Abstract
Chemokines and their receptors are involved in oncogenesis and in tumor progression, invasion, and metastasis. Various chemokines also promote cell proliferation and resistance to apoptosis of stressed cells. The chemokine CXCL8, also known as interleukin-8 (IL-8), is a proinflammatory molecule that has functions within the tumor microenvironment. Deregulation of IL-8 signaling is shown to play pivotal roles in tumorigenesis and progression. Mallory-Denk Bodies (MDBs) are prevalent in various liver diseases including alcoholic hepatitis (AH) and are formed in mice livers by feeding DDC. By comparing AH livers where MDBs had formed with normal livers, there were significant changes of IL-8 signaling by RNA sequencing (RNA-Seq) analyses. Real-time PCR analysis of CXCR2 further shows a 6-fold up regulation in AH livers and a 26-fold up regulation in the livers of DDC re-fed mice. IL-8 mRNA was also significantly up regulated in AH livers and DDC re-fed mice livers. This indicates that CXCR2 and IL-8 may be crucial for liver MDB formation. MDB containing balloon hepatocytes in AH livers had increased intensity of staining of the cytoplasm for both CXCR2 and IL-8. Over expression of IL-8 leads to an increase of the mitogen activated protein kinase (MAPK) cascade and exacerbates the inflammatory cycle. These observations constitute a demonstration of the altered regulation of IL-8 signaling in the livers of AH and mice fed DDC where MDBs formed, providing further insight into the mechanism of MDB formation mediated by IL-8 signaling in AH.
Keywords: Mallory-Denk bodies, alcoholic hepatitis, IL-8 signaling, CXCR2
Introduction
Mallory-Denk bodies (MDBs) are composed of intracellular aggregations of misfolded proteins in ballooned hepatocytes. They consist of abnormally phosphorylated, ubiquitylated, and cross-linked keratins 8 and 18 (K8/K18) and non-keratin components (French et al., 2010; Haybaeck et al., 2012). A major player that determines MDB formation is the ballooned hepatocyte. MDB-forming hepatocytes stain positive for numerous markers of preneoplasmic change (French et al., 2011). MDBs form due to the failure of the 26S proteasome protein quality control system which leads to aggresomes composed of cytokeratins (CKs) and undigested proteins such as heat shock proteins (HSPs), Ub, proteasome subunits, tubulin, and the ubiquitin-binding protein p62 (Yuan et al., 1996). It was found that the pathogenesis of MDBs is associated with the down regulation of the ufm1-conjugation system (Ufmylation) and FAT10-conjugation system (FATylation) pathways involved in protein quality control (Liu et al., 2014b). The swelling of the balloon cell cytoplasm is due to the osmotic effect of these undigested proteins. MDBs develop in the liver of DDC re-fed mice. In the DDC fed mouse model where liver cells proliferate, MDBs form and later, after DDC withdrawal (DDC primed hepatocytes), hepatocellular carcinoma (HCC) develops (Li et al., 2008; Oliva et al., 2008). Three new mechanisms of MDB formation (epigenetic mechanisms, shift from the 26S proteasome to the immunoproteasome and activation of Toll-like receptors signaling) have recently been identified (French et al., 2010). However, the detailed molecular events involved in liver MDB formation, especially in human liver disease development, remain undetermined.
Interleukin 8 (IL-8), also known as CXCL8, is a CXC-type chemokine produced in an inflammatory microenvironment. It aggravates the inflammatory state and enables cancer cells to survive and to migrate from the primary site (Matsushima et al., 1992; Rollins, 2009). IL-8 is one of the dominant transcriptional targets of inflammatory signaling mediated by NFκB, which is commonly activated in cancer cells (Gales et al., 2013). IL-8 signaling is involved in regulating the communication between different cell types such as cancer cells, endothelial cells, neutrophils, and tumor-associated macrophages within the tumor microenvironment (Vandercappellen et al., 2008). IL-8 signals primarily through the receptors CXCR1 and CXCR2, present in various types of normal as well as cancerous cells (Gales et al., 2013). The enzyme PI3-kinase (PI3K) is a principal effector of IL-8-mediated chemotaxis in neutrophils (Lane et al., 2006; Li et al., 2012). Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells (Singh et al., 2013), suggesting that IL-8 and its receptors may be attractive targets for cancer therapy. Despite these reports, the biological significance of IL-8 signaling in AH with MDB formation remains unclear.
In this study, significant changes in IL-8 signaling were observed by comparing AH livers where MDBs had formed with normal livers. These changes were obtained by RNA sequencing (RNA-Seq) analyses. The altered expression of IL-8 and CXCR2 was confirmed in the livers of DDC re-fed mice and human liver biopsies from AH livers, indicating a crucial role of IL-8 signaling during MDB formation.
Materials and Methods
Liver biopsy specimens
Human formalin-fixed paraffin-embedded (FFPE) liver biopsies from patients who had alcoholic hepatitis (AH; n=3–5) were obtained from Harbor UCLA hospital archives. In all the cases liver forming MDBs were presented. Normal control livers were used for comparison. The liver biopsies used were also used in previous studies (French et al., 2012; Liu et al., 2014a; Liu et al., 2014b). The liver biopsy sections were 4 µm thick.
Mouse liver
Diethyl 1, 4-dehydro-2, 4, 6-trimethyl-3, 5-pyridine-dicarboxylate (DDC) was used to induce the formation of Mallory-Denk bodies (MDBs) in mice. One-month-old C3H male mice were fed 0.1% DDC added to the control diet and a second group were fed control diet for 10 weeks (Li et al., 2008). The mice were then withdrawn from the drug for 1 month and re-fed DDC for 7 days as previously done (Oliva et al., 2009). Three mice were used in each of two groups as follows: 1) control, 2) DDC. DDC was re-fed for 7 days. Mice livers were placed in isopentane which is in liquid nitrogen, fast freezing the tissues. The tissues were then stored at −80°C. The livers used had been used in prior studies (Liu et al., 2014a; Liu et al., 2014b; Oliva et al., 2009). All mice were treated in a humane manner as approved by the Animal Care Committee at Harbor UCLA Laboratory BioMedical Research Institute according to the Guidelines of the National Academy of Science.
Tissue sectioning
Mice liver frozen sections were cut 5 µm thick at −20°C and immediately transferred to a micro slide box kept on dry ice and stored at −80°C. A new blade was used for each frozen sample.
RNA isolation
RNA isolation of FFPE sections of human liver biopsies was performed as previously described (Liu et al., 2014b). RNA (5µg) was treated and the quality and yield were assessed by electrophoresis using the Agilent 2100 bioanalyzer (Agilent, Palo Alto, CA, USA).
RNA sequencing (RNA-Seq)
Libraries for RNA-Seq were prepared with Nugen Ovation Human FFPE RNA-Seq Multiplex System. The workflow consists of double-stranded cDNA generation using a mixture of random and poly (T) priming, fragmentation of double stranded cDNA, end repair to generate blunt ends, adaptor ligation, strand selection via nucleotide analog-targeted degradation, InDA-C-mediated adaptor cleavage and PCR amplification to produce the final library. Different adaptors were used for multiplexing samples in one lane. Sequencing was performed on Illumina HiSeq 2500 for a single read 50 run. Data quality check was done on Illumina SAV. Demultiplexing was performed with Illumina CASAVA 1.8.2. The gene expression level was calculated using RSEM software (Li and Dewey, 2011). TPM (transcript per million) was used to normalized the gene expression. Only genes that were significantly modulated (false discovery rate (FDR)-adjusted; p-value <0.05) and more than a 2 fold change were considered differentially expressed in the AH livers compared with normal livers. The pathway and network analysis was performed using Ingenuity Pathway Analysis (IPA). IPA computes a score for each network according to the fit of the set of supplied focus genes. These scores indicate the likelihood of the focus genes to belong to a network versus those obtained by chance. A score >2 indicates a ≤ 99% confidence that a focus gene network was not generated by chance alone. The data presented here are accessible at the UCLA website (http://hpc.ucla.edu/hoffman2/file-transfer/gol.php).
Quantitative Real-time PCR
Real-time PCR was performed as previously described (Liu et al., 2014b). Primer sequences are as follows: CXCR2 (human; NM_001557) forward primer: 5’-TCTTCTTCAGGCACACTTC-3’, reverse primer: 5’-AGAACGTGGCCTCCTCTAACT-3’; CXCR2 (mouse; NM_009909) forward primer: 5'-CTGCCTTAACCCCATCATCT-3', reverse primer: 5'-GCCATGCTGAAAGACAAGAA-3'; IL-8 (human; NM_000584) forward primer: 5'-TAGCAAAATTGAGGCCAAGG-3', reverse primer: 5'-AGCAGACTAGGGTTGCCAGA-3'; IL-8 (mouse; NM_011339) forward primer: 5'-CGGCAATGAAGCTTCTGTAT-3', reverse primer: 5'-CCTTGAAACTCTTTGCCTCA-3'.
Immunohistochemical analysis
FFPE tissue slides were double stained for CXCR2 and IL-8 (Abcam Inc., Cambridge MA) with Ubiquitin (Millipore, Temecula, CA). A second set of slides was stained for CXCR2 and IL-8 (Enzo Life Sciences, Farmingdale, NY) with Ubiquitin (Millipore, Temecula, CA). CXCR2 and IL-8 were detected using donkey anti rabbit Alexa Fluor 488 (Jackson Immuno Research Laboratories Inc. West Grove, PA). Ubiquitin was detected using donkey anti mouse Alexa Fluor 594 (Jackson Labs. West Grove, PA). All slides were stained with the nuclear stain DAPI (Molecular Probes, Eugene, OR). The fluorescence intensity of stained protein of interest was measured quantitatively using a 40× objective and a standard exposure time of 800ms using a Nikon 400 fluorescent microscope with three filters (FITC-green, Texas Red, and Tri-Color), and the Nikon morphometric system.
Statistical analysis
Statistical significance was determined using the t-test and One Way ANOVA test with SigmaStat software. P< 0.05 was considered as a statistically significant difference. Regression plots were constructed using SigmaPlot software. All data were presented as the mean ±S.E.M and were representative of at least three-independent experiments done in triplicate.
Results
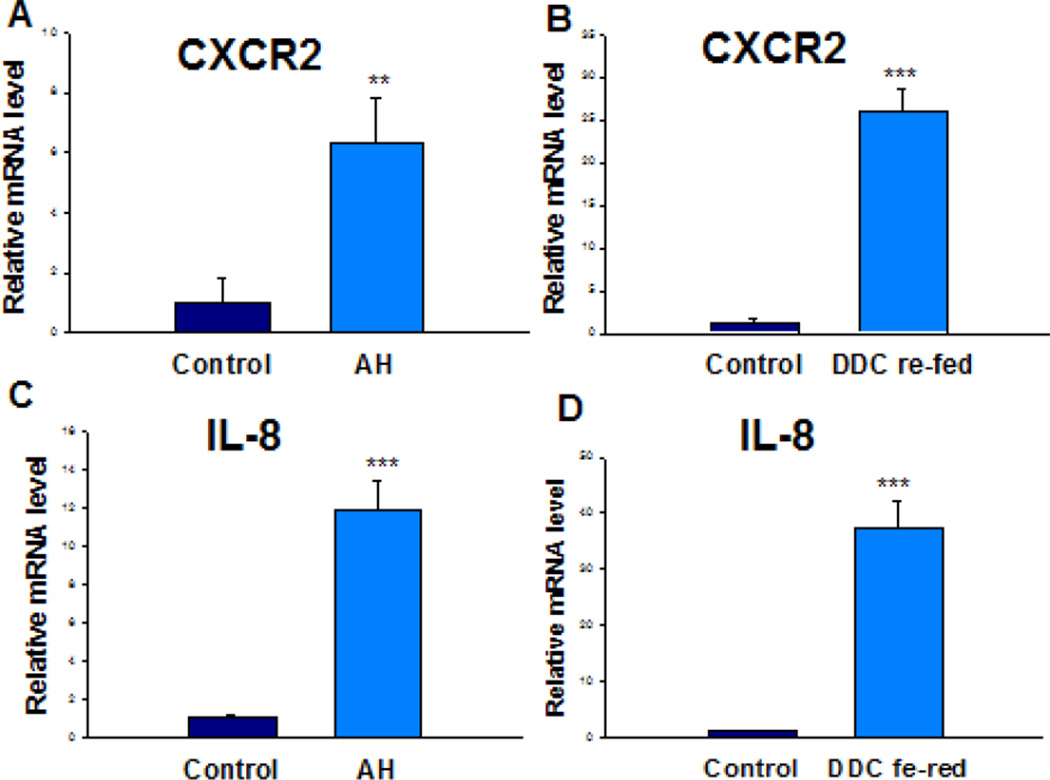
The Ingenuity Pathway Analysis (IPA) revealed the marked change of IL-8 signaling (z-score=2.309) in AH livers where MDBs had formed (Table 1). A schematic diagram of the IL-8 signaling derived using IPA was shown (Figure 1). CXCR2 and IL-8, two central elements of this pathway, were selected and quantified to verify the RNA-Seq data by qRT-PCR on all AH livers and normal livers. DDC feeding was used to induce MDB formation in mice. Hepatocyte isolation was performed from mice fed the control diet and mice re-fed DDC for 7 days as described earlier (Oliva et al., 2009). As expected, IL-8 and CXCR2 mRNAs tested were up regulated both in the livers of AH and DDC re-fed mice (Figure 2). Among them, approximately a 6-fold up regulation in AH livers and a 26-fold up regulation in DDC re-fed mice livers of CXCR2 mRNA were observed (p<0.05) (Fig. 2A and 2B). In contrast to CXCR2, IL-8 mRNA was induced to 11-fold in AH liver biopsies and 37-fold levels in livers of DDC re-fed mice (p<0.05) (Fig. 2C and 2D). Other components of this pathway including Bax, Bcl-2 and Nox4, were also up regulated to various degrees in the livers of AH patients with MDBs present (Figure 1). These data clearly suggest that these key regulators might be involved in liver MDB formation.
Table 1.
IL-8 signaling is deregulated in AH livers with MDBs.
| IPA category | Pathway −log (P-valuea) |
Gene symbol |
Molecules in dataset | Ratio |
|---|---|---|---|---|
| BRCA1-mediated | 1.41E00 | CXCR2 | Chemokine (C-X-C motif) receptor 2 | 6.56E-02 |
| NOX4 | NADPH oxidase 4 | |||
| FLT4 | Fms-related tyrosine kinase 4 | |||
| BCL-2 | B-cell CLL/lymphoma 2 | |||
| MAP2K1 | Mitogen-activated protein kinase kinase 1 | |||
| ATM | ATMserine/threonine kinase | |||
| BAX | BCL-2 associated X protein | |||
| PIK3CB | Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit beta | |||
| GNG11 | Guanine nucleotide binding protein (G protein), gamma11 | |||
| GNG2 | Guanine nucleotide binding protein (G protein), gamma 2 | |||
| GNAI1 | Guanine nucleotide binding protein (G protein), alpha inhibiting activity polypeptide 1 |
|||
| LIMK2 | LIM domain kinase 2 | |||
| PTGS2 | prostaglandin-endoperoxide synthase 2 | |||
Abbreviations: FDR, false discovery rate; IPA, ingenuity pathway analysis.
Fischer’s exact test was used to calculate the P-value, determining the probability that the association between the genes in the data set and the canonical pathway is explained by chance alone. To account for multiple canonical pathways tested by IPA, the FDR option was used (FDR<0.1).
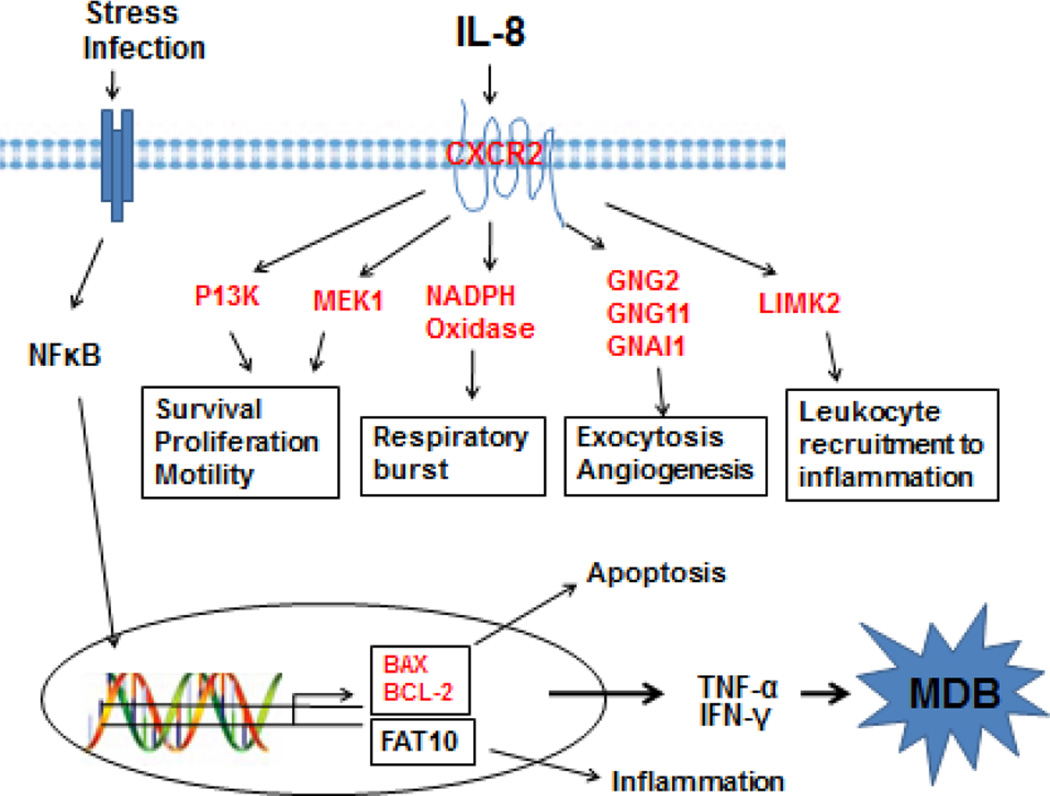
Figure 1.
Altered modulation of the IL-8 signaling pathway in AH livers with MDBs. Changed expression of the IL-8 signaling pathway was shown at the transcriptional level in AH livers compared with normal livers. The altered modulation of the IL-8 signaling pathway components contribute to the constitutive hyperactivation of this pathway, resulting the cell viability, inflammation and respiratory burst as shown in diagram. The red symbols indicate the genes with relative higher expression in alcoholic hepatitis vs normal livers.
Figure 2.
Induction of CXCR2 and IL-8 in AH livers and in the livers of DDC re-fed mice. Quantification of mRNA was carried out by SYBR real-time PCR assays. ** p<0.01 and ***p<0.001 by t-test.
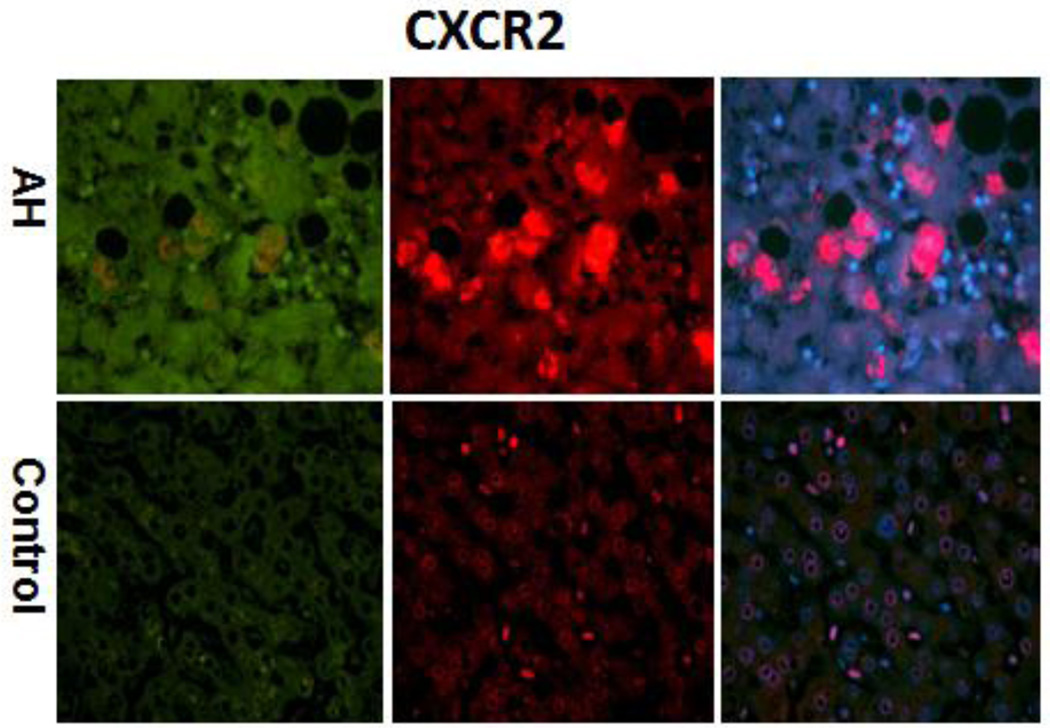
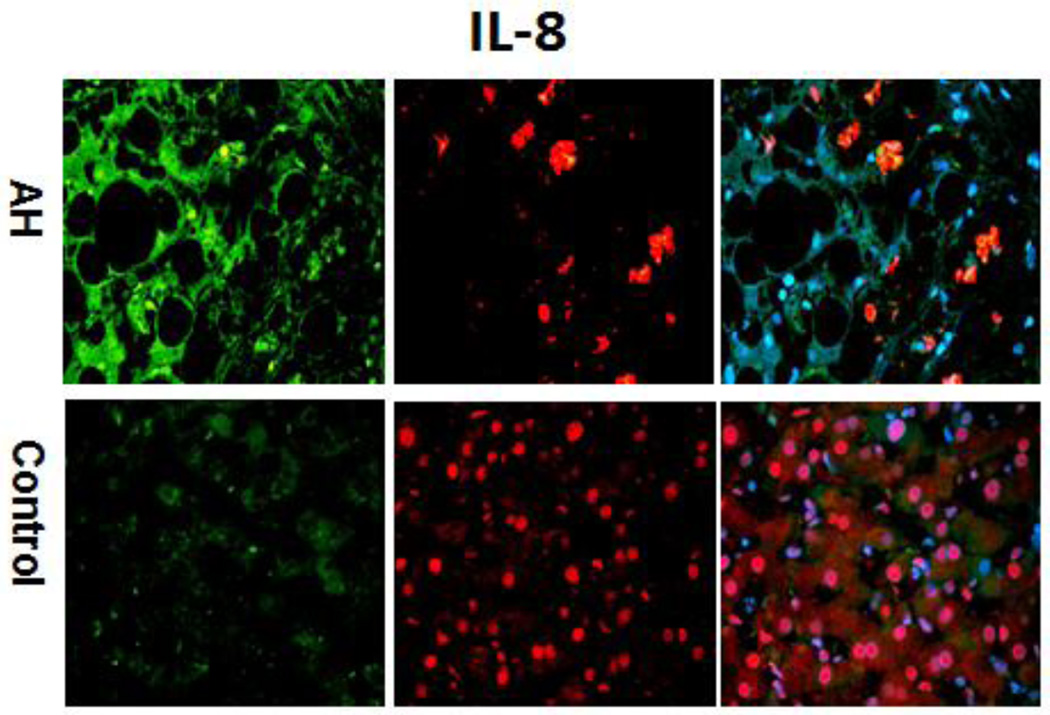
The immunohistochemical staining done on all AH livers tested in this RNA-Seq analyses showed that hepatocytes in AH liver biopsies had increased cytoplasm staining for CXCR2 (Figure 3). IL-8 also had increased staining of the cytoplasm hepatocytes of AH biopsies (Figure 4). These results suggest that the pathogenesis of liver MDB formation is linked to up regulation of the IL-8 signaling and could explain the MDB-pmn satellitosis phenomenon.
Figure 3.
The liver section from AH patients and controls were double stained with antibodies to CXCR2 (green), Ubiquitin (red) and DAPI (tricolor). AH liver sections stained with more intensity for CXCR2 compared to controls. ×324
Figure 4.
The liver sections from AH patients and controls were double stained with antibodies to IL-8 (green), Ubiquitin (red) and DAPI (tricolor). The AH liver sections stained with greater intensity for IL-8 compared to the controls. ×324
Discussion
MDBs are found in various hepatic diseases such as AH, hepatitis B, C, and HCC (Basaranoglu et al., 2011). To better understand the pathogenesis of MDBs in AH patients, it is crucial to know the development of ballooning hepatocytes. Ballooning or swelling of hepatocytes is induced by oxidative stress, which is also the main effect of alcohol exposure and its products such as oxyradicals. However, this mechanism is not fully understood. Several reports have investigated the molecular mechanisms underlying the complex pathogenesis of MDB formation in chronic hepatitis patients and in the DDC re-fed mice model (French et al., 2010; Basaranoglu et al., 2011).
In this study, an altered regulation of IL-8 signaling by a whole-genome expression analysis (RNA-Seq) was discovered in AH livers with MDBs. The up regulation of IL-8 and CXCR2 was confirmed by qRT-PCR in the livers of DDC re-fed mice, indicating a correlation of IL-8 and CXCR2 with MDB formation. This is the first study to report the changes in IL-8 and CXCR2 expression associated with MDB formation in human and mice livers. The marked increase of mRNA levels of other components of this pathway was also observed (Table 1). Taking into account the important link between IL-8 signaling and oxidative stress (Fernandes et al., 2008; Ivison et al., 2010), it is possible that IL-8 signaling may play a crucial role in oxidative stress and liver MDB formation. The immunostaining results from our lab showed that MDB-forming hapatocytes stained positive for IL-8 and CXCR2 supporting this hypothesis.
Further, NFκB, a transcription factor with pleiotropic effects and a downstream mediator of growth signaling, which controls the expression of genes that promote cell growth and survival, seems to be the main regulator controlling IL-8 gene activity (Freund et al., 2004). There is persistent NFκB activation in cancers associated with chronic inflammation. However, the inhibition of NFκB activity results in down regulation of IL-8 expression levels and inhibition of cell proliferation and metastasis (Patel et al., 2002).
Recently, the prominent up regulation expression of FAT10 in the livers of AH patients was observed (Liu et al., 2014b). FAT10 expression is induced by interferon (IFN)-γ and tumor necrosis factor α (TNFα) in tumor cells (Lukasiak et al., 2008; Ren et al., 2011) and activates NFκB, which in turn up regulates CXCR4/7 (Gao et al., 2014; Liu et al., 2014c). Interestingly, the NFκB binding site was found at the FAT10 promoter region (Zhang et al., 2006), while TNFα-dependent induction of FAT10 expression requires NFκB activation (Ren et al., 2011). The interferon sequence response element (ISRE) located on the FAT10 promoter region activates NFκB in response to IFNγ (Oliva et al., 2010). These findings indicate a potential feedback system in chronic inflammatory-associated microenvironments such as in AH. NFκB has recently been found to bind a transcript factor for both CXCR4 and CXCR7 receptors (Tarnowski et al., 2010), and CXCR4 and CXCR7 can also activate NFκB (Huang et al., 2009; Liu et al., 2014c), suggesting IL-8-mediated receptors may sustain NFκB activity by a feedback system. Our results confirmed that the IL-8 and CXCR2 mRNAs was up regulated in MDB-forming (FAT10-over-expressing) hepatocytes, indicating a potential feedback system within NFκB network. The gene expression changes induced the up regulation of IL-8 signaling may be a novel mechanism in liver MDB formation. The further elucidation of the relationship of IL-8 signaling with NFκB will provide a better understanding of chronic liver disease pathogenesis and MDB formation.
IL-8 may also regulate the activity of the mitogen activated protein kinase (MAPK) cascade in cancers, where there is a crosstalk with the epidermal growth factor receptor (EGFR) pathway through the activation of CXCR1/2. IL-8 activates the classical MAPK signaling cascade, with downstream phosphorylation of Erk1/2 in neutrophils and cancer cells (Luppi et al., 2007). MAP2K1 (MEK1) mRNA was found to be significantly up regulated in AH livers with MDBs (Figure 1) in this test. Activation of MAPK signaling is consistent with the promotion, by IL-8, of proliferation and survival for various types of cells (Luppi et al., 2007; MacManus et al., 2007). The classical cascade between Erk and MAPK signaling describes a pathway linking IL-8 to the activation of E2F and activator protein transcription factors, the main function of which is to regulate the transcription of genes (Aggarwal and Sung, 2011; Sparmann and Bar-Sagi, 2004), favoring cell proliferation and MDB formation.
In summary, our data demonstrates for the first time the gene expression changes of IL-8 signaling in the livers of AH where MDB were formed. The prominent transcription up regulation expression of IL-8 and CXCR2 were observed in FAT10 over-expressing hepatocytes, indicating that IL-8 and CXCR2 may be attractive targets for AH therapy. The data provide evidence to further understand MDB formation and the inhibition of liver cell regeneration.
Acknowledgments
This work was supported by grants from NIH (AAU01021898-03) and P50-11999 Morphology Core.
Abbreviations
- AH
alcoholic hepatitis
- CXCR1/2
human chemokine (C-X-C motif) receptor ½
- DDC
diethyl 1, 4-dehydro-2, 4, 6-trimethyl-3, 5-pyridine-dicarboxylate
- FFPE
archived formalin-fixed paraffin-embedded
- HCC
hepatocellular carcinoma
- IL-8
interleukin 8
- MDB
Mallory-Denk body
- RNA-Seq
RNA sequencing
- TNFα
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Aggarwal BB, Sung B. NF-kappaB in cancer: a matter of life and death. Cancer Discov. 2011;1:469–471. doi: 10.1158/2159-8290.CD-11-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaranoglu M, et al. Mallory-Denk Bodies in chronic hepatitis. World J Gastroenterol. 2011;17:2172–2177. doi: 10.3748/wjg.v17.i17.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AF, et al. Oxidative inactivation of the proteasome in retinal pigment epithelial cells. A potential link between oxidative stress and up-regulation of interleukin-8. J Biol Chem. 2008;283:20745–20753. doi: 10.1074/jbc.M800268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French BA, et al. Mallory-Denk bodies form when EZH2/H3K27me3 fails to methylate DNA in the nuclei of human and mice liver cells. Exp Mol Pathol. 2012;92:318–326. doi: 10.1016/j.yexmp.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW, et al. The role of innate immunity in the pathogenesis of preneoplasia in drug-induced chronic hepatitis based on a mouse model. Exp Mol Pathol. 2011;91:653–659. doi: 10.1016/j.yexmp.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SW, et al. Mallory-Denk body pathogenesis revisited. World J Hepatol. 2010;2:295–301. doi: 10.4254/wjh.v2.i8.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, et al. Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene. 2004;23:6105–6114. doi: 10.1038/sj.onc.1207815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales D, et al. The Chemokine CXCL8 in Carcinogenesis and Drug Response. ISRN Oncol. 2013;2013:859154. doi: 10.1155/2013/859154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, et al. FAT10, an ubiquitin-like protein, confers malignant properties in non-tumorigenic and tumorigenic cells. Carcinogenesis. 2014;35:923–934. doi: 10.1093/carcin/bgt407. [DOI] [PubMed] [Google Scholar]
- Haybaeck J, et al. Genetic background effects of keratin 8 and 18 in a DDC-induced hepatotoxicity and Mallory-Denk body formation mouse model. Lab Invest. 2012;92:857–867. doi: 10.1038/labinvest.2012.49. [DOI] [PubMed] [Google Scholar]
- Huang CY, et al. Stromal cell-derived factor-1/CXCR4 enhanced motility of human osteosarcoma cells involves MEK1/2, ERK and NF-kappaB-dependent pathways. J Cell Physiol. 2009;221:204–212. doi: 10.1002/jcp.21846. [DOI] [PubMed] [Google Scholar]
- Ivison SM, et al. Oxidative stress enhances IL-8 and inhibits CCL20 production from intestinal epithelial cells in response to bacterial flagellin. Am J Physiol Gastrointest Liver Physiol. 2010;299:G733–G741. doi: 10.1152/ajpgi.00089.2010. [DOI] [PubMed] [Google Scholar]
- Lane HC, et al. Cbl and Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated chemotaxis. Int Immunol. 2006;18:1315–1325. doi: 10.1093/intimm/dxl064. [DOI] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. S-adenosylmethionine prevents Mallory Denk body formation in drug-primed mice by inhibiting the epigenetic memory. Hepatology. 2008;47:613–624. doi: 10.1002/hep.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MQ, et al. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- Liu H, et al. Mallory-Denk Body (MDB) formation modulates ufmylation expression epigenetically in alcoholic hepatitis (AH) and non-alcoholic steatohepatitis (NASH) Exp Mol Pathol. 2014a;97:477–483. doi: 10.1016/j.yexmp.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. Ufmylation and FATylation pathways are downregulated in human alcoholic and nonalcoholic steatohepatitis, and mice fed DDC, where Mallory-Denk bodies (MDBs) form. Exp Mol Pathol. 2014b;97:81–88. doi: 10.1016/j.yexmp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, et al. TLR3/4 signaling is mediated via the NFkappaB-CXCR4/7 pathway in human alcoholic hepatitis and non-alcoholic steatohepatitis which formed Mallory-Denk bodies. Exp Mol Pathol. 2014c;97:234–240. doi: 10.1016/j.yexmp.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiak S, et al. Proinflammatory cytokines cause FAT10 upregulation in cancers of liver and colon. Oncogene. 2008;27:6068–6074. doi: 10.1038/onc.2008.201. [DOI] [PubMed] [Google Scholar]
- Luppi F, et al. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25–33. doi: 10.1016/j.lungcan.2006.11.014. [DOI] [PubMed] [Google Scholar]
- MacManus CF, et al. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- Matsushima K, et al. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- Oliva J, et al. Fat10 is an epigenetic marker for liver preneoplasia in a drug-primed mouse model of tumorigenesis. Exp Mol Pathol. 2008;84:102–112. doi: 10.1016/j.yexmp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, et al. Betaine prevents Mallory-Denk body formation in drug-primed mice by epigenetic mechanisms. Exp Mol Pathol. 2009;86:77–86. doi: 10.1016/j.yexmp.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva J, et al. The role of cytokines in UbD promoter regulation and Mallory-Denk body-like aggresomes. Exp Mol Pathol. 2010;89:1–8. doi: 10.1016/j.yexmp.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PS, et al. Regulation of constitutive and induced NF-kappaB activation in malignant melanoma cells by capsaicin modulates interleukin-8 production and cell proliferation. J Interferon Cytokine Res. 2002;22:427–435. doi: 10.1089/10799900252952217. [DOI] [PubMed] [Google Scholar]
- Ren J, et al. FAT10 mediates the effect of TNF-alpha in inducing chromosomal instability. J Cell Sci. 2011;124:3665–3675. doi: 10.1242/jcs.087403. [DOI] [PubMed] [Google Scholar]
- Rollins BJ. Where the confusion began: cloning the first chemokine receptors. J Immunol. 2009;183:2893–2894. doi: 10.4049/jimmunol.0990065. [DOI] [PubMed] [Google Scholar]
- Singh JK, et al. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15:210. doi: 10.1186/bcr3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Tarnowski M, et al. Regulation of expression of stromal-derived factor-1 receptors: CXCR4 and CXCR7 in human rhabdomyosarcomas. Mol Cancer Res. 2010;8:1–14. doi: 10.1158/1541-7786.MCR-09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandercappellen J, et al. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Yuan QX, et al. Mallory body induction in drug-primed mouse liver. Hepatology. 1996;24:603–612. doi: 10.1002/hep.510240324. [DOI] [PubMed] [Google Scholar]
- Zhang DW, et al. p53 negatively regulates the expression of FAT10, a gene upregulated in various cancers. Oncogene. 2006;25:2318–2327. doi: 10.1038/sj.onc.1209220. [DOI] [PubMed] [Google Scholar]