Abstract
Biopsies and ANCA testing for limited forms of granulomatosis with polyangiitis (GPA) are frequently nondiagnostic. We characterized gene expression in GPA and other causes of orbital inflammation. We tested the hypothesis that a subset of patients with nonspecific orbital inflammation (NSOI, also known as pseudotumor) mimics a limited form of GPA. Formalin-fixed, paraffin-embedded orbital biopsies were obtained from controls (n=20) and patients with GPA (n=6), NSOI (n=25), sarcoidosis (n=7), or thyroid eye disease (TED) (n=20) and were divided into discovery and validation sets. Transcripts in the tissues were quantified using Affymetrix U133 Plus 2.0 microarrays.
Distinct gene expression profiles for controls and subjects with GPA, TED, or sarcoidosis were evident by principal coordinate analyses. Compared to healthy controls, 285 probe sets had elevated signals in subjects with GPA and 1472 were decreased (>1.5-fold difference, false discovery rate adjusted p <0.05). The immunoglobulin family of genes had the most dramatic increase in expression. Although gene expression in GPA could be readily distinguished from gene expression in TED, sarcoidosis, or controls, a comparison of gene expression in GPA versus NSOI found no statistically significant differences.
Thus, forms of orbital inflammation can be distinguished based on gene expression. NSOI/pseudotumor is heterogeneous but often may be an unrecognized, localized form of GPA
Keywords: Granulomatosis with polyangiitis, orbital pseudotumor, orbit pathology, molecular pathology, gene expression profiling, microarray analysis
INTRODUCTION
Limited forms of granulomatosis with polyangiitis (GPA) are challenging to diagnose. The antineutrophil cytoplasmic antibody test is often negative in patients with limited disease.[1] Biopsy of sites such as nasal mucosa, sinus, orbit, or subglottis often fails to produce definitive diagnostic information.[1-5] Thus, in theory many patients might have a limited form of GPA that cannot be confidently diagnosed unless the inflammation extends to additional sites.
Orbital inflammation is a challenging cause of vision loss, pain, diplopia, and even blindness. The differential diagnosis is extensive and includes GPA, thyroid eye disease (TED), sarcoidosis, lymphoma, metastatic disease, histiocytosis, xanthogranuloma, and infection.[6] Many patients with orbital inflammation have a disease that defies categorization despite biopsy. Terms to describe this latter condition include orbital pseudotumor, idiopathic orbital inflammatory disease, and nonspecific orbital inflammation (NSOI).[7-13]
Transcriptomics or gene expression profiling is rapidly changing the diagnostic approach to several malignant diseases. For example, lymphomas which cannot be distinguished on the basis of histology can be differentiated on the basis of gene expression.[14-16]
In order to elucidate the pathogenesis of GPA in relation to other forms of orbital inflammation, we assembled an international consortium of ocular pathologists and orbital surgeons to collect and analyze tissues from orbital biopsies. Our data show that GPA, sarcoidosis, TED, and healthy tissue each display a distinctive pattern of gene expression. The pattern from NSOI indicates more heterogeneity, but many tissues from NSOI cannot be distinguished from GPA on the basis of gene expression.
MATERIALS AND METHODS
Centers, Biopsies, Database
This study was approved by Institutional Review Boards or Research Ethics Committees from Oregon Health & Science University, Columbia University, University of California San Diego, Wake Forest University, Medical College of Wisconsin, Mount Carmel Health System (Ohio), University of Miami, University of British Columbia, Royal Adelaide Hospital, and the King Khaled Eye Specialist Hospital. Informed consent was obtained where required by the local review board. The research adhered to the tenets of the Declaration of Helsinki. Formalin-fixed, paraffin-embedded (FFPE) samples and relevant demographic and clinical data were obtained from the participating institutions. The diagnoses of NSOI, sarcoidosis, GPA, TED, and normal were based on the clinical and histopathological information submitted by physicians from their respective institutions.
Biopsies of the orbital adipose tissue from a total of 83 subjects were studied. The age, gender, and diagnoses for subjects are summarized in Table 1. Among the 6 subjects with a diagnosis of GPA, the antineutrophil cytoplasmic antibody (ANCA) test was positive in four of five and unknown in one. Of those with a positive test, 3 had a cytoplasmic ANCA pattern. The subject with a negative ANCA had pulmonary disease in addition to the orbital disease. One other subject with GPA had renal disease, but the others had a limited form of GPA. Among the 7 subjects diagnosed with sarcoidosis, all had noncaseating granulomata present on the orbital adipose biopsy. Adenopathy was present on chest CT scan in three for whom the test was reported. In a fourth patient, granulomas were found in a confirmatory lip biopsy. In another subject, the serum angiotensin converting enzyme level was elevated, but a chest CT scan was not obtained. In 2 subjects, a clinical diagnosis of sarcoidosis was made but the details of the diagnostic work up were not available. ANCA results were available for 12 of the subjects diagnosed with NSOI. All 12 were negative. The control tissue was obtained during surgeries, such as blepharoplasty and enucleation on patients with non-inflamed orbits.
Table 1.
Ages and genders for each experimental group
| Set 1 |
Set 2 |
|||||
|---|---|---|---|---|---|---|
| Diagnosis | N | Mean age at biopsy |
Female % |
N | Mean age at biopsy |
Female % |
| NSOI | 14 | 44.0 ± 21.8 | 64.3 | 11 | 58.5 ± 24.1 | 63.6 |
| Sarcoid | 1 | 60.6 | 100 | 7* | 48.8 ± 14.7 | 71.4 |
| TED | 14 | 54.0 ± 14.7 | 78.6 | 11 | 48.6 ± 13.0 | 72.7 |
| GPA | 4 | 43.4 ± 14.3 | 75.0 | 4** | 40.0 ± 13.9 | 50.0 |
| Normal | 14 | 61.0 ± 15.6 | 64.3 | 6 | 69.7 ± 9.8 | 83.3 |
one repeated from set 1;
two repeated from set 1
Pathology Review
Two ocular pathologists (D.J.W. and H.E.G.) independently evaluated hematoxylin and eosin stained slides of all samples without reference to the indications for biopsy or other clinical information. After rendering a diagnosis in a masked fashion, the pathologists were informed of the diagnosis from the institution where tissue had been obtained. In some cases, additional stains were requested or additional clinical information was reviewed. A few cases with an ambiguous diagnosis were excluded. In all cases included in this study, a consensus diagnosis was determined by Drs. Wilson and Rosenbaum, and the contributing center’s diagnosis was accepted. Thus, a diagnosis which combined clinical and histopathological information was considered the gold standard for the purposes of this study.
Tissue Preparation and Gene Expression Profiling
RNA extraction and microarray assays were performed in the OHSU Gene Profiling Shared Resource. The tissue samples, designated as set 1 (discovery set) and set 2 (validation set), were processed at two different time points. Between the two sample sets, the RNA amplification and labeling kit was modified by the vendor (Affymetrix P/N 703088 Rev1 vs P/N 703088 Rev 3). For each specimen, multiple 10-20 μm sections were collected, and total RNA was extracted with the miRNeasy FFPE kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. RNA was amplified and labeled with SensationPlus FFPE Amplification and 3’ IVT Labeling kits (Affymetrix, Santa Clara, CA) for microarray analysis. For the majority of samples, an input of 50 ng of RNA was used, and a minimum input of 20 ng RNA was used for samples with limited RNA recoveries. Biotin-labeled cDNA targets were hybridized with a GeneChip Human Genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA), processed, and scanned according to vendor recommendations. The Human U133 Plus 2.0 array contains over 54,000 probe sets for 47,000 human transcripts and variants. Affymetrix GeneChip Command Console (AGCC) v. 3.1.1 and Affymetrix Expression Console v. 1.1 software were used for image processing and expression analysis for initial quality control, respectively. Raw and normalized gene expression microarray data are available from the GEO database (accession numbers GSE58331 and GSM1407182 through GSM1407356).
RNA quality
RNA extracted from FFPE specimens was highly fragmented as is typical of this sample type. The RNA Quality Scores (RQS) from Caliper LabChip GX (Perkin-Elmer, Waltham, MA) HT RNA assays ranged from 1.8 to 6.5 with an average of 3.0 for Set 1 samples and from 1.7 to 7.4 with an average of 2.7 for Set 2. In both sample groups the majority of the samples had an RQS between 2 and 3 with RNA fragment sizes distributed between 25 and approximately 500 bases.
Statistical Analysis
The two sets of array data were analyzed independently because samples were processed at two different time points and the amplification and labelling kit had changed. Affymetrix CEL files were normalized by the Robust Multiarray Analysis.[17] Principal coordinate analysis (PCA) was used to visualize the dissimilarity in gene expression profiles across the disease groups.[18] Linear models were fitted to test potential differences in gene expression across disease groups after controlling for sex and age at biopsy. A probe set was considered statistically significantly changed when it had at least 1.5-fold change with a false discovery rate (FDR) adjusted p-value[19] less than 0.05 in both sets. All computations were done with the R project[20] and its add-on packages: “affy”, “limma”, and “MASS”.
The Gene Set Enrichment Analysis (GSEA) program[21, 22] was used as an independent means to identify genes with enriched expression in the clusters for microarray set 2 described above. GSEA rank order analysis was based on the 40,450 probe sets that remained after excluding probe sets that had not been associated in the Affymetrix annotation with a specific gene, i.e., annotated as unknown locus, open reading frame, etc. For genes with transcripts detected by more than one probe set, the probe set with the highest signal was used for the analysis. In addition, NIH DAVID[23] was used to estimate the number of unique genes in lists of probe sets.
RESULTS
We collected FFPE orbital adipose biopsies from 83 subjects from ten centers from North America, Saudi Arabia, and Australia (Table 1). As described in the methods section, set 1 and set 2 were processed and hybridized to arrays at two different times (about 1 year apart). Therefore, we analyzed the sets independently to minimize the influence of batch variation. The two sets are also called a discovery set and a validation set, respectively, for convenience throughout the manuscript.
To visualize NSOI heterogeneity in gene expression profiles and to compare these profiles to those of other orbital inflammatory diseases, we employed a principal coordinate analysis (PCA) that allows a comparison of complex data sets. In a PCA, the relative similarity of RNA patterns of two samples is indicated by their proximity on a graph. PCA plots based on all probe sets that showed a significant difference (FDR p<0.05 and 1.5-fold difference) between at least one disease group and the normal control group are shown in Figure 1 for both data sets. Supplemental videos show these data in 3 dimensions (see Set1_PCA and Set2_PCA). The clustering of samples representing normal tissue indicates the relative similarity of the RNA patterns in the biopsies. The samples from subjects with TED are relatively homogeneous and have the closest proximity to normal samples among the four disease groups. The gene expression profiles from subjects with either sarcoidosis or GPA also tend to cluster. The gene expression profile for patients with NSOI shows more heterogeneity than the other four categories, consistent with the hypothesis that NSOI is not a single disease entity.
Figure 1. PCA reveals clustering of expression profiles of samples within each experimental group except NSOI.
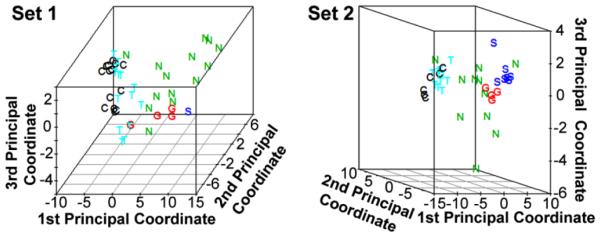
In both data sets, the NSOI samples were much more heterogeneous and not clustered. The distance between points on the plots is indicative of the difference between gene expression profiles. Rotatable views of the 3-dimensional plots are in online supplemental files (Set1_PCA and Set2_PCA). C: Control; G: GPA; N: NSOI; S: Sarcoidosis; T: TED.
The GSEA program[21, 22] was used to interrogate the gene expression patterns further. GSEA provided a means to identify expression differences in predetermined sets of genes without reliance on the FDR p-values and fold-differences used to select the probe sets employed in the creation of the PCA plots. A GSEA-generated heat map (Figure 2) shows cytokine expression profiles for orbital adipose tissues from each experimental group. Consistent with the PCA plots, a subset of the NSOI profiles are very similar to the GPA profile.
Figure 2. Orbital adipose tissues from some NSOI patients have cytokine expression profile similar to GPA patients.
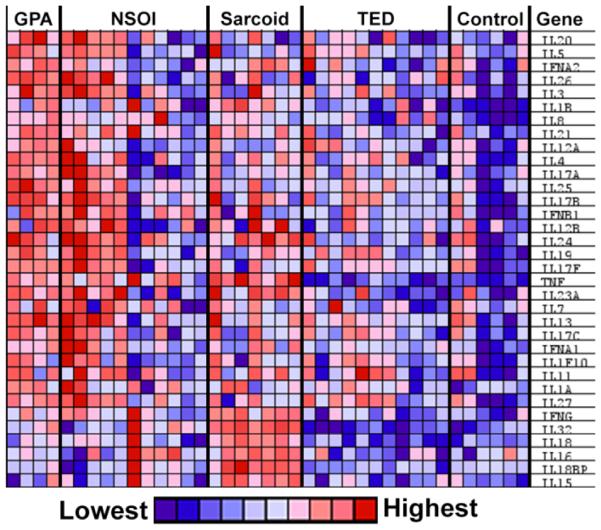
The GPA profile differs from the sarcoidosis, TED, and control profiles. A heat map of relative transcript levels was generated by GSEA from data set 2.
Disease duration might markedly affect gene expression. We divided NSOI tissues from each set into samples biopsied less than 6 months after diagnosis or greater than 18 months after diagnosis, and we were unable to identify differences based on this variable. Similarly we hypothesized that corticosteroid use would markedly affect the detection of transcripts, but we could not identify differences in gene expression based on use of corticosteroid at the time of biopsy.
Table 2 and Supplemental Table 1 (see Supplementary Data file) contain data on specific gene expression in tissue from subjects with GPA based on both the validation and discovery sets. They show 285 probe sets (for about 212 genes) with signals that were at least 1.5-fold higher, with FDR adjusted p<0.05, in tissue from the orbital fat compared to tissue from orbital adipose tissue from subjects with no known orbital disease in both sets. Most of the transcripts with the highest fold increases in expression have immunological functions including the production of antibodies and cell signaling. Table 3 and Supplemental Table 2 (see Supplementary Data file) show a similar analysis except that the supplemental table lists 1,472 probe sets (about 1,054 genes) in common to both discovery and validation sets with lower signals in GPA in the orbit compared to uninflamed controls.
Table 2.
Probe sets indicating increased expression of genes in orbital adipose tissues from subjects with GPA.
| Set 1 | Set 2 | ||||
|---|---|---|---|---|---|
| Probe Set | Gene Title | Fold Difference |
FDR P- value |
Fold Difference |
FDR P- value |
| 209875_s_at | secreted phosphoprotein 1 | 56.68 | 7.04E-04 | 54.74 | 2.51E-03 |
| 214677_x_at | immunoglobulin λ constant 1 (Mcg marker) |
9.35 | 9.63E-03 | 58.44 | 1.11E-03 |
| 216491_x_at | immunoglobulin heavy constant mu | 15.85 | 1.30E-03 | 41.14 | 3.77E-04 |
| 214669_x_at | immunoglobulin κ constant | 13.28 | 3.77E-04 | 41.10 | 7.07E-04 |
| 215379_x_at | immunoglobulin λ variable 1-44 | 8.33 | 3.30E-03 | 36.77 | 1.38E-03 |
| 209396_s_at | chitinase 3-like 1 (cartilage glycoprotein-39) |
20.75 | 2.12E-04 | 10.78 | 2.16E-02 |
| 214973_x_at | immunoglobulin heavy constant delta | 12.44 | 3.35E-04 | 15.87 | 6.54E-03 |
| 202834_at | angiotensinogen (serpin peptidase inhibitor, clade A, member 8) |
12.04 | 1.18E-03 | 8.18 | 4.06E-02 |
| 1555745_a_at | lysozyme | 9.04 | 2.47E-03 | 10.53 | 1.83E-02 |
| 1555756_a_at | C-type lectin domain family 7, member A |
10.12 | 1.38E-02 | 8.19 | 3.73E-02 |
| 211429_s_at | serpin peptidase inhibitor, clade A (α-1 antiproteinase, antitrypsin), member 1 |
11.48 | 1.75E-04 | 6.31 | 3.80E-02 |
| 205267_at | POU class 2 associating factor 1 | 10.59 | 1.47E-03 | 7.01 | 1.47E-02 |
| 219386_s_at | SLAM family member 8 | 11.25 | 8.00E-06 | 5.80 | 3.41E-02 |
| 211919_s_at | chemokine (C-X-C motif) receptor 4 | 8.67 | 1.13E-02 | 7.30 | 7.46E-03 |
| 203936_s_at | matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) |
10.40 | 2.16E-05 | 4.75 | 1.54E-02 |
| 204116_at | interleukin 2 receptor, γ | 8.62 | 9.70E-04 | 5.94 | 1.70E-02 |
| 217235_x_at | immunoglobulin λ-like polypeptide 5 | 6.24 | 7.73E-03 | 8.23 | 1.51E-02 |
| 219890_at | C-type lectin domain family 5, member A |
7.85 | 1.70E-06 | 5.68 | 6.26E-03 |
| 210072_at | chemokine (C-C motif) ligand 19 | 8.99 | 1.37E-04 | 4.07 | 4.51E-02 |
| 224342_x_at | BMS1 homolog, ribosome assembly protein (yeast) pseudogene |
6.29 | 2.66E-03 | 5.59 | 4.79E-02 |
| 222838_at | SLAM family member 7 | 5.30 | 4.72E-03 | 6.46 | 3.09E-02 |
| 1555349_a_at | integrin, β 2 (complement component 3 receptor 3 and 4 subunit) |
6.57 | 2.61E-04 | 5.06 | 7.82E-03 |
| 1558199_at | fibronectin 1 | 4.89 | 2.41E-02 | 6.07 | 6.71E-03 |
| 205798_at | interleukin 7 receptor | 6.15 | 1.21E-02 | 4.42 | 1.78E-02 |
| 213193_x_at | T cell receptor β constant 1 | 4.96 | 3.31E-03 | 5.37 | 1.81E-02 |
| 220306_at | family with sequence similarity 46, member C |
4.28 | 3.16E-02 | 6.01 | 2.99E-02 |
| 205997_at | ADAM metallopeptidase domain 28 | 6.21 | 4.19E-03 | 3.80 | 4.67E-02 |
| 1552806_a_at | sialic acid binding Ig-like lectin 10 | 5.43 | 8.53E-05 | 3.35 | 4.11E-02 |
| 233510_s_at | parvin, γ | 5.44 | 5.90E-04 | 3.20 | 5.80E-03 |
| 211881_x_at | immunoglobulin λ joining 3 | 3.10 | 4.14E-02 | 5.53 | 2.30E-02 |
| 211908_x_at | immunoglobulin κ locus | 3.52 | 1.58E-02 | 5.11 | 8.75E-04 |
| 205213_at | ArfGAP with coiled-coil, ankyrin repeat and PH domains 1 |
4.85 | 6.72E-03 | 3.48 | 1.37E-03 |
| 207794_at | chemokine (C-C motif) receptor 2 | 4.38 | 1.22E-02 | 3.40 | 1.82E-02 |
| 212657_s_at | interleukin 1 receptor antagonist | 4.06 | 8.97E-03 | 3.69 | 4.68E-03 |
| 229041_s_at | ITGB2 antisense RNA 1 | 4.05 | 1.73E-03 | 3.68 | 3.71E-02 |
| 215946_x_at | immunoglobulin λ-like polypeptide 3, pseudogene |
3.68 | 4.55E-02 | 3.78 | 2.39E-02 |
| 226658_at | podoplanin | 3.28 | 1.54E-02 | 4.13 | 2.46E-02 |
| 222858_s_at | dual adaptor of phosphotyrosine and 3-phosphoinositides |
4.07 | 1.40E-03 | 3.32 | 3.07E-02 |
| 204852_s_at | protein tyrosine phosphatase, non-receptor type 7 | 3.90 | 2.36E-04 | 3.44 | 3.27E-03 |
| 1552960_at | leucine rich repeat containing 15 | 3.66 | 2.53E-02 | 3.64 | 1.51E-02 |
| 211991_s_at | major histocompatibility complex, class II, DP α 1 |
3.21 | 2.22E-02 | 4.08 | 5.08E-03 |
| 229721_x_at | derlin 3 | 3.87 | 8.11E-03 | 3.21 | 2.40E-02 |
| 1552497_a_at | SLAM family member 6 | 3.30 | 4.60E-03 | 3.75 | 1.87E-03 |
| 224310_s_at | B-cell CLL/lymphoma 11B (zinc finger protein) |
3.65 | 4.56E-02 | 3.25 | 8.32E-04 |
| 209696_at | fructose-1,6-bisphosphatase 1 | 3.32 | 2.01E-02 | 3.57 | 2.58E-02 |
| 210423_s_at | solute carrier family 11 (proton- coupled divalent metal ion transporters), member 1 |
3.48 | 3.64E-03 | 3.32 | 1.89E-02 |
| 1553863_at | WDFY family member 4 | 2.98 | 5.10E-03 | 3.68 | 3.86E-05 |
| 219971_at | interleukin 21 receptor | 2.88 | 4.59E-02 | 3.65 | 1.61E-03 |
| 228658_at | myocardial infarction associated transcript (non-protein coding) |
3.25 | 2.39E-02 | 3.21 | 8.95E-03 |
| 202856_s_at | solute carrier family 16, member 3 (monocarboxylic acid transporter 4) |
2.76 | 8.22E-03 | 3.46 | 1.55E-03 |
Shown here are 50 probe sets with the highest fold differences compared to uninflamed control tissues and with adequate annotation. For genes interrogated by multiple probe sets, only the one with the highest fold difference is listed here. The entire list of probe sets with significantly higher signals is in Supplemental Table 1.
Table 3.
Probe sets indicating decreased expression of genes in orbital adipose tissues from subjects with GPA.
| Probe Set | Gene Title | Set 1 | Set2 | ||
|---|---|---|---|---|---|
| Fold Difference |
FDR P- value |
Fold Difference |
FDR P- value |
||
| 205913_at | perilipin 1 | −143.00 | 1.25E-04 | −121.91 | 1.11E-05 |
| 209612_s_at | alcohol dehydrogenase 1B (class I), β polypeptide |
−105.20 | 5.19E-05 | −110.27 | 8.66E-06 |
| 207175_at | adiponectin, C1Q and collagen domain containing |
−91.04 | 2.35E-04 | −67.65 | 2.89E-05 |
| 205478_at | protein phosphatase 1, regulatory (inhibitor) subunit 1A |
−54.07 | 5.49E-05 | −69.20 | 3.00E-06 |
| 1565162_s_at | microsomal glutathione S-transferase 1 | −37.42 | 1.95E-04 | −69.65 | 8.09E-07 |
| 219398_at | cell death-inducing DFFA-like effector c | −47.89 | 3.18E-05 | −52.73 | 1.65E-05 |
| 226304_at | heat shock protein, α-crystallin-related, B6 |
−13.64 | 1.57E-03 | −80.10 | 9.01E-06 |
| 203980_at | fatty acid binding protein 4, adipocyte | −43.92 | 4.28E-04 | −44.16 | 9.18E-05 |
| 225420_at | glycerol-3-phosphate acyltransferase, mitochondrial |
−48.61 | 8.73E-05 | −38.56 | 9.04E-05 |
| 201540_at | four and a half LIM domains 1 | −25.32 | 6.97E-04 | −55.33 | 2.54E-05 |
| 209699_x_at | aldo-keto reductase family 1, member C2 | −33.58 | 5.20E-06 | −42.33 | 1.65E-05 |
| 211356_x_at | leptin receptor | −24.20 | 4.22E-04 | −37.59 | 4.87E-06 |
| 209555_s_at | CD36 molecule (thrombospondin receptor) |
−34.54 | 1.22E-03 | −27.21 | 2.79E-04 |
| 202036_s_at | secreted frizzled-related protein 1 | −31.23 | 1.21E-05 | −27.05 | 2.42E-07 |
| 204151_x_at | aldo-keto reductase family 1, member C1 | −17.12 | 3.04E-05 | −40.77 | 1.76E-05 |
| 209283_at | crystallin, αB | −10.13 | 2.11E-03 | −46.48 | 4.25E-05 |
| 212097_at | caveolin 1, caveolae protein, 22kDa | −14.11 | 7.32E-04 | −39.56 | 6.29E-05 |
| 204894_s_at | amine oxidase, copper containing 3 | −30.59 | 2.46E-04 | −22.59 | 2.55E-05 |
| 219140_s_at | retinol binding protein 4, plasma | −23.29 | 6.10E-06 | −27.16 | 1.87E-05 |
| 222124_at | hypoxia inducible factor 3, α subunit | −18.41 | 5.96E-03 | −31.88 | 6.02E-05 |
| 221796_at | neurotrophic tyrosine kinase, receptor, type 2 |
−18.69 | 2.24E-04 | −30.75 | 1.31E-05 |
| 229476_s_at | thyroid hormone responsive | −20.58 | 2.24E-04 | −28.73 | 4.43E-05 |
| 203324_s_at | caveolin 2 | −12.07 | 5.31E-03 | −35.52 | 2.89E-06 |
| 203407_at | periplakin | −17.08 | 8.92E-06 | −27.81 | 1.11E-06 |
| 222513_s_at | sorbin and SH3 domain containing 1 | −10.64 | 1.67E-03 | −34.00 | 8.86E-05 |
| 205498_at | growth hormone receptor | −19.66 | 1.40E-04 | −24.45 | 7.97E-06 |
| 202016_at | mesoderm specific transcript | −19.57 | 2.23E-04 | −21.60 | 9.30E-06 |
| 204719_at | ATP-binding cassette, sub-family A (ABC1), member 8 |
−21.77 | 7.39E-04 | −18.77 | 8.67E-06 |
| 1555740_a_at | melanocortin 2 receptor accessory protein |
−19.05 | 3.34E-04 | −20.58 | 4.22E-05 |
| 212230_at | phosphatidic acid phosphatase type 2B | −13.57 | 1.30E-03 | −26.03 | 7.54E-06 |
| 210963_s_at | glycogenin 2 | −20.66 | 4.64E-04 | −18.21 | 6.38E-05 |
| 213524_s_at | G0/G1switch 2 | −13.65 | 4.73E-03 | −24.89 | 1.41E-04 |
| 222835_at | thrombospondin, type I, domain containing 4 |
−13.50 | 2.27E-03 | −24.22 | 6.56E-05 |
| 201432_at | catalase | −10.70 | 1.20E-03 | −25.30 | 2.14E-05 |
| 209763_at | chordin-like 1 | −16.42 | 9.03E-04 | −19.39 | 6.03E-06 |
| 225207_at | pyruvate dehydrogenase kinase, isozyme 4 |
−13.50 | 1.04E-02 | −21.80 | 8.69E-05 |
| 221748_s_at | tensin 1 | −9.92 | 2.77E-04 | −25.36 | 9.14E-04 |
| 225975_at | protocadherin 18 | −16.49 | 1.15E-04 | −18.31 | 3.06E-06 |
| 212741_at | monoamine oxidase A | −16.69 | 1.37E-04 | −18.01 | 2.38E-06 |
| 243585_at | ATPase type 13A5 | −14.16 | 3.23E-04 | −19.16 | 6.14E-06 |
| 219789_at | natriuretic peptide receptor C/guanylate cyclase C (atrionatriuretic peptide receptor C) |
−15.40 | 4.13E-04 | −17.67 | 7.73E-07 |
| 203851_at | insulin-like growth factor binding protein 6 |
−9.38 | 1.10E-03 | −20.16 | 3.77E-06 |
| 222484_s_at | chemokine (C-X-C motif) ligand 14 | −5.60 | 4.36E-02 | −23.53 | 1.30E-03 |
| 228224_at | proline/arginine-rich end leucine-rich repeat protein |
−6.57 | 1.93E-02 | −21.02 | 7.12E-06 |
| 211959_at | insulin-like growth factor binding protein 5 |
−9.01 | 9.98E-03 | −18.21 | 1.56E-04 |
| 206243_at | TIMP metallopeptidase inhibitor 4 | −9.02 | 4.52E-03 | −18.13 | 5.55E-05 |
| 227646_at | early B-cell factor 1 | −9.45 | 2.29E-03 | −17.15 | 1.28E-05 |
| 212071_s_at | spectrin, β, non-erythrocytic 1 | −6.58 | 2.51E-03 | −19.02 | 4.42E-04 |
| 203571_s_at | adipogenesis regulatory factor | −7.67 | 2.98E-04 | −16.93 | 3.89E-05 |
| 201525_at | apolipoprotein D | −4.64 | 7.20E-03 | −18.85 | 1.02E-03 |
Shown here are 50 probe sets with the largest negative fold differences compared to uninflamed control tissues and with adequate annotation. For genes interrogated by multiple probe sets, only the one with the largest negative fold difference is listed here. The entire list of probe sets with significantly lower signals is in Supplemental Table 2.
The expression profiles of orbital adipose tissues from the two granulomatous diseases, GPA and sarcoidosis, were also compared. Based on the same threshold of a least a 1.5-fold difference with FDR adjusted p <0.05 in both sets, we noted 54 probe sets (about 42 genes) with higher signals in GPA (Supplemental Table 3, see Supplementary Data file) and 237 probe sets (about 200 genes) with lower signals in GPA (Supplemental Table 4, see Supplementary Data file). The GPA-rich group has several cytokines expressed at relatively high levels, while the sarcoidosis group has a smaller, mostly different set of cytokine and cytokine receptor genes expressed at higher levels.
Additionally, we used Venn diagrams to depict the similarity or distinctiveness of gene expression in GPA in comparison with gene expression in healthy controls or other orbital diseases (Figure 3). Although individual patients with NSOI clearly differed from GPA, as a group, there were no probe sets that were consistently, significantly different between GPA and NSOI.
Figure 3. Venn diagrams comparing gene expression in orbital adipose from GPA patients with the other experimental groups.
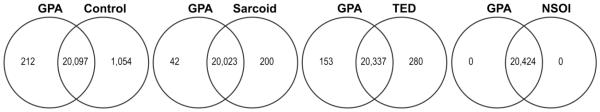
Expression levels in tissues from subjects with GPA were compared to levels in uninflamed control, sarcoidosis, NSOI, and TED orbital adipose samples. Probe sets were considered to indicate a significant difference between groups when their signals had at least a 1.5-fold change with a FDR adjusted p-value less than 0.05 in both sets. Shown are the numbers of unique genes identified by NIH DAVID from probe set lists for each category.
Although no prior study has analyzed gene expression in orbital tissue from patients with GPA, we were able to compare our results to publications on either leukocytes or nasal brushings. One group has analyzed gene expression in GPA based on isolated leukocytes.[24, 25] Seven of the probe sets that we find upregulated in orbital adipose tissue from patients with GPA relative to controls were also upregulated in leukocytes from patients with GPA. These 7 mRNAs are matrix metalloproteinase 9 (MMP9), interleukin-7 receptor (IL-7R), CD64, interleukin-1 receptor antagonist (IL-1RN), T cell receptor 1 β (TCR 1β), solute carrier protein 11A1 (SLC11A1) (also known as natural resistance associated macrophage protein-1), and Toll like receptor 2 (TLR2). In addition we noted 21 probe sets that were down regulated in both our orbital tissue specimens and the leukocytes from patients with GPA (Supplemental Table 5, see Supplementary Data file 3).
A recent report on 10 subjects with active GPA characterized gene expression in cells obtained by brushing of nasal mucosa [26]. Although this study examined a different tissue and analyzed tissue obtained by brushing rather than biopsy [26], the two analyses again offer several concordant results. Both studies implicated contributors to innate immunity including SLC11, TLR2, and TLR8. Both SLC11 and TLR2 have now been implicated in leukocytes (24, 25), nasal mucosa [26], and orbit in the pathogenesis of GPA. The nasal study found up regulation of TREM1 (triggering receptor expressed on myeloid cells) and up regulation of interleukin-1 beta and the type II IL-1 receptor, whereas our study on orbital tissue implicated TREML2 (TREM like molecule 2) and the interleukin-1 receptor antagonist. Both studies discovered an up regulation of SERPINA1 (also known as alpha 1 anti-trypsin). The nasal study found many transcripts to be down regulated but included only ten in its publication [26]. One of these, SPARCL1 (secreted protein acid rich in cysteine-like 1) or hevin is also down regulated in orbital tissue affected by GPA.
DISCUSSION
To our knowledge, this report is the first analysis of gene expression in biopsies from patients with GPA and the first report to suggest that many patients with what was once called orbital pseudotumor actually have a limited form of GPA. The analysis of gene expression strongly supports the hypothesis that NSOI is a collection of diseases with the gene expression profile often overlapping with other known entities, namely TED, sarcoidosis, or most commonly GPA.
Gene expression profiling is providing insights into other inflammatory diseases.[27] For example, gene expression profiling from peripheral blood indicates that a subset of patients with systemic lupus erythematosus express several genes regulated by type I interferons.[28] Analysis of transcripts from solid tissue has been applied to eosinophilic esophagitis[29] and giant cell myocarditis.[30] Synovium from rheumatoid arthritis, spondyloarthritis, or osteoarthritis displays distinct patterns of mRNA or protein expression.[31, 32] This study shows that a form of nonspecific inflammation can be classified on the basis of gene expression.
Our data indicate that GPA and sarcoidosis gene expression can be distinguished, but many of the tissues from subjects with NSOI have a gene expression profile indistinguishable from GPA. We did not detect a positive ANCA among these subjects with NSOI. Even among patients who are eventually diagnosed with GPA, biopsy of certain tissue sites such as the orbit, sinus, nasal mucosa, or subglottis provides definitive diagnostic information in a minority of cases.[1-5] We reported that an algorithm based on our data is more accurate than histopathology at identifying the cause of orbital inflammation.[33] We predict that molecular diagnosis will enhance the ability to diagnose GPA in mucosal sites.
Our sample population was adequate to analyze potential confounders including age, gender, disease duration, and use of corticosteroids. None of these factors could account for our findings (data not shown.)
Our report has limitations. Although we analyzed 83 tissues, they were divided among 5 separate conditions so that the diagnosis of GPA is represented by only six biopsies. The sample size, however, still allowed us to divide tissues into a discovery and validation set to demonstrate consistency in our findings. The GPA sample size was adequate to distinguish GPA clearly from sarcoidosis, TED, or controls, but not from NSOI. Furthermore, our tissue observations correlated with independent results reported for leukocytes [24, 25] or nasal brushings [26]. In addition, the upregulated transcripts which we detect are predominantly related to inflammation or response to infection. The transcripts identified by our study and previous analyses of RNA expression including TLR2 and SLC11 are especially intriguing. Many believe that bacteria such as Staphylococcus aureus act as a trigger for GPA. [34] Accordingly, the up regulation of TLR2, a receptor that recognizes bacterial cell wall, can easily be related to pathogenesis. Similarly SLC11, a molecule that transports iron, is also involved in host response to infection.[35, 36] Both our study and the report on nasal brushings from patients with GPA [26] found SPARCL1 to be down regulated. SPARCL1 deficiency has been implicated in cancer growth [37] and in abnormal wound healing [38], so it is easy to relate its relative lack to abnormal tissue repair.
We studied FFPE tissues. RNA in the blocks stored at room temperature degrades over time, and the quality of RNA that can be recovered decreases. Consequently we chose a cDNA synthesis protocol optimized for partially degraded RNA that would amplify relatively short sequences across the gene transcripts. We limited the duration that tissue could be stored until analysis. Still fresh tissue, frozen tissue, or an alternative fixation method might have yielded different results due to increased sensitivity. We limited our study to tissue from the orbit, which is composed mostly of adipose tissue in a healthy adult. NSOI or GPA can also affect the lacrimal gland (dacryoadenitis) and extra-ocular muscles (myositis). Additional studies are required to determine how results from the orbital adipose tissue compare to results from other sites. We did not validate individual levels of gene expression by a technique such as RT-PCR because we used a second data set for validation and because this report is based on patterns of gene expression, not on individual transcripts. Furthermore, we previously reported a correlation coefficient of >0.7 when we compared relative expression levels determined by our microarray procedure with levels obtained from quantitative RT-PCR.[39]
On the basis of our experience to date, we predict that molecular profiling will eventually become a routine component in the evaluation of patients with orbital inflammatory disease. B cell depletion is now accepted as an approved form of therapy for GPA[40], but B cell depletion is not effective in the treatment of TED.[41] We and others have noted success in treating orbital inflammatory disease with rituximab therapy.[42, 43] The pronounced increased expression of transcripts relating to immunoglobulins adds rationale to this approach to treatment.
Additional experience in analyzing gene expression from orbital tissues may allow even greater diagnostic subdivision. Transcriptional profiling cannot be recommended as a routine diagnostic test, but this approach shows promise in defining a small set of transcripts with implications for both diagnosis and choice of therapy.
CONCLUSIONS
In summary, this study has resulted in several novel findings. Gene expression profiling can distinguish the major causes of orbital inflammation. Although NSOI is a heterogeneous collection of diseases, some patients labeled as having NSOI may actually have a limited form of GPA. Immunoglobulin gene expression characterizes orbital tissue from patients with orbital GPA in comparison to healthy controls. Some of the transcripts with increased expression in orbital tissue from patients with GPA have also been reported to be increased in leukocytes or nasal brushings from patients with GPA. An extrapolation from this study is that the diagnostic yield from biopsies to assess the likelihood of GPA in several sites will be improved by analysis of the genes expressed in the biopsied tissue.
Supplementary Material
Highlights.
Some types of orbital inflammation are distinguishable by gene expression profiles.
Nonspecific orbital inflammation can be subdivided by analysis of gene expression.
Some NSOI gene expression profiles are indistinguishable from those of GPA.
Limited GPA in the orbit requires gene expression profiling to be identified.
ACKNOWLEDGEMENTS
We are grateful to Kristina Vartanian for her excellent technical support for the microarray work. RNA extraction and microarray assays were performed in the OHSU Gene Profiling Shared Resource.
Financial support was from NIH Grants EY020249, EY010572 and RR024140, Research to Prevent Blindness, the William and Mary Bauman Foundation, the Mas Family Foundation, and the Stan and Madelle Rosenfeld Family Trust. None of the sponsors had any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
JTR has in the past consulted for Genentech and was a co-investigator on a studied funded by Genentech to evaluate the use of rituximab for orbital inflammatory diseases.
List of abbreviations
- GPA
granulomatosis with polyangiitis
- TED
thyroid eye disease
- NSOI
nonspecific orbital inflammation
- GSEA
gene set enrichment analysis
- FFPE
formalin fixed, paraffin embedded
- PCA
principal coordinate analysis
- ANCA
anti-neutrophil cytoplasmic antibody
- NRAMP
natural resistance associated macrophage protein
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The other authors report no potential conflict of interest.
Author Contributions:
JTR conceived of the study, recruited study centers, supervised the study, reviewed the clinical diagnoses, and participated in the study design, data analysis and interpretation, and drafted the manuscript. DC helped with the study design, performed the statistical analysis, participated in data interpretation, and helped prepare the manuscript. DJW evaluated stained sections, reviewed the clinical diagnoses, and helped prepare the manuscript. HEG evaluated stained sections and helped prepare the manuscript. CAH helped with study design, supervised the RNA extraction and microarray assays, participated in data analysis, interpretation, and critical revision of the manuscript. CHS assisted in data interpretation and critical revision of the manuscript. RAD, JDN, and EAS obtained samples for analysis, gathered clinical and demographic data, and helped prepare the manuscript. CNC, JAF, DT, CA, GIH, MK, PJP, BSK, DOK, DPE, HMA, HaH, and RPY obtained IRB approval, obtained samples for analysis, gathered clinical and demographic data, and helped prepare the manuscript. SD, PP obtained samples for analysis, gathered clinical and demographic data, and helped prepare the manuscript. VAW, PJD, and DS obtained ethics committee approval, obtained samples for analysis, gathered clinical and demographic data, and helped prepare the manuscript. PS managed tissue sectioning and storage, provided administrative support, stained slides, and helped prepare the manuscript. SRP assisted in the study design and supervision, coordinated the study centers, obtained IRB approval, participated in data acquisition, analysis and interpretation, and critically revised the manuscript. All authors read and approved the manuscript.
Author Information: JTR is Chief of Arthritis and Rheumatic Diseases at the Oregon Health & Science University and Chief of Ophthalmology at the Legacy Health System Devers Eye Institute.
Contributor Information
James T. Rosenbaum, Email: rosenbaj@ohsu.edu.
Dongseok Choi, Email: choid@ohsu.edu.
David J. Wilson, Email: wilsonda@ohsu.edu.
Hans E. Grossniklaus, Email: ophtheg@emory.edu.
Christina A. Harrington, Email: harringc@ohsu.edu.
Cailin H. Sibley, Email: Sibleyc@ohsu.edu.
Roger A. Dailey, Email: daileyr@ohsu.edu.
John D. Ng, Email: ngj@ohsu.edu.
Eric A. Steele, Email: steeleer@ohsu.edu.
Craig N. Czyz, Email: dsp4000@aol.com.
Jill A. Foster, Email: fosterj@jillfoster.om.
David Tse, Email: dtse@med.miami.edu.
Chris Alabiad, Email: calabiad@med.miami.edu.
Sander Dubovy, Email: sdubovy@med.miami.edu.
Prashant K. Parekh, Email: prashant-parekh@uiowa.edu.
Gerald J. Harris, Email: GJharris@mcw.edu.
Michael Kazim, Email: mk48@columbia.edu.
Payal J. Patel, Email: ppatel811@gmail.com.
Valerie A. White, Email: Val.White@vch.ca.
Peter J. Dolman, Email: peterdolman@hotmail.com.
Bobby S. Korn, Email: bkorn@ucsd.edu.
Don O. Kikkawa, Email: dkikkawa@ucsd.edu.
Deepak P. Edward, Email: dedward@kkesh.med.sa.
Hind M. Alkatan, Email: hindkatan@yahoo.com.
Hailah al-Hussain, Email: hhussain@kkesh.med.sa.
R. Patrick Yeatts, Email: pyeatts@wfubmc.edu.
Dinesh Selva, Email: saioph@gmail.com.
Patrick Stauffer, Email: stauffep@ohsu.edu.
Stephen R. Planck, Email: plancks@ohsu.edu.
REFERENCES
- 1.Borner U, Landis BN, Banz Y, Villiger P, Ballinari P, Caversaccio M, Dubach P. Diagnostic value of biopsies in identifying cytoplasmic antineutrophil cytoplasmic antibody-negative localized Wegener's granulomatosis presenting primarily with sinonasal disease. Am J Rhinol Allergy. 2012;26:475–480. doi: 10.2500/ajra.2012.26.3825. [DOI] [PubMed] [Google Scholar]
- 2.Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener's granulomatosis. A pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol. 1990;14:555–564. doi: 10.1097/00000478-199006000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Raynaud P, Garrel R, Rigau V, Poizat F, Vic P, Cartier C, Riviere S, Baldet P, Costes V. How can the diagnostic value of head and neck biopsies be increased in Wegener's granulomatosis: a clinicopathologic study of 49 biopsies in 21 patients. Ann Pathol. 2005;25:87–93. doi: 10.1016/s0242-6498(05)86171-1. [DOI] [PubMed] [Google Scholar]
- 4.Del Buono EA, Flint A. Diagnostic usefulness of nasal biopsy in Wegener's granulomatosis. Hum Pathol. 1991;22:107–110. doi: 10.1016/0046-8177(91)90030-s. [DOI] [PubMed] [Google Scholar]
- 5.Kalina PH, Lie JT, Campbell RJ, Garrity JA. Diagnostic value and limitations of orbital biopsy in Wegener's granulomatosis. Ophthalmology. 1992;99:120–124. doi: 10.1016/s0161-6420(92)32028-7. [DOI] [PubMed] [Google Scholar]
- 6.Lutt J, Lim L, Phal P, Rosenbaum J. Orbital inflammatory disease. Semin Arthritis Rheum. 2008;37:207–222. doi: 10.1016/j.semarthrit.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Char DH, Miller T. Orbital pseudotumor. Fine-needle aspiration biopsy and response to therapy. Ophthalmology. 1993;100:1702–1710. [PubMed] [Google Scholar]
- 8.Blodi FC, Gass JDM. Inflammatory pseudotumor of the orbit. Br J Ophthalmol. 1968;52:79–93. doi: 10.1136/bjo.52.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennerdell JS, Dresner SC. The nonspecific orbital inflammatory syndromes. Surv Ophthalmol. 1984;29:93–103. doi: 10.1016/0039-6257(84)90166-8. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Wu Z, Li Y. A clinical analysis of idiopathic orbital inflammatory pseudotumor. Yan Ke Xue Bao. 2000;16:208–213. [PubMed] [Google Scholar]
- 11.Swamy BN, McCluskey PJ, Nemet A, Crouch R, Martin P, Benger R, Ghabriel R, Wakefield D. Idiopathic orbital inflammatory syndrome: clinical features and treatment outcomes. Br J Ophthlamol. 2007;91:1667–1670. doi: 10.1136/bjo.2007.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin PA, Foster CS. Etiology and management of idiopathic orbital inflammation. Am J Ophthalmol. 2004;138:1041–1043. doi: 10.1016/j.ajo.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Yuen SJ, Ruin PA. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003;121:491–499. doi: 10.1001/archopht.121.4.491. [DOI] [PubMed] [Google Scholar]
- 14.Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TFE, Bernd H-W, Cogliatti SB, Dierlamm J, Feller AC, Hansmann M-L, Haralambieva E, Harder L, Hasenclever D, Kuhn M, Lenze D, Lichter P, Martin-Subero JI, Moller P, Muller-Hermelink H-K, Ott G, Parwaresch RM, Pott C, Rosenwald A, Rosolowski M, Schwaenen C, Sturzenhofecker B, Szczepanowski M, Trautmann H, Wacker H-H, Sprang R, Loeffler M, Trumper L, Stein H, Siebert R. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 15.Bohen SP, Troyanskaya OG, Alter O, Warnke R, Botstein D, Brown PO, Levy R. Variation in gene expression patterns in follicular lymphoma and the response to rituximab. Proc Natl Acad Sci USA. 2003;100:1926–1930. doi: 10.1073/pnas.0437875100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dave SS, Fu K, Wright GW, Lam LT, LKluin P, Boerma EJ, Greiner TC, Weisenburger DD, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Delabie J, Rimsza L, Braziel RM, Grogan TM, Campo E, Jaffe ES, Dave BJ, Sanger WG, Bast M, Vose JM, Armitage JO, Connors JM, Smeland EB, Kvaloy S, Holte H, Fisher RI, MIller TP, Montserrat E, Wilson WH, Bahl M, Zhao H, Yang L, Powell J, Simon R, Chan WC, Staudt LM, Project LLMP. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354:2495–2498. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 17.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mardia KV. Some properties of classical multi-dimensional scaling. Commun Stat Theory Methods. 1978;A7:1233–1241. [Google Scholar]
- 19.Benjamini Y, Hochbert Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc, Series B. 1995;57:289–300. [Google Scholar]
- 20.The R Project for Statistical Computing. [ www.r-project.org] Accessed February 2015.
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 23.Dennis G, Jr., Sherman BT, Hosack DA, Gao JY, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 24.Alcorta D, Preston G, Munger W, Sullivan P, Yang JJ, Waga I, Jennette JC, Falk R. Microarray studies of gene expression in circulating leukocytes in kidney diseases. Exp Nephrol. 2002;10:139–149. doi: 10.1159/000049909. [DOI] [PubMed] [Google Scholar]
- 25.Alcorta DA, Barnes DA, Dooley MA, Sullivan P, Jonas B, Liu Y, Lionaki S, Reddy CB, Chin H, Dempsey AA, Jennette JC, Falk RJ. Leukocyte gene expression signatures in antineutrophil cytoplasmic autoantibody and lupus glomerulonephritis. Kidney Int. 2007;72:853–864. doi: 10.1038/sj.ki.5002371. [DOI] [PubMed] [Google Scholar]
- 26.Grayson PC, Steiling K, Platt M, Berman JS, Zhang X, Xiao J, Alekseyev YO, Liu G, Monach PA, Kaplan MJ, Spira A, Merkel PA. Defining the nasal transcriptome in granulomatosis with polyangiitis. Arthritis Rheumatol. 2015 doi: 10.1002/art.39185. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novianti PW, Roes KC, Eijkemans MJ. Evaluation of gene expression classification studies: factors associated with classification performance. PLoS One. 2014;9:e96063. doi: 10.1371/journal.pone.0096063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, Putnam PE, Franciosi JP, Garza JM, Kaul A, King EC, Collins MH, Kushner JP, Rothenberg ME. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology. 2013;145:1289–1299. doi: 10.1053/j.gastro.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassner D, Kuhl U, Siegismund CS, Rohde M, Elezkurtaj S, Escher F, Tschope C, Gross UM, Poller W, Schultheiss HP. Improved diagnosis of idiopathic giant cell myocarditis and cardiac sarcoidosis by myocardial gene expression profiling. Eur Heart J. 2014;35:2186–2195. doi: 10.1093/eurheartj/ehu101. [DOI] [PubMed] [Google Scholar]
- 31.Tilleman K, Van Beneden K, Dhondt A, Hoffman I, De Keyser F, Veys E, Elewaut D, Deforce D. Chronically inflamed synovium from spondyloarthropathy and rheumatoid arthritis investigated by protein expression profiling followed by tandem mass spectrometry. Proteomics. 2005;5:2247–2257. doi: 10.1002/pmic.200401109. [DOI] [PubMed] [Google Scholar]
- 32.Yeremenko N, Noordenbos T, Cantaert T, van Tok M, van de Sande M, Canete JD, Tak PP, Baeten D. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. 2013;65:174–185. doi: 10.1002/art.37704. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum JT, Choi D, Wilson DJ, Grossniklaus HE, Sibley CH, Harrington CA, Planck SR, Orbital Disease C. Molecular diagnosis of orbital inflammatory disease. Exp Mol Pathol. 2015;98:225–229. doi: 10.1016/j.yexmp.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadema H, Heeringa P, Kallenberg CG. Bacterial infections in Wegener's granulomatosis: mechanisms potentially involved in autoimmune pathogenesis. Curr Opin Rheumatol. 2011;23:366–371. doi: 10.1097/BOR.0b013e328346c332. [DOI] [PubMed] [Google Scholar]
- 35.Wessling-Resnick M. Nramp1 and Other Transporters Involved In Metal Withholding During Infection. J Biol Chem. 2015 doi: 10.1074/jbc.R115.643973. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrnstorfer IA, Geertsma ER, Pardon E, Steyaert J, Dutzler R. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol. 2014;21:990–996. doi: 10.1038/nsmb.2904. [DOI] [PubMed] [Google Scholar]
- 37.Isler SG, Schenk S, Bendik I, Schraml P, Novotna H, Moch H, Sauter G, Ludwig CU. Genomic organization and chromosomal mapping of SPARC-like 1, a gene down regulated in cancers. Int J Oncol. 2001;18:521–526. doi: 10.3892/ijo.18.3.521. [DOI] [PubMed] [Google Scholar]
- 38.Sullivan MM, Puolakkainen PA, Barker TH, Funk SE, Sage EH. Altered tissue repair in hevin-null mice: inhibition of fibroblast migration by a matricellular SPARC homolog. Wound Repair Regen. 2008;16:310–319. doi: 10.1111/j.1524-475X.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 39.Vartanian K, Slottke R, Johnstone T, Casale A, Planck SR, Choi D, Smith JR, Rosenbaum JT, Harrington CA. Gene expression profiling of whole blood: comparison of target preparation methods for accurate and reproducible microarray analysis. BMC Genomics. 2009;10:2. doi: 10.1186/1471-2164-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U, Group R-IR. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stan MN, Garrity JA, Thapa P, Bradley EA, Bahn RS. Randomized Double-Blind Placebo-Controlled Trial Of Rituximab For Treatment Of Graves' Ophthalmopathy. Thyroid. 2013;23(Suppl 1):A13–121. [Google Scholar]
- 42.Suhler EB, Lim LL, Beardsley RM, Giles TR, Pasadhika S, Lee ST, de Saint Sardos A, Butler NJ, Smith JR, Rosenbaum JT. Rituximab Therapy for Refractory Orbital Inflammation: Results of a Phase 1/2, Dose-Ranging, Randomized Clinical Trial. JAMA Ophthalmol. 2014;132:572–578. doi: 10.1001/jamaophthalmol.2013.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor SR, Salama AD, Joshi L, Pusey CD, Lightman SL. Rituximab is effective in the treatment of refractory ophthalmic Wegener's granulomatosis. Arthritis Rheum. 2009;60:1540–1547. doi: 10.1002/art.24454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


