Abstract
Objective
To examine long-term outcome in children with trichotillomania.
Method
We conducted follow-up clinical assessments an average of 2.8 ± 0.8 years after baseline evaluation in 30 out of 39 children who previously participated in a randomized, double-blind, placebo-controlled trial of N-acetylcysteine (NAC) for pediatric trichotillomania. Our primary outcome was change in hairpulling severity on the Massachusetts General Hospital-Hairpulling Scale (MGH-HPS) between the end of the acute phase and follow-up evaluation. We also obtained secondary measures examining styles of hairpulling, comorbid anxiety and depressive symptoms, as well as continued treatment utilization. We examined both correlates and predictors of outcome (change in MGH-HPS score) using linear regression.
Results
None of the participants continued to take NAC at the time of follow-up assessment. No significant changes in hairpulling severity were reported over the follow-up period. Subjects reported significantly increased anxiety and depressive symptoms but improvement in automatic pulling symptoms. Increased hairpulling symptoms during the follow-up period were associated with increased depression and anxiety symptoms and increased focused pulling. Older age and greater focused pulling at baseline assessment were associated with poor long-term prognosis.
Conclusions
Our findings suggest that few children with trichotillomania experience a significant improvement in trichotillomania symptoms if behavioral treatments are inaccessible or have failed to produce adequate symptom relief. Our findings also confirm results of previous cross-sectional studies that suggest an increased risk of depression and anxiety symptoms with age in pediatric trichotillomania. Increased focused pulling and older age among children with trichotillomania symptoms may be associated with poorer long-term prognosis.
Keywords: Trichotillomania, Longitudinal Studies, N-acetylcysteine, Depression, Anxiety
Trichotillomania is a psychiatric disorder characterized by recurrent hairpulling that causes noticeable hair loss and significant distress or impairment [1]. Trichotillomania has an estimated lifetime prevalence of 1% to 2% [1]. Individuals afflicted with trichotillomania suffer hair loss and irritation from repetitively pulling hair from parts of their body such as the scalp, eyelashes, eyebrows, pubic, etc.[2]. Psychological and physical effects of trichotillomania severely interfere with individuals’ social, occupational, and/or academic lives, as well as self-esteem [2]. Several studies examining short-term treatment outcome in adults and children with trichotillomania have suggested that specific behavioral treatments such as habit reversal therapy are effective across the lifespan, [3; 4] however, the evidence-based efficacy of pharmacological treatments for trichotillomania remains mixed or insufficient for any available agents [3; 5–8].
Longitudinal studies in both adults and children are critical for understanding the clinical course of trichotillomania. The current paucity of studies makes it difficult to provide useful prognostic and treatment information to patients. There are several longitudinal studies of adults with trichotillomania. Some studies have followed-up on short-term treatment trials and generally observed high rates of relapse in subjects who initially improved with either pharmacological or behavioral therapy [9; 10]. Other studies have demonstrated little long-term improvement in subjects who did not improve with initial treatment [11]. Furthermore, longitudinal studies have suggested that adults with trichotillomania who have comorbid depressive disorders, experience poorer symptom outcomes, resulting in lower self-esteem [9; 10].
To date, there are no longitudinal studies in children with trichotillomania. Several cross-sectional trichotillomania studies have examined the association between age and hairpulling characteristics. Cross-sectional data from the Trichotillomania Impact Project (TIP), an internet-based survey that examined characteristics of hairpulling across the lifespan in individuals with trichotillomania, suggest that young children with hairpulling report less awareness of pulling, fewer sites of pulling, more automatic pulling, less distress/impairment associated with hairpulling, and less comorbid anxiety and depressive symptoms compared to older children and adults with hairpulling [12; 13]. Similarly, cross-sectional analyses of children that were screened for an N-acetylcysteine trial for pediatric trichotillomania demonstrated a significant positive association of increased focused pulling and increased awareness of urges with age [14]. Treatment studies in pediatric samples have consistently reported lower rates of comorbid depression and anxiety disorders compared to adult trichotillomania cohorts. Given that there are no longitudinal outcome studies in pediatric trichotillomania, it is unknown whether age-related changes in hairpulling occur within the same individual or are attributable to different clinical characteristics in children with later-onset pulling.
The purpose of this study was to report on the long-term outcome of pediatric trichotillomania. Specifically, we sought to examine childhood prognosis in terms of continued hairpulling and comorbid anxiety and depression 1.5–4 years following the initial assessment. We also examined how the characteristics of hairpulling changed with development. Additionally, we examined baseline predictors associated with improvement in hairpulling at follow-up. In accordance with data from cross-sectional studies, we hypothesized that focused pulling, but not automatic pulling, would increase with age; comorbid anxiety and depressive symptoms would worsen with age; and older age at baseline assessment, greater focused pulling, and greater anxiety and depression symptoms at initial assessment would be associated with increased hairpulling at follow-up.
METHOD
Subjects
Eligible subjects consisted of 39 children with trichotillomania who participated in the double-blind, placebo-controlled add-on trial of NAC for pediatric trichotillomania [6]. Original inclusion criteria for the NAC trial was as follows: children and adolescents were required to 1) be 8 to 17 years of age; 2) have a primary diagnosis of trichotillomania; 3) have been pulling out their hair for at least 6 months and 4) have been on a stable medication and/or psychotherapy regimen during the course of the trial. Subjects were excluded from the original NAC trial if they 1) had bipolar disorder, psychosis, substance use disorder, developmental disorder, or mental retardation according to DSM-IV criteria as diagnosed by the primary investigator [15]; 2) had asthma that required use of an inhaler in the previous 6 months or 3) if they tested positive for either a pregnancy test or a urine drug screen. This trial failed to demonstrate any difference (Effect size=0) between NAC and placebo during the acute phase. Subjects who received placebo during the double-blind phase of this trial were given 12-weeks of NAC at study endpoint and given free clinical follow-up as necessary to manage the titration of NAC. All subjects who were originally given placebo reported taking NAC in the follow-up phase. Participants were re-contacted 1.5 years after completion of the trial through email and telephone calls. Children and parents provided an informed consent addendum under an IRB approved protocol before enrolling in the follow-up study. Families received a $50 compensation for participation in the study. If children refused to participate in the follow-up evaluation, information on current hairpulling status and treatment utilization was additionally collected from cohabitating family member. This information is included qualitatively within the manuscript but not used in the analyses.
Baseline Assessment
Ratings at the conclusion of the NAC trial (week 12) were utilized as the baseline data for the follow-up study. Clinical ratings included assessment of trichotillomania severity Massachusetts General Hospital–Hairpulling Scale (MGH-HPS) [16]; Trichotillomania Scale for Children–Child and Parent versions (TSC-C/P) [17]; National Institute of Mental Health–Trichotillomania Severity Scale (NIMH-TSS) [18]; styles of hairpulling [(Milwaukee Inventory for Styles of Trichotillomania–Child (MIST-C)] [19]; comorbid anxiety [(Multidimensional Anxiety Scale for Children (MASC)] [20]; and depressive symptoms [(Children’s Depression Inventory (CDI)] [21]. All rating scales utilized in this study were conducted by a psychiatrist with extensive experience with trichotillomania (MHB or ALW). The MGH-HPS is a seven-item, self-report scale that rates urges to pull hair, actual amount of pulling, perceived control over behavior, and distress associated with hair pulling over the past 7 days. Analysis of the MGH-HPS has demonstrated two separate factors with acceptable reliability for both: “severity” and “resistance and control.” The MGH-HPS has little research examining its psychometric properties in general and none to our knowledge in children; however it was chosen as the primary outcome for this study, as the scale has traditionally been used as a primary outcome measure in trichotillomania trials and is sensitive to change.. Additional variables that were examined as part of the predictor analysis, which were collected before the start of the NAC trial, included psychiatric history, past medication use, and past behavioral therapy.
Follow-up assessments were conducted using YaleSurvey Qualtrics and via telephone interviews. Childhood assessments included MGH-HPS; TSC-C; NIMH-TSS; MIST-C; MASC; CDI and clinical global improvement question regarding change in hairpulling since completion of the NAC trial. Parent ratings included the TSC-P as well as a survey regarding current medication use (including NAC) and treatment received in the interim. The survey asked which commonly utilized treatments for trichotillomania children were currently receiving and which treatments children had received in the past.
Data Analysis
All data analyses were conducted using SPSS version 21.0. The goals of the current analyses were to (1) report the proportion of children who were still pulling; (2) examine how the severity of hairpulling, comorbid anxiety and depression symptoms and styles of pulling changed from the conclusion of the NAC trial to follow-up; (3) examine correlates of increased hairpulling between the NAC trial and follow-up assessment and (4) examine baseline characteristics associated with more severe hairpulling at follow-up. Specifically, we hypothesized that focused, as opposed to automatic, pulling would be worse at follow-up; comorbid anxiety and depression symptoms would be worse at follow-up, and that more severe hairpulling at follow-up would be associated with an older age, increased focused pulling, and worse anxiety and depression symptoms at baseline assessment. Baseline differences between participants and non-participants in the follow-up study were assessed with either student t-test (continuous) or Fisher’s exact test (dichotomous).
Change in hairpulling severity (MGH-HPS and TSC-C/P), severity of automatic and focused pulling (MIST-C), as well as severity of anxiety (MASC) and depressive symptoms (CDI), were evaluated through paired t-tests between rating scale scores at the end of the acute phase of the NAC trial and follow-up. Dichotomous variables such as medication use (serotonin reuptake inhibitors, antipsychotic medications, alpha-2 agonists, NAC, and psychostimulant medications) were analyzed using the McNemar chi-square test and ordinal variables (number of sites of hairpulling) were evaluated using the Wilcoxon Signed Rank Test.
Correlates of increased hairpulling as assessed by change in MGH-HPS scale between the acute phase and follow-up were computed using linear regression. Variables examined included change in TSC-C/ P, CDI, MASC, MIST-C automatic and focused subscales from baseline (NAC trial endpoint) to follow-up. We also examined correlates of increased automatic and focused hairpulling on the MIST scale using similar methodology. All significant correlations between changes in hairpulling severity (MGH-HPS) were additionally adjusted for MGH-HPS score at the acute phase as this variable was significantly positively associated with change in MGH-HPS score.
Baseline predictors of change in MGH-HPS scale between the acute phase and follow-up were evaluated using linear regression. Variables examined included baseline age, duration to follow-up, comorbid conditions at baseline, medication use at baseline, and symptom severity at baseline (MGH-HPS, TSC-C/P, NIMH-HPS, MIST-C automatic and focused subscales, CDI and MASC scores). All significant findings were additionally adjusted for MGH-HPS score at the acute phase, as this variable was significantly positively associated with change in MGH-HPS score. We also conducted a backwards stepwise linear regression analysis in which all significant univariate predictors of change in MGH-HPS were entered into a single model and individually removed by significance level until all remaining terms were significant at p<0.05 level.
We used p<0.05 as our threshold for statistical significance for all statistical tests. We conducted 6 paired t-tests and 5 chi-squared tests examining changes in symptoms between endpoint and follow-up, 5 correlation tests examining correlates of increased hairpulling, and 9 linear regression analyses examining predictors of increased hairpulling at follow-up. Given the large number of statistical analyses performed, these analyses should be considered exploratory in nature for hypothesis-generating, not hypothesis-confirming, purposes and require independent replication.
RESULTS
Subjects
Of an eligible sample of 39 subjects, 30 (77%) elected to participate. Of the 9 subjects who did not participate in follow-up assessment, we were unable to locate 4 subjects and 5 were unable to participate due to time constraints. Parents of 3 of the 9 non-participants participated in the parent-interview portion of the study. All 3 of these children continued to engage in hairpulling. Table 1 compares baseline characteristics between children who did and did not participate in the follow-up interview. Clinical characteristics did not differ significantly between participants and non-participants in the follow-up study.
Table I.
Comparison between Participants and Nonparticipants in Follow-Up Assessment.
| Participants | Nonparticipants | |
|---|---|---|
| N | 30* | 9 |
| Age, mean ± SD | 13.7 ± 2.8 | 14.7 ± 2.6 |
| Female, n (%) | 26 (87%) | 8 (89%) |
| MGH-HPS | 11.4 ± 5.6 | 14.3 ± 8.5 |
| NIMH-TSS | 10.4 ± 3.5 | 12.1 ± 7.9 |
| TSC-C | 1.9 ± 0.7 | 2.3 ± 1.2 |
| TSC-P | 1.8 ± 0.7 | 2 ± 1.2 |
| MIST-C-Automatic Subscale | 13.9 ± 7.4 | 9.9 ± 6.1 |
| MIST-C-Focused Subscale | 88.7 ± 31.2 | 73.8 ± 42.7 |
| MASC | 48.1 ± 16.6 | 45 ± 17.2 |
| CDI | 8.1 ± 7.5 | 12.2 ± 10.3 |
| # Pulling sites | 1.7 ± 0.9 | 1.3 ± 0.7 |
| Treatment, current (n (%))/history (n (%)) | ||
| Antidepressants | 15 (50)/20 (67) | 4 (44)/8 (89) |
| Antipsychotic agents | 2 (7)/6 (20) | 1 (11)/2 (22) |
| Behavioral Therapy | 9 (30)/18 (60) | 1 (11)/5 (56) |
| NAC | 18 (60) | 2 (22) |
| Comorbid Disorders, current (n (%))/history (n (%)) | ||
| Depressive Disorder | 6 (20)/9 (30) | 2 (22)/4 (44) |
| Anxiety Disorder | 6 (20)/7 (23) | 2 (22)/2 (22) |
| ADHD | 3 (10)/3 (10) | 1 (11)/1 (11) |
| Skin Picking | 1 (3)/1 (3) | 0 |
| TS | 1 (3)/1 (3) | 1 (11)/2 (22) |
| OCD | 1 (3)/1 (3) | 1 (11)/1 (11) |
Abbreviations: NIMH-TSS, National Institute of Mental Health – Trichotillomania Severity Scale; MGH-HPS, Massachusetts General Hospital – Hairpulling Scale; TSC-C/P, Trichotillomania Scale for Children – Child and Parent Versions; MIST-C, Milwaukee Inventory for Styles of Trichotillomania-Child Version; CDI, Children’s Depression Inventory; MASC, Multidimensional Anxiety Scale for Children; NAC, N- Acetylcysteine; ADHD, Attention Deficit Hyperactivity Disorder; TS, Tourette’s Syndrome; OCD, Obsessive-Compulsive Disorder.
Childhood baseline evaluations took place at an average age of 13.7 ± 2.8 years. The average age for early adulthood follow-up evaluations was 16.4 ± 3.1 years. The average period between the initial and follow-up evaluations was 2.8 ± 0.8 years. Medication use and treatment utilization in the sample is depicted in Table 2. No subjects continued on N-acetylcysteine throughout the follow-up period. There were no other significant differences in treatment utilization during the follow-up period..
Table II.
Comparison between Baseline and Follow-up Measures in Follow-Up Participants.
| Baseline | Follow-up | Significance | |
|---|---|---|---|
| Age, mean ± SD | 13.7 ± 2.8 | 16.4 ± 3.1 | |
| NIMH-TSS | 10.4 ± 3.5 | 8.7 ± 4.4 | 0.46 |
| MGH-HPS | 11.4 ± 5.6 | 11.8 ± 5.7 | 0.77 |
| TSC-C | 1.9 ± 0.7 | 2.0 ± 0.8 | 0.52 |
| TSC-P | 1.8 ± 0.7 | 1.7 ± 0.8 | 0.89 |
| MIST-Automatic Subscale | 13.5 ± 7.3 | 11.4 ± 5.9 | 0.045 |
| MIST-Focused Subscale | 90.9 ± 29.4 | 91.3 ± 36.8 | 0.94 |
| CDI | 8.1 ± 7.5 | 15.2 ± 12.0 | 0.0001 |
| MASC | 48.1 ± 16.6 | 54.1 ± 18.4 | 0.009 |
| # Pulling Sites | 1.7 ± 0.9 | 1.5 ± 1.0 | 0.22 |
| Treatment, current (n (%)) | |||
| Antidepressants | 15 (50%) | 9 (30%) | 0.19 |
| Antipsychotic agents | 2 (7%) | 2 (7%) | 1 |
| NAC | 18 (60%)* | 0 | <0.0001 |
| Behavioral Therapy | 9 (30%) | 5 (18%) | 0.36 |
The primary analyses are from the end of the acute phase in the NAC trial.
Abbreviations: NIMH-TSS, National Institute of Mental Health – Trichotillomania Severity Scale; MGH-HPS, Massachusetts General Hospital – Hairpulling Scale; TSC-C,P, Trichotillomania Scale for Children – Child and Parent Versions; MIST, Milwaukee Inventory for Styles of Trichotillomania; CDI, Children’s Depression Inventory; MASC, Multidimensional Anxiety Scale for Children; NAC, N- Acetylcysteine.
All children who did not receive NAC during the double-blind phase of the trial, received NAC treatment during the follow-up period but decided to discontinue it.
Hairpulling Severity
Children with trichotillomania demonstrated no significant changes in their overall hairpulling severity over the follow-up period. Hairpulling severity on average did not differ significantly between the end of the acute phase and follow-up on the MGH-HPS (t= −0.29, p=0.77), NIMH-TSS (t=0.76, p=0.46), TSC-C (t= −0.65, p=0.52) and TSC-P (t=0.13, p=0.89). Table 2 depicts the average change in hairpulling severity during the follow-up period on the rating scales used for hairpulling during this study.
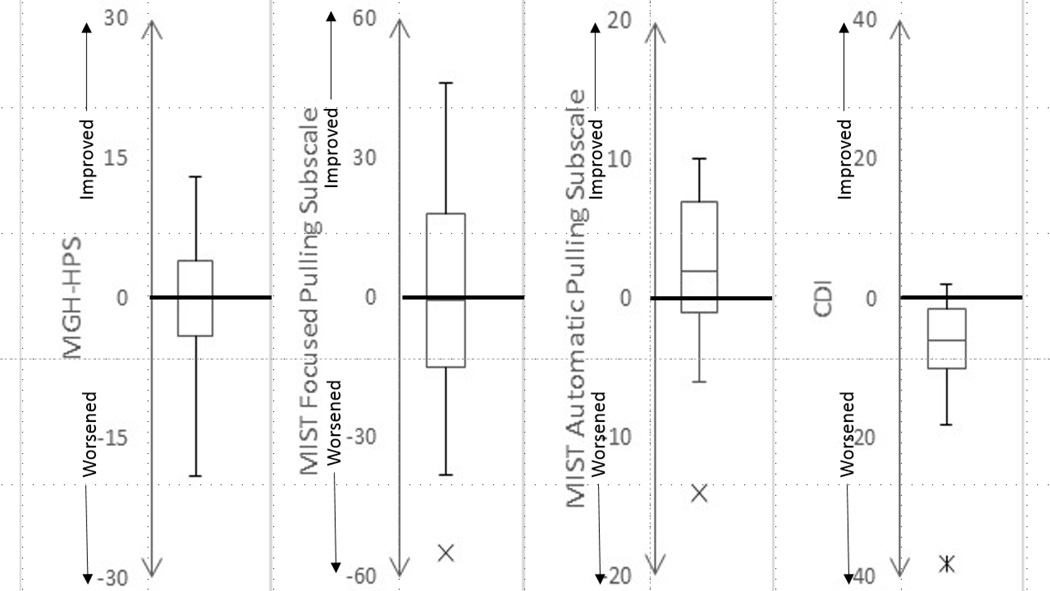
Figure 1 depicts boxplots demonstrating the distribution of change scores in hairpulling severity on the MGH-HPS experienced across the sample. On the MGH-HPS, 6% (n=2) of the sample had a significant improvement in their symptoms (MGH-HPS improvement ≥50% and at least a 5 point decrease), whereas 12% (n=4) of subjects had worse hairpulling at follow-up ((MGH-HPS worsening ≥50% and at least a 5 point increase). On the CGI, 20% (n=6) of subjects were noted to be very much or much improved during the follow-up period (CGI<3) whereas 40% (n=12) of subjects reported no change in their hairpulling. Twenty-two percent (n=7) of subjects reported their hairpulling being worse (CGI>4) during the follow-up period and 17% (n=5) of subjects reported significantly worse hairpulling (CGI>5) during the follow-up period.
Figure 1. Boxplots of Change in Symptom Severity during the Follow-Up Period.
Comorbid depression and anxiety symptoms significantly worsened during the follow-up period whereas overall hairpulling severity and severity of focused pulling did not significantly change. Automatic pulling severity significantly improved during the follow-up period. Abbreviations: MGH-HPS, Massachusetts General Hospital – Hairpulling Scale; MIST, Milwaukee Inventory for Styles of Trichotillomania; CDI, Children’s Depression Inventory; MASC, Multidimensional Anxiety Scale for Children.
Comorbid Anxiety and Depression Symptoms
Both depressive symptoms (CDI: t= −4.52, p=0.0001) and anxiety symptoms (t= −2.82, p=0.009) were significantly worse at follow-up compared to baseline evaluation.
Automatic versus Focused Hairpulling
Children at the follow-up evaluation had significantly decreased automatic (t=2.1, p<0.05), but not focused pulling (t=−0.8, p=0.94) on the MIST-C during the follow-up period (Table 2). Figure 1B and 1C depict boxplots representing the change in automatic and focused pulling over the follow-up period respectively. Children did not significantly differ in the total number of spots pulled from between baseline and follow-up (Wilcoxon Signed Rank Test = 1.22, p = 0.22). However, subjects were significantly less likely to pull from their eyelashes at follow-up (McNemar X2 = 4.0, df = 1, p = 0.04).
Clinical Correlates of Increased Hairpulling during Follow-up Period
Increased hairpulling as judged by MGH-HPS ratings during the follow-up period was significantly correlated with increased depressive symptoms (r=0.7, p<0.001), increased anxiety symptoms (r=0.46, p=0.014) and increased focused pulling (MIST-C focused pulling subscale: r=0.52, p=0.004), during the follow-up period.
Baseline Predictors of Change in Hairpulling Severity during Follow-up
More severe hairpulling at the end of the acute phase was significantly positively associated with greater improvement in MGH-HPS score during the follow-up period (β = 0.63 ± 0.18, t = 3.45, p = 0.002). Baseline age was significantly negatively associated with improvement in hairpulling during the follow-up period when hairpulling severity at the end of the acute phase was controlled for in the analysis (β = −1.2 ± 0.33, t = −3.62, p = 0.001). Older subjects experienced less improvement in hairpulling during the follow-up period. Duration of the follow-up interval was not significantly associated with improvement in hairpulling symptoms during the follow-up period (β = 1.5 ± 1.5, t = −1.0, p = 0.33).
Higher levels of focused pulling at the end of the acute phase was associated with less improvement in hairpulling severity during the follow-up period (β = −0.11 ± 0.03, t=−3.65, p = 0.001). Severity of comorbid anxiety and depression and medication use (SSRI, NAC, antipsychotics) were not associated with improvement in hairpulling symptoms during the follow-up period.
The best-fitting backward stepwise linear regression model explained 59% of the variance in improvement in hairpulling during the follow-up period and included 3 variables: (1) hairpulling severity at baseline (β = 1.03 ± 0.16, t = 6.84, p < 0.001); (2) baseline age (β = −0.84 ± 0.33, t = −2.53, p = 0.02); and (3) amount of focused pulling (β = −0.08 ± 0.03, t= −2.57, p = 0.016).
DISCUSSION
We present results from the largest longitudinal outcome study of pediatric trichotillomania conducted to date. This cohort study confirms several hypotheses suggested from previous cross-sectional studies in the area: (1) few children with trichotillomania experience a significant improvement in their symptoms if they do not respond to initial treatment; (2) focused pulling appears to worsen with age compared to automatic pulling; (3) children with trichotillomania experience increasing anxiety and depressive symptoms as trichotillomania persists; (4) worsening of comorbid anxiety and depressive symptoms is significantly correlated with changes in hairpulling severity. Our longitudinal cohort study suggested that older age in pediatric trichotillomania and more focused pulling is associated with decreased improvement of hairpulling symptoms with time.
Our longitudinal cohort study confirms several hypotheses set forth in earlier cross-sectional studies of pediatric trichotillomania. The Trichotillomania Impact Project demonstrated (1) increased hairpulling severity over time; (2) worsened social function and increased anxiety and depressive symptoms; and (3) greater awareness and reporting of urges in older children (10–17 years) as compared to younger children (0–10 years) with trichotillomania [12; 13]. A previous cross-sectional analysis, involving many individuals included in this cohort, reported increased frequency of urges/awareness of urges and increased focused pulling with age [14]. This longitudinal study demonstrated worsening anxiety and depressive symptoms within children with trichotillomania over time. Our study also demonstrated minimal improvement in hairpulling symptoms over a fairly substantial period of time after a point where subjects had sought treatment for the condition. Even if treatment was unsuccessful, due to regression to the mean, it would be reasonable to expect some improvement between baseline ratings (which were conducted after subjects enrolled in an experimental treatment for trichotillomania) and follow-up (an assessment initiated by the investigator and not related to subjects seeking treatment). The fact that we did not see substantial improvement is consistent with this condition worsening or at least persisting with time. We also demonstrated significant improvement in automatic but not focused pulling symptoms over time, which suggests that focused pulling is more likely to persist or worsen as children get older. Older age at the end of the acute phase and increased focused pulling (independent of overall hairpulling severity) were associated with poor long-term outcome. These results suggest that there may be a critical window to intervene in treating pediatric trichotillomania just prior to adolescence. During adolescence, focused pulling, as well as comorbid depressive and anxiety symptoms, increases. The worsening anxiety and depressive symptoms is correlated with changes in hairpulling severity in this longitudinal study.
This longitudinal cohort study had several limitations. With only 30 children participating in the follow-up study we had limited power to detect predictors of outcomes or correlates of hairpulling severity. Given that this study was exploratory in nature (involving multiple hypothesis testing without statistical correction), all the findings need replication in an independent and, hopefully, larger sample. Additionally, the follow-up study examined outcome at only two time points. Given that trichotillomania fluctuates in severity over time, additional assessments during the follow-up period, across shorter time intervals, may have enhanced the precision of estimates of follow-up severity. Future longitudinal studies could be improved by having more frequent assessments during the follow-up period. In addition, our sample may be slightly atypical in that many subjects in our trial (1) had received behavioral therapy for trichotillomania (but were non-responders) and (2) were referred to a tertiary care clinic for the study by local experts in trichotillomania (generally private pay clinicians) or the Trichotillomania Learning Center and thus may be (3) better educated and of higher socioeconomic status than the general population. These sample characteristics may limit the generalizability of our results. Additionally, our study requires replication as there is a strong possibility of Type I and II error as we engaged in exploratory hypothesis testing without appropriate statistical correction (e.g. a Bonferroni correction) in a small sample.
This longitudinal study appears to demonstrate that few children with trichotillomania have an improvement in symptoms with time. As children get older they tend to experience a greater proportion of focused as opposed to automatic pulling behaviors and increasing anxiety and depressive symptoms. Clinically, these results suggest that trying behavioral therapy during the pre-adolescent period, where there is less focused pulling and fewer comorbid symptoms, may be particularly important. If benefits are comparable to those achieved in previous studies [3; 4], treatment during this time period could alter psychiatric trajectory. Additionally, these results suggest that future clinical trials should account for age in their design (e.g. employing stratified randomization by age) as younger children and older adolescents have different hairpulling characteristics that may affect treatment efficacy.
Key Points.
Cross-sectional studies have suggested that trichotillomania symptoms worsen with age in children.
Prior to this study, no previous longitudinal studies have examined the course of pediatric trichotillomania.
Hair pulling symptoms do not appear to improve with time in pediatric trichotillomania.
Focused pulling appears to worsen with time as oppose to automatic pulling.
Anxiety and depression symptoms worsen with time in children with trichotillomania.
Acknowledgements
Michael Bloch gratefully acknowledges support from the National Institute of Mental Health support of the Trichotillomania Learning Center, the Yale Child Study Center Research Training Program, the National Institutes of Health 1K23MH091240, the APIRE/Eli Lilly Psychiatric Research Fellowship, the AACAP/ Eli Lilly Junior Investigator Award, NARSAD, the State of Connecticut also provided resource support via the Abraham Ribicoff Research Facilities at the Connecticut Mental Health Center. and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research. Ms Schumer, Ms, Panza, Ms. Mulqueen and Mr. Jakubovski have no acknowledgements to disclose.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Gupta MA. Emotional regulation, dissociation, and the self-induced dermatoses: clinical features and implications for treatment with mood stabilizers. Clinics in Dermatology. 2013;31(1):110–117. doi: 10.1016/j.clindermatol.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Woods DW, Flessner CA, Franklin ME, et al. The Trichotillomania Impact Project (TIP): Exploring Phenomenology, Functional Impairment, and Treatment Utilization. Journal of Clinical Psychiatry. 2006:1877–1888. doi: 10.4088/jcp.v67n1207. [DOI] [PubMed] [Google Scholar]
- 3.Bloch MH, Landeros-Weisenberger A, Dombrowski P, et al. Systematic review: pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry. 2007;62(8):839–846. doi: 10.1016/j.biopsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Franklin ME, Edson AL, Ledley DA, Cahill SP. Behavior therapy for pediatric trichotillomania: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2011;50(8):763–771. doi: 10.1016/j.jaac.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch MH. Trichotillomania across the life span. J Am Acad Child Adolesc Psychiatry. 2009;48(9):879–883. doi: 10.1097/CHI.0b013e3181ae09f3. [DOI] [PubMed] [Google Scholar]
- 6.Bloch MH, Panza KE, Grant JE, et al. N-acetylcysteine in the treatment of pediatric trichotillomania: a randomized, double-blind, placebo-controlled add-on trial. J Am Acad Child Adolesc Psychiatry. 2013;52(3):231–240. doi: 10.1016/j.jaac.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant JE, Odlaug BL, Kim SW. N-acetylcysteine, a glutamate modulator, in the treatment of trichotillomania: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2009;66(7):756–763. doi: 10.1001/archgenpsychiatry.2009.60. [DOI] [PubMed] [Google Scholar]
- 8.Rothbart R, Amos T, Siegfried N, et al. Pharmacotherapy for trichotillomania. Cochrane Database Syst Rev. 2013;11:CD007662. doi: 10.1002/14651858.CD007662.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Swedo SE, Lenane MC, Leonard HL. Long-term treatment of trichotillomania (hair pulling) N Engl J Med. 1993;329(2):141–142. doi: 10.1056/NEJM199307083290220. [DOI] [PubMed] [Google Scholar]
- 10.Keijsers GP, van Minnen A, Hoogduin CA, et al. Behavioural treatment of trichotillomania: two-year follow-up results. Behav Res Ther. 2006;44(3):359–370. doi: 10.1016/j.brat.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Keuthen NJ, Fraim C, Deckersbach T, et al. Longitudinal follow-up of naturalistic treatment outcome in patients with trichotillomania. J Clin Psychiatry. 2001;62(2):101–107. doi: 10.4088/jcp.v62n0205. [DOI] [PubMed] [Google Scholar]
- 12.Franklin ME, Flessner CA, Woods DW, et al. The child and adolescent trichotillomania impact project: descriptive psychopathology, comorbidity, functional impairment, and treatment utilization. J Dev Behav Pediatr. 2008;29(6):493–500. doi: 10.1097/DBP.0b013e31818d4328. [DOI] [PubMed] [Google Scholar]
- 13.Walther MR, Snorrason I, Flessner CA, et al. The trichotillomania impact project in young children (TIP-YC): clinical characteristics, comorbidity, functional impairment and treatment utilization. Child Psychiatry Hum Dev. 2014;45(1):24–31. doi: 10.1007/s10578-013-0373-y. [DOI] [PubMed] [Google Scholar]
- 14.Panza KE, Pittenger C, Bloch MH. Age and gender correlates of pulling in pediatric trichotillomania. J Am Acad Child Adolesc Psychiatry. 2013;52(3):241–249. doi: 10.1016/j.jaac.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 16.Keuthen NJ, O'Sullivan RL, Ricciardi JN, et al. The Massachusetts General Hospital (MGH) Hairpulling Scale: 1. development and factor analyses. Psychother Psychosom. 1995;64(3–4):141–145. doi: 10.1159/000289003. [DOI] [PubMed] [Google Scholar]
- 17.Tolin DF, Diefenbach GJ, Flessner CA, et al. The trichotillomania scale for children: development and validation. Child Psychiatry Hum Dev. 2008;39(3):331–349. doi: 10.1007/s10578-007-0092-3. [DOI] [PubMed] [Google Scholar]
- 18.Swedo SE, Leonard HL, Rapoport JL, et al. A double-blind comparison of clomipramine and desipramine in the treatment of trichotillomania (hair pulling) N Engl J Med. 1989;321(8):497–501. doi: 10.1056/NEJM198908243210803. [DOI] [PubMed] [Google Scholar]
- 19.Flessner CA, Woods DW, Franklin ME, et al. The Milwaukee Inventory for Styles of Trichotillomania-Child Version (MIST-C): initial development and psychometric properties. Behav Modif. 2007;31(6):896–918. doi: 10.1177/0145445507302521. [DOI] [PubMed] [Google Scholar]
- 20.March JS, Parker JD, Sullivan K, et al. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21(4):995–998. [PubMed] [Google Scholar]