Abstract
Schizophrenia is characterized by physical (mainly metabolic and cardiovascular) comorbidity and shortened lifespan. High sensitivity C- reactive protein (hs-CRP), an inflammatory marker of hepatic origin linked to metabolic and cardiovascular diseases and mortality in the general population, has been reported to be elevated in people with schizophrenia. However, the relationship of hs-CRP to psychiatric and medical risk factors, after controlling for potentially confounding variables such as smoking, is not well established in schizophrenia. We assessed hs-CRP levels along with various demographic, psychiatric, and metabolic measures in 88 clinically stable outpatients with schizophrenia or schizoaffective disorder and 71 age epoch-matched comparison subjects with no history of a major psychiatric illness. hs-CRP levels were significantly higher in individuals with schizophrenia than in comparison subjects. Higher hs-CRP levels in schizophrenia group were associated with female gender, more severe negative symptoms, greater medical comorbidity, and worse metabolic risk factors including BMI, fasting glucose, and hemoglobin A1c levels. hs-CRP was not related to age, race, education, smoking status, antipsychotic dosage, or cognitive impairment. Longitudinal studies are needed to investigate the relationship between hs-CRP and long-term health outcomes including metabolic syndrome, cardiovascular disease, and mortality in schizophrenia.
Keywords: Schizophrenia, Inflammation, Aging, Body mass index, hs-CRP, Metabolic disorders
1. Introduction
Schizophrenia is characterized by an attenuated life expectancy (Dickerson et al., 2014; Kirkpatrick et al., 2008). While cardiovascular and metabolic diseases are thought to be the primary contributors to this early mortality (Almeida et al., 2014; Protopopova et al., 2012), immune system dysregulation has also been proposed as a potential mechanism underlying accelerated declines in physical health in people with schizophrenia (Jones et al., 2005). C-Reactive Protein (CRP) is traditionally considered an acute phase response immune marker that is activated by pro-inflammatory cytokines and secreted by hepatocytes (Pepys and Hirschfield, 2003). CRP is also thought to be a biological marker of aging and chronic illness, and a potential indicator of chronic low grade inflammation. CRP is linked with a shorter lifespan in aging cohorts (Harris et al., 1999). Among older adults without psychiatric illnesses, CRP has been also been reported to be related to sociodemographic factors such as age, gender, education, and occupation (Demakakos et al., 2008). Elevated CRP levels have been linked to multiple physical and lifestyle factors in non- psychiatric populations such as those with obesity (Rogowski et al., 2005), metabolic syndrome (Tsimikas et al., 2006), and smoking (Tracy et al., 1997; Dietrich et al., 2007). Therefore, CRP is viewed as one of the biomarkers of aging, and is commonly assayed for aging related health outcomes, specifically intermediate prediction of cardiovascular and metabolic disease risk (Ridker, 2003; Ridker, 2007).
A meta-analysis of eight studies found that CRP levels were higher in people with schizophrenia than in comparison subjects (Miller, Culpepper, & Rapaport, 2014). CRP has been associated with more severe psychopathology (Fan et al., 2007), especially negative symptoms (Fawzi et al., 2011; Garcia-Rizo et al., 2012) and worse cognitive functioning (Dickerson et al., 2007, 2012). However, it remains unclear to what extent the elevation in CRP in schizophrenia is due to specific factors associated with directly with the disorder, including psychopathology and cognitive impairment, or to age-associated factors such as medical comorbidity, metabolic dysfunction, and physical/life style factors (such as smoking). For example, schizophrenia is associated with increased risk of higher body mass index (BMI) (Lopresti and Drummond, 2013) and cardiovascular disease (Miller et al., 2015; Sicras-Mainar et al., 2013). Notably, BMI is a significant predictor of inflammatory marker levels and psychiatric readmission (Fawzi et al., 2015; Manu et al., 2014) in schizophrenia and is closely linked to the incidence of metabolic syndrome (Saatcioglu et al., 2015). An assessment of CRP levels and metabolic risk factors may have utility in improving our understanding of metabolic dysregulation in schizophrenia. However, several of the published studies have important limitations. For example, some of these investigations lacked appropriate comparison groups or included those confounded by inadequate matching on demographic factors related to mortality, especially age and gender (Akanji et al., 2009; Dickerson et al., 2007, 2012; Fawzi et al., 2015).
The aim of the current study was to determine the relationships of high sensitivity CRP (hs-CRP) levels to demographic characteristics, current psychopathology, cognitive function, physical comorbidity, and metabolic markers in people with schizophrenia and age epoch matched comparison subjects with no history of a major psychiatric illness. We hypothesized that hs-CRP levels would be elevated in people with schizophrenia relative to the comparison group after controlling for sociodemographic factors. We also postulated that higher hs-CRP levels in the schizophrenia group would be positively associated with severity of negative symptoms, cognitive impairment, physical comorbidity, and metabolic risk factors including BMI, fasting glucose, and hemoglobin A1c (HbA1c) levels.
2. Methods
2.1 Study Participants
The present report is based on analysis of baseline data from an ongoing longitudinal study of accelerated aging in schizophrenia. Although we previously published data on predictors of self-reported happiness in a subset of this sample (Palmer et al., 2014), the current report represents our first examination of the association of hs-CRP with psychiatric and medical factors. The analyses were restricted to the participants who had a blood draw for the hs-CRP assay. Participants included 88 adult outpatients with schizophrenia (n = 65) or schizoaffective disorder (n = 23), and 71 comparison subjects with no history of major neuropsychiatric illness, matched by age epoch (26–35, 36–45, 46–55, and 56–65 years). All study participants were recruited from the greater San Diego community and interviewed using the Structured Clinical Interview for the DSM-IV-TR (SCID) (First et al., 2002). Subjects were excluded if they had 1) other current DSM-IV-TR Axis I diagnoses; 2) alcohol or other substance (other than tobacco) abuse or dependence within 3 months prior to enrollment; 3) diagnosis of dementia, mental retardation, or a major neurological disorder; 4) or any medical disability that interfered with their ability to complete the study assessments. The protocol was reviewed and approved by the UC San Diego Human Research Protections Program. All study participants provided written informed consent to participate.
2.2. Sociodemographic and clinical characteristics
Sociodemographic characteristics (age, education, gender, race/ethnicity, marital status), current smoking status), and illness-related factors (age of onset and duration of schizophrenia, daily antipsychotic medication dosages) for the study sample were ascertained through participant interview and review of available research and medical records (with the appropriate HIPAA authorization from the study participants). Antipsychotic medication daily dosages were converted to mg. chlorpromazine equivalent (CPZE), based on published standards (Andreasen, et al. 2010).
2.3 Current Psychopathology and Cognitive Functioning
Severity of psychopathology was evaluated with the interviewer-administered Scales for Assessment of Positive Symptoms and Negative Symptoms (SAPS and SANS, respectively) (Andreasen, 1983, 1984). Three global factor scores were computed from the SAPS and SANS: positive (delusions and hallucinations), negative (affective flattening or blunting, alogia, avolition-apathy, inattention, and anhedonia-asociality), and disorganized (bizarre behavior and formal thought disorder) symptoms (Toomey et al., 1997).
Our assessment of cognitive deficits focused on executive functioning as this domain may be particularly relevant to functional decline in schizophrenia (Fucetola et al., 2000; Palmer and Heaton, 2000; Wobrock et al., 2008), Participants were assessed with the following subtests from the Delis-Kaplan Executive Function System (D-KEFS) (Delis, et al., 2001): Trail Making (Letter-Number Sequencing task), Color Word Inhibition (Switching condition), and the Letter Fluency task (total F, A, and S trials). Using the SPSS 21 normal rank function, D-KEFS raw scores were converted to z-scores and coded such that higher scores represented better performance. The mean z-score for the D-KEFS tasks was used as an Executive Functioning Composite score.
2.4 Comorbidity and BMI
Medical Comorbidity
This was measured with the Total Score from the Cumulative Illness Rating Scale, which combines the presence and severity of common medical comorbidities (CIRS; Parmelee et al., 1995).
Body Mass Index (BMI)
BMI was based on assessment of participant height and weight (http://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm).
2.5 Lab Assays
Fasting Blood Glucose and A1c (Glycated or Glycosylated) Hemoglobin
These standard lab assays were done by the UC San Diego Clinical and Translational Research Institute (CTRI) lab. HbA1c refers to the percentage of hemoglobin coated with sugar, and reflects average blood sugar level for the previous two to three months. Higher HbA1c levels suggest worse blood sugar control and higher risk of diabetes and its complications.
high sensitivity C-reactive protein (hs-CRP)
hs-CRP was measured with an assay that has increased sensivity to detect CRP levels at lower ranges that may be associated with intermediate cardiovascular disease risk (Ridker, 2003; Ridker, 2007). Fasting whole blood samples were preserved with Ethylenediaminetetraacetic acid (EDTA). Following centrifugation, the plasma was stored at −80°C until assay. Hs-CRP levels were processed with a commercially available (MSD, Rockville, MD) enzyme-linked immunosorbent assay (ELISA) at the CTRI lab. Intra- and inter-assay coefficients were <5%.
2.6 Statistical Analyses
Study data were analyzed using SPSS version 24 (SPSS, Inc.; Chicago, Illinois). All study variables were examined for violation of distribution assumptions required for parametric analyses. BMI (square root) and hs-CRP (log) were transformed. Independent t-tests or Pearson chi square tests were conducted to determine significant differences in sociodemographic, clinical, and lab measures between schizophrenia and comparison groups. Pearson bivariate correlates of hs-CRP and sociodemographic, clinical, and lab measures were also performed in the two groups. Within each subject group, we employed independent t-tests to examine differences in mean levels of hs-CRP when the groups were subdivided by gender, race, marital status, or current smoking status. To explore differences between the two groups in associations of hs-CRP with metabolic risk factors, we conducted two-way ANCOVAs using diagnostic group (schizophrenia vs. comparison subjects), metabolic risk factor (BMI level), and covariates at the p < 0.1 level (gender) that were identified in the bivariate correlation analyses. Based on prior studies (Tracy et al., 1997; Dietrich et al., 2007), we also included current smoking status as a potential covariate of hs-CRP levels. Significant p values for all study variables were set at 0.05.
3. Results
3.1 Schizophrenia and Non-Psychiatric Comparison Sample Characteristics
People with schizophrenia and comparison subjects were similar in terms of current age (Table 1). Relative to the comparison sample, the schizophrenia group had lower education and higher proportions of non-Caucasians, single/never married, and current smokers. As expected, the schizophrenia group had worse psychopathology in terms of positive, negative, and disorganized symptoms, but their total SAPS scores ranged from 0 to 16 (M: 6.5; SD: 4.5) and the total SANS scores ranged from 0 to 19 (M: 7.1; SD: 4.7), indicating a generally mild level of severity for current psychopathology. The schizophrenia group also had greater executive/cognitive impairment, medical comorbidity, BMI, and blood glucose and HbA1c levels.
Table 1.
Characteristics of Schizophrenia and Non-Psychiatric Comparison Groups
| Sociodemographic | Schizophrenia (N = 88) | Non-Psychiatric Comparison Subjects (N = 71) | t or chi-square | p | ||
|---|---|---|---|---|---|---|
| M or % | SD | M or % | SD | |||
| Current Age (Years) | 49.4 | 10.8 | 50.2 | 11.5 | t = .435 | .664 |
| Education (Years) | 12.4 | 2.0 | 14.7 | 2.0 | t = 7.1 | <.001 |
| Gender (% Male) | 39.4 | - | 53.4 | - | χ2 = 3.1 | .079 |
| Race (% Caucasian) | 46.6 | - | 67.6 | - | χ2 = 7.0 | .008 |
| Marital Status (% Single, Never Married) | 62.5 | - | 29.6 | - | χ2 = 17.1 | <.001 |
| Smoking Status (% Current Smokers) | 49.4 | - | 5.6 | - | χ2 = 35.9 | <.001 |
| Age of Schizophrenia Onset (Years) | 25.8 | 11.7 | - | - | - | - |
| Duration of Illness (Years) | 23.6 | 13.3 | - | - | - | - |
| Current Antipsychotic Dose (mg CPZE/day) | 565.2 | 440.8 | - | - | - | - |
| Psychopathology and Cognition | ||||||
| Positive Symptom Factor Score | 4.3 | 3.1 | .099 | .300 | t = −12.6 | .001 |
| Negative Symptom Factor Score | 7.2 | 4.5 | 1.2 | 1.8 | t = −11.2 | .001 |
| Disorganized Symptom Factor Score | 2.3 | 1.9 | .127 | .476 | t = −10.3 | .001 |
| Executive Functioning Composite Score | -.49 | .69 | .54 | .59 | t=−9.4 | .001 |
| Comorbidity and BMI | ||||||
| CIRS Total Score | 7.1 | 5.0 | 3.1 | 3.3 | t=6.8 | .001 |
| BMI (kg/m2) | 31.0 | 8.3 | 27.7 | 7.1 | t = −3.4 | .001 |
| Lab Values | ||||||
| Fasting Blood Glucose (mg/dL) | 103.8 | 52.6 | 87.5 | 13.6 | t=2.5 | 0.013 |
| Hemoglobin A1c (%) | 6.2 | 1.2 | 5.6 | 0.3 | t=3.4 | 0.001 |
| hs-CRP (mg/L) | 4.3 | 5.4 | 2.3 | 3.8 | t = −3.9 | .001 |
Note:
BMI = Body Mass Index
CIRS = Cumulative Illness Rating Scale
CPZE = Chlorpromazine Equivalent
hs-CRP = high sensitivity C-Reactive Protein
3.2 Comparison and Correlates of hs-CRP in schizophrenia and comparison subjects
hs-CRP levels were significantly higher in people with schizophrenia than in comparison subjects. There was a significant gender difference such that women with schizophrenia had higher hs-CRP than women from the comparison group as well as men with schizophrenia (Figure 1). Gender by diagnosis group interaction was significant, F (2,155) = 3.8, p=0.047). hs-CRP levels in either group of subjects were not related to race, marital status, or current smoking status (data not shown).
Figure 1.
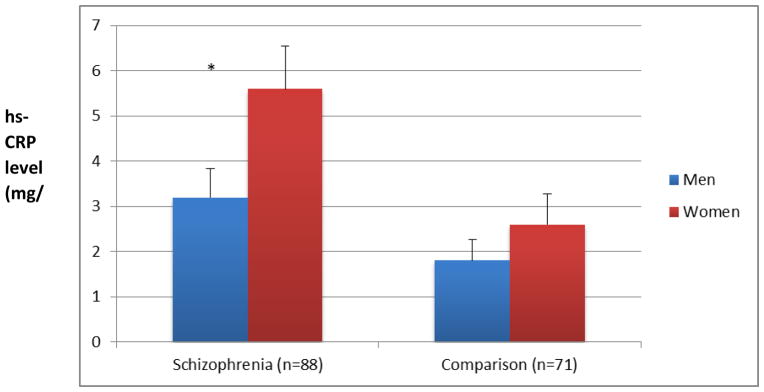
Gender by Diagnosis Associations of hs-CRP Levels (Means and Standard Bars)
Note: * Men versus women in the group with schizophrenia (t(87)=2.56, p=0.010); Men versus women in the comparison group (t(70)=0.6, p=0.500.
The bivariate correlations of hs-CRP level and study variables in schizophrenia and comparison groups are shown in Table 2. In the schizophrenia sample, elevated hs-CRP levels correlated with greater medical comorbidity, BMI, fasting blood glucose, and HbA1c levels. The only significant correlation of hs-CRP with a psychopathology variable was with severity of negative symptoms. In the comparison subjects, hs-CRP levels were positively correlated with BMI and fasting blood glucose, and inversely associated with executive function.
Table 2.
Pearson Correlates of Demographic, Clinical, and Metabolic Risk Factors and hs-CRP in Schizophrenia and Non-Psychiatric Comparison Groups
| Variables | hs-CRP (mg/L) in Schizophrenia (N = 88) | hs-CRP (mg/L) in Non-Psychiatric Comparison Subjects (N = 71) | ||
|---|---|---|---|---|
| r | p | r | p | |
| Current Age (Years) | -.087 | .420 | .090 | .455 |
| Education (Years) | -.016 | .881 | -.171 | .154 |
| Age of Schizophrenia Onset | .106 | .333 | - | - |
| Duration of Illness | -.168 | .122 | - | - |
| Current Antipsychotic Dose (mg CPZE/day) | .063 | .560 | - | - |
| Positive Symptom Factor Score | .046 | .669 | - | - |
| Negative Symptom Factor Score | .215 | .044 | - | - |
| Disorganized Symptom Factor Score | .093 | .389 | - | - |
| Executive Functioning Composite Score | -.030 | .778 | -.246 | .039 |
| CIRS Total Score | .264 | .013 | .117 | .332 |
| BMI (kg/m2) | .317 | .006 | .661 | <.001 |
| Fasting Blood Glucose (mg/dL) | .300 | .005 | .257 | .034 |
| Hemoglobin A1c (%) | .406 | .001 | -.008 | .950 |
Note:
BMI = Body Mass Index
CIRS = Cumulative Illness Rating Scale
CPZE = Chlorpromazine Equivalent
hs-CRP = high sensitivity C-Reactive Protein
To further explore the differences in associations between hs-CRP and study variables across schizophrenia and comparison groups, we conducted a two-way ANCOVA with gender [F (1,126) = 12.7, p = .001, partial eta squared = .091] and current smoking status [F(1,126) = .007, p = .932, partial eta squared <.001] as covariates. This analysis resulted in a main effect for BMI level [F (2,126) = 17.8, p <.001, partial eta squared = .218], but hs-CRP levels remained significantly higher in people with schizophrenia than in comparison subjects [F (2,126) = 7.6, p = .007, partial eta squared = .057].
4. Discussion
Our hypotheses that hs-CRP levels would be significantly higher in individuals with schizophrenia than in comparison subjects, and that the hs-CRP levels would be associated with severity of negative symptoms, greater medical comorbidity, and worse metabolic risk factors, were supported. These associations were not related to age, race, education, smoking status, or antipsychotic dosage. However, we did not find a significant association of hs-CRP with other psychopathology or with executive/cognitive impairment. Notably, there was a significant gender effect, with higher hs-CRP levels in women with schizophrenia.
It is interesting that hs-CRP was not associated with schizophrenia-related psychopathology (age of onset or duration of illness, severity of positive symptoms or disorganized symptoms, cognitive function) as much as it was to physical (CIRS) and metabolic (BMI, glucose, HbA1c) factors. The correlation with negative symptom severity, although significant, was small. Also, the hs-CRP levels did not correlate with smoking or antipsychotic dose or sociodemographic variables other than age. This might indicate that hs-CRP could be one of the (possibly direct) biomarkers of schizophrenia-related metabolic pathology.
There are several limitations of this study to consider. It was cross-sectional in nature, and therefore, restricted our ability to determine cause-and-effect relationship, if any, between hs-CRP and comorbidity or metabolic risk factors. Also, larger studies are needed to demonstrate generalizability of our current findings. We did not observe significant correlations between daily antipsychotic dosage and hs-CRP levels, although we could not measure total lifetime antipsychotic exposure. The schizophrenia and comparison groups differed on race, education, and smoking status, but these differences did not seem to affect statistical significance of hs-CRP comparisons between the two groups.
Despite the limitations, the differential gender and BMI associations with hs-CRP in schizophrenia are noteworthy. It is unclear why hs-CRP values were elevated to greater extent among women with schizophrenia. Some prior studies have reported elevated levels of CRP in women compared to men in non-psychiatric study samples (Rossi et al., 2012; Onat et al., 2012; Konishi et al., 2014). Rossi et al. (2012) suggest that leptin levels may be a moderator of gender-based differences in hs-CRP levels and adiposity in healthy comparison subjects. Anthropometric assessments and measurement of leptin levels in future gender and ethnicity-matched studies of hs-CRP would be helpful. Nonetheless, our findings suggest that gender should be considered in studies examining CRP and potentially other inflammatory markers in schizophrenia across the life course.
Longitudinal investigations are necessary for future development of new metabolic/cardiovascular disease treatment targets in schizophrenia. Research on the covariation among hs-CRP, gender, and metabolic risk factors including BMI, fasting blood glucose, and HbA1c in adults with schizophrenia may shed light on the direct and indirect influences of illness and metabolic factors on hs-CRP and other inflammatory biomarkers. This is especially pertinent to studies that may employ hs-CRP as a metabolic and cardiovascular endophenotype for interventions that include behavioral and pharmacological weight loss strategies for people with schizophrenia.
Acknowledgments
Role of Funding Source
This study was supported, in part, by NIH R01MH094151-01 (PI: Dilip V. Jeste, MD), the NIMH T32 Geriatric Mental Health Program MH019934 (PI: Dilip V. Jeste, MD), and by the Stein Institute for Research on Aging at the University of California, San Diego.
We would like to thank all of the study participants and staff.
Footnotes
Contributors
Jamie Joseph conducted literature reviews, data analyses, data interpretation, and manuscript preparation.
Colin A. Depp conducted data analyses, data interpretation, and manuscript preparation.
Averria Sirkin Martin was involved in data collection and manuscript preparation.
Rebecca Daly was involved in data collection, data management, and manuscript preparation.
Danielle K. Glorioso was involved in data collection and manuscript preparation.
Barton W. Palmer was involved with study design and manuscript preparation.
Dilip V. Jeste designed the study and was involved in manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akanji AO, Ohaeri JU, Al-Shammri S, Fatania HR. Association of blood levels of C-reactive protein with clinical phenotypes in Arab schizophrenic patients. Psychiatry research. 2009;169(1):56–61. doi: 10.1016/j.psychres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Hankey GJ, Yeap BB, Golledge J, Norman PE, Flicker L. Mortality among people with severe mental disorders who reach old age: a longitudinal study of a community-representative sample of 37,892 men. PloS one. 2014;9(10):e111882. doi: 10.1371/journal.pone.0111882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the assessment of Negative Symptoms (SANS) University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function Scale (D-KEFS): Examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Demakakos P, Nazroo J, Breeze E, Marmot M. Socioeconomic status and health: the role of subjective social status. Social science & medicine. 2008;67(2):330–40. doi: 10.1016/j.socscimed.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophrenia research. 2007;93(1–3):261–265. doi: 10.1016/j.schres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Schroeder J, Khushalani S, Yolken R. Mortality in schizophrenia: clinical and serological predictors. Schizophrenia bulletin. 2014;40(4):796–803. doi: 10.1093/schbul/sbt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophrenia research. 2012;134(1):83–88. doi: 10.1016/j.schres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Garcia R, de Pablo P, Schulze P, Hoffmann K. The effects of cigarette smoking on C-reactive protein concentrations in men and women and its modification by exogenous oral hormones in women. European journal of preventive cardiology. 2007;14(5):694–700. doi: 10.1097/HJR.0b013e328270b913. [DOI] [PubMed] [Google Scholar]
- Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert review of neurotherapeutics. 2007;7(7):789–796. doi: 10.1586/14737175.7.7.789. [DOI] [PubMed] [Google Scholar]
- Fawzi MH, Fawzi MM, Said NS. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry research. 2011;190(1):91–97. doi: 10.1016/j.psychres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Fawzi MH, Fawzi MM, Said NS, Fawzi MM, Fouad AA, Abdel-Moety H. Effect of Ramadan fasting on anthropometric, metabolic, inflammatory and psychopathology status of Egyptian male patients with schizophrenia. Psychiatry research. 2015;225(3):501–508. doi: 10.1016/j.psychres.2014.11.057. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Foley DL, Mackinnon A, Morgan VA, Watts GF, Shaw JE, Magliano DJ, Castle DJ, McGrath JJ, Waterreus A, Galletly CA. Cardiovascular risk factor associations in adults with psychosis and adults in a national comparator sample. The Australian and New Zealand journal of psychiatry. 2015 doi: 10.1177/0004867414565476. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM, Tsuang MT. Age and neuropsychologic function in schizophrenia: A decline in executive abilities beyond that observed in healthy volunteers. Biol Psychiatry. 2000;48(2):137–146. doi: 10.1016/s0006-3223(00)00240-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Bernardo M, Kirkpatrick B. Inflammatory markers in antipsychotic-naive patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatry research. 2012;198(2):212–215. doi: 10.1016/j.psychres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. The American journal of medicine. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hefner G, Shams ME, Unterecker S, Falter T, Hiemke C. Inflammation and psychotropic drugs: the relationship between C-reactive protein and antipsychotic drug levels. Psychopharmacology. 2015 doi: 10.1007/s00213-015-3976-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hinze-Selch D, Daubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophrenia bulletin. 2007;33(3):782–788. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Folsom D, Sasaki A, Mudaliar S, Henry R, Torres M, Golshan S, Glorioso DK, Jeste D. Increased Framingham 10-year risk of coronary heart disease in middle-aged and older patients with psychotic symptoms. Schizophrenia research. 2011;125(2–3):295–299. doi: 10.1016/j.schres.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AL, Mowry BJ, Pender MP, Greer JM. Immune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? Immunology and cell biology. 2005;83(1):9–17. doi: 10.1111/j.1440-1711.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophrenia bulletin. 2008;34(6):1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Parajuli RP, Takane E, Maharjan M, Tachibana K, Jiang HW, Pahari K, Inoue Y, Umezaki M, Watanabe C. Significant sex difference in the association between C-reactive protein concentration and anthropometry among 13- to 19-year olds, but not 6- to 12-year olds in Nepal. American journal of physical anthropology. 2014;154(1):42–51. doi: 10.1002/ajpa.22470. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Drummond PD. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Progress in neuro-psychopharmacology & biological psychiatry. 2013;45:92–99. doi: 10.1016/j.pnpbp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Manu P, Khan S, Radhakrishnan R, Russ MJ, Kane JM, Correll CU. Body mass index identified as an independent predictor of psychiatric readmission. The Journal of clinical psychiatry. 2014;75(6):e573–577. doi: 10.4088/JCP.13m08795. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clinical schizophrenia & related psychoses. 2014;7(4):223–230. doi: 10.3371/CSRP.MICU.020813. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Kandhal P, Rapaport MH, Mellor A, Buckley P. Total and differential white blood cell counts, high-sensitivity C-reactive protein, and cardiovascular risk in non-affective psychoses. Brain, behavior, and immunity. 2015;45:28–35. doi: 10.1016/j.bbi.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat A, Can G, Hergenç G, Uğur M, Yüksel H. Coronary disease risk prediction algorithm warranting incorporation of C-reactive protein in Turkish adults, manifesting sex difference. Nutrition, metabolism and cardiovascular diseases. 2012;(8):643–50. doi: 10.1016/j.numecd.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Osborn DP, Hardoon S, Omar RZ, Holt RI, King M, Larsen J, Marston L, Morris RW, Nazareth I, Walters K, Petersen I. Cardiovascular Risk Prediction Models for People With Severe Mental Illness: Results From the Prediction and Management of Cardiovascular Risk in People With Severe Mental Illnesses (PRIMROSE) Research Program. JAMA psychiatry. 2015;72(2):143–151. doi: 10.1001/jamapsychiatry.2014.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK. Executive dysfunction in schizophrenia. In: Sharma T, Harvey P, editors. Cognition in schizophrenia: Impairments, importance and treatment strategies. Oxford University Press; New York: 2000. pp. 52–72. [Google Scholar]
- Palmer BW, Martin AS, Depp CA, Glorioso DK, Jeste DV. Wellness within illness: happiness in schizophrenia. Schizophrenia research. 2014;159(1):151–156. doi: 10.1016/j.schres.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. Journal of clinical investigation. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopova D, Masopust J, Maly R, Valis M, Bazant J. The prevalence of cardiometabolic risk factors and the ten-year risk of fatal cardiovascular events in patients with schizophrenia and related psychotic disorders. Psychiatria Danubina. 2012;24(3):307–313. [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. Journal American college of cardiology. 2007;49(21):2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Rossi I, Bochud M, Bovet P, Paccaud F, Waeber G, Vollenweider P, Taffé P. Sex difference and the role of leptin in the association between high-sensitivity C-reactive protein and adiposity in two different populations. European journal of epidemiology. 2012;27(5):379–84. doi: 10.1007/s10654-012-9671-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Canela M, Zazpe I, Shivappa N, Hébert J, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Fitó M, Lamuela-Raventós R, Rekondo J, Fernández-Crehuet J, Fiol M, Santos-Lozano J, Serra-Majem L, Pinto X, Martínez J, Ros E, Estruch R, Martínez-González M. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. British journal of nutrition. 2015;113(6):984–95. doi: 10.1017/S0007114514004401. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatcioglu O, Kalkan M, Fistikci N, Erek S, Kilic K. Relationship Between Metabolic Syndrome and Clinical Features, and Its Personal-Social Performance in Patients with Schizophrenia. Psychiatric quarterly. 2015 doi: 10.1007/s11126-015-9384-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sicras-Mainar A, Rejas-Gutierrez J, Navarro-Artieda R, Blanca-Tamayo M. C-reactive protein as a marker of cardiovascular disease in patients with a schizophrenia spectrum disorder treated in routine medical practice. European psychiatry : the journal of the Association of European Psychiatrists. 2013;28(3):161–167. doi: 10.1016/j.eurpsy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Tay YH, Nurjono M, Lee J. Increased Framingham 10-year CVD risk in Chinese patients with schizophrenia. Schizophrenia research. 2013;147(1):187–192. doi: 10.1016/j.schres.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Toomey R, Kremen WS, Simpson JC, Samson JA, Seidman LJ, Lyons MJ, Faraone SV, Tsuang MT. Revisiting the factor structure for positive and negative symptoms: evidence from a large heterogeneous group of psychiatric patients. The American journal of psychiatry. 1997;154(3):371–377. doi: 10.1176/ajp.154.3.371. [DOI] [PubMed] [Google Scholar]
- Tracy R, Psaty B, Macy E, Bovill E, Cushman M, Cornell E, Kuller L. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arteriosclerosis, thrombosis, and vascular biology. 1997;17(10):2167–76. doi: 10.1161/01.ATV.17.10.2167. [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. Journal american college of cardiology. 2006;47 (8 Suppl):C19–31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Ecker UK, Scherk H, Schneider-Axmann T, Falkai P, Gruber O. Cognitive impairment of executive function as a core symptom of schizophrenia. World Journal of Biological Psychiatry. 2008:1–10. doi: 10.1080/15622970701849986. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. International journal of obesity. 2007;31(9):1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]


