Abstract
Background
The purpose of this study was to investigate the association between previous exposure to statins and the risk of non-Hodgkin lymphoma (NHL).
Methods
This nationwide population-based case–control study was conducted using the National Health Insurance Research Database of Taiwan. The NHL group consisted of the patients with a first-time diagnosis of NHL between 2005 and 2008. The cases of the control group were pair-matched to the NHL group according to sex, year of birth and date of NHL diagnosis (index date). The statin administration data from both groups were retrospectively collected from the index date to January 1, 1996. The cumulative defined daily dose (cDDD) was estimated to evaluate the statin exposure. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariate logistic regression.
Results
The study population was composed of 1715 NHL patients and 16942 control subjects. The analysis revealed that previous statin administration was associated with a reduced risk of subsequent NHL with an adjusted OR of 0.52 (95% CI, 0.43–0.62). Additionally, there was a dose-response relationship between statin administration and the risk of NHL. The adjusted ORs were 0.63 (95% CI, 0.46–0.86), 0.58 (95% CI, 0.42–0.79), 0.51 (95% CI, 0.38–0.67), and 0.36 (95% CI, 0.24–0.53) for the subjects with statin administrations of fewer than 28, 28 to 90, 91 to 365, and more than 365 cDDDs, respectively, relative to the subjects without any statin administration.
Conclusions
The results of this study suggest that previous statin administration is associated with a lower risk of subsequent NHL. As statins are widely used medications, the magnitude of the risk reduction may have a substantial influence on public health. Further studies to confirm our findings are warranted.
Introduction
Inhibitors of 3-hydroxy-3-methylglutary–coenzyme A reductase (HMG-CoA) are also known as statins and are common cholesterol-lowering medications that are widely used for primary and secondary prevention of cardiovascular disease and stroke[1–3].
In addition to statins’ cholesterol-lowering effect, several in-vitro and in-vivo studies have suggested that statins may have anticancer properties that are mediated via various mechanisms, such as the arrest of the cell cycle[4,5], induction of apoptosis[6–8], and inhibition of tumor growth metastasis [9–11]. Several meta-analyses and observational studies have shown that statin administration is either not associated with an increase in the development of cancer[12–15] or even associated with a decrease in risk of the development of cancer[16–20]. Regarding the administration of statins and the risk of hematologic malignancy, one meta-analysis suggested that statins may have chemopreventive effects and reported a risk reduction of 19%[21]. However, another meta-analysis did not support this result[22].
Non-Hodgkin lymphoma (NHL) is the most common hematologic malignancy in the adult population, and its incidence has increased significantly worldwide[23–25]. With respect to its pathophysiology, the development of NHL has been associated with chronic inflammatory statuses, such as chronic bacterial or viral infections, and autoimmune diseases. The association between the administration of statins and the risk of NHL was evaluated in a case-control study conducted in European countries that revealed that statin administration reduced the risk of NHL by approximately 40%[26]. However, previous epidemiologic studies have revealed that the distribution of malignant lymphomas in Asian countries differs from that in Western countries. These findings indicate that the etiologies of malignant lymphomas may exhibit racial and geographic differences[27–30]. In terms of the association between statin administration and the risk of hematologic malignancies, particularly NHL, the majority of studies have been performed in Western countries, and related data for Asian countries are relatively rare.
As numerous people use statins on a long-term basis in Taiwan, we conducted this nationwide population-based case-control study to evaluate the association between previous statin usage and the subsequent development of NHL.
Participants and Methods
Data Source
The primary data source for this study was the National Health Insurance Research Database (NHIRD) of the National Health Insurance (NHI) of Taiwan. The NHI program is a government-run, single-payer mandatory health insurance program that was initiated in 1995 and covers of all forms of medical services including inpatient and outpatient care, dental care, preventive medicine, childbirth, Chinese medicine, home care, physical therapy and rehabilitation for chronic mental illnesses. The NHIRD was built by National Health Research Institute (NHRI) of Taiwan in 1997. The content of the NHIRD includes the original claim data from the NHI and the demographic information of all NHI beneficiaries. Because more than 98% of Taiwan’s population participates in the NHI program, the NHIRD was able to provide the most comprehensive information for this study[31]. Several previous high-quality epidemiologic studies have been conducted using the NHIRD [32,33].
Identification of the NHL Group
In this study, all NHL cases are collected from the database of Registry for Catastrophic Illness Patients, a subpart of the NHIRD. The Registry for Catastrophic Illness Patients is a nationwide registration system established by the NHIRD for the patients who suffered from certain severe illnesses, such as autoimmune diseases, end-stage renal disease and malignant diseases. The patients with above major diseases are able to apply for catastrophic illness registration to waive all co-payments of the NHI program when seeking medical services for the catastrophic illness. The approval for a certificate of catastrophic illness involves a strict review process conducted by the Department of Health of Taiwan. For patients with NHL, the certificates of catastrophic illness were issued once the lymphoma was proved by pathology. The database of Registry for Catastrophic Illness Patients includes information, such as diagnostic codes in the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) format, the date of the diagnosis of catastrophic illness, the date of death, dates of each clinic visit, data of prescriptions, amounts of expenditure, and inpatient/outpatient claims, for all the beneficiaries with catastrophic illnesses from 1996 to 2008.
The patients with NHL were identified using the ICD-9-CM codes of the NHL (i.e., 200.0–200.8, 202.0–202.9). The NHL group was composed of patients aged 20 years and older who were first diagnosed with NHL between the 1st of January 2005 and the 31st of December 2008. The index dates of the NHL group were taken as the dates on which NHL was diagnosed. The administrative data for the NHL group were composed of all of the original outpatient and inpatient care data since 1996. We excluded patients who had antecedent or co-existing malignant diseases (140.0–199.1) or HIV infection (042) that were diagnosed before the index date.
Identification of the Control Group
The data for the control group were retrieved from the Longitudinal Health Insurance Database 2005 (LHID2005), which is a subpart database of NHIRD that contains randomly selected representative data from 1,000,000 patients among all NHI enrollees from the year 2005 registry that were selected by a systematic sampling method for research purposes. According to the report of the NHIRD, there were no significant differences in the demographic information of the LHID2005 sample and the entire NHI database. This database is composed of all of the original medical claims related to the 1,000,000 enrollees’ historical outpatient and inpatient care under the NHI program from 1996 to 2008.
The control group was composed of patients who were hospitalized with diagnoses unrelated to statin administration, including orthopedic conditions, trauma (excluding wrist and hip fractures), and other conditions (acute infection, hernia, kidney stones, and cholecystitis). We excluded patients with wrist and hip fractures that were diagnosed before the index date because previous articles have shown that statin users are at a reduced risk of osteoporosis [34–36]. The patients with a previous cancer diagnosis or HIV infection were also excluded.
The patients in the control group were randomly pair-matched to the patients with NHL according to sex, year of birth and index date (10 controls for every NHL case). The index dates (i.e., the date of hospital admission) of the control subjects were within the same month of the index dates of their matched NHL subjects.
Evaluation of Previous Statin Exposure
To evaluate the association between the statin dosage and NHL, the data, including the date the statin was prescribed, the daily dose, and the number of days administered, were collected. All subjects in both groups were traced back to evaluate the previous statin usages from the index date to January 1, 1996. We utilized the defined daily dose (DDD), which is recommended by the World Health Organization as a unit for measuring the prescribed amount of a drug. The DDD was calculated according to the following formula: (total amount of drug)/(amount of drug in a DDD) = number of DDDs. To evaluate the durations of exposures, the cumulative DDDs (cDDDs) were calculated to measure the combinations of doses and days of the statins (specifically, lovastatin, pravastatin, rosuvastatin, fluvastatin, simvastatin, and atorvastatin) from the 1st of January 1996 to the index date.
Identification of Potential Confounders
We identified the documented risk factors for NHL as the potential confounders that were recorded between the index date and the 1st of January 1996, which included autoimmune disease (rheumatoid arthritis (714), systemic lupus erythematosus (710.0) and sicca syndrome (710.2))[37], hepatitis B infection (070.2, 070.3, V02.61) [38], hepatitis C infection (070.7, 070.41, 070.44, 070.51, 070.54, V02.62) [39,40] and diabetes mellitus (250) [41,42]. Some confounders associated with statin administration, such as hypertension (201–204) and hyperlipidemia (272), were also added for adjustment.
In terms of environmental factors, we adjusted for the urbanization level. According to the standards established by the NHRI, all 365 towns in Taiwan are stratified into 5 levels with level 1 representing the most urbanized areas. The criteria for determining the urbanization level included the density of population (persons/km2), the number of physicians (per 100,000 people), the percentage of workers in the agricultural field, the percentage of residents with a college education, and the percentage of the elderly people (over 65 years of age).
We also identified the following medications that could have potentially confounded the association between the use of statins and the risk of cancer: nonsteroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2) inhibitors, aspirin, angiotensin-converting enzyme inhibitors (ACEI), and other lipid-lowering drugs (including fibrate, niacin, bile acid-binding resins, and others). The subjects who had taken the above-mentioned medications for at least one prescription during the 1-year period prior to the index date were defined as users.
Statistical Analysis
The chi-square test (χ2 test) was performed to compare the frequencies of each of the categorical variables. To evaluate the association between the statin and NHL, the study population was categorized as non-users (subjects without prescriptions for any statin at any time between the index date and the 1st of January 1996) and statin users (subjects with at least one prescription for statin during the same period). The statin users were further categorized into four groups based on the cDDDs (<28, 28 to 90, 91 to 365, and > 365 cDDDs). A multivariate logistic regression was performed to estimate the relative magnitude in relation to statin administration. The odds ratios (ORs) and their 95% confidence intervals (95% CIs) were calculated using statin non-users as the reference. The NHL risk analyses were adjusted for gender, age, urbanization level, diabetes mellitus, HBV infection, HCV infection, hypertension, hyperlipidemia, autoimmune diseases (RA, SLE and sicca syndrome), and the use of ACEI, NSAID, COX-2 inhibitors and aspirin, fibrate and other lipid-lowering drugs. The analyses were performed using the SAS statistical package (version 9.3, SAS Institute, Cary, NC). All of the statistical tests were two-sided. Values of P < 0.05 were considered statistically significant.
Ethical Approval
The NHIRD is a completely de-identified and encrypted database for use in research only. This study was conducted in accordance with the Declaration of Helsinki. The study was also reviewed and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-EXEMPT-20140027).
Results
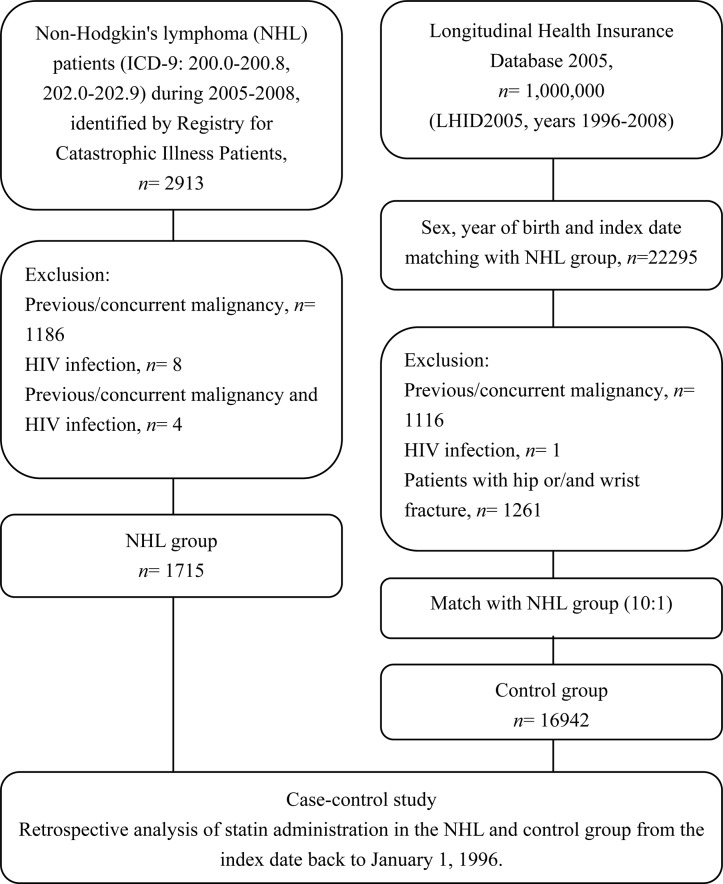
Between 2005 and 2008, 2913 patients were diagnosed with NHL. After excluding the patients with previous malignancies and HIV infection, a total of 1715 patients with NHL were enrolled in this study. After matching for sex, year of birth and index date, we randomly selected 16,942 control subjects, approximated 91.95% as 10:1 matched with the NHL patients. The research design of this study is illustrated in Fig 1.
Fig 1. Flow chart summarizing the data acquisition for the NHL and control groups.
The demographic data for each of the groups are listed in Table 1. In terms of the medical histories and risk factors for lymphoma, significantly higher rates of HBV infection, HCV infection, diabetes mellitus, hypertension, hyperlipidemia, rheumatoid arthritis, systemic lupus erythematosus and sicca syndrome were present in the NHL group. Regarding the selected medical conditions, the NHL group exhibited a significantly lower rate of prior statin administration and higher rates of NSAID and COX-2 inhibitors administration. There were no significant differences in the usages of ACEI, aspirin or other lipid-lowering agents between these two groups (Table 2).
Table 1. Demographic characteristics of the NHL group and the matched control group.
| Variables | Total number (n = 18657) | NHL group (n = 1715) | Control group (n = 16942) | P-value |
|---|---|---|---|---|
| Age (mean ± SD) | 58.76±16.53 | 58.99±16.62 | 58.73±16.52 | 1.000 |
| 20–49 years of age (n, %) | 5421(29.06) | 493(28.75) | 4928 (29.09) | |
| 50–59 years of age (n, %) | 4010(21.49) | 365 (21.28) | 3645 (21.51) | |
| 60–69 years of age (n, %) | 3556(19.06) | 325 (18.95) | 3231 (19.07) | |
| 70–79 years of age (n, %) | 3755(20.13) | 347(20.23) | 3408 (20.12) | |
| ≥ 80 years of age (n, %) | 1915(10.26) | 185 (10.79) | 1730 (10.21) | |
| Male sex (n, %) | 10640 (57.03) | 979 (57.08) | 9661(57.02) | 1.000 |
| Urbanization level * | <0.001 | |||
| 1 (n, %) | 4680 (25.08) | 412 (24.02) | 4268 (25.19) | |
| 2 (n, %) | 1439 (7.71) | 94 (5.48) | 1345 (7.94) | |
| 3 (n, %) | 773 (4.14) | 64 (3.73) | 709 (4.18) | |
| 4 (n, %) | 8041 (43.10) | 672 (39.18) | 7369 (43.49) | |
| 5 (n, %) | 3652(19.57) | 469 (27.35) | 3183 (18.79) | |
| Medical diseases | ||||
| HBV (n, %) | 188 (1.00) | 59 (3.44) | 129 (0.76) | <0.001 |
| HCV (n, %) | 85 (0.46) | 22 (1.28) | 63 (0.37) | <0.001 |
| DM (n, %) | 1510 (8.09) | 256 (14.93) | 1254 (7.40) | <0.001 |
| Hypertension (n, %) | 3541 (18.98) | 524 (30.55) | 3017 (17.81) | <0.001 |
| Hyperlipidemia (n, %) | 1474 (7.90) | 202 (11.78) | 1272 (7.51) | <0.001 |
| RA (n, %) | 134 (0.72) | 37 (2.16) | 97 (0.57) | <0.001 |
| SLE (n, %) | 17 (0.09) | 10 (0.58) | 7 (0.04) | <0.001 |
| Sicca syndrome (n, %) | 65 (0.35) | 15 (0.87) | 50 (0.29) | <0.001 |
*72 subjects, including 4 in the NHL group and 68 in the control group, were not stratified. HBV, hepatitis B virus; HCV, hepatitis C virus; DM, diabetes mellitus; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus
Table 2. Histories of drug exposure of the NHL matched control groups.
| Variables | Total number (n = 18657) | NHL group (n = 1715) | Control group (n = 16942) | P-value |
|---|---|---|---|---|
| Any statin use (n, %) | 2760 (14.79) | 200 (11.66) | 2560 (15.11) | <0.001 |
| Statin | ||||
| Lovastatin (n, %) | 1090 (5.84) | 80 (4.66) | 1010 (5.96) | 0.029 |
| Pravastatin (n, %) | 526 (2.82) | 34 (1.98) | 492 (2.90) | 0.028 |
| Rosuvastatin (n, %) | 334 (1.79) | 17 (0.99) | 317 (1.87) | 0.009 |
| Fluvastatin (n, %) | 568 (3.04) | 34 (1.98) | 534 (3.15) | 0.007 |
| Simvastatin (n, %) | 935 (5.01) | 62 (3.62) | 873 (5.14) | 0.005 |
| Atorvastatin (n, %) | 1205 (6.46) | 90 (5.25) | 1115 (6.58) | 0.032 |
| Other Drugs | ||||
| Aspirin (n, %) | 2607 (13.97) | 232 (13.53) | 2375(14.02) | 0.577 |
| NSAID (n, %) | 11069(59.33) | 1215 (70.85) | 9854(58.16) | <0.001 |
| ACEI (n, %) | 1630 (8.74) | 144 (8.40) | 1486 (8.77) | 0.601 |
| COX-2 inhibitor (n, %) | 2181 (11.69) | 248 (14.46) | 1933 (11.41) | <0.001 |
| Fibrate and other lipid-lower agents (n, %) | 573 (3.07) | 45 (2.62) | 528 (3.12) | 0.259 |
NSAID, non-steroidal anti-inflammatory drug; ACEI, angiotensin-converting enzyme inhibitor; COX-2, cyclooxygenase-2
The association between previous statin exposure and the risk of subsequent NHL was analyzed (Table 3). The numbers of subjects with any previous statin usage in the NHL and control groups were 200 (11.66%) and 2560 (15.11%), respectively. The analysis revealed that previous exposure to any statin was associated with a reduced risk of NHL with a crude OR of 0.74 (95% CI, 0.64–0.87). Additionally, there was a trend toward a decreased in the crude ORs for the NHL risk following the stratification of the users by the cumulative statin doses. After adjusting for the possible confounders (i.e., age, gender, urbanization level, diabetes mellitus, hypertension, hyperlipidemia, HBV infection, HCV infection, RA, SLE, sicca syndrome and the use of other lipid-lowering agents), the adjusted ORs remained significantly decreased (adjusted OR: 0.52; 95% CI, 0.43–0.62). When the statin users were further categorized by their cumulative doses, the inverse association between the administration of statins and the risk of NHL was found to be more significant at the higher cumulative doses of statins. The adjusted ORs were 0.63 (95% CI, 0.46–0.86), 0.58 (95% CI, 0.42–0.79), 0.51 (95% CI, 0.38–0.67), and 0.36 (95% CI, 0.24–0.53) for the cDDDs of statins of fewer than 28, 28 to 90, 91 to 365, and more than 365, respectively.
Table 3. Crude and adjusted odds-ratios of non-Hodgkin lymphoma (NHL) associated with previous statin administration during the follow-up period in the study cohort.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| NHL group (n = 1715) | Control group (n = 16942) | Crude OR | 95% CI | P-value | Adjusted* OR | 95% CI | P-value | |
| Overall | ||||||||
| Any statin use (vs. non-user) | 200 | 2560 | 0.74 | 0.64–0.87 | <0.001 | 0.52 | 0.43–0.62 | <0.001 |
| Cumulative use (vs. non-user) | ||||||||
| User (< 28 cDDDs) | 48 | 560 | 0.81 | 0.60–1.10 | 0.177 | 0.63 | 0.46–0.86 | 0.004 |
| 28–90 cDDDs | 51 | 599 | 0.81 | 0.60–1.08 | 0.151 | 0.58 | 0.42–0.79 | <0.001 |
| 91–365 cDDDs | 68 | 845 | 0.76 | 0.59–0.98 | 0.036 | 0.51 | 0.38–0.67 | <0.001 |
| >365 cDDDs | 33 | 556 | 0.56 | 0.40–0.80 | 0.002 | 0.36 | 0.24–0.53 | <0.001 |
OR, odds ratio; CI, confidence interval; cDDD, cumulative defined daily dose.
*Adjusted for age, gender, urbanization level, HBV infection, HCV infection, autoimmune diseases (RA, SLE and sicca syndrome), diabetes mellitus, hypertension, hyperlipidemia, and the uses of ACEI, NSAID, COX-2 inhibitors and aspirin, fibrate and other lipid-lowering drugs.
Discussion
In this population-based case-control study, we retrospectively analyzed the histories of statin administration in the NHL and control groups. The results indicated that previous statin administration was associated with a 48% reduction in the NHL risk compared with the patients who did not take any statins after controlling for the potential confounders. Additionally, this study also revealed a trend toward a greater NHL risk reduction with longer-term and more frequent prescriptions of statins. An early population-based case-control study conducted by Fortuny J et al. in Europe reported a risk reduction magnitude of 40%, which agrees with our results[26]. Unlike our study, that study did not find a greater reduction in the risk of lymphoma in the patients with prolonged statin administration durations. This difference may potentially have been caused by the different confounding variables that were adjusted for in these two studies. Moreover, the number of cases and the percentage of statin users were higher in our study possibly because statins may have been more frequently prescribed in recent years.
The details of the mechanisms by which statin use may decrease the risk of NHL are not well understood, but some potential mechanisms may support the results of the present study. First, statins have been shown to exhibit anti-inflammatory properties [43,44]. Previous research has shown that chronic inflammation can lead to downstream oncogenic mutations in signaling pathways that have been linked to the development of various tumors, including lymphomas[45–47]. Second, statins have various antitumor properties related to many cancers. A previous animal model study revealed that atorvastatin can inactivate the RAS and ERK1/2 signaling pathways and inhibit the expression of MYC oncogene, which are thought to be closely associated with the development of malignant lymphomas[48]. Another study indicated that statins exert anti-lymphoma effects via the induction of apoptosis, which is associated with an increased generation of reactive oxygen species, activation of p38 and suppression of the AKT and ERK pathways[49]. Additionally, previous studies have revealed that patients who adhere to chronic preventive medicine are more likely to seek healthier lifestyles and preventive medical services, which may also partly explain the lower risk of NHL in long-term statin users[50,51].
This nationwide population-based case-control study has some strengths. First, our study used a computerized database that is population-based and highly representative. Additionally, because approximately 98% of the residents of Taiwan are of Han Chinese ethnicity, the potential confounds due to heterogeneous genetic backgrounds may have been reduced. Moreover, the diagnoses of NHL are believed to be correct due to the validation by the Registry for Catastrophic Illness Patients. Second, the present study demonstrated the dose-dependent nature of the effects of statins in the chemoprevention of NHL by analyzing the cumulative DDDs. Furthermore, we selected the control group from only the patients who had been hospitalized to ensure that the index dates were the same between the NHL and control groups. Because each of the groups in this study had similar durations of investigation, the potential association bias was minimized. Third, statins are available only by prescription in Taiwan. The data regarding statin administrations were collected from a historical database; therefore, the prescription data from before the dates of NHL diagnoses were all available, and thus recall bias was avoided.
This study also has some limitations. First, although we adjusted for the potential confounders in the statistical analysis, a number of possible unmeasured confounding variables, such as Epstein-Barr virus and Helicobacter pylori infection statuses, which are associated with NHL, were not available in this database. Second, we excluded the patients with HIV infection because of the very small number of cases. According to data from the Centers for Disease Control of Taiwan, the rate of new annual cases of HIV remained relatively low until the end of 2005. However, a previous case-control study revealed that statin administration may reduce the risk of NHL in HIV-positive patients [52]. Third, because we were unable to contact the patients directly about their use of statins due to the anonymization of their identification numbers, it is possible that we overestimated the administration of statins because we presumed that all of the prescribed statins were actually taken by the patients. Finally, we analyzed the data only in terms of the statins that were administered after 1996, and information about the prescription of statins prior to 1996 was not available. This lack of information may have resulted in the underestimation of the cDDDs and dose-response effects.
In summary, the results of our study revealed that previous statin administration was associated with a significant 48% reduction in the development of subsequent NHL. Additionally, the chemopreventive effects of statins increased with prolonged usage durations and increased dosages. Because statins are commonly prescribed and widely used medications, the magnitude of the risk reduction may have a substantial affect on public health. Additional studies, particularly prospective randomized trials, are warranted to confirm our findings.
Acknowledgments
The authors are grateful for the assistance of the Department of Internal Medicine and Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 2. Amarenco P, Labreuche J, Lavallee P, Touboul PJ (2004) Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke 35: 2902–2909. [DOI] [PubMed] [Google Scholar]
- 3. Hebert PR, Gaziano JM, Chan KS, Hennekens CH (1997) Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 278: 313–321. [PubMed] [Google Scholar]
- 4. Carlberg M, Dricu A, Blegen H, Wang M, Hjertman M, Zickert P, et al. (1996) Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme a reductase and cell growth. J Biol Chem 271: 17453–17462. [DOI] [PubMed] [Google Scholar]
- 5. Keyomarsi K, Sandoval L, Band V, Pardee AB (1991) Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res 51: 3602–3609. [PubMed] [Google Scholar]
- 6. Yu X, Pan Y, Ma H, Li W (2013) Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncol Res 20: 351–357. 10.3727/096504013X13657689382897 [DOI] [PubMed] [Google Scholar]
- 7. Wong WW, Dimitroulakos J, Minden MD, Penn LZ (2002) HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16: 508–519. [DOI] [PubMed] [Google Scholar]
- 8. Dimitroulakos J, Marhin WH, Tokunaga J, Irish J, Gullane P, Penn LZ, et al. (2002) Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia 4: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schointuch MN, Gilliam TP, Stine JE, Han X, Zhou C, Gehrig PA, et al. (2014) Simvastatin, an HMG-CoA reductase inhibitor, exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer. Gynecol Oncol 134: 346–355. 10.1016/j.ygyno.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Wang Z, Li Y, Li W, Chen Y (2013) Simvastatin prevents proliferation and bone metastases of lung adenocarcinoma in vitro and in vivo. Neoplasma 60: 240–246. 10.4149/neo_2013_032 [DOI] [PubMed] [Google Scholar]
- 11. Islam M, Sharma S, Kumar B, Teknos TN (2013) Atorvastatin inhibits RhoC function and limits head and neck cancer metastasis. Oral Oncol 49: 778–786. 10.1016/j.oraloncology.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vinogradova Y, Coupland C, Hippisley-Cox J (2011) Exposure to statins and risk of common cancers: a series of nested case-control studies. BMC Cancer 11: 409 10.1186/1471-2407-11-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haukka J, Sankila R, Klaukka T, Lonnqvist J, Niskanen L, Tanskanen A, et al. (2010) Incidence of cancer and statin usage—record linkage study. Int J Cancer 126: 279–284. 10.1002/ijc.24536 [DOI] [PubMed] [Google Scholar]
- 14. Dale KM, Coleman CI, Henyan NN, Kluger J, White CM (2006) Statins and cancer risk: a meta-analysis. JAMA 295: 74–80. [DOI] [PubMed] [Google Scholar]
- 15. Kaye JA, Jick H (2004) Statin use and cancer risk in the General Practice Research Database. Br J Cancer 90: 635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi M, Zheng H, Nie B, Gong W, Cui X (2014) Statin use and risk of liver cancer: an update meta-analysis. BMJ Open 4: e005399 10.1136/bmjopen-2014-005399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Tang W, Wang J, Xie L, Li T, He Y, et al. (2014) Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control 25: 237–249. 10.1007/s10552-013-0326-6 [DOI] [PubMed] [Google Scholar]
- 18. Jespersen CG, Norgaard M, Friis S, Skriver C, Borre M (2014) Statin use and risk of prostate cancer: a Danish population-based case-control study, 1997–2010. Cancer Epidemiol 38: 42–47. 10.1016/j.canep.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 19. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ (2004) The risk of cancer in users of statins. J Clin Oncol 22: 2388–2394. [DOI] [PubMed] [Google Scholar]
- 20. Blais L, Desgagne A, LeLorier J (2000) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: a nested case-control study. Arch Intern Med 160: 2363–2368. [DOI] [PubMed] [Google Scholar]
- 21. Yi X, Jia W, Jin Y, Zhen S (2014) Statin use is associated with reduced risk of haematological malignancies: evidence from a meta-analysis. PLoS One 9: e87019 10.1371/journal.pone.0087019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonovas S, Filioussi K, Tsantes A, Sitaras NM (2007) Use of statins and risk of haematological malignancies: a meta-analysis of six randomized clinical trials and eight observational studies. Br J Clin Pharmacol 64: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adamson P, Bray F, Costantini AS, Tao MH, Weiderpass E, Roman E (2007) Time trends in the registration of Hodgkin and non-Hodgkin lymphomas in Europe. Eur J Cancer 43: 391–401. [DOI] [PubMed] [Google Scholar]
- 24. Groves FD, Linet MS, Travis LB, Devesa SS (2000) Cancer surveillance series: non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst 92: 1240–1251. [DOI] [PubMed] [Google Scholar]
- 25. Katanoda K, Yako-Suketomo H (2008) Comparison of time trends in Hodgkin and non-Hodgkin lymphoma incidence (1973–97) in East Asia, Europe and USA, from cancer incidence in five continents Vol. IV-VIII. Jpn J Clin Oncol 38: 391–393. 10.1093/jjco/hyn037 [DOI] [PubMed] [Google Scholar]
- 26. Fortuny J, de Sanjose S, Becker N, Maynadie M, Cocco PL, Staines A, et al. (2006) Statin use and risk of lymphoid neoplasms: results from the European Case-Control Study EPILYMPH. Cancer Epidemiol Biomarkers Prev 15: 921–925. [DOI] [PubMed] [Google Scholar]
- 27. Chen WL, Tsai WC, Chao TY, Sheu LF, Chou JM, Kao WY, et al. (2010) The clinicopathological analysis of 303 cases with malignant lymphoma classified according to the World Health Organization classification system in a single institute of Taiwan. Ann Hematol 89: 553–562. 10.1007/s00277-009-0870-z [DOI] [PubMed] [Google Scholar]
- 28. Muller AM, Ihorst G, Mertelsmann R, Engelhardt M (2005) Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 84: 1–12. [DOI] [PubMed] [Google Scholar]
- 29. Sukpanichnant S (2004) Analysis of 1983 cases of malignant lymphoma in Thailand according to the World Health Organization classification. Hum Pathol 35: 224–230. [DOI] [PubMed] [Google Scholar]
- 30.(2000) The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int 50: 696–702. [DOI] [PubMed] [Google Scholar]
- 31. Hsing AW, Ioannidis JP (2015) Nationwide Population Science: Lessons From the Taiwan National Health Insurance Research Database. JAMA Intern Med. 10.1001/jamainternmed.2015.3540 [DOI] [PubMed] [Google Scholar]
- 32. Chiu HF, Ho SC, Chen CC, Yang CY (2011) Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol 106: 894–898. 10.1038/ajg.2010.475 [DOI] [PubMed] [Google Scholar]
- 33. Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC (2013) Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 31: 1514–1521. 10.1200/JCO.2012.44.6831 [DOI] [PubMed] [Google Scholar]
- 34. Jadhav SB, Jain GK (2006) Statins and osteoporosis: new role for old drugs. J Pharm Pharmacol 58: 3–18. [DOI] [PubMed] [Google Scholar]
- 35. Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H (2000) HMG-CoA reductase inhibitors and the risk of fractures. JAMA 283: 3205–3210. [DOI] [PubMed] [Google Scholar]
- 36. Rejnmark L, Olsen ML, Johnsen SP, Vestergaard P, Sorensen HT, Mosekilde L (2004) Hip fracture risk in statin users—a population-based Danish case-control study. Osteoporos Int 15: 452–458. [DOI] [PubMed] [Google Scholar]
- 37. Smedby KE, Baecklund E, Askling J (2006) Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev 15: 2069–2077. [DOI] [PubMed] [Google Scholar]
- 38. Engels EA, Cho ER, Jee SH (2010) Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol 11: 827–834. 10.1016/S1470-2045(10)70167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gasztonyi B, Par A, Szomor A, Battyany I, Nagy A, Kereskai L, et al. (2000) Hepatitis C virus infection associated with B-cell non-Hodgkin's lymphoma in Hungarian patients. Br J Haematol 110: 497–498. [PubMed] [Google Scholar]
- 40. Mizorogi F, Hiramoto J, Nozato A, Takekuma Y, Nagayama K, Tanaka T, et al. (2000) Hepatitis C virus infection in patients with B-cell non-Hodgkin's lymphoma. Intern Med 39: 112–117. [DOI] [PubMed] [Google Scholar]
- 41. Mitri J, Castillo J, Pittas AG (2008) Diabetes and risk of Non-Hodgkin's lymphoma: a meta-analysis of observational studies. Diabetes Care 31: 2391–2397. 10.2337/dc08-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J (2012) Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood 119: 4845–4850. 10.1182/blood-2011-06-362830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4: 977–987. [DOI] [PubMed] [Google Scholar]
- 44. Pruefer D, Makowski J, Schnell M, Buerke U, Dahm M, Oelert H, et al. (2002) Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation 106: 2104–2110. [DOI] [PubMed] [Google Scholar]
- 45. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13: 759–771. 10.1038/nrc3611 [DOI] [PubMed] [Google Scholar]
- 46. Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, et al. (2005) Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A 102: 14825–14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Balkwill F, Charles KA, Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7: 211–217. [DOI] [PubMed] [Google Scholar]
- 48. Shachaf CM, Perez OD, Youssef S, Fan AC, Elchuri S, Goldstein MJ, et al. (2007) Inhibition of HMGcoA reductase by atorvastatin prevents and reverses MYC-induced lymphomagenesis. Blood 110: 2674–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qi XF, Zheng L, Lee KJ, Kim DH, Kim CS, Cai DQ, et al. (2013) HMG-CoA reductase inhibitors induce apoptosis of lymphoma cells by promoting ROS generation and regulating Akt, Erk and p38 signals via suppression of mevalonate pathway. Cell Death Dis 4: e518 10.1038/cddis.2013.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brookhart MA, Patrick AR, Dormuth C, Avorn J, Shrank W, Cadarette SM, et al. (2007) Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol 166: 348–354. [DOI] [PubMed] [Google Scholar]
- 51. Patrick AR, Shrank WH, Glynn RJ, Solomon DH, Dormuth CR, Avorn J, et al. (2011) The association between statin use and outcomes potentially attributable to an unhealthy lifestyle in older adults. Value Health 14: 513–520. 10.1016/j.jval.2010.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chao C, Xu L, Abrams DI, Towner WJ, Horberg MA, Leyden WA, et al. (2011) HMG-CoA reductase inhibitors (statins) use and risk of non-Hodgkin lymphoma in HIV-positive persons. AIDS 25: 1771–1777. 10.1097/QAD.0b013e328349c67a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.