Abstract
Gait variability measures have been linked to fall risk in older adults. However, challenging walking tasks may be required to elucidate increases in variability that arise from subtle age-related changes in cognitive processing and sensorimotor function. Hence, the study objective was to investigate the effects of visual perturbations, increased cognitive load, and narrowed step width on gait variability in healthy old and young adults. Eleven old (OA, 71.2 ± 4.2 years) and twelve young (YA, 23.6 ± 3.9 years) adults walked on a treadmill while watching a speed-matched virtual hallway. Subjects walked: 1) normally, 2) with mediolateral visual perturbations, 3) while performing a cognitive task (serial seven subtractions), and 4) with narrowed step width. We computed the mean and variability of step width (SW and SWV, respectively) and length (SL, SLV) over one three minute trial per condition. Walking normally, old and young adults exhibited similar SWV and SLV. Visual perturbations significantly increased gait variability in old adults (by more than 100% in both SWV and SLV), but not young adults. The cognitive task and walking with narrowed step width did not show any effect on SWV or SLV in either group. The dramatic increase in step width variability when old adults were subjected to mediolateral visual perturbations was likely due to increased reliance on visual feedback for assessing whole body position. Further work is needed to ascertain whether these findings may reflect sub-clinical balance deficits that could contribute to the increased fall risk seen with advancing age.
Keywords: virtual reality, aging, optical flow, dynamic balance, dual task
1. Introduction
Approximately one-third of adults over 65 fall annually with a majority of these falls occurring during locomotion [1]. The underlying causes of these falls presumably arise from a number of physiological factors including reduced sensory acuity [2, 3], diminished executive function [4], reduced cognitive capacity [5], decreased muscle strength [6, 7], and slowed neuromuscular function [6]. Although these phenomena are common features of aging, the manner in which they affect balance function during walking is not well understood. For example, even if an individual scores in the normal range for all of these physiological areas, subtle age-related changes may disrupt old adults' ability to maintain balance in a challenging environment and contribute to a first fall.
Prior studies have used gait variability to characterize balance during walking [8-10]. In fact, substantial differences in gait variability (e.g. standard deviation or coefficient of variation of stride time, step width or step length over many steps) have been reported between old adults with and without a history of falls [11, 12]. Compromised mediolateral balance is particularly relevant as it requires fine sensorimotor control of foot placement from step to step [8]. However, healthy old adults often exhibit gait variability similar to young adults during normal, unencumbered walking [4]. Hence, gait variability during normal walking may not be sufficiently robust to identify old adults at risk of a first fall.
In the literature, challenging walking task paradigms have been used to elucidate the influence of age-related changes on walking performance [9-11, 13-16]. These challenging walking tasks are often designed to target the functional consequences of aging on sensory, cognitive and/or neuromuscular function. For example, the use of altered lighting conditions, vibrating insoles or virtual environments can manipulate sensory feedback and alter gait variability [8, 14, 17]. Attention-demanding tasks during walking, such as mathematical calculations, have also been shown to preferentially increase gait variability in old adults [10, 13, 15]. Finally, manipulating foot placement using balance beams or obstacles may reveal differences in neuromuscular function, reflecting an inability to cope with challenges present in the physical environment [16, 18]. These additional task requirements have shown promise to expose age-related differences in balance during walking; however, their comparative effects are not yet well understood.
The purpose of this study was to investigate the effects of perturbed visual feedback, increased cognitive load, and narrowed step width demands on gait variability in healthy old and young adults. We first hypothesized that during unencumbered walking, old and young adults would walk with similar step width variability (SWV) and step length variability (SLV). Second, with the addition of challenging task requirements, SWV and SLV would increase more in old adults than young adults. Our clinical motivation was to identify relative increases in old adults' SWV and SLV that point to opportunities for early diagnosis of age-related balance deficits.
2. Methods
2.1 Subjects and experimental protocol
Eleven old adults (mean ± standard deviation; age: 71.2 ± 4.2 years, height: 1.64 ± 0.06 m, mass: 66.9 ± 9.6 kg, 10 female) and twelve young adults (age: 23.6 ± 3.9 years, height: 1.69 ± 0.25 m, mass: 70.7 ± 11.3 kg, 7 female) participated in this study. Subjects were included if they walked without an assistive device, were free of orthopedic injuries in the prior six months, had no neurological injury or pathology, and met the American College of Sports Medicine cardiovascular guidelines for exercise. Additionally, subjects could not have experienced an unexpected fall [19] in the previous six months. They were also required to score in the normal range on the Dynamic Gait Index (DGI) [20], a series of eight walking tasks (eg. turning one's head while walking, changing speeds, navigating stairs) each worth up to three points. Individuals scoring below 19 are considered to be at an increased risk of falling. The old adults in this study scored an average of 23.8 ± 0.6 on the DGI. All subjects provided written informed consent as per the University of Wisconsin-Madison Health Science Institutional Review Board.
At the beginning of each session, subjects walked along a 10 m walkway at a comfortable pace. We used the average of two times taken to traverse the middle 6 m of the walkway to prescribe subjects' treadmill speed. During the treadmill familiarization period, three old adults reported being unsure that they could complete the treadmill walking trials at their over-ground speed. We therefore reduced the treadmill speed by 10% for these subjects. To ensure subject safety, old adults used a harness during treadmill testing that was adjusted such that it would prevent a full fall but was slack enough to allow free movement around the treadmill surface without providing substantial haptic feedback. Subjects then completed a series of four 3-minute walking trials on a split-belt instrumented treadmill (Bertec, Columbus, OH) presented in a random order. The four conditions included normal walking (Normal), mediolateral visual perturbations (Visual), a cognitive challenge (Cognitive), and narrow step width (Narrow), each described in detail below. All tasks were performed with the subject facing a semi-circular rear-projection screen, which displayed a virtual hallway moving at the same speed as the treadmill (Figure 1a). This system was described in more detail by O'Connor, et al. [8]. To investigate the confounding effects of walking speed, young adults repeated all conditions at 80% of their preferred speed.
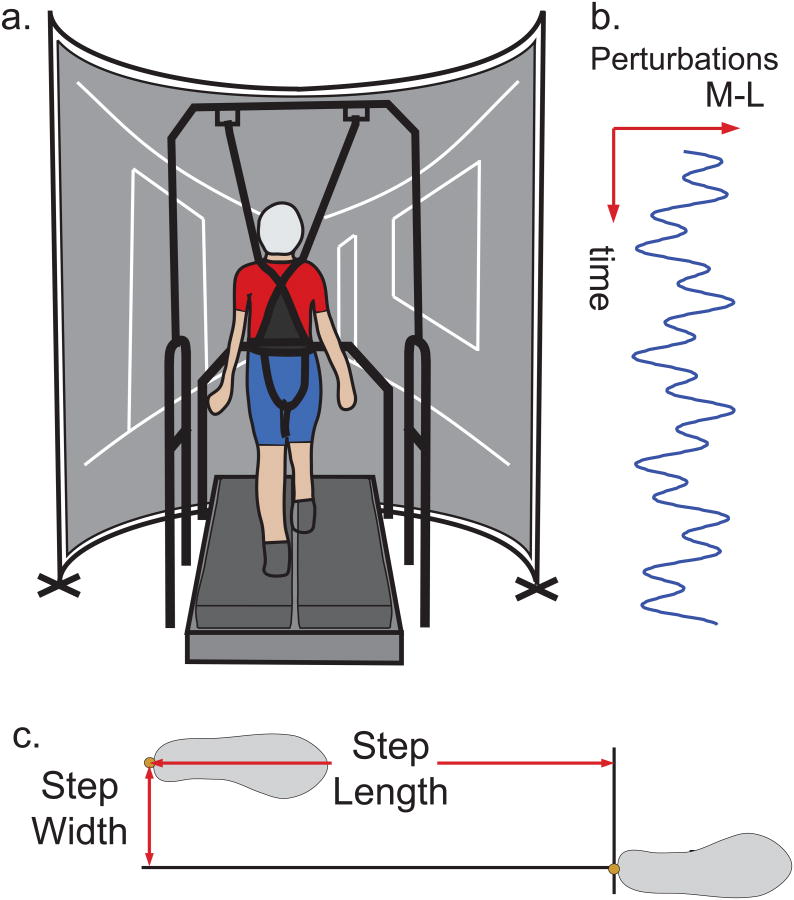
Figure 1.
Experimental setup. 1a. Subject walks on a split-belt treadmill surrounded by a semicircular projection screen. A rear-projected virtual hallway moved at the same speed as the treadmill. Old adults wore a harness during testing that was adjusted to prevent falls but still allow free movement around the treadmill surface. 1b. During the visual perturbation trial, we added a mediolateral perturbation consisting of a sum of sinusoids to the virtual hallway motion. 1c. Step width and step length were calculated from heel kinematic data for both left-right and right-left steps.
For the normal walking condition, subjects were asked to walk normally while watching the virtual hallway. During the visual perturbation condition, a continuous mediolateral motion consisting of the sum of two sinusoids (0.135 and 0.0442 Hz) with 0.175 m amplitudes was added to the speed-matched virtual hallway (Figure 1b) [8, 17]. This mediolateral motion was applied such that the fore-ground translated at the full amplitude of the perturbation while the end of the hall remained nearly stationary. This ensured that perturbations challenged walking balance and not control of heading. During the cognitive challenge condition, subjects walked while counting backwards by sevens starting at a prescribed random three-digit number [4]. In the event that subjects reached zero before the trial was complete, they continued from a new prescribed three-digit number. For the narrow step width task [16], subjects were asked to place each step on the 1 cm gap separating the two treadmill belts as if they were on a balance beam. During this trial, subjects were allowed to look at their feet as needed.
2.2 Measurements and data analysis
Three dimensional pelvic and foot kinematics were recorded at 100 Hz using a passive motion capture system (Motion Analysis, CA) to track retro-reflective markers placed on the sacrum and both heels. Kinematic data were low-pass filtered at 8 Hz using a 4th order Butterworth filter. We then identified heel strikes from peaks in the fore-aft position of the heel markers relative to the sacral marker [21]. We computed right-left and left-right step width (SW) values from consecutive mediolateral heel positions averaged over a period from 12-25% of the gait cycle (heel-strike to heel-strike), corresponding to mid-stance prior to heel-rise [22], (Figure 1c). Step length (SL) was computed as the relative fore-aft position of successive heel markers at 20% of each gait cycle plus the treadmill translation over the duration of that step [17]. We characterized gait variability as the standard deviation of step width and step length over all steps performed within a 3-minute trial (old: 345+31 steps, young: 332 ± 17 steps). All mean and variability metrics were normalized to subject leg length (%LL).
2.3 Statistical Analysis
We first confirmed normal distribution using a Kolmogorov-Smirnov test (STATISTICA, StatSoft, Tulsa, OK). We used a repeated measures ANOVA with a Tukey Honest Significant Differences (HSD) post-hoc test to test for significant effects of age (old vs. young) and condition (Normal, Cognitive, Visual, Narrow) on mean step width, mean step length, SWV and SLV using an α<0.05 criterion. Corrections for heterogeneous variances, determined from Levene's Test, were used when necessary. Within groups, we focused on comparisons between normal and challenging task conditions. Between groups, we focused on comparisons between young and old adults under the same task conditions. We used t-tests to investigate significant effects of speed (80%, 100% preferred) on step width and length metrics in young adults for each condition (Normal, Cognitive, Visual, Narrow).
3. Results
There were no significant differences in normalized preferred walking speed on the treadmill between old (1.24 m/s or 1.68 ± 0.12 LL/s) and young (1.36 m/s or 1.68 ± 0.19 LL/s) adults (leg-length normalized, T-Test, F1,21 = 2.44, p = 0.928). Group values for SW, SL, SWV, and SLV are summarized in Table 1 along with the ANOVA results.
Table 1.
Group mean (± standard deviation) values for SW, SL, SWV, and SLV during each condition are listed for old and young adults walking at preferred speed. Group and condition main effects or interactions from a repeated measures ANOVA are reported.
| Metric | Group | Normal | Visual | Cognitive | Narrow | Effect Group | DoF | F | p |
|---|---|---|---|---|---|---|---|---|---|
| SW | Old | 16.52 (1.17) | 18.75 (1.37) | 16.3 (1.46) | 5.57 (0.95) | Group | 1, 21 | - | 0.569 |
| Young | 16.68 (1.12) | 18.05 (1.32) | 17.94 (1.40) | 1.14 (0.91) | Condition | 3, 63 | 155.35 | <0.001 | |
| Group × Condition | 3, 63 | 5.21 | 0.003 | ||||||
| SL | Old | 88.06 (2.06) | 83.44 (2.27) | 88.22 (2.16) | 88.92 (2.39) | Group | 1, 21 | - | 0.304 |
| Young | 90.13 (1.98) | 89.67 (2.17) | 89.43 (2.07) | 91.89 (2.29) | Condition | 3, 63 | 10.88 | <0.001 | |
| Group × Condition | 3, 63 | 5.13 | 0.003 | ||||||
| SWV | Old | 3.53 (0.30) | 8.92 (1.00) | 3.62 (0.28) | 4.45 (0.38) | Group | 1, 21 | 12.05 | 0.002 |
| Young | 2.93 (0.29) | 4.02 (0.96) | 3.01 (0.27) | 3.18 (0.36) | Condition | 3, 63 | 20.20 | <0.001 | |
| Group × Condition | 3, 63 | 9.14 | <0.001 | ||||||
| SLV | Old | 2.92 (0.20) | 6.73 (0.77) | 3.13 (0.22) | 3.15 (0.29) | Group | 1, 21 | 8.79 | 0.007 |
| Young | 2.58 (0.19) | 3.46 (0.73) | 2.45 (0.21) | 2.85 (0.28) | Condition | 3, 63 | 17.85 | <0.001 | |
| Group × Condition | 3, 63 | 7.12 | <0.001 |
3.1 Average Step Width and Step Length
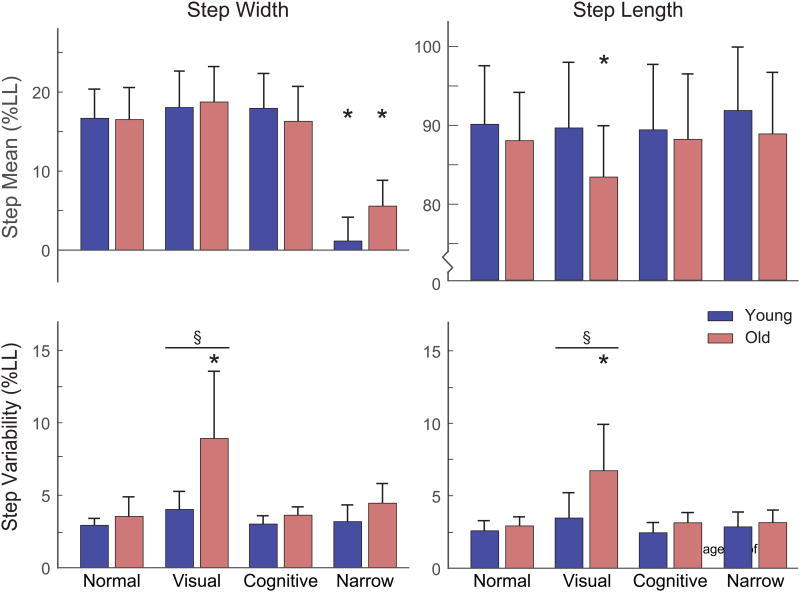
Compared to walking normally, old adults walked with 5% shorter steps during the visual perturbation condition (Tukey, p < 0.001) (Figure 3). Additionally, and by design, both young and old adults walked with significantly narrower steps during the narrow SW condition compared to normal walking (OA: 66% reduction on average, YA: 93% reduction, Tukey, p's < 0.001).
Figure 3.
Summary step placement results for all subjects. Group mean ± standard deviation are represented. An asterisk (*) denotes a significant within-group difference between a challenging condition and normal walking (p < 0.01). A section sign (§) and horizontal line denote a significant between-group difference (p < 0.01) within a condition.
3.2 Variability of Step Width and Step Length
Old and young adults exhibited similar step width variability (SWV, OA vs. YA, 3.5 ± 1.4 %LL vs. 2.9 ± 0.5 %LL, Tukey, p = 0.996) and step length variability (SLV, 2.9 ± 0.6 %LL vs. 2.6 ± 0.7 %LL, Tukey, p = 0.998) during normal walking (Figure 2, Figure 3). During the visual perturbation condition, young adults did not exhibit a significant change SWV (Tukey, p = 0.898) or SLV (Tukey, p = 0.718) relative to normal walking. However, old adults exhibited greater SWV and SLV than young adults (Tukey, p's < 0.001) in the visual perturbation condition, with both variability metrics increasing significantly relative to the normal walking condition (+152% and +131% increase in mean SWV and SLV, respectively, Tukey, p's < 0.001). Compared to walking normally, neither group exhibited significant changes in SWV or SLV during the cognitive task (Tukey; p's ≥ 0.999) or the narrow step width condition (Tukey; p's ≥ 0.888).
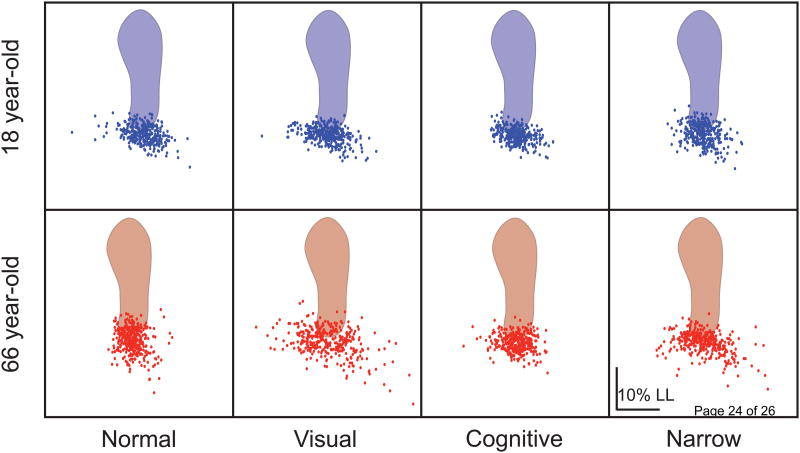
Figure 2.
The distribution of step width and step length values about the mean of two representative subjects plotted for each walking trial. Each gray dot represents the step width and step length for a single step, measured from heel markers. Scatter along the x-axis and y-axis represents variability in SW and SL, respectively. The scatter of SW and SL values is fairly tight for both subjects in all but the visual perturbation trial. Note the wide range of step placements for the old adult in the visual condition.
3.3 Effect of walking speed
When young adults walked at 80% preferred speed (1.08 ± 0.09 m/s or 1.35 ± 0.19 LL/s), SW and SLV were very similar for each condition to walking at preferred speed (T-test, p's ≥ 0.372). SWV was also not significantly different between speeds (T-test, p's ≥ 0.107) (Figure 4). Only step length was significantly affected when young adults walked more slowly (-14% decrease in average SL; T-test, p's < 0.001).
Figure 4.
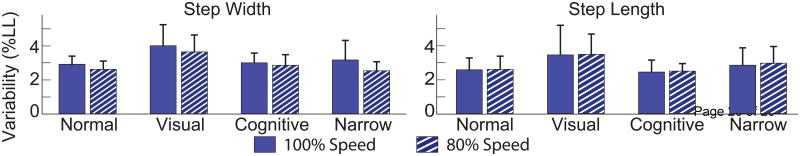
Young adults performed all trials at 100% and 80% of their preferred overground walking speed. Walking slower elicited shorter steps (p's < 0.001) for each condition, but there were no differences in step width variability or step length variability.
4. Discussion
Gait variability has emerged over the last two decades as a promising metric for characterizing dynamic balance [8-10] and altered variability has been linked to old adults with a history of falls [4, 11, 12]. At the same time, the need for challenging tasks to distinguish between old and young adults has also been highlighted [9-11, 13-16]. As hypothesized in this study, SWV and SLV were statistically indistinguishable between healthy young and healthy old adults with no falls history during normal, unencumbered walking. Further, in partial support of our second hypothesis, walking with mediolateral visual perturbations induced changes in SWV and SLV that were able to distinguish old and young adults. This observation suggests that aging may bring a greater reliance on visual feedback to maintain balance during walking, an observation that is consistent with postural control studies [23-25] and has broad relevance for both balance assessment and the design of falls prevention programs. Surprisingly, and in contrast with our second hypothesis, we did not see any difference in SWV or SLV between old and young adults in response to either an increased cognitive load or narrow step placement demands.
Why do old adults seemingly exhibit such heightened sensitivity to aberrant visual feedback during gait? To understand this, it is first imperative to recognize the importance of lateral step placement to the maintenance of balance in walking. Biomechanical models of gait have shown that frontal plane balance is inherently unstable, but easily stabilized by choosing where to step relative to the body center of mass kinematics [8, 26]. To accomplish this, multi-modal sensory feedback is needed to assess the whole body kinematic state, with sensory integration and sensorimotor processing then critical to executing step placement. Thus, it seems that old adults place a greater reliance, or increased gain, on visual feedback to assess the body's kinematic state, perhaps compensating for age-related decrements in vestibular function [3] and somatosensory feedback from the periphery [2, 27]. There is also evidence that old adults may be particularly reliant on peripheral vision [23], which was stimulated to a large degree by the visual perturbations used in this experiment. Alternatively, old adults may be less capable of down-weighting aberrant visual information [24, 25]. In either scenario, old adults would incorrectly assess their kinematic state based on the flawed visual information leading to an inappropriate step placement relative to the center of mass. Incorrect step placement can then further compromise mediolateral balance, requiring subsequent recovery steps to restore balance, thereby contributing to the increase in step width variability observed here. Interestingly, we previously found that the visual perturbation frequencies permeate old adult's center of mass motion [28]. That old adults' gait patterns entrain to one or both of the frequencies used in the perturbed visual flow further supports the idea that they were relying upon the aberrant visual feedback in their sensorimotor control of walking.
Contrary to prior studies, the cognitive task in this study did not induce changes in SWV or SLV in old adults [9, 10, 13, 15]. Our study is therefore in line with the study by Springer, et al. which found that a cognitive task affected gait variability in old adults with a history of falls, but did not affect old adults with no history of falls [4]. That study also found that impaired executive function was strongly associated with increased swing time variability in old adults with a history of falls. One interpretation of these findings is that cognitive challenges interfere with central regulation of periodic movement patterns. The old adults in this study may not have been affected by the cognitive task because the specific activity (serial 7 subtractions) was not sufficiently challenging to disrupt this regulation in the healthy old population tested in this study.
During the narrow step width condition both groups were able to effectively narrow their step width when asked. On average, old adults had a wider step width during this task but the difference between groups was not significant (p = 0.204). We also did not find significant effects of this task on SWV or SLV. These conclusions are in contrast with prior studies [16], though our variability measures are comparable to the values reported by Dean et al. [18].
Slower absolute walking speeds are often seen with increasing age and have been tied to a variety of health issues, including fall risk [29]. However, deleterious effects are most frequently found in individuals with a preferred walking speed below 1.0 m/s, nearly 20% slower than the old adults in this study [30]. Additionally, the old adults in this study did not show any significant differences in normalized walking speed from young adults. Thus, gait speed would seem insufficient to explain the differences in gait variability we observed between groups. Further, when young adults were asked to voluntarily reduce their walking speed, we observed no significant changes in their gait variability metrics. Thus, the age-related effects we observed most likely reflect robust age-related differences in the control of balance.
There are some limitations of this study that are relevant to consider in interpreting our findings. First, we only considered one specific visual perturbation consisting of a mediolateral rotation of the visual field. Other studies have shown that young adult gait variability depends on visual perturbation direction, magnitude, and frequency content [8, 17], such that further study is needed to determine the visual conditions to which old adults may be most sensitive. Second, the reliability of gait variability has been questioned by some in the gait community [31], with others suggesting that the precision and robustness of gait variability can be improved by using longer trials to allow the inclusion of larger numbers of steps [32-34]. Accordingly, our experiments were performed on a treadmill which permitted the collection of 300+ steps/trial to characterize gait variability. However, while the use of a treadmill allowed us to collect large numbers of steps, treadmill walking regularizes walking speed and may induce gait variability values that differ from those seen overground [12, 35]. Finally, to ensure their safety, the old adults in the study used a harness while walking on the treadmill. While the harness was adjusted to ensure that subjects' movement was not restricted, it is still possible that tactile feedback from the harness could have influenced our results.
Further work is needed to determine whether the visual perturbation technique used in this study could be used to identify subtle sensory deficits that could eventually lead to falls in old adults. Adding clinical metrics of sensory acuity to future studies would allow us to investigate whether the increase in gait variability induced by visual perturbations may arise from deficits in, for example, peripheral sensation. Further, it will be important to extend the data set to include old adults with clinical balance deficits and/or a history of falls. These future studies could lead to the development of a low-cost battery of tests to enable early detection of balance deficits and targeted falls prevention programs in old adults.
In summary, we have shown that perturbed visual feedback was sufficient to induce greater gait variability in healthy old adults relative to young adults but that increased cognitive load and narrow step width demands were ineffective in delineating the two groups. The dramatic increase in step width variability when old adults were subjected to mediolateral visual perturbations was likely due to increased reliance on visual feedback for assessing whole body position. Further work is needed to ascertain whether these findings may reflect sub-clinical balance deficits that could contribute to the increased fall risk seen with advancing age.
Research Highlights.
Investigated effects of challenging walking tasks in old (OA) and young (YA).
Variability of step width (SWV) and step length (SLV) characterized subjects.
Visual perturbation induced OA vs. YA differences in SWV and SLV.
A cognitive task and narrow step width did not induced changes in SWV or SLV.
Only visual perturbation induced variability differences vs. normal walking.
Acknowledgments
The authors would like to acknowledge Holly Schoenberg and Michael Schmidt. This work was funded by NIH F31AG046945 and NIH F32AG044904.
Footnotes
Conflict of Interest: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Carrie Francis, Jason Franz, Shawn O'Connor, and Darryl Thelen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tromp AM, Pluijm SMF, Smit JH, Deeg DJH, Bouter LM, Lips P. Fall-risk screening test: A prospective study on predictors for falls in community-dwelling elderly. Journal of Clinical Epidemiology. 2001;54(8):837–844. doi: 10.1016/s0895-4356(01)00349-3. [DOI] [PubMed] [Google Scholar]
- 2.Skinner HB, Barrack RL, COOK SD. Age-related decline in proprioception. Clinical orthopaedics and related research. 1984;184:208–211. [PubMed] [Google Scholar]
- 3.Sloane PD, Baloh RW, Honrubia V. The vestibular system in the elderly: clinical implications. American journal of otolaryngology. 1989;10(6):422–429. doi: 10.1016/0196-0709(89)90038-0. [DOI] [PubMed] [Google Scholar]
- 4.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: The role of aging, falls, and executive function. Movement Disorders. 2006;21(7):950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 5.Li SC, Lindenberger U, Sikström S. Aging cognition: from neuromodulation to representation. Trends in cognitive sciences. 2001;5(11):479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- 6.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46(3):451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. Journal of neurophysiology. 2009;102(3):1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait & posture. 2008;27(3):431–439. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubost V, Kressig RW, Gonthier R, Herrmann FR, Aminian K, Najafi B, Beauchet O. Relationships between dual-task related changes in stride velocity and stride time variability in healthy older adults. Human movement science. 2006;25(3):372–382. doi: 10.1016/j.humov.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J NeuroEngineering and Rehabilitation. 2005;2(21) doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Archives of Physical Medicine and Rehabilitation. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 13.Priest AW, Salamon KB, Hollman JH. Age-related differences in dual task walking: a cross sectional study. Journal of NeuroEngineering and Rehabilitation. 2008;5 doi: 10.1186/1743-0003-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thies SB, Richardson JK, Ashton-Miller JA. Effects of surface irregularity and lighting on step variability during gait∷ A study in healthy young and older women. Gait & posture. 2005;22(1):26–31. doi: 10.1016/j.gaitpost.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait & Posture. 2007;26:113–119. doi: 10.1016/j.gaitpost.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Schrager MA, Kelly VE, Price R, Ferrucci L, Shumway-Cook A. The effects of age on medio-lateral stability during normal and narrow base walking. Gait & posture. 2008;28(3):466–471. doi: 10.1016/j.gaitpost.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Connor SM, Xu HZ, Kuo AD. Energetic cost of walking with increased step variability. Gait & posture. 2012;36(1):102–107. doi: 10.1016/j.gaitpost.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. Biomedical Engineering, IEEE Transactions on. 2007;54(11):1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- 19.Gibson M, Andrew R, Isaacs B, Radebaugh T, Worm-Petersen J. The prevention of falls in later life. A report of the Kellogg International Work Group on prevention of falls by the elderly. Danish Medical Bulletin. 1987;34(S4):1–24. [PubMed] [Google Scholar]
- 20.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the Probability for Falls in Community-Dwelling Older Adults. Physical Therapy. 1997;77(8):812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 21.Zeni J, Jr, Richards J, Higginson J. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait & posture. 2008;27(4):710–714. doi: 10.1016/j.gaitpost.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry J, Burnfield JM. Gait Analysis: Normal and Pathological Function. SLACK Incorporated; Thorofare, NJ: 2010. [Google Scholar]
- 23.Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. Journal of Gerontology. 1989;44(4):M118–M127. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- 24.Horak FB, Shupert CL, Mirka A. Components of postural dyscontrol in the elderly: a review. Neurobiology of aging. 1989;10(6):727–738. doi: 10.1016/0197-4580(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 25.Jeka JJ, Allison LK, Kiemel T. The dynamics of visual reweighting in healthy and fall-prone older adults. Journal of motor behavior. 2010;42(4):197–208. doi: 10.1080/00222895.2010.481693. [DOI] [PubMed] [Google Scholar]
- 26.Yang W, Sirinivasan M. Stepping in the direction of the fall: the next foot placement can be predicted from current upper body state in steady-state walking. Biol Lett. 2014;10(20140405) doi: 10.1098/rsbl.2014.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins S, Waked E, McClaran J. Proprioception and stability: foot position awareness as a function of age and footware. Age and Ageing. 1995;24(1):67–72. doi: 10.1093/ageing/24.1.67. [DOI] [PubMed] [Google Scholar]
- 28.Franz JR, Francis CA, Allen MS, O'Connor SM, Thelen DG. Advanced Age Brings a Reliance on Visual Feedback to Maintain Balance During Walking. Human Movement Science. doi: 10.1016/j.humov.2015.01.012. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(8):844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 30.Van Kan GA, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini L, Gillette-Guyonnet S, Inzitari M. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 31.Lord S, Howe T, Greenland J, Simpson L, Rochester L. Gait variability in older adults: a structured review of testing protocol and clinimetric properties. Gait & posture. 2011;34(4):443–450. doi: 10.1016/j.gaitpost.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Hollman JH, Childs KB, McNeil ML, Mueller AC, Quilter CM, Youdas JW. Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait & posture. 2010;32(1):23–28. doi: 10.1016/j.gaitpost.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait & Posture. 2004;20(1):26–29. doi: 10.1016/S0966-6362(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 34.Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson's disease? Towards an optimal testing protocol. Gait & posture. 2013;37(4):580–585. doi: 10.1016/j.gaitpost.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Dingwell J, Cusumano J, Cavanagh P, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. Journal of biomechanical engineering. 2001;123(1):27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]