Abstract
Purpose
Selective serotonin reuptake inhibitors such as escitalopram are commonly used to treat patients with Autism Spectrum Disorder (ASD), but there are individual differences in treatment response and tolerability. CYP2C19 encodes the primary enzyme responsible for escitalopram metabolism and we investigated whether polymorphisms in CYP2C19 were related to symptoms and dosing in a pharmacogenetic study of ASD.
Methods
Participants completed the Aberrant Behavior Checklist-Community Version (ABC-CV) weekly for 6 weeks. Escitalopram was initiated at a dose of 2.5 mg qd with weekly increases to 20 mg unless intolerable side-effects occurred. Three CYP2C19 metabolizer groups, including ultrarapid, extensive, and reduced metabolizers, were examined in relation to symptom improvement and tolerated dose.
Results
ABC-CV scores improved over the course of treatment (p<0.0001). There were no differences identified in the rate of improvement across metabolizer groups for the ABC-CV-irritability subscale, which was the primary outcome for clinical symptoms. There was a trend for a metabolizer group by time interaction with respect to dose (p=0.10). This interaction was driven by the linear rate of change from week 1 to study endpoint between the reduced metabolizers and ultrarapid metabolizer groups (p=0.05). Post hoc analyses identified significant differences in the rate of dose escalation between ultrarapid metabolizers and extensive metabolizers and for ultrarapid metabolizers compared to reduced metabolizers (p’s<0.04), whereby ultrarapid metabolizers exhibited a slower rate of change in dose over time.
Conclusion
CYP2C19 ultrarapid metabolizers were associated with reduced tolerance to a fixed titration schedule of open label escitalopram in this ASD study sample. Possible explanations may involve the altered kinetics of faster metabolizers or previously unknown activities of escitalopram metabolites.
Keywords: Escitalopram, CYP2C19, Autism Spectrum Disorder
Introduction
Autism Spectrum Disorder (ASD) is characterized by impairments in social communication, restricted and repetitive behaviors, interests, or activities [1, 2]. ASD is a common disorder of childhood onset, with recent estimates suggesting a prevalence of approximately 1 in 68 children in the United States [3]. Behavioral interventions are commonly used as first line treatments for ASD [4] but 35%–56% of patients are treated with psychotropic medications [5, 6]. Clinical symptoms commonly requiring intervention include irritability, aggressive behaviors, and hyperactivity [7]. The exact mechanisms underlying the pathophysiology of these features are not unequivocally defined, but differences in serotonergic function have been identified in ASD relative to comparison populations [8]. The two most commonly prescribed medication classes [5, 6] for these symptoms include antipsychotics and selective serotonin (5HT) reuptake inhibitors (SSRIs) that are both known to modulate aspects of 5HT signaling via effects at 5HT receptors and/or the serotonin transporter [7]. Antidepressants do not have FDA indications for the treatment of ASD, but utilization estimates in this population range from 13–25%[5, 6] with SSRIs specifically ranging from 7–15.6% [9, 10].
Studies of SSRIs in ASD (autistic disorder, Asperger disorder, pervasive developmental disorder NOS) [11–14] as well as those focused specifically on autistic disorder [14] have most commonly focused on the treatment of repetitive behaviors with mixed results for these symptoms [11–14] with the largest study having been negative [13]. Regarding irritability symptoms that are frequently a target of pharmacotherapy, open label investigations of SSRIs in ASD [15–18], as well as randomized controlled trials (RCTs) in ASDs or autistic disorder [13, 14]have suggested potential efficacy. Interestingly, the largest RCT of an SSRI (citalopram) to date identified that 23%–38% of all participants reported adverse effects of aggression, irritability, or increased energy/activation during titration, although irritability was reduced in treated compared to placebo patients at the end of the study [13]. These adverse effects were also reported in earlier studies of SSRIs [15, 18]. Observations of beneficial responses to SSRIs countered with the risks for adverse effects as well as heterogeneous results across studies have led to hypotheses that there may be subgroups of patients more likely to respond or have side effects and pharmacogenetic investigations of these phenomena are warranted.
Pharmacogenetic investigations of SSRIs may involve both pharmacodynamic and pharmacokinetic contributors. The serotonin transporter and serotonin-2A genes (SLC6A4 and HTR2A, respectively) have been investigated in pharmacogenetic studies of ASD with mixed results [16, 17]. To our knowledge, genetic variation in drug metabolism has not been extensively studied in relation to SSRI treatment outcomes in ASD. Clinical observations of dose sensitivity in some patients [15] and genetic variation in the drug metabolism pathway for some SSRIs support a need for this type of investigation.
Escitalopram (S-CT), while not formally indicated for the treatment of ASD, is an example of an SSRI that may be used for the treatment of irritability and repetitive behavior symptoms in ASD patients in the clinical setting. Escitalopram is hepatically metabolized to metabolites that are known to have lower serotonin transporter affinity than the parent drug [19]. The actions of these metabolites beyond binding at other serotonin, dopamine, and norepinephrine receptors and/or transporters [19] have not been extensively investigated. The CYP2C19 enzyme along with CYP2D6 and CYP3A4 are known to influence the bioconversion of S-CT to S-desmethylcitalopram (S-DCT) [20]. Inhibitor studies as well as genetic studies have identified that blocking or upregulating the CYP2C19 pathway has a significant influence on the ratio of S-CTP:S-DCT, while the effects of modifying CYP2D6 and CYP3A4 pathways appear to be less pronounced in this regard [20, 21]. Currently the product labeling for citalopram (racemic S-CT+R-CT) as well as escitalopram contain language indicating that genetic metabolizer status may be important for dosing [22]. Most pharmacogenetic data thus far for CYP2C19 and escitalopram have resulted from studies of major depressive disorder in adults. Whether these findings are relevant to patients with ASD is not known.
We examined the relationship between genetic variants known to influence the metabolic activity of CYP2C19 and symptom response, behavioral side effects, and tolerance of a predefined dose titration schedule in patients with ASD who enrolled in two previous open label treatment studies. To our knowledge this is the first study to examine CYP2C19 pharmacogenetics in ASD.
Methods
Study design
We conducted a candidate gene pharmacogenetic study investigating associations of CYP2C19 genetic variants with symptoms, tolerability, and dosing outcomes to the SSRI escitalopram in ASD. Study samples from two escitalopram pharmacogenetic studies (5-HTTLPR of the serotonin transporter target) using similar enrollment, assessment, and treatment strategies were combined for this pharmacogenetic analysis [17] [16].
Participants
Participants (n=89) 4–45 years of age were recruited through the Developmental Disorders Clinic and the Neurodevelopmental Psychopharmacology Clinic at the University of Chicago and University of Illinois at Chicago Institute for Juvenile Research. Inclusion criteria for this pharmacogenetic analysis included a confirmed diagnosis of ASD including Autism, Asperger disorder or Pervasive Developmental Disorder not otherwise specified according to DSM-IV-TR [1]. Diagnoses were made subsequent to a psychiatric exam by a child psychiatrist (TO, FN, EC) using DSM-IV-TR criteria as well as assessments including the Autism Diagnostic Interview-Revised (ADI-R) [23, 24], and the Autism Diagnostic Observation Schedule-Generic [25], or Autism Diagnostic Observation Schedule 2nd Edition (ADOS-2) [26]. Additional inclusion criteria for this combined pharmacogenetic analysis included a minimum score of 12 on the Aberrant Behavior Checklist – Community Version Irritability Subscale (ABC-CV) [27] to represent patients with significant irritability for whom pharmacotherapy might be considered in the clinical setting. Age-appropriate cognitive/developmental tests were administered to assess verbal and non-verbal IQ. These tests included the Differential Ability Scales, First and Second Editions [28, 29], the Mullen Scales of Early Learning (MSEL) [30], the Wechsler Abbreviated Scale of Intelligence (WASI) [31], and the Peabody Picture Vocabulary Test [32, 33]. Participants were free of other serious medical or neurological conditions. Additionally, participants had not received prior treatment with either escitalopram or citalopram and were free of other psychoactive medications at the time of study enrollment.
Outcome Measures
The ABC-CV irritability subscale (ABC-CV-Irr) was chosen as the primary outcome variable for assessing clinical symptoms. ABC-CV total scores as well as other subscales were reserved for secondary analyses. The ABC-CV is a 58-item assessment with severity ratings of 0 (not problematic) to 3 (severely problematic) for each question. Five subscales (Inappropriate Speech, Irritability, Hyperactivity, Lethargy, and Stereotypy) along with Total scores are assessed. Irritability was chosen a priori as the primary outcome measure because patients with symptom severity in this domain are the ones most commonly requiring pharmacotherapy. Irritability represents both a target symptom for improvement as well as a marker of dose related adverse effects in some patients who exhibit activation and symptom exacerbation in the context of exposure to antidepressant medications [17, 18]. The ABC-CV was completed weekly by parents and caregivers for the duration of study assessments.
We also examined dosing/titration trajectory over the course of study as an outcome due to the structured nature of this aspect of the study. Final doses as well as well as dose changes over time were examined.
The studies included in this pharmacogenetic analysis were approved by the University of Chicago and the University of Illinois at Chicago Institutional Review Boards. Informed consent/permission was obtained from the parent or guardian of the study participants if they were minors or decisionally impaired, or from adult study subjects able to consent for themselves.
Treatment
The study was designed as a forced titration, open label examination of escitalopram monotherapy with an examination of symptom response and dose titrations across 6 weeks. All subjects, caregivers, and investigators were aware of the drug and dose. Investigators were blind to genotype results until after treatment was completed. Participants were initiated on a dose of 2.5 mg escitalopram at the beginning of week 1 of the study followed by weekly increases to 5, 10, 15, and finally 20 mg po qd. If participants experienced adverse effects, the titration escalation was altered to maintain a tolerated dose [16, 18]. The first of the two treatment studies used in this pharmacogenetic analysis was a 10 week study of escitalopram in the treatment of ASD [17, 18]. In that study it was recognized that no further improvements in the ABC-CV-Irr were observed from 6–10 weeks [18]. Thus the follow-up study was designed as a 6-week investigation [16] with assessments and dosing strategies completed for the purposes of further assessing the clinical benefit of escitalopram as well as pharmacogenetic analyses of participants from both studies using the first 6 weeks of treatment from Owley et al merged with the 6-week study of Najjar et al.
Genotyping and genetic analyses
Whole blood was used as the source for DNA. All subjects had blood drawn prior to starting the medication and genotyping was completed after all participants completed treatment. Genotyping for CYP2C19 was completed using Pyrosequencing as published previously [34]. These assays were previously validated in our laboratory (JRB) against Sangar Sequencing (data not shown) to validate assay performance.
The three variants selected (rs4244285, rs4986893, and rs12248560) are the defining SNPs for the CYP2C19*2, CYP2C19*3, and CYP2C19*17 alleles, respectively [35]. These are the most commonly observed variants related to reduced (*2 or *3) as well as increased (*17) CYP2C19 enzymatic activity. The currently recognized drug metabolizer categories for CYP2C19 are based on the following diplotypes: Extensive metabolizer (*1/*1), intermediate metabolizer (*1/*2 or *1/*3), poor metabolizer (*2/*2, or *2/*3, or *3/*3), and ultrarapid metabolizer (*1/*17 or *17/*17) [Scot SA 2012]. In our study population there was only n=1 *2/*2 poor metabolizer and the response and tolerability characteristics did not differ from intermediate metabolizers. Thus our three metabolizer groups for analyses were extensive metabolizers (EM), reduced metabolizers (RM; poor metabolizer+intermediate metabolizers), and ultrarapid metabolizers (UM). The minor allele frequencies (MAF) for the rs4244285, and rs12248560 variants were 0.13, and 0.20 respectively with genotype distributions that did not deviate from Hardy-Weinberg Equilibrium. All participants were homozygous CC (no variant) for rs4986893, which is known to be a rare SNP in most non-Asian populations [35].
Statistics
Differences between the three drug metabolizer groups in baseline characteristics were examined using between-subjects analysis of variance (ANOVA) for continuous variables and Chi square tests for categorical variables (Table 1).
Table 1.
Demographic and Baseline Clinical Data
| Characteristics (N) | All Participants (N=89) | Reduced Metabolizers (N=23) | Extensive Metabolizers (N=40) | Ultrarapid Metabolizers (N=26) | p-value |
|---|---|---|---|---|---|
| Gender (%) | 0.18 | ||||
| Male | 70 (78.7) | 19 (82.6) | 28 (70) | 23 (88.5) | |
| Female | 19 (21.3) | 4 (17.4) | 12 (30) | 3 (11.5) | |
| Age, mean ±SD (range), months | 136.7 ±66.9 (54–532) | 135.7 ±53.9 (54–286) | 140.4 ±84.3 (71–532) | 132.1 ±45.4 (73–272) | 0.89 |
| Weight, mean ±SD (range), kg | 47.4 ±20.1 (19–114) | 45.5 ±22.3 (19–104.3) | 47.4 ±19.9 (21.8–108) | 49.3 ±19.2 (22.7–114) | 0.81 |
| Tanner Stage (%) | 0.90 | ||||
| Prepubertal (Tanner Stage <3) | 60 (67.4) | 19 (82.6) | 28 (70) | 17 (65.4) | |
| Postpubertal (Tanner Stage ≥3) | 29 (32.6) | 4 (17.4) | 12 (30) | 9 (34.6) | |
| Race/Ethnicity (%) | 0.52, 0.70* | ||||
| African American | 13 (14.6) | 4 (17.4) | 5 (12.5) | 4 (15.4) | |
| African American/Hispanic | 1 (1.1) | 0 (0) | 1 (2.5) | 0 (0) | |
| Asian | 4 (4.5) | 1 (4.3) | 3 (7.5) | 0 (0) | |
| Caucasian/Hispanic | 4 (4.5) | 2 (8.7) | 1 (2.5) | 2 (7.7) | |
| Caucasian/Non-Hispanic | 65 (73) | 15 (62.5) | 30 (75) | 20 (76.9) | |
| Best estimate diagnosis (%) | 0.16 | ||||
| ASD | 60 (67.4) | 15 (65.2) | 28 (70) | 17 (65.4) | |
| Autism | 29 (32.6) | 8 (34.8) | 12 (30) | 9 (34.6) | |
| Cognitive, mean ±SD (range) | |||||
| Verbal IQ (N=79) | 76.7±31.7 (11–141) | 77.4±28.4 (18–118) | 77.8±33.2 (11–141) | 74.6±33.2 (14–125) | 0.97 |
| Non-Verbal IQ (N=89) | 83.2±31.7 (21–146) | 79.0±26.7 (30–130) | 88.6±30.0 (21–146) | 78.3±37.3 (25–134) | 0.34 |
| ABC-CV Total Score | 74.6±24.5 (33–138) | 72.2±24.9 (35–133) | 73.6±24.9 (33–138) | 78.3±24.0 (37–132) | 0.65 |
| ABC-CV Irritability Subscale | 21.6±7.0 (12–43) | 23.3±6.9 (14–43) | 20.3±5.9 (12–31) | 22.5±8.5 (12–42) | 0.24 |
ABC-CV, aberrant behavior checklist – community version; ASD, autism spectrum disorder; IQ, intelligence quotient.
p=0.70 represents comparisons of race (Caucasian, African American, Asian) and p=0.52 represents comparisons of Hispanic or non-Hispanic Ethnicity across metabolizer groups.
Analysis of ABC-CV-Irr and Dose Titrations
A series of mixed effects regression (MRM) analyses (random intercept and slope) [36] were conducted to examine differences in the linear rates of change from baseline (pre-treatment) to study endpoint (6-week assessment) between the metabolizer groups on the primary (ABC-CV-Irr) and secondary symptom outcome measures (ABC-CV Total; ABC-CV remaining subscales). Independent predictors included metabolizer group (class variable), time, and the metabolizer group by time interaction. The primary predictor of interest was the metabolizer group by time interaction. For dose-titrations, we only examined differences in the linear rates of change from week 1 to study endpoint given that dosing of medication was uniformly 2.5 mg qd at week 1. Follow-up MRMs were conducted when the metabolizer group by time interaction was p≤0.10 (enabled the examination of trends). These analyses were aimed at identifying the time point at which the metabolizer groups began to differ. For these analyses, the independent predictors included dummy variables for metabolizer groups, dummy variables for each assessment point (week 1, 2, 3, 4, 5, 6 vs. baseline; for dose titration week 2, 3, 4, 5, 6, vs. week 1) and the two-way interactions. The primary predictors of interest here were the two-way interactions. All analyses controlled for age and sex which were predefined covariates in our data analysis plan and consistent with other clinical analyses of this study sample [17] [16]. We evaluated the influence of demographic variables including race (dichotomized as Caucasian or non-Caucasian), pubertal status [pre-pubertal defined as a Tanner Stage (TS) <3 or age <144 months if TS was not assessed; post-pubertal was defined as a TS ≥3 or age ≥144 months if TS was not assessed], weight, final dose (for ABC-CV-Irr models), and non-verbal IQ (for ABC-CV-Irr models), but these factors were not included in the final models as they did not influence the pattern or significance of the pharmacogenetic findings. SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Significance for main effect testing and post hoc analyses was set at p<0.05. A repeated measures design with 1 within subjects factor (7 time points) and 1 between subjects factor (3 groups) with 25 subjects per group achieved 73% power to test the time x group interaction if a Geisser-Greenhouse Corrected F Test is used with a 5% significance level and the actual effect standard deviation is 0.85 (an effect size of 0.49). A minimum of 30 per group is necessary to achieve 83% power to test the interaction.
Results
Symptom Improvement
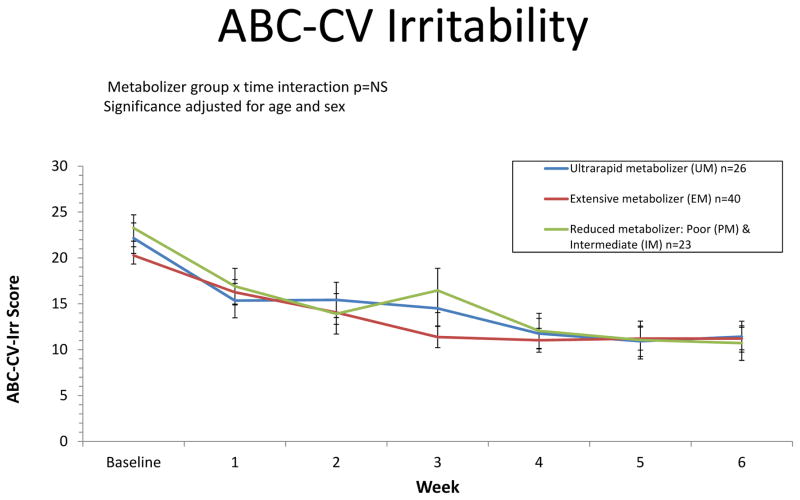
Eighty-four of the 89 subjects completed the 6 week dose titration and assessment phase of this study. There were no significant differences in demographic and baseline clinical variables across CYP2C19 metabolizer groups (Table 1). The primary analysis of ABC-CV-Irr identified overall symptom improvement from baseline to the study endpoint (week 6) (p<.0001). All metabolizer groups exhibited significant improvement (p’s<.0001) with no significant differences in the magnitude of improvement from baseline to endpoint (6 weeks) across metabolizer groups (p=0.39) (Figure 1). Secondary analyses of other ABC-CV total and subscale scores identified symptom improvement from baseline to endpoint for Hyperactivity, Inappropriate Speech, Lethargy, Stereotypy and Total Scores over the course of follow-up (p’s<0.001). There were no significant differences in the magnitude of improvement from baseline to endpoint between the metabolizer groups for the other ABC-CV measures assessed (p’s>0.18).
Figure 1. ABC-CV Irritability.
Aberrant Behavior Checklist – Community Version (ABC-CV) assessments were completed weekly over the course of the study. Results shown are stratified by CYP2C19 metabolizer groups.
Dosing and Titration
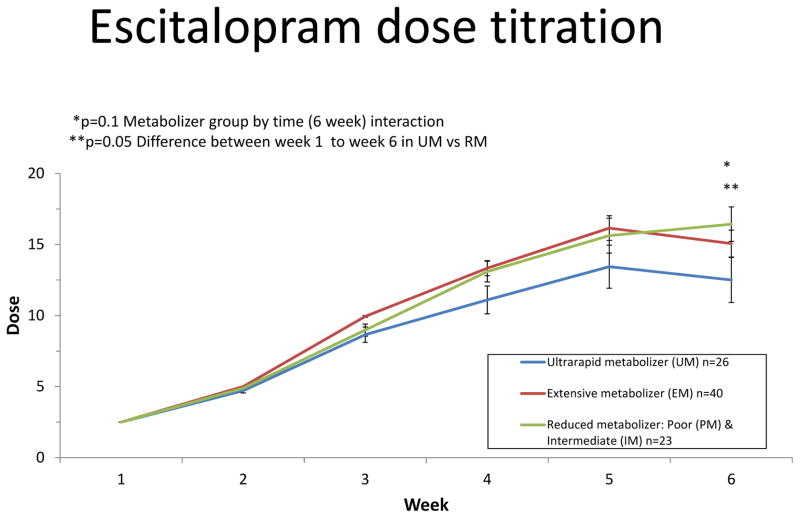
Of the 84 completers, 44 finished the predefined titration schedule for the study to 20 mg. Final doses (mean+/−SD mg/d) across metabolizer groups were ultrarapid metabolizers (12.5±7.8), extensive metabolizers (15.4±6.4), and reduced metabolizers (16.7±5.8) (F=1.47 2df p=0.26 adjusted for age and sex). Similar patterns of final dose were observed across males and females. There was a trend for a metabolizer group by time interaction with respect to dose (p=0.10) (Figure 2). This interaction was driven by differences in the linear rate of change from week 1 to study endpoint between the reduced metabolizers and ultrarapid metabolizer groups (p=0.05). There also was a trend for the extensive metabolizers and ultrarapid metabolizer groups to show a difference in the linear rate of change in dose from week 1 to endpoint (p=0.09). However, there was no significant differences in the rate of change from week 1 to study endpoint between the extensive metabolizers and reduced metabolizers groups (p=0.60). Follow-up analyses indicated differences in the slope of dosing change starting from week 4 and continuing through week 6 in the ultrarapid metabolizers compared to extensive metabolizers (week 4 vs week 1 p=0.04; week 5 vs week 1 p=0.016; week 6 vs week 1 p=0.02) indicating differences in the trajectory of dose escalation/titration such that ultrarapid metabolizers exhibited a slower rate of change in dose over time. The differences in the slope of dosing change was evident at week 6 when comparing the ultrarapid metabolizers to reduced metabolizers (p=0.0025).
Figure 2. Escitalopram dose titration.
Participants were initiated on a dose of 2.5 mg escitalopram at the beginning of week 1 of the study followed by weekly increases to 5, 10, 15, and finally 20 mg po qd. If participants experienced adverse effects, the titration escalation was altered to maintain a tolerated dose. Results shown are stratified by CYP2C19 metabolizer groups. Significance values represent differences in the linear rate of dosing change from week 1 to time of follow-up assessments. Follow-up analyses indicated differences in the slope of dosing change starting from week 4 and continuing through week 6 in the ultrarapid metabolizers compared to extensive metabolizers (week 4 vs week 1 p=0.04; week 5 vs week 1 p=0.016; week 6 vs week 1 p=0.02). The difference in the slope of dosing change was evident at week 6 when comparing the ultrarapid metabolizers to reduced metabolizers (p=0.0025).
Discussion
In this pharmacogenetic study of escitalopram for the treatment of ASD, genetically-defined metabolizer status for CYP2C19 was assessed for relationships with clinical response as well as dosing during 6 weeks of study. Clinical symptoms as measured by the ABC-CV rating scale improved over the course of treatment and the magnitude or rate of improvement did not differ significantly across genotype groups. In an examination of tolerance to the titration schedule used in the study, secondary analyses identified that ultrarapid metabolizers had a slower rate of dosing change compared to other groups. To our knowledge this study represents the first to examine CYP2C19 pharmacogenetics of escitalopram in ASD.
Pharmacogenetic studies of escitalopram or citalopram (racemic mixture of R-(−)-citalopram and S-(+)-citalopram) to date have predominantly been conducted in the context of treatment studies in adults with depression [37, 38] or as a part of pharmacokinetic studies of healthy controls [39]. Pharmacogenetic studies of pharmacokinetic parameters have identified reasonably clear and replicated relationships between genetically defined metabolizer groups and overall exposure as well as other pharmacokinetic parameters [39] although the subsequent link to symptom improvement in patients has not been reliable [37, 38]. This disparity perhaps underscores the importance of examining such outcomes in the context of fixed dose or predefined titration studies which are arguably more likely to elucidate genotype group differences than flexible dose designs where ‘the art’ of antidepressant dosing by the clinician may obfuscate such effects.
To this end the differences observed across genotype groups in the present study were related to the tolerance of the titration schedule, although the findings were not in the hypothesized direction. The finding that ultrarapid metabolizers had slower rates of dose increases and trends toward lower 6 week doses is intriguing given that most would anticipate that reduced or poor metabolizers would be most likely to exhibit dose-related adverse effects and in turn be more likely to experience difficulties with a set titration scheme. Additionally, the disparity between identifying pharmacogenetic findings of dosing data but not irritability measures is also intriguing given that self-reported irritability was an indicator for altering the dose escalation sequence.
Our understanding of these relationships may benefit from careful consideration of what is known (and unknown) about the pharmacokinetic characteristics of escitalopram and its metabolites, as well as additional studies closely examining pharmacogenetic and pharmacokinetic findings in ASD using structured dosing designs. The pharmacokinetic parameters of antidepressant medications are often different in children and adolescents as compared to adults [40]. In the case of escitalopram and citalopram, the half-lives appear to be approximately 30% (~10hr) shorter in adolescents (~19hr as compared to ~29hr in adults), which may correspond to an increased clearance, shorter time to peak concentration, and lower overall exposure (as measured by area under the concentration curve) [40]. One would expect that these differences would be even more pronounced in those who are ultrarapid metabolizers, where drug clearance may be increased and half-lives are likely to be shorter. Shorter half-lives are generally associated with lower steady-state concentrations, but also with a shorter time to steady-state. The notion of “start low and go slow” with respect to antidepressant titration has long been appreciated, particularly in treatment of anxiety-spectrum disorders as well as children and adolescents [41] where patients are thought to be more sensitive to the activating properties of SSRIs. Accommodation to a dose increase is an important factor in this process and our observations of a lack of tolerance to dose increases in faster metabolizers may be hypothesized to be associated to a faster time to steady-state as opposed to the final steady-state concentration itself. This would be consistent with subjective reports of exacerbated irritability in some patients soon after a dose increase that was not captured by subsequent ABC-CV-Irr ratings. This phenomenon has been previously described in cases presented as part of an earlier treatment study of fluoxetine for ASD and mental retardation whereby some patients were noted to experience side effects after beginning therapy or a dose increase, but these effects abated over time or after returning to a previously tolerated dose [15].
Additional pharmacokinetic and pharmacogenetic studies may help further our understanding of the findings presented herein. The clinical relevance of escitalopram metabolites S-DCT and S-didesmethylcitalopram (S-DDCT) are not well described. Although thought to be less clinically relevant than the parent compound, the effects beyond serotonin, dopamine, and norepinephrine neurotransmitters have not been extensively examined [19]. Ultrarapid metabolizers do not appear to accumulate S-DCT concentrations disproportionately as compared to extensive metabolizers [42, 43], although less has been described with respect to S-DDCT. Additional pharmacogenomic approaches may also be useful. These include the investigation of other potential candidate genes, assessments of de novo copy number variants (CNVs) or single nucleotide variants (SNVs), as well as use of genome-wide association based polygenic analyses which are being used in disease risk or phenotype characterization studies.
The results presented herein must be interpreted in the context of the limitations of our study. Our ability to adequately explain results related to dosing and titration is constrained by the lack of serum concentrations and pharmacokinetic data which were not collected as part of this study. The sample size is modest, which limits our power to exhaustively examine interactions between genotypes and demographic variables, although these were relatively evenly distributed across metabolizer groups and not identified as major contributors to dosing and symptom outcomes. Placebo response measured dichotomously across RCTs of antipsychotics and antidepressants in ASD has ranged from 10–34%, with placebo response more likely in patients with higher levels of disruptive behaviors, mood/autism symptoms, and reported caregiver strain[44]. Due to the open label study design of the present study, we are unable to assess placebo response which may have influenced our ability to detect pharmacogenetic associations with clinical improvement over time. Finally, while we chose the most commonly observed and examined variants in CYP2C19, there may be other rare variants that also influence the activity of this enzyme [35].
Nonetheless the findings presented here represent novel observations using a controlled titration scheme, which is unique in pharmacogenetic studies. Additionally this is the first to examine CYP2C19 pharmacogenetics in ASD patients treated with escitalopram. The results of faster metabolizers being less tolerant of the forced titration protocol are contrary to expectations and underscore the need for follow up studies in this area with a particular focus on the collection of ancillary pharmacokinetic data examining serum concentrations of escitalopram and its metabolites. Given the growing excitement regarding the potential clinical utility of pharmacogenetic information, these findings in ASD highlight the importance of studying drug outcomes across disease states where drugs may be used. SSRI antidepressants such as escitalopram are used in many patients besides adults with major depressive disorder, in whom the bulk of pharmacogenetic analyses have been conducted. Thus we must give careful consideration to the generalization of pharmacogenetic relationships.
Acknowledgments
Source of funding: This work has been supported by NICHD/NINDS/NIEHS P50HD055751 (EHC), NIMH P50MH094267 (EHC), K08MH083888 (JRB), K23092696 (MWM), K01MH098798 (LHR), K23MH082121 (SJ), K01MH064539 (TO)
Shitalben Patel, Kathleen Hennessy, Zengping Hao and Kelley Moore provided expert technical assistance.
Footnotes
Conflicts of interest:
J.R.B is on a scientific advisory board for Physician’s Choice Laboratory Services and Ortho-McNeil Janssen, E.H.C has been a consultant for a Seaside Therapeutics multi-site clinical trial. SJ serves as a part of a Roche Pharmaceuticals multi-site clinical trial. The remaining authors report no disclosures.
References
- 1.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision: DSM-IV-TR. [Google Scholar]
- 2.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, D.C: American Psychiatric Association; 2013. DSM-5. [Google Scholar]
- 3.CDC. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002) 2014;63(2):1–21. [PubMed] [Google Scholar]
- 4.Volkmar F, Siegel M, Woodbury-Smith M, King B, McCracken J, State M. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2014;53(2):237–57. doi: 10.1016/j.jaac.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Mandell DS, Morales KH, Marcus SC, Stahmer AC, Doshi J, Polsky DE. Psychotropic medication use among Medicaid-enrolled children with autism spectrum disorders. Pediatrics. 2008;121(3):e441–8. doi: 10.1542/peds.2007-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg RE, Mandell DS, Farmer JE, Law JK, Marvin AR, Law PA. Psychotropic medication use among children with autism spectrum disorders enrolled in a national registry, 2007–2008. J Autism Dev Disord. 2010;40(3):342–51. doi: 10.1007/s10803-009-0878-1. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan G, McCracken JT. Psychopharmacology of autism spectrum disorders. Pediatr Clin North Am. 2012;59(1):175–87. xii. doi: 10.1016/j.pcl.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(6):919–29. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(6):571–9. doi: 10.1089/cap.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan SL, Nicholas JS, Carpenter LA, King LB, Garrett-Mayer E, Charles JM. High prescription drug use and associated costs among Medicaid-eligible children with autism spectrum disorders identified by a population-based surveillance network. Ann Epidemiol. 2012;22(1):1–8. doi: 10.1016/j.annepidem.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582–9. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- 12.Hollander E, Soorya L, Chaplin W, Anagnostou E, Taylor BP, Ferretti CJ, et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. AJ Psychiatry. 2012;169(3):292–9. doi: 10.1176/appi.ajp.2011.10050764. [DOI] [PubMed] [Google Scholar]
- 13.King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66(6):583–90. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDougle CJ, Naylor ST, Cohen DJ, Volkmar FR, Heninger GR, Price LH. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry. 1996;53(11):1001–8. doi: 10.1001/archpsyc.1996.01830110037005. [DOI] [PubMed] [Google Scholar]
- 15.Cook EH, Jr, Rowlett R, Jaselskis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry. 1992;31(4):739–45. doi: 10.1097/00004583-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Najjar F, Owley T, Mosconi MW, Jacob S, Hur K, Guter SJ, et al. Pharmacogenetic study of serotonin transporter and 5HT2A genotypes in autism. J Child Adolesc Psychopharmacol. 2015 doi: 10.1089/cap.2014.0158. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owley T, Brune CW, Salt J, Walton L, Guter S, Ayuyao N, et al. A pharmacogenetic study of escitalopram in autism spectrum disorders. Autism Res. 2010;3(1):1–7. doi: 10.1002/aur.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owley T, Walton L, Salt J, Guter SJ, Jr, Winnega M, Leventhal BL, et al. An open-label trial of escitalopram in pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2005;44(4):343–8. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- 19.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2–3):249–58. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 20.Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281–90. doi: 10.2165/00003088-200746040-00002. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Schaid DJ, Desta Z, Kubo M, Batzler AJ, Snyder K, et al. Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br J Clin Pharmacol. 2014;78(2):373–83. doi: 10.1111/bcp.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drozda K, Muller DJ, Bishop JR. Pharmacogenomic testing for neuropsychiatric drugs: current status of drug labeling, guidelines for using genetic information, and test options. Pharmacotherapy. 2014;34(2):166–84. doi: 10.1002/phar.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 24.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview, Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 25.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Services; 2001. [Google Scholar]
- 26.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule. 2. Torrance, CA: Western Psychological Services; 2012. (ADOS-2) Manual (Part I): Modules 1–4. [Google Scholar]
- 27.Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89(5):485–91. [PubMed] [Google Scholar]
- 28.Elliott CD. Differential Abilities Scales. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 29.Elliott CD. Differential abilities scales. 2. San Antonio, TX: Pearson; 2007. (DAS-II) [Google Scholar]
- 30.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. AGS Edition. [Google Scholar]
- 31.Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 32.Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 4. Minneapolis MN: Pearson; 2007. [Google Scholar]
- 33.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 34.Kim KA, Song WK, Kim KR, Park JY. Assessment of CYP2C19 genetic polymorphisms in a Korean population using a simultaneous multiplex pyrosequencing method to simultaneously detect the CYP2C19*2, CYP2C19*3, and CYP2C19*17 alleles. J Clin Pharm Ther. 2010;35(6):697–703. doi: 10.1111/j.1365-2710.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- 35.Scott SA, Sangkuhl K, Shuldiner AR, Hulot JS, Thorn CF, Altman RB, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenetics and genomics. 2012;22(2):159–65. doi: 10.1097/FPC.0b013e32834d4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- 37.Hodgson K, Tansey K, Dernovsek MZ, Hauser J, Henigsberg N, Maier W, et al. Genetic differences in cytochrome P450 enzymes and antidepressant treatment response. J Psychopharmacol (Oxf) 2014;28(2):133–41. doi: 10.1177/0269881113512041. [DOI] [PubMed] [Google Scholar]
- 38.Mrazek DA, Biernacka JM, O’Kane DJ, Black JL, Cunningham JM, Drews MS, et al. CYP2C19 variation and citalopram response. Pharmacogenetics and genomics. 2011;21(1):1–9. doi: 10.1097/fpc.0b013e328340bc5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang M, Tybring G, Dahl ML, Lindh JD. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: a systematic review and meta-analysis. Clin Pharmacokinet. 2014;53(9):801–11. doi: 10.1007/s40262-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 40.Findling RL, McNamara NK, Stansbrey RJ, Feeny NC, Young CM, Peric FV, et al. The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J Child Adolesc Psychopharmacol. 2006;16(1–2):131–45. doi: 10.1089/cap.2006.16.131. [DOI] [PubMed] [Google Scholar]
- 41.Kodish I, Rockhill C, Ryan S, Varley C. Pharmacotherapy for anxiety disorders in children and adolescents. Pediatr Clin North Am. 2011;58(1):55–72. x. doi: 10.1016/j.pcl.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Ohlsson Rosenborg S, Mwinyi J, Andersson M, Baldwin RM, Pedersen RS, Sim SC, et al. Kinetics of omeprazole and escitalopram in relation to the CYP2C19*17 allele in healthy subjects. Eur J Clin Pharmacol. 2008;64(12):1175–9. doi: 10.1007/s00228-008-0529-z. [DOI] [PubMed] [Google Scholar]
- 43.Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008;83(2):322–7. doi: 10.1038/sj.clpt.6100291. [DOI] [PubMed] [Google Scholar]
- 44.King BH, Dukes K, Donnelly CL, Sikich L, McCracken JT, Scahill L, et al. Baseline factors predicting placebo response to treatment in children and adolescents with autism spectrum disorders: a multisite randomized clinical trial. JAMA pediatrics. 2013;167(11):1045–52. doi: 10.1001/jamapediatrics.2013.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]