Abstract
The Varroa mite (Varroa destructor) is implicated as a major disease factor in honey bee (Apis mellifera) populations worldwide. Honey bees are extensively relied upon for pollination services, and in countries such as New Zealand and Australia where honey bees have been introduced specifically for commercial pollinator services, the economic effects of any decline in honey bee numbers are predicted to be profound. V. destructor established in New Zealand in 2000 but as yet, Australia remains Varroa-free. Here we analyze the history of V. destructor invasion and spread in New Zealand and discuss the likely long-term impacts. When the mite was discovered in New Zealand, it was considered too well established for eradication to be feasible. Despite control efforts, V. destructor has since spread throughout the country. Today, assessing the impacts of the arrival of V. destructor in this country is compromised by a paucity of data on pollinator communities as they existed prior to invasion. Australia’s Varroa-free status provides a rare and likely brief window of opportunity for the global bee research community to gain understanding of honey bee-native pollinator community dynamics prior to Varroa invasion.
Keywords: Apis mellifera, Bombus, Deformed wing virus, Pollinator communities, Varroa destructor
Introduction
The arrival of the Varroa honey bee mite, Varroa destructor, in New Zealand has had far-reaching consequences for honey bee populations and pastoral agriculture. In this paper, we present for the first time, an analysis of the history of the interception, the regulatory response, and the subsequent invasion of the mite in New Zealand within the framework of realized and potential implications for the country in terms of ecosystem services to agriculture, the likely impacts on other pollinators, introduced and native, managed and wild, and reflect on unforeseen consequences of Varroa invasion. In addition, the lack of data and missed opportunities for research in New Zealand and conversely the unique research opportunity that presents itself in Australia, currently Varroa-free, are highlighted. As the arrival of V. destructor in Australia at some stage in the future seems likely, we emphasize here the urgent and strategically important need, and the opportunity for comprehensive ecological analyses of pollinator communities before and after its establishment.
The significance of pollinators and the Varroa threat
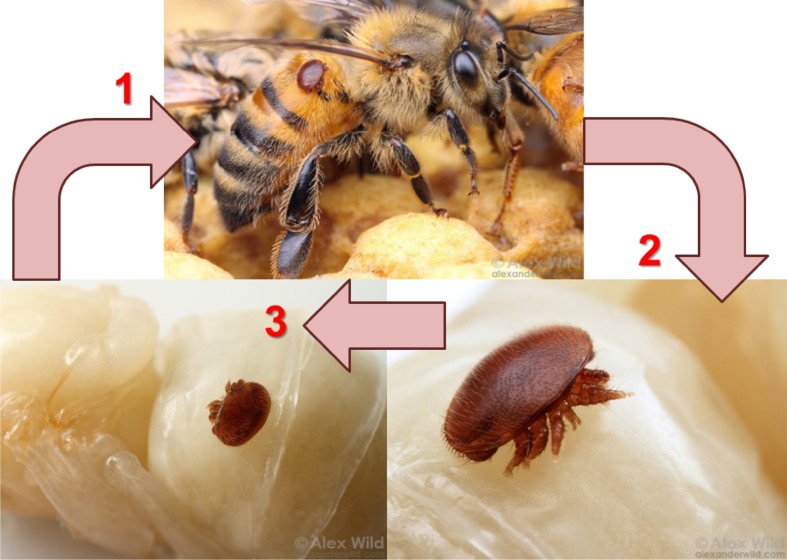
Pollination services provided by insects have a worldwide economic value conservatively estimated to be at least US$215 billion per year (Gallai et al. 2009). Of specific importance is the role insects play in agriculture, where over 35 % of global crops are dependent on pollination by wild and managed bees (Klein et al. 2007). The most relied upon pollinator for agriculture is the honey bee (Apis mellifera), including both the wild and domesticated stocks (Potts et al. 2010). As a result of its critical importance to agriculture and its economic significance, the western honey bee (A. mellifera) has been studied more intensively than any other insect pollinator. Honey bee populations face multiple threats worldwide (Rosenkranz et al. 2010; Williams et al. 2010; Mondet et al. 2014; McMenamin and Genersch 2015), one of the most significant of which is the parasitic Varroa mite, V. destructor, and its associated viruses. Varroa mites reproduce within honey bee brood cells and feed on the hemolymph of the bee (Fig. 1). The reproductive phase of the Varroa mite’s life cycle takes place within honey bee brood cells. A mated female mite invades a brood cell containing a 5th instar bee larva, and about 60 h after the cell is sealed, the mite lays its first egg. This egg usually develops into a male and is followed by additional eggs that develop into females (Ritter 1981). The nymphal mites grow and with assistance from the maternal parent, they feed on the hemolymph of the developing bee. Once the Varroa male is sexually mature, he inseminates the young female mites in the cell, and upon emergence of the adult bee from the brood cell, the maternal parent and newly mated female offspring exit the cell and transfer rapidly onto nurse bees. In the absence of controls, infestations typically result in colony death within 4 years, although colonies may succumb in as little as 8 months (Rosenkranz et al. 2010).
Fig. 1.
Generalized Life Cycle of Varroa destructor. Mites on adult honey bee and pupa. 1 Initial invasion often begins with mite transfer via adult bees inside or outside the colony. 2 The mites then invade brood cells, feeding on the larvae, and laying eggs. 3 Mites hatch and reproduce within the cell. Mated female mites leave to find new hosts. Photographs by Alex Wild
Varroa destructor evolved with the Asian honey bee (Apis cerana), but in the mid-twentieth century expanded its host range to include the European A. mellifera. From the initial host-range expansion in Asia, V. destructor is thought to have spread across Russia into Europe by 1967 (as cited in Rosenkranz et al. 2010). As European A. mellifera lacked resistance to V. destructor, there was resultant wide-scale mortality of commercial and wild honey bee colonies. Further, mites were discovered in Brazil in 1972 and the United States in 1987 (Oldroyd 1999). African populations of honey bees in the Americas show behaviors and traits that allow mite populations to stabilize below lethal levels (Martin and Medina 2004).
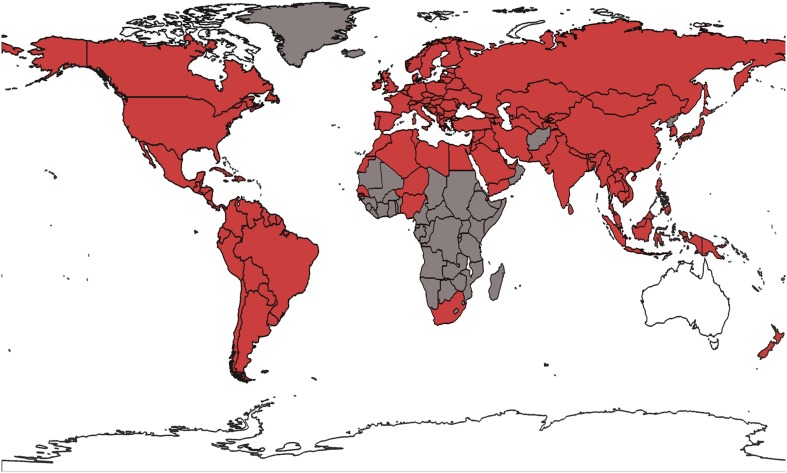
New Zealand and Australia were the last major beekeeping countries free of this parasitic mite until the year 2000, when V. destructor mites were discovered in honey bee colonies near Auckland in the North Island of New Zealand (Fig. 2) (Ministry of Agriculture and Forestry 2002a). This incursion marked the start of Varroa’s invasion in New Zealand. V. destructor has since spread throughout both main islands, reaching southernmost mainland regions in the last few years (Ministry for Primary Industries 2013). The impact of this invasion and concurrent commercial costs is estimated to be between NZ$365 and 661 million over 35 years (Ministry of Agriculture and Forestry 2002b). Only offshore areas such as the Chatham Islands, 800 km to the east of the South Island of New Zealand, remain without V. destructor, leaving Australia as the last major beekeeping country in the world reportedly free of this destructive honey bee parasite (Mark and Cliff 2001; Cunningham et al. 2002). We suggest here that there exists a globally important opportunity in Australia and New Zealand to gain fundamental insights into pollinator interactions as well as how best to mitigate the effects of honey bee decline on pollination services. New Zealand and Australia can offer an internationally valuable contrast as these two countries share a relatively recent history of Apis and Bombus introduction for agriculture and horticulture, significant overlap among economically important crops and native plant genera, and a shared biogeographical history (Waters and Craw 2006). New Zealand and Australia also have a culture of scientific and governmental co-operation, including sharing of biosecurity information. Although the native bee faunas of the two countries differ significantly in species richness and taxonomic diversity, all native New Zealand bees are likely derived from Australian colonizers (Donovan 2007). The recent spread of Varroa in New Zealand is seriously impacting agriculture and beekeeping operations and as yet, the long-term effects of feral honey bee losses are unknown. In order to maintain ecosystem functions and services, it is highly desirable that pollinator interactions are far better understood before and after V. destructor invasion and that these interactions are monitored closely in New Zealand and Australia to assess changes in community equilibria before and after V. destructor invasion.
Fig. 2.
The current approximate global distribution of Varroa destructor. Countries in white are free of V. destructor. Countries shaded in gray are data-deficient, or represent countries where African honey bees demonstrate some resistance to V. destructor
Pollination by honey bees
Pollination by wild bees (including feral honey bees) has been found to be complementary to that of managed honey bees in over 40 crop systems in 19 countries, including Australia and New Zealand (Garibaldi et al. 2013). Wild bees provide ecosystem services that are free, efficient, and independent of managed bees (Ricketts 2004). Varroa invasion most likely has a greater impact on feral honey bees than on managed honey bee populations, because in the case of feral bees there is no opportunity for mite control via treatment with miticides. In Europe, estimated densities of honey bee colonies suggest that managed honey bees significantly supplement wild populations (Jaffé et al. 2010). Since the discovery of V. destructor in the North Island of New Zealand and its subsequent spread to the South Island in 2006, feral honey bee populations across the country have declined dramatically (Howlett and Donovan 2010). This loss of feral honey bees may ultimately have more of an impact on pollination services than Varroa infestation of managed hives (Garibaldi et al. 2013). Understanding the contribution that insect pollinators other than managed and feral honey bees make to agriculture and conservation is an important first step in determining their compensatory potential in the face of the current decline in honey bee populations (Potts et al. 2010; Rader et al. 2012). The maintenance of ecosystem services is often tied to the protection of natural habitat, which however can be compromised by economic development (Kremen et al. 2002). In the context of feral and managed honey bee losses, studying the roles that unmanaged insects play can inform land managers and encourage the maintenance of habitats that support pollinator web resiliency and diverse pollinator assemblages (Garibaldi et al. 2013).
In countries where feral introduced honey bees co-exist with native bees, there is also the possibility that the loss of feral honey bees may have a positive conservation outcome for native bee species. While resource competition between honey bees and native bees has yet to be identified in New Zealand, there have been very few studies undertaken to evaluate the degree of overlap between honey bees and native bees in this country (Howlett and Donovan (2010). A review of competitive interactions between honey bees and native bees across multiple continents, however, indicates that honey bees can have a negative effect on native bees (Paini 2004), but nowhere in the world has experimental manipulation of pollinator assemblages and resources utilization been conducted before and after V. destructor invasion. Thus, the manner of response by native pollinator assemblages to the decline of honey bees, as well as their ability to provide compensatory pollination services, remains largely unknown.
Invasion and response in New Zealand
Varroa destructor was identified in Auckland, New Zealand’s largest metropolitan area, on 11th April, 2000. Upon identification, the New Zealand Government’s Ministry of Agriculture and Forestry (MAF), now Ministry for Primary Industries (MPI), undertook an initial survey of the immediate area which determined that the degree of infestation around the detected cluster of infected hives suggested it had been present undetected for 3–5 years (Ministry of Agriculture and Forestry 2002a). The then Minister for Biosecurity is quoted as saying “the most likely route was through the illegal importation of queen bees by a New Zealand beekeeper, either by post or as personal luggage (Ministry of Agriculture and Forestry 2002a).” However, the scenario of accidental arrival via container ship was not discounted.
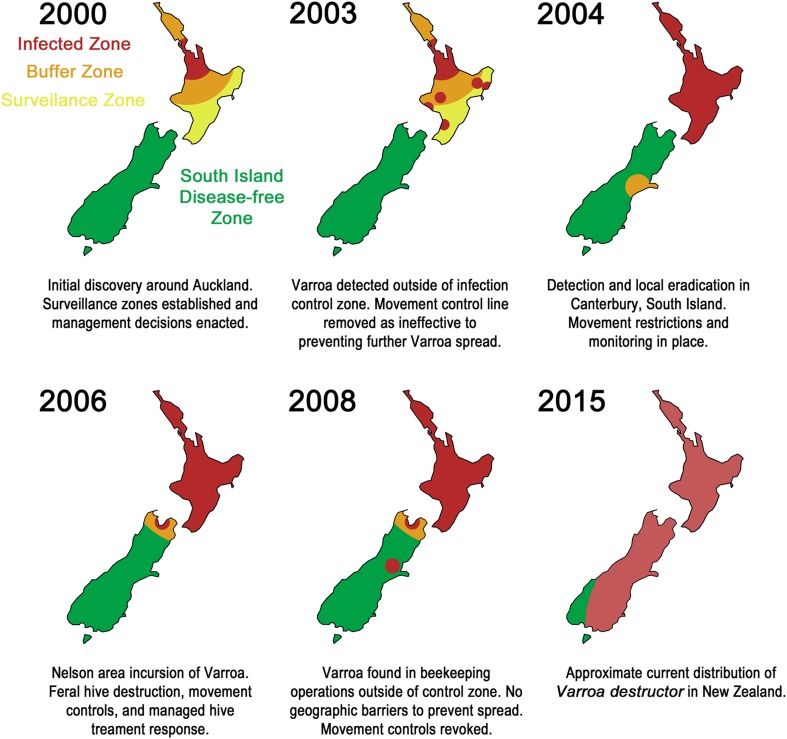
Between April and June 2000, surveys of managed colonies within an infected zone were sampled by treating hives with miticides and checking for dead mites. Several zones were established throughout the North and South Islands for prioritization (Fig. 3). The infected zone was centered on the Auckland area and surrounded by buffer zones to the north and south of the infected zone. The south-eastern portion of the North Island was declared a surveillance zone. The South Island was declared a disease-free zone, and any recently imported bees were placed under movement restrictions. Results of the survey found extensive Varroa infestations in the infected zone. Beekeeper movement of hives was determined to have contributed to local spread to the north and south of Auckland, as well as into several buffer zone sites. The extent of the affected locations and the timeframe involved in the pattern of infestation strongly suggested that feral honey bee populations were also contributing to spread to the buffer zones. Methods for controlling infested feral honey bee colonies within the North Island were examined in June of 2000 with a view to a possible eradication attempt (Benard et al. 2001), but in July, the Government announced that eradication was unlikely to succeed and that no attempt would be made to eradicate the mite. While the costs of eradication were considered economically worthwhile, the decision was made on the basis that eradication was not technically feasible (Ministry of Agriculture and Forestry 2002a).
Fig. 3.
Establishment of zones of control and subsequent spread of Varroa destructor in New Zealand since 2000
As V. destructor continued to spread rapidly throughout the North Island the following year, control efforts were halted and steps were taken to develop a strategy for keeping the South Island Varroa-free (Ministry of Agriculture and Forestry 2003a). This strategy included widespread surveillance and monitoring, as well as continued restrictions on the movement of hives from the North Island to South Island (Ministry of Agriculture and Forestry 2003b). Despite this strategy, in June 2004, a single mite was found in the Canterbury region of the South Island (Ministry of Agriculture and Forestry 2004b). Further testing of hives at the apiary and within a ten kilometer radius found no additional mites in the over 700 hives sampled (Ministry of Agriculture and Forestry 2004a). The Ministry of Agriculture and Forestry took the approach of destroying 40 hives at the Canterbury detection point in an attempt to prevent the spread of V. destructor in case miticide treatments, and monitoring of local hives still allowed low numbers to evade detection (Ministry of Agriculture and Forestry 2004c). However, V. destructor was detected at apiaries in the Nelson region of the South Island in June 2006. A controlled area declaration was put in place, and movements of beekeeping materials were immediately restricted (Ministry of Agriculture and Forestry 2006a). Surveys around the infected apiaries were initiated as well as destruction of feral bee colonies in the surrounding areas (Ministry of Agriculture and Forestry 2006b). Throughout 2007, Biosecurity New Zealand conducted a series of workshops to educate beekeepers on Varroa detection and control. They also continued monitoring and movement restrictions around the affected areas of the South Island (Ministry of Agriculture and Forestry 2007). However, in 2008, mites were found in hives outside of the controlled area and a new controlled zone was then established around the new infestation (Ministry of Agriculture and Forestry 2008b). Following several subsequent detections outside the expanded controlled area, Biosecurity New Zealand decided to revoke all movement controls regarding the Varroa mite, citing widespread infestations, and a lack of geographic barriers preventing further spread (Ministry of Agriculture and Forestry 2008a). In 2009, the MAF funded South Island Varroa response program was ended as mite populations were found beyond the controlled area. The size of beekeeping operations, duration of exposure, and scope of invasion outside the controlled area made any further control attempts unlikely to succeed. This decision marked the end of the South Island Varroa response program (Ministry for Primary Industries 2013).
Economic effects of Varroa in New Zealand
The economic impact of V. destructor was predicted to be felt most directly by the agricultural industries that utilize pollination both from managed and feral honey bees. In addition to direct losses, the number of managed hives was expected to decrease as the number of hobbyist beekeepers declined due to the increased hive mortality and additional costs of maintenance. Indeed, after V. destructor invaded New Zealand, the number of registered hives remained steady, but the number of registered beekeeping enterprises collapsed by half and has not completely recovered 15 years later (Ministry for Primary Industries 2014). Total costs for New Zealand were estimated to be between NZ$365 and 661 million calculated for an indicative 35-year time span (Ministry of Agriculture and Forestry 2002b). On both islands, pastoral impacts were estimated to account for 78 % of costs, horticultural and arable 15 %, and beekeeping approximately 7 % (Ministry of Agriculture and Forestry 2002b). Sectors affected and examples of increasing costs include (1) The pastoral sector, with increased costs associated with the need for increases in nitrogen fertilizer application due to a reduction of pollination and the subsequent decrease in seed set of nitrogen-fixing plants (i.e., clover), clover reseeding, and associated production loss; (2) Horticultural and arable sectors, with increases in pollination charges and reductions in crop yields, increases in numbers of hives per hectare to replace lost pollinators, increases in pollination costs due to higher demand, and associated reductions in crop yields; (3) the beekeeping sector, with increased management costs, increases in pollination rental fees, reduction in small scale bee keepers, and increases in the number of pollinator hives supplied to the arable sector (Ministry of Agriculture and Forestry 2000, 2002b). Unfortunately, extensive economic data directly associated with honey bee losses over time are lacking in New Zealand, but if industry reports are to be followed, extrapolating minimal Varroa treatments to every commercial honey bee hive would be at least NZ$12.5 m nationwide in 2014 alone (Ministry for Primary Industries 2014). Even ignoring the increasing risk of V. destructor becoming resistant to treatment, any potential further decreases in the supply of pollinator services could cause the associated indirect costs of Varroa’s introduction to rise.
Secondary effects of Varroa: A viral threat for other bees?
In economic assessments of the impact of honey bee decline in pastoral settings, bumblebees (Bombus spp.) have been considered as compensating pollinators; however, their populations may also be at risk from the spread of Varroa (Ministry of Agriculture and Forestry 2000). Bumblebees are not susceptible to Varroa (Carvell 2002), but there is a potential link in the form of deformed wing virus (DWV), which also infects bumblebees (Genersch et al. 2006). Deformed wing virus is a potentially fatal virus transmitted by V. destructor, acting synergistically to cause multiple physical deformations (Genersch et al. 2006). The prevalence of DWV in honey bee populations in Europe has been closely linked to Varroa infestation. The same seems to be true in New Zealand, where the prevalence of honey bees infected with DWV has been shown to be greater in areas with a longer history of Varroa presence, closely following the invasion front (Mondet et al. 2014). In European bee communities, DWV in bumblebee populations has been shown to correlate with the presence of DWV in honey bees, strongly suggesting pathogen spillover (Genersch et al. 2006; Fürst et al. 2014). In addition to bumblebees, solitary bee species are likely to be infected by honey bee viruses when in proximity to apiaries (Ravoet et al. 2014). If similar interactions are occurring in New Zealand, there may be major implications in terms of additional costs for the pastoral economy from reductions in compensatory pollination of clover by bumblebees. The role of clover as a nitrogen-fixer reduces the need for fertilization of pasture (Ledgard et al. 2001), and if additional nitrogen fertilization is required for New Zealand’s pastoral dependent economy, there will be greater costs both economically and environmentally (Barnett and Pauling 2005). The additional problems with increases in fertilizer applications may also exacerbate the current concerns over the impacts of eutrophication in New Zealand watersheds (Parliamentary Commissioner for the Environment 2013). Alterations in plant–pollinator networks and pollination services resulting from V. destructor‘s establishment in New Zealand are already the cause for concern, and bumblebee losses due to deformed wing virus would further exacerbate the problem. Novel diseases introduced to the native solitary bee community may have additional unforeseen consequences by altering pollinator syndromes if bee abundances change. The detection of deformed wing virus and diseases associated with this virus in non-Apis bees (Genersch et al. 2006), is an excellent example of potential secondary effects of Varroa invasion that may result in more complex community changes and greater economic losses than originally hypothesized.
Ecological effects of Varroa in New Zealand
The impact of V. destructor on the pastoral industry reflects one of the primary reasons social bees were brought to New Zealand. Honey bees and bumblebees were introduced to New Zealand in the 19th century for pollination of clover (Trifolium) species, in particular, which are an essential component of New Zealand pastoral systems. Honey bees and bumblebees possess medium length tongues that are able to access floral rewards of clover, whereas short-tongued native bees typically cannot (Donovan 1980). Introduced social bees have thus played an important role in the pollination of horticultural and agricultural crops since their introduction (Huryn 1995; Howlett and Donovan 2010).
There are approximately 40 species of bee in New Zealand, 28 of which are native, solitary, short-tongued species in the families Colletidae and Halictidae that are not susceptible to the Varroa mite (Donovan 2007). The significance of the role of New Zealand native bees as pollinators has not been well studied (Campbell et al. 2010; Bischoff et al. 2013). Since their introduction, social bees have expanded their range throughout the country and have been recorded foraging extensively on a diverse array of native and introduced plant species (Huryn 1995; Donovan 2007). Native bees have likewise been noted to be extensive users of introduced plants (Donovan 2007). In the Remarkables mountains, central South Island, it has been observed that over 90 % of native bee visits are to introduced plants that honey bees are also known to utilize (Jay Iwasaki, unpublished data). The decline in honey bee populations could leave native bees with access to a higher proportion of resources and thus positively affect native bee populations through greater resource availability compared to when honey bees were present. It has been suggested that native bee species may have the potential to offset honey bee ecosystem service losses across New Zealand (Rader et al. 2012). However, the shorter seasonal abundance and short tongue length of native compared with introduced bees casts doubt on the ability of native bees to compensate completely for the loss of honey bees. While experiments studying pollination by wild bees have been conducted in New Zealand, with positive results for some crops such as Brassica rapa (Rader et al. 2009), investment in more in-depth examination of the importance of non-Apis pollinators is required. Currently, little is known about changes to plant–pollinator networks and pollination services in New Zealand post-Varroa, highlighting missed research opportunities and the need for intensification of ecological study in these areas.
Varroa in Australia: A research agenda
Varroa destructor is now established in most regions of New Zealand, while Australia is currently considered Varroa-free. Since 1992, there has been an average of one border detection of Apiscerana bees in Australia per year, which are often in association with Varroa spp. mites. It is possible that V. destructor is present already in association with Apis cerana, but has yet to be detected. It is also considered likely that there have been undetected arrivals, reflecting a high probability of an unknown V. destructor establishment event (Barry et al. 2010). If Australia follows the patterns shown in other countries, once V. destructor is detected, the potential for successful eradication is low. Australian honey bee populations are highly susceptible to V. destructor, and it is likely that significant losses will be detected among feral and commercial honey bee populations (Rinderer et al. 2013). In addition to the negative physiological effects of V. destructor, the introduction of a vector of hymenopteran viruses may also represent a threat to other bees. Deformed wing virus has not yet been detected in Australia (John Roberts, CSIRO, personal communication, 25 February 2015). As in New Zealand (Mondet et al. 2014), V. destructor’s 2007 arrival in Hawaii coincided with a significantly increased prevalence of DWV in associated areas (Martin et al. 2012). It seems likely that the risk would be the same for Australian populations of honey bees and potentially, native bees as well. A report by the Rural Industries Research and Development Corporation estimated that pollination services to Australia by honey bees alone were worth between 0.6 and 1.7 billion AUS$. Subsequent effects of direct losses could reach over 2 billion AUS$ and affect 11 000 jobs (Gordon and Davis 2003). With this in mind, the Australian Department of Agriculture, Fisheries and Forestry (DAFF) has implemented a comprehensive Varroa management strategy that focuses on the use of domesticated and non-domesticated pollinators other than the honey bee, underscoring the importance of pollination research that relates to invasion ecology, competitive release, and alternative pollinators (Department of Agriculture, Fisheries 2011). In addition, a recent CSIRO biosecurity analysis reiterated the V. destructor threat as one of its top “megashock” threats to be prepared for in the coming decades, further underlining the significance (CSIRO 2014). With the threat of Varroa, the role that native bees play in Australian pollination services is becoming of increasing interest and importance (Blanche et al. 2006; Batley and Hogendoorn 2009). In Australia, as in New Zealand and worldwide, managing landscapes to enhance densities of native bees and other alternative pollinators may help reduce reliance on honey bee pollination in some systems. While the arrival of V. destructor in Australia will undoubtedly be damaging economically, the loss of feral honey bees may have a positive impact on the conservation of native bees. Honey bees were introduced to Australia in 1822 and feral populations occur throughout the country. High densities of up to 77 feral colonies per km2 have been recorded, reflecting high degrees of invasion success and the potential for competition with native bees (Oldroyd et al. 1997). Evidence suggests that honey bees compete with native bees for resources across Australia, indicating a potential capacity for increased population sizes of native bees if honey bees decline (Paini 2004). While Australia has a much higher diversity of bees than New Zealand (in total, over 1600 species vs. 40), a large proportion of the native bees in Australia belong to the same families that comprise the native bee community assemblage in New Zealand (Colletidae, Halictidae) (Batley and Hogendoorn 2009). As in New Zealand, Australian native bees are threatened by loss of habitats and resources as a result of urban and agricultural development (Batley and Hogendoorn 2009). It is clear that studies examining pollinator interactions and pollination services pre- and post-Varroa will need a relatively long temporal dataset in order to account for annual and seasonal variations in weather and flowering patterns. Research on whether or not deformed wing virus can infect native bee populations, as in Europe, is also critical information for understanding the net negative or positive effect on native bees in Australia. For these reasons, the need for additional monitoring projects to be conducted and expanded is crucial. In Australia researchers have devoted significant resources for studying diverse bee communities (Paton 1993; Kingston and McQuillan 1998; Hingston and McQuillan 1999; Paini 2004; Paini and Roberts 2005; Blanche et al. 2006; Batley and Hogendoorn 2009; Cunningham et al. 2013; Popic et al. 2013), and the relative paucity of bee diversity in New Zealand underscores the importance of the Australian perspective.
Value of Varroa-free islands: Arks and laboratories
Tasmania and the Chatham Islands provide potential havens for the preservation of Varroa-free honey bee populations, and offer research opportunities that differ from those provided by mainland Australia and New Zealand. Bombus terrestris, which is absent from the Australian mainland, was introduced to Tasmania in 1992 (Hingston et al. 2002). Several Bombus species including B. terrestris have been present in New Zealand since the 19th Century, but none are present on the Chatham Islands. Like the Chatham Islands of New Zealand, Tasmania also lacks Varroa species and as islands, both are further insulated from invasion. Goulson et al. (2002) examined whether exotic bumblebees and honey bees compete with native bees in Tasmania. They observed significant negative correlations between honey bee and native bee abundance, but no evidence of bumblebees directly displacing native bees. In addition, despite any immediate impact on native bees, bumblebee pollination in Tasmania may facilitate the spread of invasive plants, emphasizing the complexity of invasion dynamics. New Zealand and Tasmania share a similar biogeography, and the invasive ecology of other introduced species present in both New Zealand and Tasmania emphasizes the potential for comparative studies (Stout et al. 2002). Comparative studies of pollinator networks in New Zealand and Tasmania may offer the best possibilities for reducing confounding factors given similarities in climate, plant groups, and bee communities.
Missed opportunities in New Zealand
Invasive species must pass through at least three stages before they can have an economic or ecological impact: introduction, establishment, and spread (Lockwood et al. 2013). Varroa in New Zealand was not detected upon introduction, but rather had been present in the country and had experienced some local expansion around the initial establishment site over perhaps 3–5 years (Ministry of Agriculture and Forestry 2000). The perceived extent of regional establishment into wild honey bee populations within that time led to the conclusion of eradication being technically infeasible (Ministry of Agriculture and Forestry 2002b). Additional factors that are associated with V. destructor, such as the impact of deformed wing virus and its potential for infection of other bee species, were not included in the initial economic assessment of Varroa’s introduction. The extent of infection and geographic complexity would have ensured a wild reservoir of V. destructor, regardless of commercial eradication for both the North initial introduction and the subsequent South Island detections (Ministry of Agriculture and Forestry 2002b). The multiple detections of V. destructor in the South Island suggest incomplete monitoring and/or possible movement despite controls would have continued to negate quarantine efforts during the initial phase of establishment. Once V. destructor was detected in South Island feral honey bee populations, any further controls were futile.
The difficulty of eradication warrants the approach of emphasizing early detection coupled with a rapid integrated control scheme, being taken by the Australian Department of Agriculture, Fisheries and Forestry. As Varroa’s arrival is taken as inevitable, efforts are also focused on mitigation should establishment occur. As V. destructor has yet to be documented in Australia, there is an unparalleled opportunity to document and research the impact of Varroa invasion and consequent decline in honey bee populations. This research is globally significant as it provides a unique situation to assess the impact of honey bee decline not only on pollination services to agriculture before Varroa and associated virus introductions, but also on the foraging dynamics and ecology of a wide spectrum of pollinators.
It is also critical to understand what resistance factors are relevant in honey bee hives that have survived infestation in New Zealand and in the future, Australia. Although Varroa has had a devastating impact on both commercial and unmanaged honey bee populations, natural tolerance and resistance are widespread traits in countries with Varroa and its associated viruses (Le Conte et al. 2007; Rosenkranz et al. 2010). Understanding what factors contribute to resistance can help in selectively breeding resistant honey bees and successfully allowing selective pressures to evolve tolerances (Harbo and Harris 2001). This approach is especially important in the face of miticide resistance wherever such products are used (Johnson et al. 2010).
The Varroa mite has decimated significant numbers of honey bee colonies where it has become established (Rosenkranz et al. 2010). In some areas where Varroa has arrived, prior negative effects on native bees due to competition for floral resources by honey bees had been of concern (Paini 2004; Martin et al. 2012). There is also the possibility that past competition with honey bees may have driven vulnerable native bees to extinction, or selected for bee communities that demonstrate robustness in the face of honey bee competition. Thus, there may be circumstances whereby the loss of honey bees could represent a gain for native bees.
In New Zealand, competition between honey bees and native bees has not been well studied. While some localized experimental data suggest honey bees and native bees do not compete for resources (Jay Iwasaki, unpublished data), there have been no studies of the differences in native bee populations before and after Varroa introduction to demonstrate any landscape level effects. In contrast, there are multiple studies in Australia that show negative consequences of honey bee presence for native bees and birds (Paini 2004). The missed research opportunities pre-Varroa in New Zealand highlight the importance of documenting conditions throughout Australia to better understand the effects of introduced honey bees on native fauna that may occupy similar niches to the honey bee.
Regardless of whether the establishment of Varroa may provide a resource benefit for native bees in Australia (the last major beekeeping country free of Varroa), recent discoveries that associated Varroa pathogens such as deformed wing virus can affect non-host species (e.g., bumblebees and native bees in Europe) suggest a negative net effect (Fürst et al. 2014). In addition, honey bees are integral pollinators and represent billions of dollars of ecosystem services and further losses could threaten food security (Potts et al. 2010). The concerns about honey bee decline and the general pollinator decline worldwide highlight the inherent vulnerability of depending on one pollinator species and bring into focus the importance of management strategies that integrate and enhance the ecosystem services provided by a diverse pollinator community (Garibaldi et al. 2013).
It is abundantly clear that around the world many biological invasions have negatively affected native plant and animal communities. It is also clear that the spread of novel parasites and pathogens can have severe impacts on pollinator species that may have significant unforeseen consequences decades later. What is less clear in New Zealand is the response of native pollinator communities to the spread of a parasite and novel viruses. This research gap exists because baseline data were not obtained prior to Varroa arrival in the country. In New Zealand, comparative studies of disease prevalence among native bee and bumblebee populations in the North Island and South Island should be given a high priority. Results could be indicative of the potential epidemiological consequences for Australian bee communities. The need for greater understanding of pollinator dynamics before and after the establishment of Varroa, and pollinator community changes in the aftermath of invasion provide a strong argument for establishing, maintaining, and expanding studies of biotic interactions and community dynamics across Australia at the earliest stage possible.
Acknowledgments
We would like to thank several anonymous reviewers for input on an earlier version of this manuscript. We would also like to thank Ken Miller for help with Figure design, as well as Alexander Wild, for allowing use of his images in journal articles free of license fees.
Biographies
Jay M. Iwasaki
is a PhD candidate in Ecology at the University of Otago studying competitive interactions between native and introduced bees in New Zealand.
Barbara I. P. Barratt
is an insect ecologist who focuses on biosafety of biological control, biosecurity, native grassland biodiversity, community ecology, and conservation.
Janice M. Lord
is a botanist interested in the evolution of plant–animal mutualisms and plant reproductive strategies, with a particular focus on plant pollination systems in cold climates and novel communities.
Alison R. Mercer
is a neuroethologist who focuses on neural mechanisms that underlie learning, memory, and stress responses in the honey bee.
Katharine J. M. Dickinson
is principally a plant ecologist with research interests that include plant–animal interactions.
Contributor Information
Jay M. Iwasaki, Phone: 64 21 0843 9883, Email: jay.iwasaki@otago.ac.nz
Barbara I. P. Barratt, Phone: 64 3 489 9061, Email: barbara.barratt@agresearch.co.nz
Janice M. Lord, Phone: 64 3 479 5131, Email: janice.lord@botany.otago.ac.nz
Alison R. Mercer, Phone: 64 3 479 7961, Email: alison.mercer@otago.ac.nz
Katharine J. M. Dickinson, Phone: 64 3 479 9059, Email: kath.dickinson@botany.otago.ac.nz
References
- Barnett J, Pauling J. The environmental effects of New Zealand’s free-market reforms. Environment, Development and Sustainability. 2005;7:271–289. doi: 10.1007/s10668-005-7316-0. [DOI] [Google Scholar]
- Barry, S., D. Cook, R. Duthie, D. Clifford, and D. Anderson. 2010. Future Surveillance Needs for Honeybee Biosecurity. Rural Industries Research and Development Corporation 10/107.
- Batley M, Hogendoorn K. Diversity and conservation status of native Australian bees. Apidologie. 2009;40:347–354. doi: 10.1051/apido/2009018. [DOI] [Google Scholar]
- Benard H, Bolger P, Stone M, Thornton R. The outbreak of Varroa destructor in New Zealand bees: Delimiting survey results and management options. Surveillance. 2001;28:3–5. [Google Scholar]
- Bischoff M, Campbell DR, Lord JM, Robertson AW. The relative importance of solitary bees and syrphid flies as pollinators of two outcrossing plant species in the New Zealand alpine. Austral Ecology. 2013;38:169–176. doi: 10.1111/j.1442-9993.2012.02389.x. [DOI] [Google Scholar]
- Blanche KR, Ludwig JA, Cunningham SA. Proximity to rainforest enhances pollination and fruit set in orchards. Journal of Applied Ecology. 2006;43:1182–1187. doi: 10.1111/j.1365-2664.2006.01230.x. [DOI] [Google Scholar]
- Campbell DR, Bischoff M, Lord JM, Robertson AW. Flower color influences insect visitation in alpine New Zealand. Ecology. 2010;91:2638–2649. doi: 10.1890/09-0941.1. [DOI] [PubMed] [Google Scholar]
- Carvell C. Habitat use and conservation of bumblebees (Bombus spp.) under different grassland management regimes. Biological Conservation. 2002;103:33–49. doi: 10.1016/S0006-3207(01)00114-8. [DOI] [Google Scholar]
- Le Conte Y, de Vaublanc G, Crauser D, Jeanne F, Rousselle J-C, Bécard J-M. Honey bee colonies that have survived Varroa destructor. Apidologie. 2007;38:566–572. doi: 10.1051/apido:2007040. [DOI] [Google Scholar]
- CSIRO . Australia’s biosecurity future: Preparing for future biological challenges. Canberra: CSIRO; 2014. [Google Scholar]
- Cunningham SA, FitzGibbon F, Heard T. The future of pollinators for Australian agriculture. Australian Journal of Agricultural Research. 2002;53:893–900. doi: 10.1071/AR01186. [DOI] [Google Scholar]
- Cunningham SA, Schellhorn NA, Marcora A, Batley M. Movement and phenology of bees in a subtropical Australian agricultural landscape. Austral Ecology. 2013;38:456–464. doi: 10.1111/j.1442-9993.2012.02432.x. [DOI] [Google Scholar]
- Department of Agriculture, Fisheries, and Forestry . A honey bee industry and pollination continuity strategy should Varroa become established in Australia. Auckland: Australian Government; 2011. [Google Scholar]
- Donovan BJ. Interactions between native and introduced bees in New Zealand. New Zealand Journal of Ecology. 1980;3:104–116. [Google Scholar]
- Donovan, B. J. 2007. Apoidea (Insecta: Hymenoptera). In Fauna of New Zealand 57. Landcare Research.
- Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature. 2014;506:364–366. doi: 10.1038/nature12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallai N, Salles J-M, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological Economics. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- Garibaldi L, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, Kremen C, Carvalheiro LG, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339:1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- Genersch E, Yue C, Fries I, de Miranda JR. Detection of Deformed wing virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. Journal of Invertebrate Pathology. 2006;91:61–63. doi: 10.1016/j.jip.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Gordon, J., and L. Davis. 2003. Valuing honeybee pollination. Rural Industries Research and Development Corporation. 03/077.
- Goulson D, Stout JC, Kells AR. Do exotic bumblebees and honeybees compete with native flower-visiting insects in Tasmania? Journal of Insect Conservation. 2002;6:179–189. doi: 10.1023/A:1023239221447. [DOI] [Google Scholar]
- Harbo JR, Harris JW. Resistance to Varroa destructor (Mesostigmata: Varroidae) when mite-resistant queen honey bees (Hymenoptera: Apidae) were free-mated with unselected drones. Journal of Economic Entomology. 2001;94:1319–1323. doi: 10.1603/0022-0493-94.6.1319. [DOI] [PubMed] [Google Scholar]
- Hingston A, McQuillan P. Displacement of Tasmanian native megachilid bees by the recently introduced bumblebee Bombus terrestris (Linnaeus, 1758) (Hymenoptera: Apidae) Australian Journal of Zoology. 1999;47:59–65. doi: 10.1071/ZO98016. [DOI] [Google Scholar]
- Hingston AB, Marsden-Smedley J, Driscoll DA, Corbett S, Fenton J, Anderson R, Plowman C, Mowling F, et al. Extent of invasion of Tasmanian native vegetation by the exotic bumblebee Bombus terrestris (Apoidea: Apidae) Austral Ecology. 2002;27:162–172. doi: 10.1046/j.1442-9993.2002.01179.x. [DOI] [Google Scholar]
- Howlett B, Donovan B. A review of New Zealand’s deliberately introduced bee fauna: Current status and potential impacts. New Zealand Entomologist. 2010;33:92–101. doi: 10.1080/00779962.2010.9722196. [DOI] [Google Scholar]
- Huryn VMB. Use of native New Zealand plants by honey bees (Apis mellifera L.): A review. New Zealand Journal of Botany. 1995;33:497–512. doi: 10.1080/0028825X.1995.10410621. [DOI] [Google Scholar]
- Iwasaki, J. Unpublished data, Department of Botany, University of Otago, Dunedin, New Zealand.
- Jaffé R, Dietemann V, Allsopp MH, Costa C, Crewe RM, Dall’olio R, de la Rúa P, El-niweiri MAA, et al. Estimating the density of honeybee colonies across their natural range to fill the gap in pollinator decline censuses. Conservation Biology. 2010;24:583–593. doi: 10.1111/j.1523-1739.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- Johnson RM, Ellis MD, Mullin CA, Frazier M. Pesticides and honey bee toxicity—USA. Apidologie. 2010;41:312–331. doi: 10.1051/apido/2010018. [DOI] [Google Scholar]
- Kingston AB, McQuillan PB. Does the recently introduced bumblebee Bombus terrestris (Apidae) threaten Australian ecosystems? Australian Journal of Ecology. 1998;23:539–549. doi: 10.1111/j.1442-9993.1998.tb00764.x. [DOI] [Google Scholar]
- Klein A-M, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proceedings of the Royal Society: Biological Sciences. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C, Williams NM, Thorp RW. Crop pollination from native bees at risk from agricultural intensification. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgard SF, Sprosen MS, Penno JW, Rajendram GS. Nitrogen fixation by white clover in pastures grazed by dairy cows: Temporal variation and effects of nitrogen fertilization. Plant and Soil. 2001;299:177–187. doi: 10.1023/A:1004833804002. [DOI] [Google Scholar]
- Lockwood JL, Hoopes MF, Marchetti MP. Invasion ecology. London: Wiley; 2013. [DOI] [PubMed] [Google Scholar]
- Mark G, Cliff V. Control of Varroa (a guide for New Zealand Beekeepers) Willington: New Zealand Ministry of Agriculture and Forestry; 2001. [Google Scholar]
- Martin SJ, Medina LM. Africanized honeybees have unique tolerance to Varroa mites. Trends in Parasitology. 2004;20:113–114. doi: 10.1016/j.pt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GE, Powell M, Nikaido S, Schroeder DC. Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- McMenamin AJ, Genersch E. Honey bee (Apis mellifera) colony losses and associated viruses. Current Opinion in Insect Science. 2015;2:1–9. doi: 10.1016/j.cofs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Ministry for Primary Industries. 2013. Varroa mite. Retrieved 26 March, 2015, from http://www.biosecurity.govt.nz/pests/varroa.
- Ministry for Primary Industries. 2014. 2014 Apiculture Monitoring Report. doi:10.1111/j.1747-0080.2007.00199.x.
- Ministry of Agriculture and Forestry . Varroa in New Zealand: Economic impact assessment. Auckland: Ministry of Agriculture and Forestry; 2000. [Google Scholar]
- Ministry of Agriculture and Forestry . Management of biosecurity risks: Case studies. Willington: Ministry of Agriculture and Forestry; 2002. [Google Scholar]
- Ministry of Agriculture and Forestry . Review of Varroa economic impact assessment: Recommendations on revision. Canberra: Ministry of Agriculture and Forestry; 2002. [Google Scholar]
- Ministry of Agriculture and Forestry. 2003a. Major changes to Varroa movement controls. Retrieved 26 March, 2015, from https://web.archive.org/web/20130208175132/http://mpi.govt.nz/news-resources/news/major-changes-to-varroa-movement-controls.aspx.
- Ministry of Agriculture and Forestry. 2003b. Varroa pest management strategy outlined. Retrieved 26 March, 2015, from https://web.archive.org/web/20130208174554/http://mpi.govt.nz/news-resources/news/varroa-pest-management-strategy-outlined.aspx.
- Ministry of Agriculture and Forestry. 2004a. No further finds of Varroa in South Island. Retrieved 26 March, 2015, from https://web.archive.org/web/20130208185754/http://mpi.govt.nz/news-resources/news/no-further-finds-of-varroa-in-south-island.aspx.
- Ministry of Agriculture and Forestry. 2004b. South Island Varroa update. Retrieved 26 March, 2015, from https://web.archive.org/web/20130208185805/http:/mpi.govt.nz/news-resources/news/south-island-varroa-update.aspx.
- Ministry of Agriculture and Forestry. 2004c. Varroa investigation continues. Retrieved 26 March, 2015, from https://web.archive.org/web/20130206220404/http://mpi.govt.nz/news-resources/news/varroa-investigation-continues.
- Ministry of Agriculture and Forestry. 2006a. Nelson Varroa bee mite incursion—Update 3. Retrieved 26 March, 2015, from https://web.archive.org/web/20130216161132/http://mpi.govt.nz/news-resources/news/nelson-varroa-bee-mite-incursion-update-3.aspx.
- Ministry of Agriculture and Forestry. 2006b. Varroa update 15. Retrieved 26 March, 2015, from https://web.archive.org/web/20130216170505/http://mpi.govt.nz/news-resources/news/varroa-update-15.aspx.
- Ministry of Agriculture and Forestry. 2007. Varroa update 18. Retrieved 26 March, 2015, from https://web.archive.org/web/20130216171009/http://mpi.govt.nz/news-resources/news/varroa-update-18.aspx.
- Ministry of Agriculture and Forestry. 2008a. MAF biosecurity to revoke varroa movement controls. Retrieved 26 March, 2015, from https://web.archive.org/web/20130216180955/http://mpi.govt.nz/news-resources/news/maf-biosecurity-to-revoke-varroa-movement-controls.aspx.
- Ministry of Agriculture and Forestry. 2008b. New South Island controlled area for Varroa bee mite. Retrieved 26 March, 2015, from https://web.archive.org/web/20130216172053/http://mpi.govt.nz/news-resources/news/new-south-island-controlled-area-for-varroa-bee-mi.aspx.
- Mondet F, de Miranda JR, Kretzschmar A, Le Conte Y, Mercer AR. On the front line: Quantitative virus dynamics in honeybee (Apis mellifera L.) Colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathogens. 2014;10:1–15. doi: 10.1371/journal.ppat.1004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd B. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends in Ecology & Evolution. 1999;14:312–315. doi: 10.1016/S0169-5347(99)01613-4. [DOI] [PubMed] [Google Scholar]
- Oldroyd BP, Thexton E, Lawler S, Crozier R. Population demography of Australian feral bees (Apis mellifera) Oecologia. 1997;111:381–387. doi: 10.1007/s004420050249. [DOI] [PubMed] [Google Scholar]
- Paini D, Roberts J. Commercial honey bees reduce the fecundity of an Australian native bee. Biological Conservation. 2005;123:103–112. doi: 10.1016/j.biocon.2004.11.001. [DOI] [Google Scholar]
- Paini DR. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: A review. Austral Ecology. 2004;29:399–407. doi: 10.1111/j.1442-9993.2004.01376.x. [DOI] [Google Scholar]
- Parliamentary Commissioner for the Environment. 2013. Water quality in New Zealand: Land use and nutrient pollution. Retrieved 26 March, 2015, from http://www.pce.parliament.nz/assets/Uploads/PCE-Water-quality-land-use-web-amended.pdf.
- Paton DC. Honeybees in the Australian environment. BioScience. 1993;43:95–103. doi: 10.2307/1311970. [DOI] [Google Scholar]
- Popic TJ, Wardle GM, Davila YC. Flower-visitor networks only partially predict the function of pollen transport by bees. Austral Ecology. 2013;38:76–86. doi: 10.1111/j.1442-9993.2012.02377.x. [DOI] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: Trends, impacts and drivers. Trends in Ecology & Evolution. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Rader R, Howlett BG, Cunningham SA, Westcott DA, Newstrom-Lloyd LE, Walker MK, Teulon DAJJ, Edwards W. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. Journal of Applied Ecology. 2009;46:1080–1087. doi: 10.1111/j.1365-2664.2009.01700.x. [DOI] [Google Scholar]
- Rader R, Howlett BG, Cunningham SA, Westcott DA, Edwards W. Spatial and temporal variation in pollinator effectiveness: Do unmanaged insects provide consistent pollination services to mass flowering crops? Journal of Applied Ecology. 2012;49:126–134. doi: 10.1111/j.1365-2664.2011.02066.x. [DOI] [Google Scholar]
- Ravoet J, De Smet L, Meeus I, Smagghe G, Wenseleers T, de Graaf DC. Widespread occurrence of honey bee pathogens in solitary bees. Journal of Invertebrate Pathology. 2014;122:55–58. doi: 10.1016/j.jip.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Ricketts T. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conservation Biology. 2004;18:1262–1271. doi: 10.1111/j.1523-1739.2004.00227.x. [DOI] [Google Scholar]
- Rinderer TE, Oldroyd BP, Frake AM, de Guzman LI, Bourgeois L. Responses to Varroa destructor and Nosema ceranae by several commercial strains of Australian and North American honeybees (Hymenoptera: Apidae) Australian Journal of Entomology. 2013;52:156–163. doi: 10.1111/aen.12003. [DOI] [Google Scholar]
- Ritter Wolfgang. Varroa disease of the honeybee Apis mellifera. Bee World. 1981;62:141–153. doi: 10.1080/0005772X.1981.11097838. [DOI] [Google Scholar]
- Roberts J. Email communication. Canberra: CSIRO ecosystem Sciences/Biosecurity Flagship; 2015. [Google Scholar]
- Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. Journal of Invertebrate Pathology. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Stout JC, Kells AR, Goulson D. Pollination of the invasive exotic shrub Lupinus arboreus (Fabaceae) by introduced bees in Tasmania. Biological Conservation. 2002;106:425–434. doi: 10.1016/S0006-3207(02)00046-0. [DOI] [Google Scholar]
- Waters JM, Craw D. Goodbye Gondwana? New Zealand biogeography, geology, and the problem of circularity. Systematic Biology. 2006;55:351–356. doi: 10.1080/10635150600681659. [DOI] [PubMed] [Google Scholar]
- Williams GR, Tarpy DR, VanEngelsdorp D, Chauzat MP, Cox-Foster DL, Delaplane KS, Neumann P, Pettis JS, et al. Colony collapse disorder in context. BioEssays. 2010;32:845–846. doi: 10.1002/bies.201000075. [DOI] [PMC free article] [PubMed] [Google Scholar]