Abstract
This cross-sectional study was intended to examine health effects of 678 male workers employed during an 8-yr period from 2000 to 2007 at 36 municipal and private waste incineration plants in Japan. Blood samples were obtained for analysis of concentrations of dioxins including coplanar polychlorinated biphenyls (coplanar PCBs) and evaluation of health effects. Health effects including diabetes were surveyed via a physician’s interview or clinical data from blood samples. There was a certain difference in serum concentrations of polychlorinated dibenzofurans (PCDFs) between the incinerator workers and Japanese general population, although no differences in the concentrations of total dioxins or polychlorinated dibenzo-p-dioxins (PCDDs) were found between the two groups. A few positive correlations between serum levels of PCDDs and PCDFs and the results of laboratory and physiological tests were found, but coplanar PCBs showed significant relations with 14 parameters of the tests. The background serum levels of PCDDs, PCDFs and total dioxins were significantly associated with the prevalence of diabetes. No essential differences in serum concentrations of total dioxins and in prevalence of diabetes between our subjects and the general population suggested that the incinerator workers were marginally exposed to dioxins in the workplace without any recognizable adverse health effects.
Keywords: Dioxin, Waste incinerator worker, Diabetes mellitus, Hypertension
Introduction
Many studies have been performed about health effects of persistent organic pollutants (POPs) including dioxins. Early investigations on dioxins were conducted with respect to highly exposed groups such as residents of Seveso1), Yusho2) or Yucheng3) patients, veterans of Operation Ranch Hand Service in the Vietnam War4), employees of pesticide factories5) and waste incinerator workers6,7,8,9,10,11,12). These investigations also revealed that dioxins influence human health, for example, carcinogenicity, immunological abnormalities, liver dysfunction and complex interactions with the endocrine systems13,14,15,16). It was also noteworthy that the increased levels of tetrachlorodibenzo-p-dioxin (TCDD) exposure in veterans of Operation Ranch Hand in the Vietnam war17, 18), in American trichlorophenol (TCP) production workers19) and in German BASF workers20) were closely associated with increased prevalence of diabetes. In addition, a positive link between POPs and diabetes was reported in general population-based studies. Longnecker et al.21), studying the association of background level dioxin exposure with the prevalence of diabetes for 1,197 retired Air Force veterans who never had contact with dioxin-contaminated herbicides and whose serum levels of dioxins were within the range of the background levels seen in the USA, deliberately concluded that the association of dioxins with diabetes may be due to reasons other than causality, although a causal contribution cannot be wholly dismissed. Lee et al.22) reported that the prevalence of diabetes was positively associated with six POPs including polychlorinated dioxins based on analysis of data for 2,016 adult participants in the National Health and Nutrition Examination Survey from 1999 to 2002. Uemura et al.23) showed a significant association between serum concentrations of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and dioxin-like polychlorinated biphenyls (DL-PCBs) and the prevalence of metabolic syndrome including glucose intolerance among the general population in Japan. Because diabetes is a major threat to public health worldwide24), evaluation of the contributions of the risk factors arising from the living and occupational environments to type 2 diabetes is urgently needed, in addition to evaluation of those of traditional risk factors of lifestyle, obesity and physical inactivity.
In 1997, a national survey of dioxins in emission gases from the municipal waste incineration plants in Japan revealed that some of incineration facilities emitted high levels of dioxins25). In particular, the emission level for one of the facilities in Osaka was reported to be 180 ng-TEQ/Nm3, which far exceeded the national guideline level of 5 ng/Nm3, and the plant was found to be contaminating the soil near the incineration plant with 8,500 pg TEQ/g of dioxins26). The Japan Industrial Safety and Health Association (JISHA) subsequently established the “Research and Investigation Committee on the Assessment of Exposure to Dioxins among Workers in the Waste Incineration Industry” to investigate occupational exposure to dioxins and the health status of workers engaged in municipal and private waste incineration facilities in Japan from 1999 to 2007.
For the purpose of analyzing the data obtained by the aforementioned Committee, the present study first examined total serum dioxin levels and their isomer patterns of employees working for waste incineration plants in Japan in comparison with those of the general population living in the urban, farming and fishing areas of Japan in a survey by the Ministry of the Environment, Japan (MOEJ)27, 28). This MOEJ survey was compiled as “The Survey on the Exposure to Dioxins and Other Chemical Compounds in Humans” during a 9-yr period spanning 2002 to 2010 to clarify the relationship between the body burden and dietary intake of dioxins and other POPs and their related factors in the Japanese general population.
Second, the correlations between serum concentrations of dioxins and the results of laboratory and physiological tests were analyzed. Third, we also analyzed significant associations between serum concentrations of PCDDs, PCDFs, coplanar polychlorinated biphenyls (coplanar PCBs) and total dioxins and the prevalences of four major diseases, diabetes, hypertension, hyperlipidemia and liver dysfunction. All the data including the present results were compiled from the Report of the Research and Investigation Committee on the Assessment of Exposure to Dioxins among Workers in the Waste Incineration Industry29) and data compiled and subsequently investigated by the Committee in the fiscal years 2006 and 2007.
Subjects and Methods
Study population
The subjects participating in the Committee’s survey were the employees working mainly inside the waste incineration facilities during a 9-yr period from 1999 to 2007. Since we did not analyze some of coplanar PCBs in 1999, the subjects participating in 1999 were excluded from the present study. Six hundred ninety-eight male workers participated in this survey spanning 2000 to 2007; these individuals worked for 36 municipal and private waste incineration plants in Japan. We excluded 20 workers, because we could not obtain samples from them. Ultimately, the study subjects comprised a total of 678 male workers. After the purpose and methods of this survey were explained to all the subjects, written informed consent was obtained from them. The protocol of the present study was approved by the Ethics Review Committee of the JISHA.
Clinical examinations
A questionnaire on lifestyle, present or past illness and medical history was handed out to each subject in advance and was collected on the day of medical examination. We first conducted blood cell counts and physical examinations for all the participants to exclude anemic subjects, because we had to take a 180-ml blood sample. Then, physiological and clinical examinations were performed by physicians, and diagnosis of skin conditions such as chloracne was performed by dermatologists, and an interview concerning occupational history was performed by occupational physicians. Tests for blood cells counts, blood chemistry and immunological function were conducted, and serum concentrations of dioxins were measured. Blood count and blood chemistry tests were analyzed at Rousai Hospitals. Immunological function tests were entrusted to SRL Co. Inc (Tokyo, Japan). LDH data measured before 2003 were adjusted with those after 2004, because the analytical method for LDH was changed after 2004. Since some portions of the subjects participated in the present survey after having breakfast or lunch and since blood glucose varied greatly depending on the time of eating or drinking, we used HbA1c as the criterion for evaluation of diabetes instead of fasting blood glucose. The HbA1c data obtained by the Japan Diabetes Society (JDS) procedure were standardized into those of the National Glycohemoglobin Standardization Program (NGSP) with the following approximation expression30).
| HbA1c (NGSP,%)=1.02×HbA1c (JDS,%)+0.25% |
Subjects were defined as having diabetes if they reported that they had been diagnosed with it by a physician or if they had a serum concentration of HbA1c at or above 6.5%31). Hypertension, hyperlipidemia and liver dysfunction were also examined for all the subjects. These three diseases were defined as follow.
Hypertension: Subjects were defined as having hypertension if they reported that they had been diagnosed with it or if they had a systolic blood pressure at or above 140 mmHg or a diastolic blood pressure at or above 90 mmHg.
Hyperlipidemia: Subjects were defined as having hyperlipidemia if they reported that they had been diagnosed with it by a physician or if they had a serum concentrations of total cholesterol and triglyceride at or above 220 mg/dl and 150 mg/dl, respectively, or a serum concentration of HDL cholesterol at or below 40 mg/dl.
Liver dysfunction: Subjects were defined as having liver disease if they reported that they had been diagnosed with it by a physician or if they had serum concentrations of AST, ALT and GGT at or above 40IU/l, 45IU/l and 80IU/l, respectively. Patients who had been diagnosed as having chronic viral hepatitis were excluded from the present analysis of liver dysfunction regardless of the observed hepatic indicators.
The definitions for the prevalences of the four diseases except liver dysfunction were the same as those for the disease prevalences among the Japanese general population compiled in the National Health and Nutrition Examination Survey in Japan32). Significant associations between serum concentrations of dioxin congeners and the prevalences of these four diseases were further analyzed by adjusting for age, survey year, body mass index (BMI), smoking habit and alcohol consumption.
Exposure assessment
Classification by job history
Workers were divided into groups (groups I to IV) based on the following job categories according to the result of interviews conducted by occupational physicians6).
-
I:
workers whose jobs did not involve work inside an incineration facility.
-
II:
workers whose jobs did involve work inside an incineration facility but only in handling of solidified fly ash and slag or nonflammable residues.
-
III:
workers whose jobs involved helping with incineration-related work inside an incineration facility but did not take part in work assigned for category IV.
-
IV:
workers whose jobs mainly involved operation and maintenance of an incinerator including a furnace, electric dust collector and wet scrubber inside an incineration facility.
All the workers of groups III and IV were asked to wear respirators, gloves and goggles during their working hours.
Blood dioxin measurements
Analysis of serum concentrations of dioxins was carried out according to the analytical method described in the interim report issued by the Ministry of Welfare (MOW), Japan33). Blood samples were collected into a transfusion bag containing heparin sodium solution and were divided into two portions: one for the blood test for examination of health effects and the other for analysis of dioxins by Otsuka Assay Laboratory (Tokushima, Japan) after centrifugation. The analytical method for dioxins was as follows: Lipids were extracted from plasma with a solution of saturated ammonium sulfate and ethanol:hexane solution after addition of an internal standard of 13C-labeled mixed dioxin isomer solution containing 10 pg each of PCDDs and PCDFs and 20 pg each of coplanar PCBs as a clean-up spike. The extracted hexane layer was washed with distilled water, and was treated with anhydrous sodium sulfate and evaporated to dryness, and the lipid weight was then measured. After elution with hexane again, sulfate acid was added and the layer was removed repeatedly until it became transparent. The hexane layer was washed with distilled water again and was treated with anhydrous sodium sulfate and evaporated until a small volume of residue was obtained. The residue was applied to a multilayer silica column and silica gel column, and analysis of PCDDs, PCDFs and coplanar PCBs was carried out by gas chromatography-high resolution mass spectrometry (GC-MS) after addition of an internal standard of 13C-labeled mixed dioxin isomer solution which contained 10 pg of PCDDs and PCDFs and 20 pg each of coplanar PCBs as a syringe spike. The quantitation limit for TCDD, Tetra-CDF (TCDF), penta-CDDs (PeCDDs) and PeCDFs was set as 1 pg/g lipid, that for hexa-CDDs (HxCDDs), HxCDFs, hepta-CDDs (HpCDDs) and HpCDFs as 2 pg/g lipid, that for octa-CDD (OCDD) and OCDF as 4 pg/g lipid and that for coplanar-PCBs as 10 pg/g lipid. If the measured value was less than the quantitation limit, a half of the limit was used for calculation.
Statistical analysis
Serum concentrations of dioxin isomers expressed as pg/g lipid were converted into the toxic equivalency (TEQ) value and expressed as pg TEQ/g lipid according to the WHO TEF method34). In order to compare isomer patterns of serum dioxins of the participating incinerator workers, we used the serum concentrations of dioxin isomers in the male and female general population living in urban, farming and fishing areas of Japan from the survey by the MOEJ27). The MOEJ’s dioxin concentrations were averaged over a 9-yr period from 2002 to 2010, and were expressed as arithmetic means. The partial correlation coefficients between the parameters of laboratory and physiological tests and the serum concentrations of dioxins, adjusting for age, survey year, BMI, smoking habit and alcohol consumption, were analyzed. We also performed multiple regression analysis to adjust for age, survey year, BMI, smoking habit and alcohol consumption as confounders. Relations between serum concentrations of dioxin congeners and prevalences of diabetes, hypertension, hyperlipidemia and liver dysfunction were analyzed by logistic regression analysis.
Statistical analysis was conducted with the SPSS software, ver.11.5 (SPSS Inc., Chicago, IL, USA).
Results
Study population
Table 1 shows demographic characteristics of the subjects participating in the present study. Approximately 90% of the subjects were classified into group III or IV, indicating that the study population consisted predominantly of the workers who engaged in incineration-related jobs and in operation and maintenance of an incinerator and related equipment. Mean working duration in incineration-related jobs was 12.4 yr. Ninety-two percent of the subjects wore respirators during their working hours. About 80% of the subjects were alcohol drinkers and smokers including ex-smokers. Fifty-three percent of the subjects had hyperlipidemia, 45% had hypertension, 31% had liver dysfunction, and 6.6% had diabetes.
Table 1. Demographic characteristics of the study population.
| Number of plants | 36 | |||
| Number of subjects | 678 | |||
| Working duration (yr)1 | 12.4 ± 9.4 | |||
| Age (yr)1 | 43.1 ± 11.1 | |||
| Percent body fat (%)1 | 22.9 ± 61 | |||
| Body mass index1 | 24.0 ± 3.3 | |||
| Smoking habit | n | Ratio | ||
| Nonsmoker | 121 | 17.8% | ||
| Smoker | 556 | 82.0% | ||
| Ex-smoker | 165 | 24.3% | ||
| Current smoker | 390 | 57.5% | ||
| Unknown | 1 | 0.1% | ||
| Alcohol consumption | n | Ratio | ||
| Nondrinker | 126 | 18.6% | ||
| Drinker | 551 | 81.3% | ||
| 2–3 times/month | 96 | 14.2% | ||
| 2–3 times/wk | 220 | 32.4% | ||
| Everyday | 225 | 33.2% | ||
| Unknown | 10 | 1.5% | ||
| Job categories2 | n | Ratio | ||
| I | 39 | 5.8% | ||
| II | 11 | 1.6% | ||
| III | 70 | 10.3% | ||
| IV | 550 | 81.1% | ||
| Unknown | 8 | 1.2% | ||
| Personal protective equipment | n | Rate | ||
| Respirators | 625 | 92.2% | ||
| Goggles | 413 | 60.9% | ||
| Gloves | 480 | 70.8% | ||
| Number of diseases | n | Rate | ||
| Hypertension | 304 | 44.8% | ||
| Diabetes mellitus | 45 | 6.6% | ||
| Hyperlipidemia | 362 | 53.4% | ||
| Liver dysfunction | 211 | 31.1% | ||
1The values represent arithmetic means ± SD. 2Job categories I to IV are described in Subjects and Methods.
Table 2 shows the numbers of the subjects having the four major diseases and their demographic characteristics when the serum concentrations of dioxin congeners were classified into four quartiles at the designated cutoff values. The quartile-categorized serum concentrations of dioxin congeners tended to increase with an increase in age and working duration. The numbers of the subjects having these four diseases tended to increase with an increase in the quartile-categorized serum concentrations of dioxin congeners.
Table 2. Number of diseases and characteristics of the subjects according to quartiles of serum concentrations of dioxin congeners.
| Cut-off value (pg TEQ/g lipid) |
n | Working duration (yr) |
Age (yr) | Percent body fat (%) |
BMI | Smoking habit | Alcohol consumption | Number of diseases2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoker | Drinker | HTN | DM | HL | LDF | ||||||||
| Total Dioxins | |||||||||||||
| Q11 | <9.76 | 169 | 9.23 ± 7.7 | 35.3 ± 10.5 | 21.6 ± 6.5 | 23.0 ± 3.4 | 143 | 123 | 50 | 3 | 65 | 35 | |
| Q2 | ≥9.76 | 170 | 12.2 ± 9.4 | 41.8 ± 10.3 | 23.8 ± 5.8 | 24.6 ± 3.6 | 141 | 138 | 66 | 4 | 94 | 50 | |
| Q3 | ≥13.70 | 170 | 12.7± 9.2 | 46.7 ± 8.9 | 23.1 ± 5.5 | 24.3 ± 3.1 | 132 | 145 | 90 | 16 | 105 | 66 | |
| Q4 | ≥21.67 | 169 | 15.5 ± 10.0 | 48.7 ± 9.7 | 22.8 ± 4.8 | 24.2 ± 2.8 | 140 | 145 | 98 | 22 | 98 | 60 | |
| PCDDs | |||||||||||||
| Q1 | <4.34 | 169 | 9.4 ± 7.9 | 36.6 ± 11.1 | 22.1 ± 6.7 | 23.2 ± 3.5 | 136 | 126 | 54 | 3 | 65 | 35 | |
| Q2 | ≥4.34 | 170 | 11.8 ± 8.9 | 42.0 ± 10.4 | 23.0 ± 5.3 | 24.1 ± 3.5 | 148 | 136 | 76 | 7 | 98 | 54 | |
| Q3 | ≥6.09 | 170 | 13.5 ± 9.7 | 45.8 ± 9.5 | 23.6 ± 5.2 | 24.5 ± 3.1 | 135 | 146 | 80 | 10 | 103 | 63 | |
| Q4 | ≥8.98 | 169 | 15.1 ± 9.9 | 48.0 ± 10.0 | 22.6 ± 5.2 | 24.3 ± 3.6 | 137 | 143 | 94 | 25 | 96 | 59 | |
| PCDFs | |||||||||||||
| Q1 | <2.16 | 169 | 10.1 ± 8.3 | 36.2 ± 10.1 | 22.9 ± 6.7 | 23.4 ± 3.6 | 136 | 126 | 52 | 3 | 69 | 47 | |
| Q2 | ≥2.16 | 170 | 11.6 ± 9.0 | 42.8 ± 10.3 | 22.8 ± 5.8 | 24.3 ± 3.1 | 151 | 143 | 73 | 5 | 99 | 50 | |
| Q3 | ≥3.10 | 170 | 13.4 ± 9.9 | 45.2 ± 10.2 | 23.2 ± 5.9 | 24.2 ± 3.1 | 134 | 141 | 87 | 15 | 105 | 57 | |
| Q4 | ≥4.51 | 169 | 14.6 ± 9.6 | 48.1 ± 10.2 | 22.4 ± 4.5 | 24.2 ± 2.8 | 135 | 141 | 92 | 22 | 89 | 57 | |
| Coplanar PCBs | |||||||||||||
| Q1 | <2.60 | 169 | 9.3 ± 7.6 | 34.3 ± 10.0 | 21.7 ± 6.5 | 23.2 ± 3.5 | 144 | 119 | 46 | 2 | 68 | 2 | |
| Q2 | ≥2.60 | 170 | 12.2 ± 9.5 | 42.4 ± 10.0 | 23.3 ± 5.7 | 24.1 ± 3.3 | 144 | 142 | 69 | 9 | 91 | 53 | |
| Q3 | ≥4.45 | 169 | 12.3 ± 8.9 | 45.6 ± 9.3 | 23.3 ± 5.4 | 24.3 ± 3.2 | 127 | 139 | 84 | 14 | 97 | 63 | |
| Q4 | ≥7.30 | 170 | 15.9 ± 10.1 | 50.0 ± 8.7 | 23.0 ± 5.2 | 24.4 ± 3.0 | 141 | 151 | 105 | 20 | 106 | 66 | |
1Q1, Q2, Q3 and Q4 stand for the 1st, 2nd, 3rd and 4th quartiles, respectively. 2HTN: hypertension; DM: diabetes mellitus; HL: hyperlipidemia; LDF: liver dysfunction
Blood dioxin levels
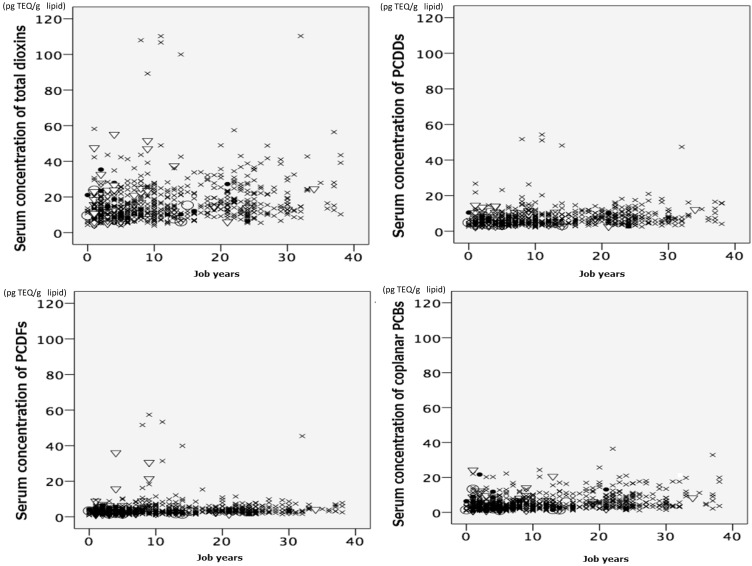
Table 3 shows the serum concentrations of dioxin isomers in the incinerator workers expressed as both medians and minimal and maximal values, and Table 4 shows arithmetic mean concentrations of dioxin isomers averaged over the 9-yr period according to age group of the incinerator workers, with reference to those in each age group of the Japanese general population compiled by the MOEJ27). We used the arithmetic means in Table 4, because the MOEJ reported the dioxin concentrations as arithmetic means. Median and arithmetic mean serum concentration of total dioxins were 13.7 and 17.2 pg TEQ/g lipid for the incinerator workers, respectively, while the arithmetic mean concentration of total dioxins was 19.4 pg TEQ/g lipid for the Japanese general population, indicating no difference between the two groups. On the other hand, the ratios of PCDDs, PCDFs and coplanar PCBs to the total dioxins were 42.6%, 23.9% and 33.4% for the incinerator workers, respectively, while the corresponding ratios were 41.8%, 17.6% and 40.6% for the Japanese general population. It was thus found that the arithmetic mean PCDFs of our subjects were significantly higher than those of the Japanese general population, although no significant difference in the mean PCDDs or coplanar PCBs between the two groups was found. Figure 1 shows that the serum concentrations of total dioxins in groups III and IV increased in spite of a short duration of employment (job years), suggesting that the workers were exposed to high levels of dioxins as compared with the workers in groups I and II.
Table 3. Distribution of serum concentrations of dioxin isomers in Japanese incinerator workers.
| Dioxins | TEF | Median | Min | Max | |
|---|---|---|---|---|---|
| (pg TEQ/g lipid) | |||||
| PCDDs | 6.08 | 1.88 | 54.27 | ||
| 2,3,7,8-TCDD | 1 | 0.50 | 0.50 | 6.10 | |
| 1,2,3,7,8-PeCDD | 1 | 3.40 | 0.50 | 35.40 | |
| 1,2,3,4,7,8-HxCDD | 0.1 | 0.10 | 0.05 | 2.90 | |
| 1,2,3,6,7,8-HxCDD | 0.1 | 1.48 | 0.27 | 11.75 | |
| 1,2,3,7,8,9-HxCDD | 0.1 | 0.24 | 0.10 | 3.38 | |
| 1,2,3,4,6,7,8-HpCDD | 0.01 | 0.09 | 0.00 | 2.00 | |
| OCDD | 0.0003 | 0.03 | 0.00 | 3.30 | |
| PCDFs | 3.10 | 0.85 | 57.36 | ||
| 2,3,7,8-TCDF | 0.1 | 0.05 | 0.05 | 0.62 | |
| 1,2,3,7,8-PeCDF | 0.03 | 0.02 | 0.00 | 0.19 | |
| 2,3,4,7,8-PeCDF | 0.3 | 2.10 | 0.36 | 27.24 | |
| 1,2,3,4,7,8-HxCDF | 0.1 | 0.25 | 0.10 | 7.63 | |
| 1,2,3,6,7,8-HxCDF | 0.1 | 0.34 | 0.10 | 13.29 | |
| 1,2,3,7,8,9-HxCDF | 0.1 | 0.10 | 0.10 | 1.04 | |
| 2,3,4,6,7,8-HxCDF | 0.1 | 0.10 | 0.10 | 6.84 | |
| 1,2,3,4,6,7,8-HpCDF | 0.01 | 0.03 | 0.00 | 3.13 | |
| 1,2,3,4,7,8,9-HpCDF | 0.01 | 0.01 | 0.00 | 0.33 | |
| OCDF | 0.0003 | 0.00 | 0.00 | 0.01 | |
| Coplanar PCBs | 4.45 | 0.72 | 36.41 | ||
| Non-ortho PCBs | |||||
| PCB77 | 0.0001 | 0.00 | 0.00 | 0.02 | |
| PCB81 | 0.0003 | 0.00 | 0.00 | 0.01 | |
| PCB126 | 0.1 | 3.00 | 0.50 | 31.13 | |
| PCB169 | 0.03 | 0.84 | 0.15 | 7.38 | |
| Mono-ortho PCBs | |||||
| PCB105 | 0.00003 | 0.03 | 0.00 | 0.30 | |
| PCB114 | 0.00003 | 0.01 | 0.00 | 0.08 | |
| PCB118 | 0.00003 | 0.17 | 0.00 | 1.26 | |
| PCB123 | 0.00003 | 0.00 | 0.00 | 0.02 | |
| PCB156 | 0.00003 | 0.09 | 0.00 | 0.76 | |
| PCB157 | 0.00003 | 0.02 | 0.00 | 0.15 | |
| PCB167 | 0.00003 | 0.03 | 0.00 | 0.19 | |
| PCB189 | 0.00003 | 0.01 | 0.00 | 0.11 | |
| Total dioxins | 13.70 | 3.74 | 110.32 | ||
Table 4. Arithmetic means of serum concentrations of dioxin isomers (pg TEQ/g lipid) for each age group of incinerator workers compared with the reference data obtained from the MOEJ report27).
| Dioxins | This study | Japanese general population1 | p3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr old) | Ratio2 | Age (yr old) | Ratio2 | ||||||||||||||
| 20–29 (n=100) |
30–39 (n=155) |
40–49 (n=199) |
50–59 (n=184) |
>60 (n=40) |
Total (n=678) |
20–29 (n=333) |
30–39 (n=451) |
40–49 (n=496) |
50–59 (n=566) |
>60 (n=347) |
Total (n=2,264) |
||||||
| PCDDs | 4.29 | 6.07 | 8.07 | 9.20 | 8.86 | 7.35 | 42.6% | 5.04 | 5.60 | 7.48 | 10.62 | 12.01 | 8.10 | 41.8% | |||
| 2,3,7,8-TCDD | 0.66 | 0.76 | 0.95 | 1.14 | 1.13 | 0.93 | 5.4% | 0.42 | 0.52 | 0.69 | 1.38 | 1.56 | 0.91 | 4.7% | |||
| 1,2,3,7,8-PeCDD | 2.45 | 3.26 | 4.48 | 4.84 | 4.79 | 4.02 | 23.3% | 3.18 | 3.43 | 4.55 | 6.43 | 7.35 | 4.96 | 25.6% | |||
| 1,2,3,4,7,8-HxCDD | 0.12 | 0.16 | 0.20 | 0.19 | 0.18 | 0.18 | 1.0% | 0.05 | 0.06 | 0.11 | 0.19 | 0.23 | 0.13 | 0.6% | ** | ||
| 1,2,3,6,7,8-HxCDD | 1.01 | 1.56 | 1.87 | 2.15 | 1.94 | 1.75 | 10.2% | 1.08 | 1.26 | 1.66 | 2.00 | 2.17 | 1.63 | 8.4% | * | ||
| 1,2,3,7,8,9-HxCDD | 0.17 | 0.26 | 0.34 | 0.37 | 0.30 | 0.30 | 1.8% | 0.16 | 0.18 | 0.26 | 0.35 | 0.39 | 0.27 | 1.4% | * | ||
| 1,2,3,4,6,7,8-HpCDD | 0.09 | 0.12 | 0.12 | 0.14 | 0.11 | 0.12 | 0.7% | 0.11 | 0.11 | 0.14 | 0.18 | 0.19 | 0.15 | 0.8% | |||
| OCDD | 0.03 | 0.04 | 0.05 | 0.08 | 0.05 | 0.05 | 0.3% | 0.03 | 0.04 | 0.06 | 0.09 | 0.10 | 0.07 | 0.3% | |||
| PCDFs | 2.24 | 3.30 | 4.79 | 4.87 | 5.50 | 4.12 | 23.9% | 2.14 | 2.21 | 3.00 | 4.44 | 5.44 | 3.40 | 17.6% | ** | ||
| 2,3,7,8-TCDF | 0.06 | 0.07 | 0.08 | 0.09 | 0.10 | 0.08 | 0.4% | 0.06 | 0.06 | 0.09 | 0.12 | 0.13 | 0.09 | 0.5% | |||
| 1,2,3,7,8-PeCDF | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.1% | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.0% | ** | ||
| 2,3,4,7,8-PeCDF | 1.52 | 2.02 | 2.99 | 3.20 | 3.59 | 2.64 | 15.3% | 1.51 | 1.68 | 2.29 | 3.40 | 4.24 | 2.59 | 13.4% | |||
| 1,2,3,4,7,8-HxCDF | 0.24 | 0.31 | 0.45 | 0.37 | 0.39 | 0.36 | 2.1% | 0.21 | 0.15 | 0.22 | 0.32 | 0.38 | 0.25 | 1.3% | ** | ||
| 1,2,3,6,7,8-HxCDF | 0.30 | 0.48 | 0.70 | 0.56 | 0.63 | 0.55 | 3.2% | 0.25 | 0.24 | 0.31 | 0.44 | 0.51 | 0.34 | 1.8% | ** | ||
| 1,2,3,7,8,9-HxCDF | 0.12 | 0.11 | 0.11 | 0.11 | 0.12 | 0.11 | 0.6% | 0.03 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.0% | † | ||
| 2,3,4,6,7,8-HxCDF | 0.13 | 0.25 | 0.32 | 0.26 | 0.31 | 0.26 | 1.5% | 0.04 | 0.05 | 0.07 | 0.13 | 0.15 | 0.09 | 0.4% | ** | ||
| 1,2,3,4,6,7,8-HpCDF | 0.06 | 0.08 | 0.12 | 0.08 | 0.08 | 0.09 | 0.5% | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.1% | ** | ||
| 1,2,3,4,7,8,9-HpCDF | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.1% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | † | ||
| OCDF | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | † | ||
| Coplanar PCBs | 2.23 | 3.79 | 6.01 | 8.28 | 9.12 | 5.76 | 33.4% | 3.40 | 4.39 | 6.78 | 11.12 | 14.03 | 7.85 | 40.6% | |||
| Non-ortho PCBs | |||||||||||||||||
| PCB77 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | † | ||
| PCB81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | † | ||
| PCB126 | 1.70 | 2.86 | 4.40 | 5.90 | 6.33 | 4.17 | 24.2% | 2.56 | 3.32 | 5.15 | 8.63 | 10.86 | 6.03 | 31.2% | |||
| PCB169 | 0.59 | 0.76 | 1.05 | 1.33 | 1.56 | 1.02 | 5.9% | 0.60 | 0.75 | 1.11 | 1.66 | 2.07 | 1.23 | 6.3% | |||
| Mono-ortho PCBs | |||||||||||||||||
| PCB105 | 0.02 | 0.03 | 0.05 | 0.07 | 0.07 | 0.05 | 0.3% | 0.02 | 0.03 | 0.05 | 0.08 | 0.10 | 0.06 | 0.3% | |||
| PCB114 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.1% | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.02 | 0.1% | |||
| PCB118 | 0.10 | 0.16 | 0.24 | 0.33 | 0.37 | 0.23 | 1.4% | 0.13 | 0.17 | 0.27 | 0.43 | 0.57 | 0.31 | 1.6% | |||
| PCB123 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.0% | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.0% | † | ||
| PCB156 | 0.05 | 0.06 | 0.11 | 0.14 | 0.17 | 0.10 | 0.6% | 0.04 | 0.06 | 0.10 | 0.16 | 0.21 | 0.11 | 0.6% | |||
| PCB157 | 0.01 | 0.02 | 0.03 | 0.03 | 0.04 | 0.02 | 0.1% | 0.01 | 0.02 | 0.03 | 0.04 | 0.06 | 0.03 | 0.2% | |||
| PCB167 | 0.01 | 0.02 | 0.04 | 0.05 | 0.06 | 0.03 | 0.2% | 0.02 | 0.03 | 0.04 | 0.07 | 0.10 | 0.05 | 0.3% | |||
| PCB189 | 0.06 | 0.07 | 0.12 | 0.15 | 0.18 | 0.01 | 0.1% | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.1% | |||
| Total dioxins | 8.77 | 13.16 | 18.86 | 22.35 | 23.49 | 17.23 | 100.0% | 10.58 | 12.19 | 17.25 | 26.18 | 31.47 | 19.35 | 100.0% | |||
1Serum concentrations of dioxins in the male and female general population living in all areas of Japan obtained from the MOEJ report27). 2Isomer ratios indicate the ratios of dioxin isomers to total dioxins in both this study and the MOEJ report. 3p was computed using the paired t-test for comparison of arithmetic means between this study and the MOEJ survey (*p<0.05, **p<0.01). †The t-test was not applied because the arithmetic mean value in the MOEJ survey was zero.
Fig. 1.
Scatterplots of the serum concentrations of dioxins and job duration for each job category in the Japanese waste incinerator workers. Job categories I, II, III and IV are indicated by a closed circle (●), open circle (○), triangle (▽) and cross (×), respectively.
Health effect assessment
Physicians and dermatologists reported no remarkable clinical findings on physical examination or diagnosis of the skin for the subjects in the present study. Table 5 shows the results of laboratory and physiological tests and correlations of their parameters with the serum concentrations of dioxin congeners. Significant partial correlations were found between the serum concentration of PCDDs and HbA1c and PHA, between the serum concentration of PCDFs and TP, Alb, HbA1c, PHA and PHA-SI, between the serum concentration of total dioxins and DBP, PLT, TP, AST, GGT and AMY, and between the serum concentration of coplanar PCBs and SBP, DBP, PLT, TP, Alb, T-bil, AST, ALT, LAP, GGT, AMY, Fe, BUN, UA, Cr, TG and BS.
Table 5. Correlations between serum concentrations of dioxin congeners and parameters of laboratory and physiological tests in the subjects working for waste incineration facilities.
| Median | Min | Max | Pearson’s correlation coefficient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCDDs | PCDFs | Coplanar PCBs | Total dioxins | |||||||||
| Physiological test | ||||||||||||
| SBP (mmHg) | 133 | 74 | 233 | –0.0097 | 0.0118 | 0.0985 | * | 0.0375 | ||||
| DBP (mmHg) | 81 | 40 | 127 | 0.0488 | 0.0695 | 0.1435 | ** | 0.0961 | * | |||
| Heart rate (count/min) | 75 | 48 | 135 | 0.0324 | –0.0347 | 0.0570 | 0.0251 | |||||
| Blood cell count | ||||||||||||
| WBC (count/μl) | 6,800 | 3,220 | 14,100 | 0.0306 | –0.0049 | –0.0646 | –0.0143 | |||||
| RBC (×104 count/μl) | 483 | 357 | 629 | 0.0077 | –0.0309 | 0.0110 | –0.0061 | |||||
| Hb (g/dl) | 15.3 | 11.9 | 19.8 | 0.0256 | –0.0130 | 0.0725 | 0.0360 | |||||
| HT (%) | 44.8 | 31.7 | 58.8 | 0.0093 | –0.0256 | 0.0276 | 0.0058 | |||||
| PLT (×104 count/μl) | 23.8 | 3.8 | 43.8 | –0.0593 | –0.0951 | –0.1067 | ** | –0.0946 | * | |||
| Blood chemistry test | ||||||||||||
| TP (g/dl) | 7.2 | 5.9 | 9.2 | 0.0613 | 0.0932 | * | 0.1024 | ** | 0.0864 | * | ||
| Alb (g/dl) | 4.7 | 3.8 | 5.8 | 0.0233 | 0.0802 | * | 0.1002 | ** | 0.0686 | |||
| T-bil (mg/dl) | 0.6 | 0.2 | 5.1 | –0.0246 | 0.0439 | 0.0909 | * | 0.0402 | ||||
| AST (IU/l) | 23 | 11 | 131 | 0.0262 | 0.0168 | 0.1610 | ** | 0.0818 | ||||
| ALT (IU/l) | 25 | 6 | 224 | 0.0331 | –0.0100 | 0.1507 | ** | 0.0663 | ||||
| LDH (IU/l)1 | 216 | 96 | 1,174 | 0.0080 | 0.0176 | 0.0461 | 0.0188 | |||||
| LDH (IU/l)2 | 170 | 119 | 363 | –0.0673 | –0.0098 | –0.0706 | –0.0633 | |||||
| ALP (IU/l) | 219 | 83 | 730 | –0.0523 | –0.0680 | –0.0373 | –0.0595 | |||||
| LAP (IU/l) | 55 | 23 | 192 | 0.0313 | –0.0090 | 0.1193 | ** | 0.0585 | ||||
| GGT (IU/l) | 38 | 10 | 1,133 | 0.0530 | 0.0060 | 0.1946 | ** | 0.1033 | ** | |||
| AMY (IU/l) | 70 | 25 | 231 | –0.0503 | –0.0746 | –0.0817 | * | –0.0777 | * | |||
| CK (IU/l) | 119 | 35 | 3,087 | 0.0508 | 0.0703 | 0.0428 | 0.0598 | |||||
| Fe (μg/dl) | 101 | 21 | 277 | 0.0314 | 0.0506 | 0.0922 | * | 0.0608 | ||||
| BUN (mg/dl) | 13 | 6 | 24 | 0.0227 | 0.0506 | 0.0881 | * | 0.0579 | ||||
| UA (mg/dl) | 5.7 | 2.7 | 10.7 | 0.0250 | –0.0263 | 0.0884 | * | 0.0336 | ||||
| Cr (mg/dl) | 0.8 | 0.4 | 1.5 | –0.0530 | –0.0209 | –0.0863 | * | –0.0629 | ||||
| T-ch (mg/dl) | 199 | 99 | 385 | 0.0329 | –0.0232 | 0.0517 | 0.0166 | |||||
| HDL-ch (mg/dl) | 53 | 17 | 131 | –0.0184 | 0.0090 | 0.0197 | 0.0006 | |||||
| TG (mg/dl) | 121 | 31 | 2363 | 0.0155 | –0.0523 | 0.0998 | * | 0.0317 | ||||
| BS (mg/dl) | 95 | 63 | 446 | 0.0440 | –0.0306 | 0.1042 | ** | 0.0559 | ||||
| HbA1c (%) | 4.8 | 2.1 | 11.8 | 0.0917 | * | 0.0961 | * | 0.0525 | 0.0906 | |||
| Immunological function test | ||||||||||||
| PHA (cpm) | 38,774 | 2,275 | 60907 | –0.0791 | * | –0.1121 | ** | –0.0102 | –0.0702 | |||
| ConA (cpm) | 32,095 | 1,595 | 64,400 | –0.0148 | 0.0064 | 0.0091 | –0.0027 | |||||
| PHA-SI3 | 122.2 | 4.0 | 606.2 | –0.0279 | –0.1140 | ** | 0.0208 | –0.0330 | ||||
| ConA-SI4 | 99.5 | 2.8 | 334.8 | 0.0028 | –0.0542 | –0.0320 | 0.0007 | |||||
| NKACT (%) | 38.0 | 5.0 | 79.0 | –0.0062 | 0.0269 | 0.0416 | 0.0238 | |||||
| CD3 (%) | 72.6 | 43.1 | 89.2 | –0.0407 | –0.0344 | –0.0509 | –0.0510 | |||||
| CD4 (%) | 45.2 | 14.9 | 74.6 | –0.0202 | –0.0569 | –0.0449 | –0.0405 | |||||
| CD8 (%) | 29.3 | 10.5 | 60.8 | 0.0146 | 0.0661 | 0.0295 | 0.0339 | |||||
| CD4/CD8 ratio | 1.6 | 0.3 | 5.6 | –0.0190 | –0.0699 | –0.0406 | –0.0413 | |||||
| CD19 (%) | 8.6 | 0.5 | 25.9 | 0.0417 | 0.0421 | –0.0380 | 0.0121 | |||||
| CD56 (%) | 16.1 | 3.1 | 48.7 | –0.0183 | 0.0369 | 0.0600 | 0.0448 | |||||
Adjusted for age, survey year, BMI, smoking habits and alcohol consumption. *p<0.05, **p<0.01. 1,2The LDH data measured at Rousai Hospitals were adjusted as described in Subjects and Methods. 3,4SI indicates the Stimulation Index. SBP: Systolic blood pressure; DBP: Diastolic blood pressure; WBC: White blood cell; RBC: Red blood cell; Hb: Hemoglobin; Ht: Hematocrit; PLT: Platelet; TP: Total protein; Al: Albumin; T-bil: Total bilirubin; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; LDH: Lactate dehydrogenase; ALP: Alkaline phosphatase; LAP: Leucine aminopeptidase; GGT: Gamma-glutamyltransferase; AMY: Amylase; CK: Creatine kinase; Fe: Ferrum (iron); BUN: Blood urea nitrogen; UA: Urinary acid; Cr: Creatinine; T-cho: Total cholesterol; HDL-ch: High-density lipoprotein cholesterol; TG: Triglyceride; BS: Blood sugar; HbA1c: Hemoglobin-A1c; PHA: Lymphocyte blastoid transformation by Phytohemaggutinin; ConA: Lymphocyte blastoid transformation by Concanavalin A; NKACT: Natural killer cell activity; CD: Cluster of differentiation
As shown in Table 6, multiple regression analysis revealed significant partial regression coefficients between PCDFs and GGT and T-ch, and between total dioxins and PLT, while there were no significant partial regression coefficients between PCDDs and any parameter. On the other hand, coplanar PCBs were significantly correlated with 14 parameters of the laboratory and physiological tests.
Table 6. Significant partial regression coefficients computed using multiple regression analysis.
| Laboratory or physiological test | Total dioxins | |||
| B | 95% CIs | p | ||
| Lower | Upper | |||
| PLT (×104 count/µl) | –0.002 | –0.003 | 0.000 | <0.05 |
| Laboratory or physiological test | PCDFs | |||
| B | 95% CIs | p | ||
| Lower | Upper | |||
| GGT (IU/l) | –0.012 | –0.023 | 0.000 | <0.05 |
| T-ch (mg/dl) | –0.003 | –0.006 | 0.000 | <0.05 |
| Laboratory or physiological test | Coplanar PCBs | |||
| B | 95% CIs | p | ||
| Lower | Upper | |||
| SBP (mmHg) | 0.003 | 0.001 | 0.006 | <0.01 |
| DBP (mmHg) | 0.004 | 0.001 | 0.006 | <0.01 |
| Hb (g/dl) | 0.001 | 0.000 | 0.003 | <0.05 |
| PLT (×104 count/µl) | –0.005 | –0.009 | –0.001 | <0.05 |
| TP (g/dl) | 0.001 | 0.000 | 0.002 | <0.05 |
| AST (IU/l) | 0.013 | 0.006 | 0.019 | <0.01 |
| ALT (IU/l) | 0.014 | 0.005 | 0.023 | <0.01 |
| LAP (IU/l) | 0.006 | 0.002 | 0.009 | <0.01 |
| GGT (IU/l) | 0.026 | 0.014 | 0.039 | <0.01 |
| BUN (mg/dl) | 0.005 | 0.001 | 0.009 | <0.05 |
| TG (mg/dl) | 0.015 | 0.005 | 0.025 | <0.01 |
| BS (mg/dl) | 0.006 | 0.002 | 0.010 | <0.01 |
| HbA1c (%) | 0.002 | 0.000 | 0.004 | <0.05 |
| CD3 (%) | –0.003 | –0.005 | 0.000 | <0.05 |
B: partial regression coefficient; CIs: confidence intervals. Adjusted for age, survey year, BMI, smoking habit and alcohol consumption.
p was computed using the Wald test.
Table 7 shows the results of logistic regression analysis for dose-response relationships between the serum concentrations of PCDDs, PCDFs, total dioxins or coplanar PCBs and the prevalences of diabetes and hypertension. Statistically significant associations between dioxins and diabetes or hypertension were found for two criteria: a significant difference in adjusted odds ratios (ORs) was found between the first and fourth quartile and a significant trend in ORs was found by the Jonckheere test. The serum levels of PCDDs, PCDFs and total dioxins were significantly associated with the prevalence of diabetes. Although the adjusted OR in the 4th quartile for coplanar PCBs was marginally significant compared with the reference value, we judged that the prevalence of diabetes was not significantly associated with coplanar PCBs. In addition, the serum levels of PCDFs and total dioxins were significantly associated with the prevalence of hypertension. On the other hand, no significant association of PCDDs, PCDFs or total dioxins was found with prevalences of hyperlipidemia or liver dysfunction, while coplanar PCBs were significantly associated with these three diseases (data not shown).
Table 7. Adjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) of the quartiles of serum concentrations of dioxin congeners to prevalence of diabetes mellitus and hypertension.
| Diabetes mellitus | Hypertension | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORs | 95% CIs | p | ORs | 95% CIs | p | |||||
| Lower | Upper | Lower | Upper | |||||||
| PCDDs | ||||||||||
| Q1: | 1.00 | 1.00 | ||||||||
| Q2: | 2.33 | 0.50 | 10.86 | 0.28 | 1.35 | 0.84 | 2.19 | 0.22 | ||
| Q3: | 3.18 | 0.71 | 14.23 | 0.13 | 1.16 | 0.71 | 1.91 | 0.55 | ||
| Q4: | 6.70 | 1.61 | 27.91 | <0.01 | 1.66 | 1.00 | 2.78 | 0.05 | ||
| p for trend | <0.05 | <0.05 | ||||||||
| PCDFs | ||||||||||
| Q1: | 1.00 | 1.00 | ||||||||
| Q2: | 1.56 | 0.31 | 7.77 | 0.59 | 1.34 | 0.82 | 2.19 | 0.24 | ||
| Q3: | 4.20 | 0.99 | 17.81 | 0.05 | 1.74 | 1.06 | 2.87 | <0.05 | ||
| Q4: | 5.69 | 1.32 | 24.53 | <0.05 | 1.90 | 1.12 | 3.25 | <0.05 | ||
| p for trend | <0.05 | <0.05 | ||||||||
| Coplanar PCBs | ||||||||||
| Q1: | 1.00 | 1.00 | ||||||||
| Q2: | 3.30 | 0.60 | 18.04 | 0.17 | 1.26 | 0.76 | 2.09 | 0.37 | ||
| Q3: | 4.84 | 0.91 | 25.68 | 0.06 | 1.66 | 0.99 | 2.79 | 0.05 | ||
| Q4: | 5.36 | 1.00 | 28.72 | 0.05 | 2.31 | 1.33 | 4.02 | <0.01 | ||
| p for trend | <0.05 | <0.05 | ||||||||
| Total dioxins | ||||||||||
| Q1: | 1.00 | 1.00 | ||||||||
| Q2: | 1.28 | 0.24 | 6.85 | 0.77 | 1.01 | 0.62 | 1.66 | 0.96 | ||
| Q3: | 4.44 | 1.04 | 18.94 | <0.05 | 1.62 | 0.97 | 2.70 | 0.07 | ||
| Q4: | 4.98 | 1.17 | 21.17 | <0.05 | 1.92 | 1.12 | 3.28 | <0.05 | ||
| p for trend | <0.05 | <0.05 | ||||||||
Adjusted for age, survey year, BMI, smoking habit and alcohol consumption. Q1, Q2, Q3 and Q4 stand for the 1st, 2nd, 3rd and 4th quartiles, respectively. p was computed using the Wald test. p for trend was computed using the Jonckheere trend test.
Comparison with former study groups
As shown in Table 8, it was notable that there was no essential difference in the serum concentrations of total dioxins in each age group between this study and the Japanese general population reported by the MOEJ when the populations were classified into 5 different groups of age. Furthermore, the prevalence of diabetes for each age group in this study was similar to that in the Japanese general population according to the National Health and Nutrition Survey. On the other hand, the prevalences of hypertension for younger workers in this study were significantly higher than those for the corresponding age groups of the Japanese general population, although there were no significant differences in the prevalences of hypertension in older individuals and individuals of all ages between this study and the Japanese general population. It should be pointed out that in the present study, no essential difference in the prevalence of diabetes was found for individuals younger than 70 yr of age between this study and the US general population according to the National Health and Nutrition Examination Survey22)(data not shown).
Table 8. The prevalences of diabetes and hypertension in each age group of Japanese incinerator workers compared with those of the Japanese general population.
| Age (yr) | Concentration of serum total dioxin (pg TEQ/g lipid) | Diabetes | Hypertension | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| This study | Japanese general population1 | This study | Japanese general population2 | This study | Japanese general population2 | |||||||||||||||
| Median | Min | Max | Median | Min | Max | n | Rate | n | Rate | p | Rate | n | Rate | p | ||||||
| ≤29 | 8.3 | 3.9 | 32.6 | 11 | 0.8 | 54 | 98 | 1.0% | 114 | 0.0% | 0.28 | 23.5% | 122 | 6.6% | <0.01 | |||||
| 30–39 | 12.0 | 3.7 | 106.7 | 12 | 0.1 | 45 | 156 | 2.6% | 212 | 0.5% | 0.09 | 34.0% | 220 | 19.1% | <0.01 | |||||
| 40–49 | 14.4 | 5.3 | 100.0 | 17 | 1.5 | 95 | 201 | 5.5% | 207 | 4.8% | 0.05 | 46.3% | 214 | 35.5% | <0.05 | |||||
| 50–59 | 18.6 | 5.5 | 110.3 | 26 | 1.1 | 130 | 184 | 12.0% | 350 | 13.1% | 0.70 | 60.9% | 363 | 59.2% | 0.35 | |||||
| 60–69 | 21.3 | 7.2 | 110.2 | 31 | 6.9 | 120 | 39 | 17.9% | 389 | 14.7% | 0.58 | 59.0% | 411 | 66.7% | 0.33 | |||||
| Total | 13.7 | 3.7 | 110.3 | 19 | 0.1 | 130 | 678 | 6.6% | 1,272 | 9.0% | 0.07 | 44.8% | 1,330 | 46.2% | 0.55 | |||||
1Serum concentrations of dioxins in the male and female general population living in urban, farming and fishing areas of Japan obtained from the MOEJ reports27). 2The prevalences of diabetes and hypertension in the male Japanese general population were quoted from National Health and Nutrition Survey in 200632). p was computed with the χ2 test
Discussion
We conducted a cross-sectional analysis to evaluate occupational exposure of Japanese incinerator workers to dioxins including coplanar PCBs and the association between the dioxin exposure and their adverse health effects. About 90% of the subjects participating in the present study were classified into two different categories of jobs: that is, they were either assigned for incineration-related jobs inside an incineration facility (group III) and for operation and maintenance of an incinerator including furnace, electric dust collector and wet scrubber (group IV)6). The workers in these two groups were thought to be at high risk of excessive exposure to fly ash or slag containing high levels of dioxins compared with the general population. Indeed, a small number of the incinerator workers of group III or IV were exposed to high levels of dioxins in spite of a short exposure duration (Fig. 1).
The arithmetic mean serum concentration of PCDFs for the incinerator workers was significantly higher than that for the Japanese general population27). The present finding was consistent with those reported by Schecter et al.8) and Päpke et al.9), who reported that the serum concentration of PCDFs of incinerator workers was significantly higher than their controls. Kumagai et al.10,11,12) reported the increases in the serum concentrations of HxCDF and HpCDF and TEQ of PCDFs of incinerator workers, suggesting that the workers had inhaled dust containing PCDDs and PCDFs during their work. In the present study, most of the serum concentrations of dioxin isomers without coplanar PCBs tended to be higher in young workers compared with young individuals in the Japanese general population. This inference is compatible with the findings reported to date: Shih et al.35) reported significantly increased serum levels of PCDD/Fs in the incinerator workers after a month of maintenance work. Kumagai et al.36) also reported much higher serum levels of PCDD/Fs including 1,2,3,4,6,7,8-HpCDF in the incinerator workers than in control subjects, suggesting occupational exposure to fly ash and dust containing PCDD/Fs during work at the incineration plant. Taking into consideration that the patterns of the serum concentrations of PCDF isomers of the incinerator workers were different from those of the general population, a possibility of occupational exposure to dioxins emitted from the waste incinerator could not be totally ruled out. However, the arithmetic mean serum concentration of total dioxins was 17.2 pg TEQ/g lipid, which was at the same level as that of the general population conducted by the MOEJ survey27) (19.4pg TEQ/g lipid).
Significant partial correlations between the serum concentrations of dioxin isomers and several parameters of laboratory and physiological tests were found. To examine the correlations between them further, we performed multiple regression analysis. Most of the correlations disappeared with exception of the correlations between PCDFs and GGT and T-ch, and between total dioxins and PLT. Regarding the possibility of toxicologically and clinically meaningful effects, these results should be interpreted carefully with reference to the literature reported to date13). We also found significant correlations between coplanar PCBs and 14 parameters of laboratory and physiological tests. It was previously reported that adverse health effects of coplanar PCBs were skin rash, chloracne, liver disturbances, immunosuppressive changes, neurological and unspecific psychological or psychosomatic effects, and that these health outcomes resulted primarily from exposure to high levels of these PCBs in the workplace13). It was also reported in 1992 that human exposure to background levels of PCBs was not associated with any diseases13). However, Lee et al.37) recently reported that exposure of the general population to background levels of PCBs and other POPs may contribute to development of insulin resistance, leading to type 2 diabetes, obesity, dyslipidemia and cardiovascular diseases. Although the positive correlations of coplanar PCBs with 14 parameters of laboratory and physiological tests were found in the present study, occupational exposure of incinerator workers to coplanar PCBs may contribute little to their health outcomes as compared with possible effects arising from the environmental exposure, because the serum concentrations of coplanar PCBs in our subjects of incinerator workers were very close to those of the Japanese general population.
A significant association of the serum concentrations of PCDDs, PCDFs and total dioxins with the prevalences of diabetes was found in the present study, although the prevalence of diabetes was 6.6% for our subjects, which was close to the prevalence of 9.0%32) for the Japanese general population in the same age ranges (Table 8). This was consistent with the results of three cross-sectional studies, one on the Japanese general population23), one on the US general population in the US National Health and Examination Survey22), and one on the Air Force veterans who never had contact with dioxin-contaminated herbicide21). It should be pointed out in the present study that no essential difference in the prevalence of diabetes in individuals younger than 70 yr of age was also found between this study and the US general population according to the National Health and Nutrition Examination Survey22). Since the above three studies21,22,23) and our study were all cross-sectional, the causality of the dose-response relationship remains unknown. In contrast to the significant association of serum levels of dioxins with diabetes found in the large population exposed to background levels of dioxins, however, it is intriguing that no clear dose-response relationship between serum levels of TCDD and diabetes was found in two cohort studies, one on the US trichlorophenol production workers exposed to high levels of TCDD38) and one on women residing in an area of Seveso highly contaminated with TCDD39). Two recent reports support the causal relation of TCDD with diabetes from the standpoint of molecular mechanisms: De Tata40) proposed a mechanistic pathway initiated by the binding of aryl hydrocarbon receptors (AhRs) with TCDD in the pancreatic beta cell as a relevant and sensitive target of dioxin cytotoxicity leading to impairment of insulin secretion. Lee et al.41) reported that the AhR transactivation activity was linearly related with the parameters of metabolic syndrome such as type 2 diabetes in a population. Therefore, the present findings of our cross-sectional study provide additional evidence of the reported significant association17,18,19,20,21,22,23) of dioxins exposure with the prevalence of diabetes. The present findings derived from the TEF/TEQ approach also suggest that the TEQ-converted values of dioxin congeners are useful for health assessment of humans exposed to complex mixtures of PCDD, PCDF and coplanar-PCB isomers at environmentally relevant levels, even if the conger patterns are different.
The significant association of PCDFs and total dioxins with hypertension found in the present study is apparently compatible with the findings of Uemura et al.23), who found that serum dioxins were associated with features of metabolic syndrome such as glucose intolerance, hypertension and elevated triglycerides among the Japanese general population. Moreover, we found that the prevalence of hypertension for younger workers in this study tended to be higher than that in the Japanese general population, although there was no difference in the prevalence of hypertension in individuals of all ages between this study (44.8%) and the Japanese general population (46.2%). Further epidemiological and experimental toxicology studies will be needed to examine the possible association of human exposure to low levels of dioxins with the prevalence of hypertension.
There were no essential differences in the prevalences of the four major diseases between the total number of the incinerator workers recruited in the present study and the Japanese general population32, 42), or in the serum concentrations of PCDD, PCDF and coplanar PCB congeners between the incinerator workers and the Japanese general population. These results can be taken as indicating that occupational exposure to the present levels of dioxins may not have contributed to health outcomes in the incinerator workers. Furthermore, the significant association of dioxins with the prevalence of diabetes could be related to environmental exposure to dioxins ubiquitously present in food and the environment rather than to occupational exposure to dioxins in the waste incineration facility workplace.
Assessment of exposure to dioxins based on the measured serum lipid concentration expressed as pg TEQ/g lipid has an advantage over conventional exposure assessment based on intake of dioxins because the measured serum lipid level of dioxins aggregates exposure from all routes and media, which individually may be difficult to quantify because of low concentrations.
There were some limitations in the present study. First, since the present epidemiological study was cross-sectional, the causality of the dose-response relationship between the prevalence of diabetes and serum concentrations of dioxins was beyond the scope of the present purpose. Second, we did not exclude patients with type 1 diabetes because no medical information about the physician-diagnosed type 1 diabetes could be obtained. Besides, we were not able to get the reliable data for fasting serum glucose level, because some portion of the subjects participated in blood sampling after having finished breakfast or lunch. Blood insulin was not measured in the present survey. Third, since no essential difference in the serum concentrations of dioxins between the incinerator workers and the Japanese general population was found here, we were not able to draw a definite conclusion regarding whether or not the incinerator workers were exposed occupationally to excessive levels of dioxins. Many local governments actively implement regulatory and administrative countermeasures that require municipal waste incineration plants to reduce the generation of dioxins and emission of them into the environment, owing to heightened social and public concerns over health outcomes related to dioxins in recent decades in Japan. These countermeasures were extended to effective prevention of occupational exposure to dioxins in the incineration facilities by means of wearing respirators, goggles, gloves and protective clothing.
Conclusion
The present assessment of exposure to dioxins revealed that the incinerator workers were marginally exposed to dioxins in the workplace. Although some laboratory and physiological test results were significantly correlated with serum concentrations of dioxin congeners, these findings should be carefully interpreted. We can conclude that a significant association between PCDDs, PCDFs and total dioxins and the prevalence of diabetes was observed in the present study, which was consistent with the findings obtained from three cross-sectional studies on serum levels of dioxins in the general population. However, the similarities in the prevalences of diabetes between the incinerator workers and the Japanese general population in the same ranges of age suggest that occupational exposure of the incinerator workers to the present levels of dioxins might contribute little to the adverse health effects of dioxins.
Acknowledgments
The present study was based on a survey that was carried out under close consultation and evaluation by the members of the “Research and Investigation Committee on the Assessment of Exposure to Dioxins among Workers in the Waste Incineration Industry”, which was organized by the Japan Industrial Safety and Health (JISHA) and entrusted by the Ministry of Health, Labour and Welfare. The Committee, chaired by Tsutomu Takata (JISHA) and vice-chaired by Haruhiko Sakurai (JISHA), consisted of the following members: Shoichi Asahi (University of Occupational and Environmental Health, Japan (UOEH)), Heihachiro Arito (JISHA), Takao Iida (Kitakyushu Life Science Center), Iwao Uchiyama (Kyoto University Graduate School of Engineering), Kazuhiro Nomura (Tokyo Rosai Hospital), Toshihiro Kawamoto (UOEH), Suminori Kono (Kyushu University Graduate School of Medicine), Yasutaka Ogawa (National Institute of Occupational Safety and Health, Japan (JNIOSH)), Isamu Tanaka (UOEH), Tsuguya Fukui (St. Luke’s International Hospital) and Shaw Watanabe (National Institute of Health and Nutrition). The cooperation of the aforementioned Committee members is highly appreciated. The staff of the National Institute of Occupational Safety and Health, Japan, Rousai Hospitals throughout the whole country and JISHA are greatly appreciated for their efforts and contributions to this survey. The Committee’s secretariat consisted of Mitsuhiro Kudo (Chief), Kenya Yamamoto (study director) and Yoko Aiba. Ippei Mouri and Naomi Hisanaga (JNIOSH) helped with the survey. The authors are deeply indebted to Dr. Akira Ogami, Professor of UOEH, Institute of Industrial Ecological Sciences, UOEH, for his sustained encouragement, invaluable discussion, and improvement of the manuscript throughout the present study.
References
- 1.Mocarelli P, Patterson DG, Jr, Marocchi A, Needham LL. (1990) Pilot study (phase II) for determining polychlorinated dibenzo-p-dioxin (PCDD) and polychlorinated dibenzofuran (PCDF) levels in serum of Seveso, Italian residents collected at the time of exposure: future plan. Chemosphere 20, 967–74. [Google Scholar]
- 2.Masuda Y. (1996) Approach to risk assessment of chlorinated dioxins from Yusho PCB poisoning. Chemosphere 32, 583–94. [DOI] [PubMed] [Google Scholar]
- 3.Chen PH, Hites RA. (1983) Polychlorinated biphenyl and dibenzofurans retained in the tissues of a deceased patient with Yucheng in Taiwan. Chemosphere 12, 1507–16. [Google Scholar]
- 4.The Centers for Disease Control Veterans Health Studies (1988) Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin levels in US Army Vietnam-era veterans. JAMA 260, 1249–54. [PubMed] [Google Scholar]
- 5.Fingerhut MA, Sweeney MH, Patterson DG, Jr, Piacitelli LA, Morris JA, Marlow DA, Hornung RW, Cameron LW, Connally LB, Needham LL, Halperin WE. (1989) Levels of 2,3,7,8-tetrachlorodibenzo- p-dioxin in the serum of U.S. chemical workers exposed to dioxin contaminated products: Interim results. Chemosphere 19, 835–40. [Google Scholar]
- 6.Takata T. (2003) Survey on the health effects of chronic exposure to dioxins and its accumulation on workers of a municipal solid waste incinerator, rural part of Osaka Prefecture, and the results of extended survey afterwards. Ind Health 41, 189–96. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura K, Kikuchi Y, Watanabe S, Waechter G, Sakurai H, Takada T. (2000) Health effects of chronic exposure to polychlorinated dibenzo-P-dioxins (PCDD), dibenzofurans (PCDF) and coplanar PCB (Co-PCB) of municipal waste incinerator workers. J Epidemiol 10, 262–70. [DOI] [PubMed] [Google Scholar]
- 8.Schecter A, Päpke O, Ball M, Lis A, Brandt-Rauf P. (1995) Dioxin concentrations in the blood of workers at municipal waste incinerators. Occup Environ Med 52, 385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Päpke O, Ball M, Lis A. (1993) Potential occupational exposure of municipal waste incinerator workers with PCDD/PCDF. Chemosphere 27, 203–9. [Google Scholar]
- 10.Kumagai S, Koda S, Miyakita T, Yamaguchi H, Katagi K, Yasuda N. (2000) Polychlorinated dibenzo-p-dioxin and dibenzofuran concentrations in the serum samples of workers at continuously burning municipal waste incinerators in Japan. Occup Environ Med 57, 204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai S, Koda S, Miyakita T, Ueno M. (2002) Polychlorinated dibenzo-p-dioxin and dibenzofuran concentrations in serum samples of workers at intermittently burning municipal waste incinerators in Japan. Occup Environ Med 59, 362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai S, Koda S, Oda H. (2003) Exposure evaluation of dioxins in municipal waste incinerator workers. Ind Health 41, 167–74. [DOI] [PubMed] [Google Scholar]
- 13.Environmental Health Criteria 140 (1992) Polychlorinated Biphenyls and Terphenyls. International Programme on Chemical Safety. http://www.inchem.org/documents/ehc/ehc/ehc140.htm #9.2.4.1., Accessed September 16, 2014.
- 14.Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. (1998) The Seveso studies on early and long-term effects of dioxin exposure: a review. Environ Health Perspect 106 Suppl 2, 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA. (1998) Dioxin exposure and non-malignant health effects: a mortality study. Occup Environ Med 55, 126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arisawa K, Takeda H, Mikasa H. (2005) Background exposure to PCDDs/PCDFs/PCBs and its potential health effects: a review of epidemiologic studies. J Med Invest 52, 10–21. [DOI] [PubMed] [Google Scholar]
- 17.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. (1997) Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology 8, 252–8. [DOI] [PubMed] [Google Scholar]
- 18.Michalek JE, Pavuk M. (2008) Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. J Occup Environ Med 50, 330–40. [DOI] [PubMed] [Google Scholar]
- 19.Calvert GM, Sweeney MH, Deddens J, Wall DK. (1999) Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med 56, 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott MG, Zober A, Germann C. (1994) Laboratory results for selected target organs in 138 individuals occupationally exposed to TCDD. Chemosphere 29, 2423–37. [DOI] [PubMed] [Google Scholar]
- 21.Longnecker MP, Michalek JE. (2000) Serum dioxin level in relation to diabetes mellitus among Air Force veterans with background levels of exposure. Epidemiology 11, 44–8. [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR., Jr2006) A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care 29, 1638–44. [DOI] [PubMed] [Google Scholar]
- 23.Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Dakeshita S, Nii K, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T. (2009) Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Environ Health Perspect 117, 568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO) (2011) Diabetes Programme: Facts and Figures. http://www.who.int/diabetes/facts/en/. Accessed October 27, 2014.
- 25.Japan Ministry of Welfare (MOW) (1997) Report on improved circumstances about waste incineration facilities emitting gases containing higher than 80ng TEQ/Nm3 (in Japanese). www.env.go.jp/ recycle/kosei_press/h980921a.html. Accessed September 16, 2014.
- 26.Japan Industrial Safety and Health Association (JISHA) (1998) Report of Committee on Investigation of Health Effects due to Exposure to Dioxins in a Toyono-gun Waste Incineration Plant. Edited by Ministry of Labour (MOL), Japan, Division of Hazardous Chemical Investigation, 1–102, JISHA, Tokyo (in Japanese). [Google Scholar]
- 27.Ministry of the Environment, Japan (MOEJ) (2010) Report of A Survey on the Accumulation of Dioxins and Other Chemical Compounds in Humans. Office of Environmental Risk Assessment, Division of Environmental Health, Environmental Policy Bureau, MOEJ, March, 2011 (in Japanese).
- 28.Nakamoto M, Arisawa K, Uemura H, Katsuura S, Takami H, Sawachika F, Yamaguchi M, Juta T, Sakai T, Toda E, Mori K, Hasegawa M, Tanto M, Shima M, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Satoh H. (2013) Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health 86, 849–59. [DOI] [PubMed] [Google Scholar]
- 29.Japan Industrial Safety and Health Association (JISHA)(2007) Report of Research and Investigation Committee on the Assessment of Exposure to Dioxins among Workers at Waste Incineration Industry including Comprehensive Report of Health Status during a Period from 1999 to 2005. Edited by JISHA, Tokyo (in Japanese). [Google Scholar]
- 30.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus Related Laboratory Testing of Japan Diabetes Society (2012) International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 3, 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (2011) Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. Abbreviated Report of a WHO Consultation, 3–9. [PubMed]
- 32.Ministry of HealthLabor and Welfare (MHLW) (2006) National Health and Nutrition Examination Survey in Japan. Edited and published by Life-style Related Diseases Control Section, General Affairs Division, Health Service Bureau, MHLW (in Japanese). [Google Scholar]
- 33.Ministry of Welfare (MOW) Japan (1999) Interim Report on Human Blood Concentrations of Dioxins in a Study of Human Exposure Condition to Dioxins and Human Health, 1–15, MOW Tokyo (in Japanese). [Google Scholar]
- 34.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. (2006) The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93, 223–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih TS, Chen HL, Wu YL, Lin YC, Lee CC. (2006) Exposure assessment of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in temporary municipal-waste-incinerator maintenance workers before and after annual maintenance. Chemosphere 64, 1444–9. [DOI] [PubMed] [Google Scholar]
- 36.Kumagai S, Koda S. (2005) Polychlorinated dibenzo-p-dioxin and dibenzofuran concentrations in serum samples of workers at an infectious waste incineration plant in Japan. J Occup Environ Hyg 2, 120–5, quiz D6–7. [DOI] [PubMed] [Google Scholar]
- 37.Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR., Jr2011) Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS ONE 6, e15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steenland K, Calvert G, Ketchum N, Michalek J. (2001) Dioxin and diabetes mellitus: an analysis of the combined NIOSH and Ranch Hand data. Occup Environ Med 58, 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warner M, Mocarelli P, Brambilla P, Wesselink A, Samuels S, Signorini S, Eskenazi B. (2013) Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso women’s health study. Environ Health Perspect 121, 906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Tata V. (2014) Association of dioxin and other persistent organic pollutants (POPs) with diabetes: epidemiological evidence and new mechanisms of beta cell dysfunction. Int J Mol Sci 15, 7787–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HK. (2011) Mitochondrial dysfunction and insulin resistance: the contribution of dioxin-like substances. Diabetes Metab J 35, 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makino S .(2008) The report of the survey about prevalence of the general health check up in Tokyo. Edited by Association of Occupational Health Service Providers, Tokyo Sanpo 2, 37, 2–5 (in Japanese). [Google Scholar]