Engraved on the Eiffel Tower in Paris are the names of 72 scientists, engineers, and mathematicians, including Lavoisier, Laplace, and Coulomb. Among them is Paul Broca’s in recognition of his contributions to neurology (see Schiller’s [1979] biography entitled Paul Broca: Founder of French Anthropology, Explorer of the Brain).
In neuroscience, his name is most directly associated with speech and language. Broca was very interested in the localization of brain function, and his observations of patient Tan led him to propose that language was dependent on the left inferior frontal lobe – a region subsequently called Broca’s area. As told by Broca (1861, p. 236), “At the time of his admission, Tan was perfectly able-bodied and intelligent” and “understood almost everything that was said to him.” But “he could no longer pronounce more than a single syllable, which he ordinarily repeated twice at a time; whenever a question was asked of him, he would always reply tan, tan, in conjunction with quite varied expressive gestures.” Tan’s brain lesion was on the left hemisphere of the prefrontal cortex (discovered upon autopsy following his death). Broca concluded that “All this permits, however, the belief that, in the present case, the lesion of the frontal lobe was the cause of the loss of speech.” Broca’s 1861 report can be regarded as the most important clinical paper in the history of cortical localization (Finger, 1994; p. 58).
Broca’s interests were broad and varied. Among others, he was concerned with understanding the overall layout and macroscopic organization of the mammalian cerebral hemispheres – that is, cortex which he and others at the time referred to as the “mantle of the hemisphere”. In 1878, he published a paper entitled Anatomie comparée des circonvolutions cérébrales: Le grand lobe limbique et la scissure limbique dans la série des mammifères (“Comparative anatomy of the cerebral circumvolutions: The great limbic lobe and the limbic fissure in the mammalian series”) (Broca, 1878). Its impact on neuroscience has been enormous, even though an English translation has not been available. We are thus thrilled that the entirety of the paper, over one hundred pages in the original print by the journal Revue d’Anthropologie (Anthropology Review), is reproduced in this issue of the Journal of Comparative Neurology.
The great limbic lobe
Macroscopically, the mammalian cerebrum can be described in terms of the major lobes (most clearly in primates): occipital, temporal, parietal, frontal, and insular. The major subdivisions are most discernible along the lateral, external surface of the brain (with the exception of the insula, of course, which, is covered by the “lid” [operculum] of the frontal and parietal lobes). From the medial surface of the brain the large-scale organization is less clear-cut. In fact, for Broca, a type of “super lobe” is to be recognized: Le grand lobe limbique. Why grand and why limbique?
Broca described the existence of a “great cerebral system” (Broca1) that encircles the limbus (or edge) of the hemisphere. As defined by Broca, the limbic lobe was not a “simple” lobe, but a great lobe in so far as it constituted a “primary division, a fundamental division that is more than a lobe, and furthermore encompasses several lobes” (p. X). In other words, the term “lobe” was not sufficient to describe it, which prompted him to denote it as the great limbic lobe. For Broca, the great limbic lobe could be compared to a racquet, as most clearly evident in the case of the otter brain (Figure 1).
Figure 1.
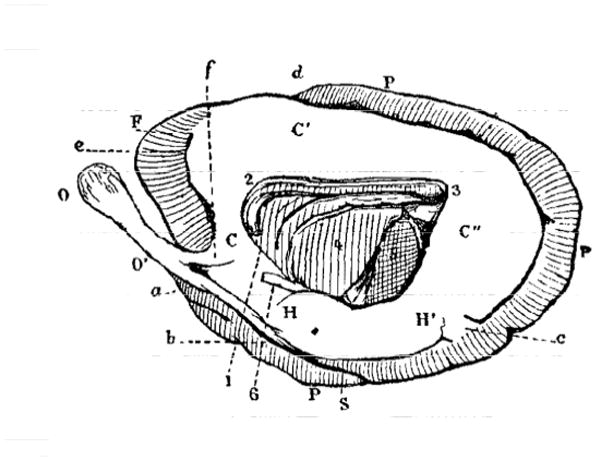
The otter brain as seen medially. The great limbic lobe is left in white; the remaining parts are hatched. Left is anterior; right is posterior. Broca suggested that the overall shape could be compared to that of a racquet. C, C′, and C″ indicate what Broca called the lobe of the corpus callosum. O is the olfactory lobe and O′ is the olfactory peduncle. Note the small proportion comprising the frontal lobe (F), and the large parietal lobe (P). For a complete set of abbreviations, see Fig. 1 in the original paper. Reproduced from Broca (1878; Figure 1).
Although several other researchers had commented on the internal surface of the cortex, Broca set himself the task of systematically understanding the organization of the medial surface with respect to the overall organization of the hemispheres. That is, Broca was interested in understanding the organization of the cortex from a comparative perspective across as many mammalian species as possible.
Thus, Broca’s overall strategy was to study the organization of the cerebral hemisphere across diverse mammalian lines. His first step was to describe the organization of the otter brain. His choice was carefully considered for expository purposes as it allowed the reader to become “familiar with the formation, the relationships, and the connections of the great limbic lobe” (p. X). Importantly, this allowed readers subsequently to “easily study the variations present in the mammalian series” (p. X). He then turned to lissencephalic (that is, smooth) brains of osmatic mammals (“mammals in which the supremacy of the sense of smell is substantiated by the advanced development of the olfactory system”, p. X). Next, he described gyrencephalic (that is, with discernable convolutions) brains of osmatic mammals, and then brains of aquatic mammals. Putting all the necessary pieces together, he was then in a position finally to tackle the primate brain. The thorough examination of more than 30 species prior to describing the primate brain was required because the “brain model of primates is completely different from the model of other mammals” (p. X). It is important to emphasize, however, that Broca correctly ascertained that “all parts of the primate brain have analogous parts in other brains and vice versa” (p. X; although neuroscientists would now read this passage without hesitation, this statement was quite controversial around the time that Broca was writing). Although he highlighted important differences across species, he believed “the various specialized models are merely derived from this general model” (p. X). In fact, clearly he viewed his contribution as helping establish the “continuity of the mammalian series” (Broca) – whereas he felt others had not been successful.
Broca thus concluded that the great limbic lobe exists in all mammals, though with “varying degrees of distinctness, size, and completion” (Broca). At the broadest level, he subdivided the cortical hemispheres into two components: 1) the great limbic lobe, comprising the bulk of the medial surface of the cortex, and demarcated by the “limbic fissure” (Broca; now referred to as cingulate sulcus in primates and most species); and 2) the “mass of the convolutions” (Broca), which formed the rest of the mantle. And although the great limbic lobe was present in lissencephalic brains, its “distinction and utterly unique nature” (Broca) became most obvious in gyrencephalic brains. It was in this latter case that two different parts could be most clearly discerned.
Working through the brain of more than 30 mammals2–3 allowed Broca to characterize what seems to be one of his central aims: the primate brain (Figure 2). The careful observations he made in the first six sections of the paper allowed him to reach the following point: “We now have all the components we need to ascertain the distinctive characteristics of the primate brain” (p. X). He described multiple changes to the primate brain, three of which can be highlighted: 1) the olfactory lobe was rudimentary and reduced to a small bulb; 2) the cingulate gyrus (Broca’s lobe of the corpus callosum) was atrophied in its anterior part but not in its posterior portion (thus, it increases in size from front to back); and 3) the “enormous development of the frontal lobe” (Broca).
Figure 2.
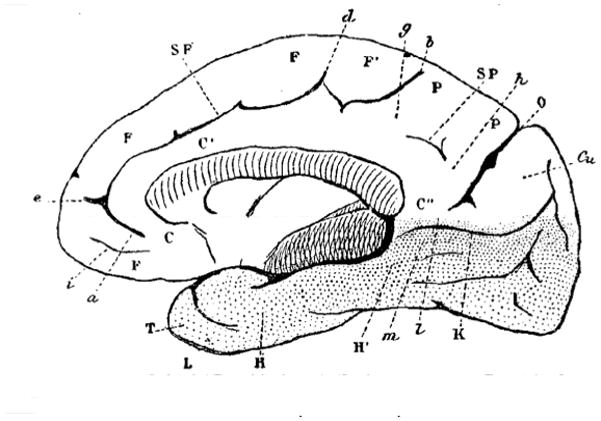
Medial surface of the yellow baboon brain. C, C′, and C″ indicate what Broca called the convolution or lobe of the corpus callosum. For a complete set of abbreviations, please refer to the original figure. Reproduced from Broca (1878; Figure 35).
In all, the primate brain was to be distinguished from that of other mammals by a fundamental property: “the predominance of the frontal lobe” (Broca). This “frontal dominance” (Broca) was accompanied by another important change that was more directly linked to the great limbic lobe: the atrophy of the olfactory system. Critically, these two changes were not incidental but reflected the fact that “a true correlation … exists between the enlargement of the frontal lobe in primates and the inverse evolution of the great limbic lobe” (p. X).
To understand why these coupled changes were so important to Broca, we need to realize that these structural changes had fundamental consequences for the understanding of mental function. This is because the sense of smell was, for Broca, a bestial sense, which was to be contrasted to vision, whose value “to an animal is the value of its intelligence” (Broca). Commenting on the “lowest vertebrates” (Broca), he proposed that “the intellectual involvement needed in exercising the sense of smell is rather slight, and the part of the hemisphere where this occurs likely ranks low in the cerebral hierarchy” (p. X). Critically, “this part of the hemisphere constitutes the great limbic lobe in mammals” (p. X; italics in the original). Furthermore, he proposed that, in primates, “intelligence … gained supremacy over this bestial sense … by two concurrent anatomical events: the advanced development of the frontal lobe, and the atrophy of the olfactory lobe.” (p. X).
The impact of these ideas, so starkly stated by Broca, cannot be overstated. For Broca, during evolution, “as the brain was perfected” (Broca), parts of the brain (the frontal lobe) that are needed for the “highest intellectual functions” (Broca) became dominant, while the great limbic lobe which is more directly linked to the sense of smell “retreated and largely atrophied” (Broca). We can thus understand why the project of elucidating the organization of the brain’s major lobes was so important to him. And, most important, subsequent to Broca, it was relatively easy to link limbic parts of the brain with emotion (often equated with the bestial or irrational side), and the lateral parts of the brain with cognition (the rational side).
From Broca’s great limbic lobe to the limbic brain
The limbic system is one of the most enduring and influential models in all of neuroscience. Standard medical textbooks teach it and versions of the system are presented in basic neuroscience texts. How did the field go from Broca’s concept of the great limbic lobe to the limbic system as integral to the emotional brain? We will not recapitulate the history of the concept as much has been written about it (Brodal, 1981; Kotter and Meyer, 1992; LeDoux, 1996; Pessoa, 2008). Here, we only comment briefly on the major outlines of this history.
A major step from Broca’s great limbic lobe to the limbic system was the important role James Papez (1937) attributed to the cingulate gyrus in his “mechanism of emotion” (Papez). His circuit-level theory of emotion included multiple brain regions in addition to the cingulate gyrus, including the hypothalamus, hippocampus, anterior thalamus, and their interconnections. The most substantial clinical studies summarized by Papez involving cingulate lesions described impairments of loss of memory, drowsiness/stupor, and change in personality or character. Papez also highlighted a study by Annitage and Meagher (1933) that “… listed as first among the mental symptoms loss of spontaneity in emotion, thought and activity.” (p. 736). Another study of a patient with cingulate lesion described initial symptoms of “fright, followed by a hysterical fit” (p. 737); when the patient improved “the patient was emotional, irritable and depressed”. Papez conceded that evidence regarding functions of the cingulate was scant and that most of the evidence was based on damage to the corpus callosum that involved substantial portions of the surrounding cortex. Nevertheless, he goes on to suggest the following:
Cogent argument can be drawn from such evidence in support of the view that the gyrus cinguli is the seat of the dynamic vigilance by which environmental experiences are endowed with an emotional consciousness. (p. 737)
The next major step from Broca’s great limbic lobe to the limbic system passes through the work of Paul MacLean. In particular, in attempting to extend Papez’s theory of emotion, MacLean (1949) proposed the idea of the visceral brain, which he linked to the “limbic lobe of Broca” (MacLean) in the first figure of the paper. Figure 3 reproduces MacLean’s figure and summarizes some of the main components of the visceral brain, which he considered to be the “old brain”. Notably, MacLean emphasized the existence of “strong connections between the old brain (rhinencephalon [literally, nose brain]) and the hypothalamus” (p. 339). This latter structure was viewed as central to emotion given the work by Bard and Cannon (among others) and its proposed role as “the head ganglion of the autonomic nervous system”4. MacLean proposed that the phylogenetically old brain was largely concerned with visceral and emotional functions, which was to be contrasted to the “new” parts of the brain (neocortex/neopallium):
… though our intellectual functions are carried on the newest and mostly highly developed part of the brain, our affective behavior continues to be dominated by a relatively crude and primitive system. This situation provides a clue to understanding the difference between what we ‘feel’ and what we ‘know’ (p. 351).
Figure 3.
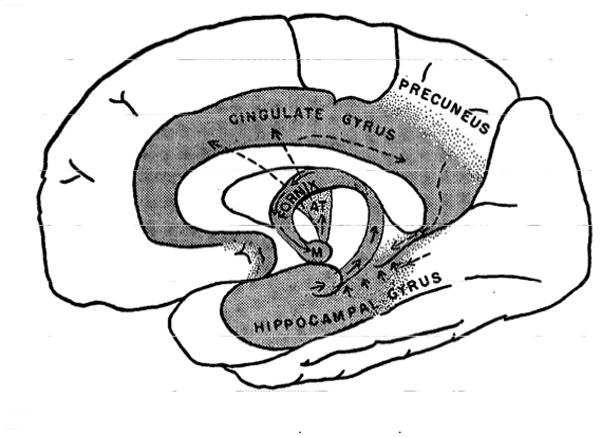
MacLean’s visceral brain. The shaded area represents, according to MacLean, “what was formerly known as the limbic lobe of Broca” (caption of MacLean’s Figure 1; p. 340). Reproduced from MacLean (1949; Figure 1) with permission.
A few years later, MacLean explicitly called the visceral brain the limbic system: “The limbic system is comprised of the cortex contained in the great limbic lobe of Broca … together with its subcortical cell stations” (MacLean, 1952; p. 407).
MacLean’s limbic system establishes an emotional brain that is largely segregated from the brain that supports reason, echoing a dichotomy with a long history in Western thinking, and which still propels much work in brain and mind research today. A competing view, of course, is that emotion and cognition are much more highly integrated (Pessoa, 2013).
The limbic system concept of MacLean, which builds heavily on the ideas by Papez, continued to evolve in the subsequent decades. But we see that, though connected to the original ideas by Broca, the limbic system idea was an attempt to understand the underpinnings of the emotional brain. Broca, on the other hand, sought to understand the general principles of macroscopic organization of the cerebral hemispheres. But the connection between the two should also be evident. Broca described two brains; the great limbic lobe, and the other comprising the convolutions that make up the rest of the cortex. He suggested that the great limbic lobe ranked low in the hierarchy of cortical regions and that it was connected to the bestial sense of smell. In contrast, the “other brain” was important for intelligence.
The limbic system concept propelled the quest for unravelling the underpinnings of the emotional brain to the forefront of brain research. But, as much as the term is used in neuroscience, it is plagued with problems (Brodal, 1981; Kotter and Meyer, 1992; LeDoux, 1996; Pessoa, 2008). Foremost, a stable concept for a limbic system has failed to materialize, and the term is frequently used in a circular fashion – what is deemed to be the emotional brain at a particular point in time is referred to as the limbic brain, and vice versa. Clearly, this type of usage does not provide a productive framework to advance our understanding of the organization of emotion in the brain, and in particular its relationship to other mental functions, most notably, cognition.
Many commentators have provided persuasive arguments against the limbic system concept (in the context of the emotional brain). Here, we briefly touch upon an aspect that is less commonly discussed but which is critically important. Many versions of the limbic system subscribe to antiquated notions of an “old” emotional system that is largely autonomous. The brain has, of course, what can be considered old systems. However, these do not exist within a “layered” architecture, with newer structures simply added on top of old ones, such that the ones on top control the ones at the bottom (cf. the triune brain of MacLean [1990]). Evolutionary changes to brain circuits are such that “new” systems are embedded within “old” ones. This interweaving creates a web of structural and functional coupling in a way that blurs “old” and “new”.
To illustrate this idea, consider the hypothalamus, a centerpiece of traditional emotion models, which has been conceptualized in terms of “descending” systems, as when described as the “head ganglion” of the autonomic nervous system. However important the hypothalamus may be for descending control, a significant recent insight is that the mammalian cerebral cortex and the hypothalamus share massive bidirectional connections (Risold et al., 1997; Swanson, 2000; Figure 4). And whereas hypothalamic contributions to descending control of bodily functions are well documented, its contributions to ascending processing are poorly understood. Notably, the hypothalamus has widespread projections to all sectors of prefrontal cortex not only in rats but also macaque monkeys (Rempel-Clower and Barbas, 1998). Given the role of the hypothalamus as an important component of the autonomic nervous system, this pattern of connectivity implies that the hypothalamus has the ability to influence processing throughout prefrontal cortex. Notably, this includes lateral prefrontal cortex, which is important for cognitive function.
Figure 4.
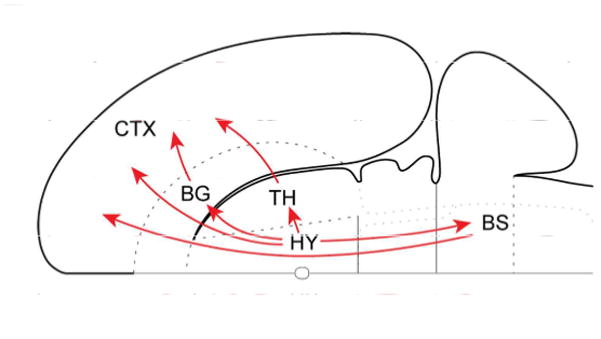
Hypothalamic ascending connectivity. Summary of the four major pathways from the hypothalamus to cerebral cortex schematized on a flattened representation of the rat brain. The basal ganglia here refer to the basal forebrain and the amygdala complex. Note that one of the indirect connections first descends to the brainstem. BG, basal ganglia; BS, brainstem; CTX, cortex; HY, hypothalamus; TH, thalamus. Reproduced with permission from Risold et al., 1997.
The amygdala, a staple of modern-day models of emotional processing, is another structure whose connectivity blurs the distinction between “old” and “new”. As many as 1,000 separate pathways connect the amygdala to cortical and subcortical regions (Petrovich et al., 2001). The connectivity is all the more notable given that it involves all cortical lobes (although with varying degrees of strength). In all, the amygdala is an extensively interconnected “connector hub” (Guimera and Nunes Amaral, 2005). Importantly, its influence is not restricted to regions to which it is strongly directly connected (Pessoa, 2014), as illustrated by the robust “functional connections” between the amygdala and lateral prefrontal cortex in both macaque monkeys and humans (Birn et al., 2014).
The example of the hypothalamus and the amygdala illustrate (see also Pessoa [2013], chapter 9) that the old/new conceptualization that is central to many versions of the limbic system is rather impoverished. To fully understand the “emotional” brain, it is necessary to understand the “cognitive” brain, and vice versa. This approach is also required to understand the full ramifications of psychopathologies such as the anxiety disorders and depression, whose impact on mental function encompasses both emotional and cognitive dimensions (see, for example, Crocker et al., 2013). In the end, viewing brain evolution in terms of new systems being embedded within old ones such that their interweaving blurs old and new is not simply metaphorical. Indeed, multivariate statistical and computational techniques are starting to elucidate the interdependence of multiple brain systems, based on both anatomical (Modha and Singh, 2010; Bota et al., 2015) and functional brain data (Kinnison et al., 2002; McMenamin et al., 2014).
Conclusion
Broca’s 1878 paper is a tour de force that has had an immense impact on neuroscience. The absence of an accessible English translation of the paper has been a regrettable omission that prevented non-French speakers from a deeper appreciation of Broca’s insights. The translation published in this issue of the journal finally corrects this omission.
Acknowledgments
Grant sponsor: National Institute of Mental Health (R01 MH071589 to L.P.).
We thank Mihai Sirbu and Nicole Friedman for assistance with references and figures.
Footnotes
We place Broca’s name adjacent to short quotes from the 1878 paper to make the source explicit (we do the same for Papez and MacLean below). For longer quotes, we provide the pages from his 1878 paper.
As phrased by Broca (without Linnaean classification): Antelope, baboon (yellow baboon), badger, bat, babirusa, boar, camel, cat, cattle, beaver, deer (roe), deer (Java mouse-deer), dog, dog (shepherd and ratter), dolphin, elephant, ferret, fox, goat, gibbon, gorilla, hedgehog, horse, human (including fetuses between three-five months and older than five months), mandrill, marmoset, marmot, marten, monkey (capuchin, saki, squirrel, and woolly monkeys), otter, pig, porpoise, rabbit, sea lion, seal, sheep, sloth, steer, tapir, weasel, and wolf.
Despite Broca’s great contributions to science, he was not immune to prejudices of the day. He notes that “…vestige of the limbic fissure (limbic sulcus) tends to disappear in humans. … In most Whites, it disappears altogether; … I have found that this sulcus in all the Negro brains that I have studied so far…” (p. X). Regrettably, he goes so far as to state that “The presence of the limbic sulcus at the tip of the temporal lobe should be considered a sign of inferiority in humans” (p. X).
This is a direct quote from MacLean, who cites a physiology textbook of the time. This expression, which is still frequently quoted in many neuroscience textbooks, and used in one form or another in research papers and reviews, appears to be originally from Sherrington.
CONFLICT OF INTERESTS STATEMENT
The authors have no conflict of interest to disclose.
ROLE OF AUTHORS
LP and PRH wrote the paper.
Contributor Information
Luiz Pessoa, Department of Psychology, University of Maryland, College Park, Maryland 20742.
Patrick R. Hof, Fishberg Department of Neuroscience and Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, New York 10029
Literature Cited
- Annitage G, Meagher R. Gliomas of the Corpus callosum. Z Neurol. 1933;146:454–488. [Google Scholar]
- Birn RM, Shackman AJ, Oler J, Williams LE, McFarlin DR, Rogers GM, Shelton SE, Alexander AL, Pine DS, Slaterry MJ, Davidson RJ, Fox AS, Kalin NH. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014;198:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M, Sporns O, Swanson LW. Architecture of the cerebral cortical association connectome underlying cognition. P Natl Acad Sci USA. 2015;112:E2093–E2101. doi: 10.1073/pnas.1504394112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, ramollissement chronique et destruction partielle du lobe antérieur gauche du cerveau. Bull Soc Anthropol. 1861;2:235–238. [Google Scholar]
- Broca P. Anatomie comparée des circonvolutions cérébrales: le grande lobe limbique et la scissure limbique dans la série des mammifères. Rev D’Anthropol. 1878;1:385–498. [Google Scholar]
- Brodal A. Neurological anatomy in relation to clinical medicine. Oxford University Press; New York: 1981. [Google Scholar]
- Crocker LD, Heller W, Warren SL, O’Hare AJ, Infantolino ZP, Miller GA. Relationships among cognition, emotion, and motivation: implications for intervention and neuroplasticity in psychopathology. Frontiers in human neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger S. Origins of neuroscience: a history of explorations into brain function. Oxford University Press; New York: 1994. [Google Scholar]
- Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnison J, Padmala S, Choi JM, Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. J Neurosci. 2012;32:8361–8372. doi: 10.1523/JNEUROSCI.0821-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter R, Meyer N. The limbic system: a review of its empirical foundation. Behav Brain Res. 1992;522:105–127. doi: 10.1016/s0166-4328(05)80221-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain. New York: Simon & Schuster; 1996. [Google Scholar]
- McMenamin BW, Langeslag SJ, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. Psychosomatic disease and the ‘visceral brain’: recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (visceral brain) Electroenceph Clin Neurophysiol. 1952;44:407–418. doi: 10.1016/0013-4694(52)90073-4. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: Role in paleocerebral functions. New York: Plenum Press; 1990. [DOI] [PubMed] [Google Scholar]
- Modha DS, Singh R. Network architecture of the long-distance pathways in the macaque brain. P Natl Acad Sci USA. 2010;107:13485–13490. doi: 10.1073/pnas.1008054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch Neurology Psych. 1937;38:725–743. [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;92:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L. The cognitive-emotional brain: from interactions to integration. Cambridge: MIT Press; 2013. [Google Scholar]
- Pessoa L. Precis of the cognitive-emotional brain. Behav Brain Sci. 2014:1–66. doi: 10.1017/S0140525X14000120. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Schiller F. Paul Broca: Founder of French antropology, explorer of the brain. Berkeley: University of California Press; 1979. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]


