocol without contacting the stainless steel disc. The hydrating fluid from these specimens was also collected, and the hydoxyproline content quantified via HPLC. The collagen that was released to the hydrating bath in these non-contact specimens was assumed to originate from degradation at the cut surfaces and debris from machining during specimen fabrication and to be common to all specimens. Therefore, in order to determine the amount of hydroxyproline in the hydrating solutions that was due solely to wear, the average amount measured in the non-contact specimens was subtracted from that of the wear specimens.
CYTOTOXICITY
Porcine stifles from approximately 1 year old animals were obtained from a local abattoir (Martins Meats) and were dissected under sterile conditions within 6 h of slaughter. Once the joint capsule was breached, a 6 mm biopsy punch and clean scalpel blade were used to extract full-thickness cartilage explants from both femoral condyles. Explants were rinsed sequentially in Betadine® (Purdue Products, LLC, Stamford, CT) and PBS solutions and then were cultured in medium consisting of Dulbecco's Modified Eagle's medium, 10% FBS and 1% Penicillin/Streptomycin in a humid environment at 37°C and 5% CO2.
The viability of chondrocytes in the cartilage explants was evaluated 24 h after genipin treatment with a Live/Dead Viability/Cytotoxicity kit (Molecular Probes, Eugene, OR). Explants were incubated in PBS supplemented with 0, 2 or 10 mM genipin for 15 min. Afterwards, they were returned to genipin-free media for 23 h and 45 min. Explants were then incubated in Live/Dead assay reagents in media for 30 min to stain live cells green (1 μM Calcein AM) and dead cells red (2 μM Ethidium-1 Homodimer) and washed in PBS prior to imaging with a Nikon A1R-MP Confocal microscope. To evaluate the amount of red autofluorescence due to the genipin crosslinks,31 additional samples that had been exposed to genipin but not to the Live/Dead assay reagents were also imaged under identical conditions.
STATISTICS
One way ANOVA with Tukey's post-hoc test and a post-test for linear trend were performed to compare the effects of the different genipin treatments on tissue modulus, wear, and friction, with statistical significance set at p < 0.05 (Graphpad Prism Software, La Jolla CA). Slopes and y-intercepts from the linear regression of the hydroxyproline data and tissue depth from the collagenase digestion were also analyzed with a one-way ANOVA and Tukey's post-hoc test. Data are presented as mean ± SEM.
RESULTS
VISCOELASTIC PROPERTIES
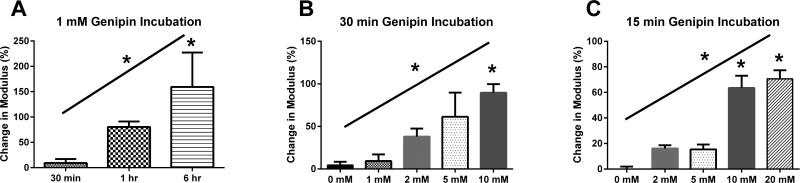
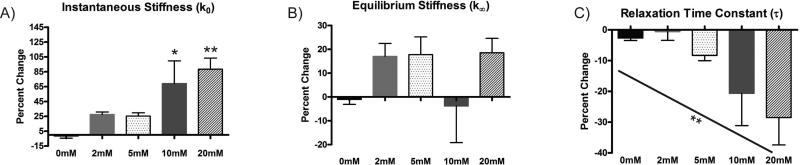
Genipin caused an increase in the unloading stiffness that was dependent on both the genipin concentration and incubation time. In the 1 mM genipin solution, the unloading stiffness increased in a time-dependent fashion to 159% of control after the 6 h treatment (Figure 1A). Similarly, increases in genipin concentration resulted in dose-dependent increases in the cartilage stiffness for both 30 min and 15 min incubation times. A 30 min incubation in 10 mM genipin solutions produced significant increases in the unloading stiffness (Figure 1B), while the same was true for 15 min incubations in 10 and 20 mM genipin. (Figure 1C). In the SLS analysis of the 15 minute incubations, the average R2 value for all curve fits was 0.984 ± 0.006. There was a significant increase in the instantaneous stiffness k0 with the 10 and 20 mM genipin compared to the 0 mM treatment (Figure 2A). The equilibrium stiffness k∞ did not significantly change with genipin concentration (p = 0.2022 for the one-way ANOVA; Figure 2B), while there was a dose-dependent decrease in the relaxation time constant (Figure 2C).
Figure 1.
Change in unloading stiffness with genipin treatment. (A) Effect of incubation time with 1 mM genipin solution. (B) Effect of genipin concentration during 30 min incubation. (C) Effect of genipin concentration during 15 min incubation. * over bar indicates significant difference from control; * over inclined line indicates significant linear trend. *p < 0.05.
Figure 2.
Change in SLS model properties with 15 min genipin incubation: (A) instantaneous stiffness, k0, (B) equilibrium stiffness, k∞, and (C) relaxation time constant, τ. * over bar indicates significant difference from control; * over inclined line indicates significant linear trend. *p < 0.05 and **p<0.01.
COLLAGENASE DIGESTION
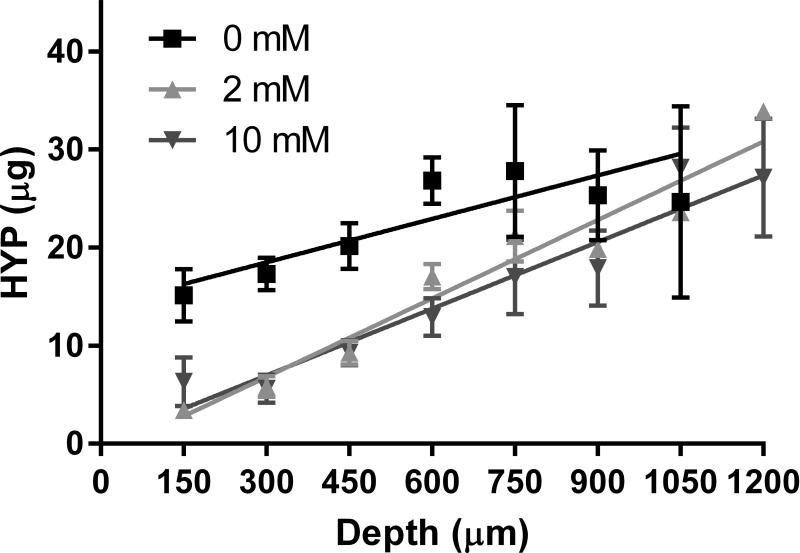
In all groups, the cartilage section that was the least digested was from the articular surface, with increasing amounts of hydroxyproline released from progressively deeper sections (Figure 3). In the specimens crosslinked in 2 and 10 mM genipin solutions, the amount of hydroxyproline that was released at the articular surface was less than half that in the controls, while the amount that was released at the greatest depths was similar between groups. A linear regression relating the amount of digested hydroxyproline to the depth of the tissue was conducted for each specimen. Interestingly, the y-intercepts for the 2 mM and 10 mM genipin treatments were significantly lower than that of the control but were not different from one another (Table 1).
Figure 3.
Amount of digested hydroxyproline due to collagenase through the depth of the tissue and linear regressions for each treatment.
Table 1.
Average and standard deviations of the slope, y-intercept and R for the linear regression to the amount of hydroxyproline digested through the depth of the tissue for each genipin crosslinking protocol.
| Slope | Y-intercept | R2 | |
|---|---|---|---|
| 0 mM | 0.0196 ± 0.0171 | 12.2 ± 6.74 | 0.695 ± 0.251 |
| 2 mM | 0.0247 ± 0.00388 | −0.537 ± 2.08** | 0.816 ± 0.131 |
| 10 mM | 0.0230 ± 0.00109 | −0.0171 ± 3.92** | 0.798 ± 0.140 |
* indicates a significant difference from the corresponding 0 mM control
(p < 0.01.)
COEFFICIENTS OF FRICTION
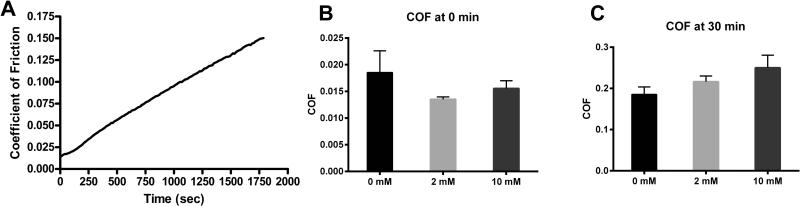
In all specimens the COF after 30 min of loading was significantly greater than the initial COF, but did not appear to have reached equilibrium (Figure 4A). There was no significant difference between the COF of the control and genipin treated groups at either the beginning or end of the test (p = 0.3992 and 0.1746 for the one-way ANOVA of the 0 and 30 min data, respectively; Figure 4B and 4C).
Figure 4.
(A) Representative COF over the 30 minute test. (B) COF initially and after (C) 30 minutes of loaded reciprocal motion.
WEAR
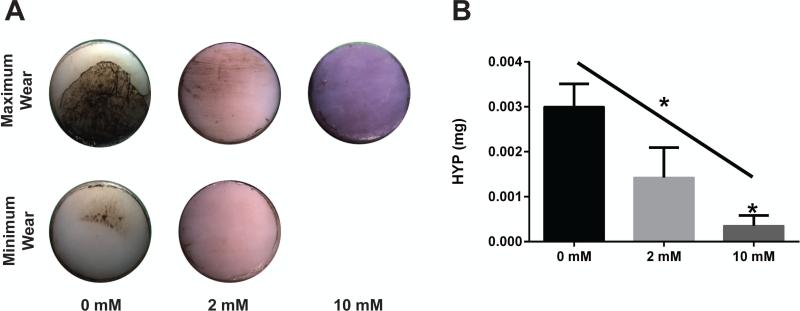
After wear testing, all four control samples showed varying degrees of visible wear, with two specimens demonstrating clear signs of wear at the half-way point of the wear test. In the 2 mM genipin treated specimens, half had minimal wear and the other half had no visible wear when imaged with india ink. No visible wear was present in any of the 10 mM genipin treated specimens (Figure 5A). Because genipin-treated specimens became blue in color, similar to that reported for other tissues,42 the india ink-stained areas could not be quantified to distinguish variations in wear between the groups. Analysis of the hydroxyproline content released to the lubricating fluid was consistent with observations of wear and demonstrated linear dependence on the degree of crosslinking (Figure 5B). The 2 mM genipin treatment reduced the average wear to about one half that of the control group, and 10 mM genipin protocol significantly decreased the collagen released to the hydrating bath due to wear to approximately 10% of control.
Figure 5.
(A) Minimum and maximum visible wear for the three groups. No visible wear was observed in any specimen in the 10 mM group so only the maximum wear image is presented. Color differences are due to genipin crosslinking. (B) Hydroxyproline content released to the hydrating bath due to wear per specimen. * over bar indicates significant difference from control; * over inclined line indicates significant linear trend. *p < 0.05.
CELL VIABILITY
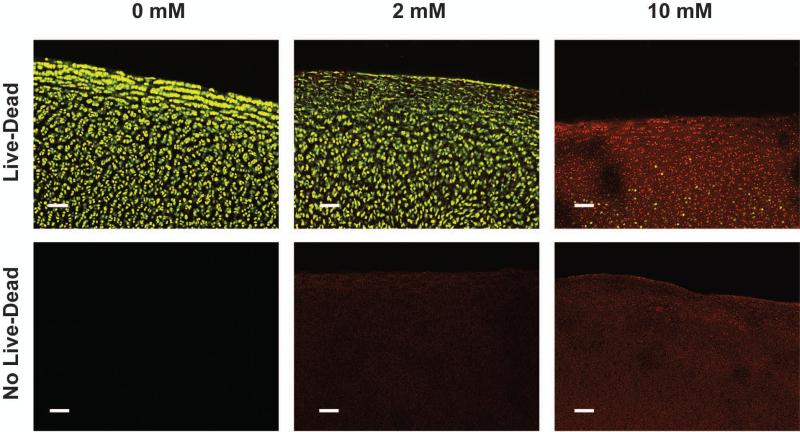
Chondrocyte viability was maintained in the control and 2 mM genipin groups, but showed a marked decrease at the 10 mM concentration (Figure 6). In the superficial zone, the intensity of the green Calcein AM staining of the live cells was decreased in the 2 mM specimen as compared to the controls, suggesting decreased cell metabolism. Due to the autofluorescence of genipin, a dose-dependent red background fluorescence is observed in the genipin-treated cartilage matrices without the addition of Live/Dead assay reagents.
Figure 6.
Top row: Representative confocal images of Live/Dead staining of the three groups. Bottom row: Confocal images without Live/Dead stain illustrate the red autofluorescence of genipin crosslinks. Scale bar = 100 μm.
DISCUSSION
This study investigated a means of crosslinking the collagen network of articular cartilage in a clinically relevant time span to strengthen the tissue and to improve its resistance to chemical and mechanical degradation. The results indicate the potential therapeutic value of this technique, as the genipin treatments significantly reduced the rate of collagen degradation by collagenase at the cartilage articular surface and also decreased the rate of mechanical wear in a dose dependent manner without affecting the coefficient of friction. Potential limitations of this crosslinking agent were also demonstrated; namely, that there is a tradeoff between chondrocyte viability and the improved wear resistance of cartilage. The specimens treated with 10 mM genipin showed significantly less wear, but also a pronounced loss of cell viability as compared to the untreated controls. The data also suggest that a favorable trade-off may be achieved by the proper genipin dose, as the 2 mM genipin treatment protected cartilage against collagenase degradation equally well as those treated with the 10 mM solution and reduced the wear rate of cartilage without appearing to impact chondrocyte viability. However, even at 2 mM, genipin decreased the intensity of Calcein AM staining at the articular surface, suggesting a lowered cell metabolism. This observation is consistent with a prior report that genipin suppresses chondrocyte metabolism and alters gene expression at non-toxic doses.32 Further studies are necessary to optimize genipin concentration and to assess the long-term effects of the genipin treatment on chondrocyte behavior.
The resistance to collagenase digestion was most improved at the articular surface and was essentially unchanged in the deep zone, likely because genipin did not fully diffuse through the depth of the cartilage during the 15 minute treatment. Additionally, the viscoelastic properties of the tissue were determined with a relatively shallow 70 μm indentation; the properties throughout the depth also may not have been significantly affected by the crosslinking treatments. As wear is primarily a surface phenomenon, improvements to resistance to chemical degradation and mechanical damage are most critical at the articular surface. In addition, limiting protection to the surface may maintain the native properties of the deeper tissue and the osteochondral interface, thereby preserving normal joint function.
The instantaneous stiffness increased with increasing genipin concentration, consistent with the unloading stiffness, while the equilibrium stiffness did not significantly change. In indentation testing, the tissue deformation under the indenter is similar to that in confined compression, where the strain during stress relaxation is initially greatest at the articular surface; over time as the stress relaxes, the strain becomes more uniform throughout the depth.43 Therefore, the instantaneous stiffness primarily reflects the material properties at the articular surface, the location of the greatest strain, while equilibrium stiffness is more of a composite measurement taken through the depth of the tissue. These observations may explain the results of the current study, in which the instantaneous stiffness increased with crosslinking, perhaps because collagen crosslinking is greatest at the articular surface (as indicated by the decreased collagenase digestion at that location) where the instantaneous stiffness is measured. On the other hand, the equilibrium stiffness may not have significantly changed with crosslinking because the complete depth of the tissue was not fully crosslinked.
The decreasing relaxation time constant with increasing genipin concentration indicates that crosslinking influences time-dependent material behavior of cartilage. The relaxation time constant is analogous to the characteristic diffusion time τp in linear poroelastic theory, which is directly proportional L2/(Hk), where L is the characteristic length over which fluid flows, H is the longitudinal modulus, and k is the hydraulic permeability.44 Therefore, the decreased stress relaxation time constant may be directly related to an increased longitudinal modulus, consistent with the increased tissue stiffness, as the indentation depth (and therefore the magnitude of L) did not vary with crosslinking, nor was permeability likely to be significantly altered. Although the SLS model provides a basic understanding of how cartilage time-dependent and -independent properties are changing with crossling, further studies are necessary to fully determine the effect of genipin crosslinking on cartilage mechanical properties.
Because the frictional response of cartilage is dependent on the interstitial fluid load support,45 a potential concern was that altering the stiffness of the solid matrix of cartilage would alter the interstitial fluid pressure and subsequently the COF. However, crosslinking treatments did not influence the initial COF (Figure 4B) and no statistically significant differences were observed after 30 min (Figure 4C). This is similar to what has been reported in cartilage that had been crosslinked in glutaraldehyde after a 7 day incubation8 and also for crosslinked hydrogels.46 The data show a non-significant trend of increased COF associated with increased crosslinking at 30 min (Figure 4C). It is possible that this trend may have become significant if the experiment had been run for a longer time, but such testing conditions would not be physiologically relevant. Because the COF is unchanged (or perhaps slightly increased) with the crosslinking treatments, the decrease in the wear can be attributed solely to the strengthening of the tissue. While the stiffness and not the strength of the tissue was measured here, previous studies have shown that intermolecular crosslinks also increase the strength of cartilage.10
In conclusion, the results of this study suggest that collagen crosslinking via genipin may be a promising preventative treatment to slow the progression of cartilage degeneration. The crosslinking treatments are effective at enhancing the cartilage's resistance to both chemical degradation, particularly at the articular surface, and to damage due to mechanical wear. These enhancements were achieved without sacrificing the coefficient of friction of the tissue or, in the case of the 2 mM treatment, chondrocyte viability. If the beneficial effects of this in vitro treatment could be brought into the clinical setting, the combination of reduced degradation due to chemical digestion and reduced damage due to mechanical wear could be expected to significantly slow the advancement of OA. Such a treatment could be particularly effective when used prophylactically following joint trauma, where a single event can lead to secondary OA.47-50 Additionally, a treatment that increases the wear resistance of cartilage may be a viable option to render cartilage more tolerant to articulation against materials other than native cartilage, such as in the case of hemiarthroplasty. Finally, the short (15 minute) duration of exposure would facilitate the clinical application of such a treatment. Genipin treatments may be limited, however, to clinical applications where the joint is open, as intra-articular injection would crosslink all the tissues of the joint, including the ligaments and synovium. Further studies will be required to assess the safety and efficacy of genipin crosslinking in vivo, as well as to determine the beneficial effect of genipin on damaged and diseased cartilage.
ACKNOWLEDGEMENTS
The authors thank Kevin Labus and John Cochran for preliminary studies that led to this research. The research was supported by the US Army Medical Research & Materiel Command W81XWH-07-066 and the Wendell F. Bueche Fellowship in Engineering (MEM), NIH grant AR047702 (SBT) and the Department of Veterans’ Affairs (SBT). The authors have no interests which might be perceived as posing a conflict or bias.
Footnotes
Author Contributions Statement: MEM contributed to the study design, performed the research, collected data, analyzed and interpreted the data, and drafted the manuscript. CMB performed research, collected data, analyzed and interpreted the data, and contributed to the manuscript. MLJ conducted research, collected data, analyzed and interpreted the data, and contributed to the manuscript. TCO was involved in the design of the study, the critical review of the manuscript and in acquiring funding for the study. SBT participated in the conception of the study, the interpretation of the data, and contributed to the manuscript. DRW contributed to the study conception and design, the analysis and interpretation of the data, manuscript preparation, and in acquiring funding for the study. All authors approved the final version of the manuscript.
REFERENCES
- 1.Cicuttini FM, Baker JR, Spector T. The association of obesity with osteoarthritis of the hand and knee in women: A twin study. J Rheumatol. 1996;23:1221–1226. [PubMed] [Google Scholar]
- 2.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–99. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis & Rheumatism. 2009;60:3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 4.Backus JD, Furman BD, Swimmer T, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. Journal of Orthopaedic Research . 2010 doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res. 1994;12:451–63. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- 6.Lipshitz H, Etheredge R, 3rd, Glimcher MJ. In vitro studies of the wear of articular cartilage--III. the wear characteristics of chemical modified articular cartilage when worn against a highly polished characterized stainless steel surface. J Biomech. 1980;13:423–36. doi: 10.1016/0021-9290(80)90036-6. [DOI] [PubMed] [Google Scholar]
- 7.Radin EL, Swann DA, Paul IL, Mcgrath PJ. Factors influencing articular cartilage wear in vitro. Arthritis & Rheumatism. 1982;25:974–980. doi: 10.1002/art.1780250810. [DOI] [PubMed] [Google Scholar]
- 8.Oungoulian SR, Hehir KE, Zhu K, et al. Effect of glutaraldehyde fixation on the frictional response of immature bovine articular cartilage explants. J Biomech. 2014;47:694–701. doi: 10.1016/j.jbiomech.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. the age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen AC, Temple MM, Ng DM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–7. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- 11.DeGroot J, Verzijl N, Wijk W, et al. Age related decrease in susceptibility of human articular cartilage to matrix metalloproteinase–mediated degradation: The role of advanced glycation end products. Arthritis & Rheumatism. 2001;44:2562–2571. doi: 10.1002/1529-0131(200111)44:11<2562::aid-art437>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Huang LLH, Sung HW, Tsai CC, Huang DM. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J Biomed Mater Res. 1998;42:568–576. doi: 10.1002/(sici)1097-4636(19981215)42:4<568::aid-jbm13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Yao CH, Liu BS, Hsu SH, Chen YS, Tsai CC. Biocompatibility and biodegradation of a bone composite containing tricalcium phosphate and genipin crosslinked gelatin. Journal of Biomedical Materials Research Part A. 2004;69:709–717. doi: 10.1002/jbm.a.30045. [DOI] [PubMed] [Google Scholar]
- 14.Tsai C, Huang R, Sung H, Liang HC. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J Biomed Mater Res. 2000;52:58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Sung H, Chen C, Huang R, Hsu J, Chang W. In vitro surface characterization of a biological patch fixed with a naturally occurring crosslinking agent. Biomaterials. 2000;21:1353–1362. doi: 10.1016/s0142-9612(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Tsai CC, Liang HC, Sung HW. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials. 2002;23:2447–2457. doi: 10.1016/s0142-9612(01)00379-9. [DOI] [PubMed] [Google Scholar]
- 17.Sung H, Liang I, Chen C, Huang R, Liang H. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J Biomed Mater Res. 2001;55:538–546. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. Journal of Biomedical Materials Research Part A. 2003;64:427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 19.Sung HW, Huang RN, Huang LLH, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. Journal of Biomaterials Science, Polymer Edition. 1999;10:63–78. doi: 10.1163/156856299x00289. [DOI] [PubMed] [Google Scholar]
- 20.Muzzarelli RA. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr Polym. 2009;77:1–9. [Google Scholar]
- 21.Bispo VM, Mansur AA, Barbosa-Stancioli EF, Mansur HS. Biocompatibility of nanostructured chitosan/poly (vinyl alcohol) blends chemically crosslinked with genipin for biomedical applications. Journal of biomedical nanotechnology. 2010;6:166–175. doi: 10.1166/jbn.2010.1110. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Chang W, Lin K, Sung H. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: In vitro and in vivo studies. Journal of Biomedical Materials Research Part A. 2003;65:271–282. doi: 10.1002/jbm.a.10476. [DOI] [PubMed] [Google Scholar]
- 23.Yao C, Liu B, Chang C, Hsu S, Chen Y. Preparation of networks of gelatin and genipin as degradable biomaterials. Mater Chem Phys. 2004;83:204–208. [Google Scholar]
- 24.Bigi A, Cojazzi G, Panzavolta S, Roveri N, Rubini K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials. 2002;23:4827–4832. doi: 10.1016/s0142-9612(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 25.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12:2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 26.Moffat KL, Marra KG. Biodegradable poly (ethylene glycol) hydrogels crosslinked with genipin for tissue engineering applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;71:181–187. doi: 10.1002/jbm.b.30070. [DOI] [PubMed] [Google Scholar]
- 27.Mwale F, Iordanova M, Demers CN, et al. Biological evaluation of chitosan salts cross-linked to genipin as a cell scaffold for disk tissue engineering. Tissue Eng. 2005;11:130–140. doi: 10.1089/ten.2005.11.130. [DOI] [PubMed] [Google Scholar]
- 28.Ko C, Wu C, Huang H, Chu I. Genipin cross-linking of type II collagen-chondroitin sulfate-hyaluronan scaffold for articular cartilage therapy. Journal of Medical and Biological Engineering. 2007;27:7. [Google Scholar]
- 29.Yan L, Wang Y, Ren L, et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. Journal of Biomedical Materials Research Part A. 2010;95:465–475. doi: 10.1002/jbm.a.32869. [DOI] [PubMed] [Google Scholar]
- 30.Lima EG, Tan AR, Tai T, et al. Genipin enhances the mechanical properties of tissue-engineered cartilage and protects against inflammatory degradation when used as a medium supplement. Journal of Biomedical Materials Research Part A. 2009;91:692–700. doi: 10.1002/jbm.a.32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundararaghavan HG, Monteiro GA, Lapin NA, et al. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. Journal of Biomedical Materials Research Part A. 2008;87:308–320. doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Lau TT, Loh WL, Su K, Wang DA. Cytocompatibility study of a natural biomaterial crosslinker—Genipin with therapeutic model cells. Journal of Biomedical Materials Research Part B: Applied Biomaterials . 2011 doi: 10.1002/jbm.b.31786. [DOI] [PubMed] [Google Scholar]
- 33.McGann ME, Bonitsky CM, Ovaert TC, Wagner DR. The effect of collagen crosslinking on the biphasic poroviscoelastic cartilage properties determined from a semiautomated microindentation protocol for stress relaxation. Journal of the mechanical behavior of biomedical materials. 2014;34:264–272. doi: 10.1016/j.jmbbm.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Sneddon IN. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 35.Oliver WC, Pharr GM. Improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. [Google Scholar]
- 36.Cheng L, Xia X, Yu W, Scriven L, Gerberich W. Flat-punch indentation of viscoelastic material. Journal of Polymer Science Part B: Polymer Physics. 2000;38:10–22. [Google Scholar]
- 37.Oyen ML, Cook RF, Stylianopoulos T, et al. Uniaxial and biaxial mechanical behavior of human amnion. J Mater Res. 2005;20:2902–2909. [Google Scholar]
- 38.Leong P, Morgan E. Measurement of fracture callus material properties via nanoindentation. Acta biomaterialia. 2008;4:1569–1575. doi: 10.1016/j.actbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutson PR, Crawford ME, Sorkness RL. Liquid chromatographic determination of hydroxyproline in tissue samples. Journal of Chromatography B. 2003;791:427–430. doi: 10.1016/s1570-0232(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 40.McGann ME, Vahdati A, Wagner DR. Methods to assess in vitro wear of articular cartilage. Proc Inst Mech Eng Part H J Eng Med. 2012;226:612–622. doi: 10.1177/0954411912447014. [DOI] [PubMed] [Google Scholar]
- 41.Bell C, Ingham E, Fisher J. Influence of hyaluronic acid on the time-dependent friction response of articular cartilage under different conditions. Proc Inst Mech Eng Part H J Eng Med. 2006;220:23. doi: 10.1243/095441105X69060. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Lim J, Bhoo S, Paik Y, Hahn T. Colorimetric determination of amino acids using genipin from< i> gardenia jasminoides</i>. Anal Chim Acta. 2003;480:267–274. [Google Scholar]
- 43.Wang CC, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J Biomech. 2001;34:75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 44.Nia HT, Han L, Li Y, Ortiz C, Grodzinsky A. Poroelasticity of cartilage at the nanoscale. Biophys J. 2011;101:2304–2313. doi: 10.1016/j.bpj.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–70. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman ME, Furey MJ, Love BJ, Hampton JM. Friction, wear, and lubrication of hydrogels as synthetic articular cartilage. Wear. 2000;241:129–135. [Google Scholar]
- 47.Borrelli J, Zaegel MA, Martinez MD, Silva MJ. Diminished cartilage creep properties and increased trabecular bone density following a single, sub-fracture impact of the rabbit femoral condyle. Journal of Orthopaedic Research. 2010;28:1307–1314. doi: 10.1002/jor.21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haut R, Ide T, De Camp C. Mechanical responses of the rabbit patello-femoral joint to blunt impact. J Biomech Eng. 1995;117:402. doi: 10.1115/1.2794199. [DOI] [PubMed] [Google Scholar]
- 49.Newberry WN, Mackenzie CD, Haut RC. Blunt impact causes changes in bone and cartilage in a regularly exercised animal model. Journal of orthopaedic research. 1998;16:348–354. doi: 10.1002/jor.1100160311. [DOI] [PubMed] [Google Scholar]
- 50.Newberry W, Garcia J, Mackenzie C, Decamp C, Haut R. Analysis of acute mechanical insult in an animal model of post-traumatic osteoarthrosis. J Biomech Eng. 1998;120:704–709. doi: 10.1115/1.2834882. [DOI] [PubMed] [Google Scholar]