Abstract
Objective
Schizophrenia patients exhibit impaired working and episodic memory, but this may represent generalized impairment across memory modalities or performance deficits restricted to particular memory systems in subgroups of patients. Furthermore, it is unclear whether deficits are unique from those associated with other disorders.
Method
Healthy controls (n=1101) and patients with schizophrenia (n=58), bipolar disorder (n=49) and attention-deficit-hyperactivity-disorder (n=46) performed 18 tasks addressing primarily verbal and spatial episodic and working memory. Effect sizes for group contrasts were compared across tasks and the consistency of subjects’ distributional positions across memory domains was measured.
Results
Schizophrenia patients performed poorly relative to the other groups on every test. While low to moderate correlation was found between memory domains (r=.320), supporting modularity of these systems, there was limited agreement between measures regarding each individual’s task performance (ICC=.292) and in identifying those individuals falling into the lowest quintile (kappa=0.259). A general ability factor accounted for nearly all of the group differences in performance and agreement across measures in classifying low performers.
Conclusions
Pathophysiological processes involved in schizophrenia appear to act primarily on general abilities required in all tasks rather than on specific abilities within different memory domains and modalities. These effects represent a general shift in the overall distribution of general ability (i.e., each case functioning at a lower level than they would have if not for the illness), rather than presence of a generally low-performing subgroup of patients. There is little evidence that memory impairments in schizophrenia are shared with bipolar disorder and ADHD.
Keywords: psychosis, working memory, episodic memory, cognition, bipolar disorder
1. Introduction
Memory impairment is a core feature of schizophrenia (Kahn and Keefe, 2013) related to functioning and prognosis (Green et al., 2004). Patients with schizophrenia and their first-degree relatives demonstrate impairment in working and episodic memory (Agnew-Blais and Seidman, 2012; Aleman et al., 1999; Forbes et al., 2009; Snitz et al., 2006; Trandafir et al., 2006) and both working memory (Glahn et al., 2003) and episodic memory are moderately heritable (Finkel and McGue, 1993; Owens et al., 2011). Thus, memory impairments may represent a biomarker of schizophrenia; however, questions about the generality of these deficits remain to be addressed.
First, despite group level memory impairment in schizophrenia, measures of memory performance are limited as individualized diagnostic classifiers (Glahn et al., 2007; Kern et al., 2011). It is unclear whether deficits across memory domains and modalities (e.g., working vs. episodic, verbal vs. visuospatial) reflect generalized impairment (Gold and Dickinson, 2013), a specific subgroup of patients exhibiting neurocognitive deficits in multiple domains (McDermid Vaz and Heinrichs, 2002), or different subsets of patients displaying deficits in different domains (Karlsgodt et al., 2011). Previous research in a large schizophrenia sample found that cognitive impairment was best explained by a single deficit factor (Keefe et al., 2006); however, this study did not include controls and so could not directly asses how patterns found in patients compare to typical cognitive structure. A model including executive functioning, memory and processing speed best discriminates schizophrenia from controls (Lam et al., 2014), which supports the theory that patients with schizophrenia are broadly cognitive impaired, but this study included relatively independent cognitive tasks and the structure within multiple memory-related tasks has not been measured. Additionally, more refined, cognitive neuroscience-based tasks might better identify discrete neurocognitive subsystems that are impaired in patient groups (Carter and Barch, 2007).
Second, it is unclear whether memory deficits associated with schizophrenia represent biomarkers of risk processes shared with other diagnostic syndromes, such as bipolar disorder (Kurtz and Gerraty, 2009) or attention-deficit-hyperactivity-disorder (ADHD)(Martinussen et al., 2005). While memory impairments in ADHD are likely more circumscribed (Castel et al., 2011), impairments in bipolar disorder may be closer to those found in schizophrenia, particularly among cases with psychotic symptoms (Glahn et al., 2006; Hill et al., 2013). Thus, it is important to assess the structure of cognitive dysfunction across diagnostic boundaries (Cuthbert and Insel, 2010).
This study sought to clarify the distribution and covariation of impairments across domains of memory in patients with schizophrenia and to determine to the extent to which these impairments are shared with bipolar disorder and ADHD. The psychiatric comparison groups allow us to examine memory impairment in schizophrenia in the context of individuals who are hypothesized to share genetic risk architecture with schizophrenia. A large reference sample of community volunteers (n=1101) was collected to provide robust estimation of the normative distributions of performance on all measures, which included both established neuropsychological tasks and experimental tasks designed to isolate theoretically separable aspects of working and episodic memory functioning (Carter et al., 2008).
We hypothesized that among patients with schizophrenia, distributions on all measures of memory performance would be shifted downward compared with those of the reference population and that there would be consistency in terms of where particular patients scored in the distributions across domains and modalities. We further hypothesized that there would be a similar distributional shift and cross-distributional consistency among bipolar cases with psychotic features, but not among non-psychotic bipolar patients or subjects with ADHD.
2. Methods
2.1 Subjects
The study was approved by the Institutional Review Boards of UCLA and Yale University and participants provided written informed consent. Subjects were recruited via the UCLA Consortium for Neuropsychiatric Phenomics (www.phenomics.ucla.edu). 1101 healthy controls (CON) without history of psychosis or ADHD and no current mood or anxiety disorders were studied, as well as 58 schizophrenia (SCZ) patients, 49 bipolar (BP) patients, and 46 ADHD patients. Participants, aged 21–50, were recruited by community advertisements from the Los Angeles area, identified as “White, Not of Hispanic or Latino Origin” or “Hispanic or Latino, of Any Race” and completed at least 8 years of education. (Other racial and ethnic groups were not recruited to minimize confounding planned genetic analyses in the broader study.) Participants were screened for neurological disease, head injury with loss of consciousness or cognitive sequelae, or substance dependence within past 6 months. Subjects were excluded if urinalysis results were positive for drugs of abuse on the day of testing.
2.2 Clinical and Cognitive Assessment
Participants were interviewed using the SCID-IV (First et al., 1995) and patient groups were rated on the Hamilton Psychiatric Rating Scale for Depression (HAM-D) (Hamilton, 1960), the Scale for the Assessment of Positive and Negative Symptoms (SAPS and SANS) (Andreasen, 1983a; 1983b) and the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962). Participants in the BP group were also differentiated into those with history of psychosis, determined by report of positive psychotic symptoms during the SCID interview, and those without history of psychosis. Interviewers were trained to criterion levels of reliability, which for the SCID involved meeting kappa scores of .85 or greater on diagnosis and .75 or greater on symptom and algorithm decisions(Ventura et al., 1998). For symptom assessment scales interviewers were trained to a criterion ICC = .80 or greater (Ventura et al., 1995).
The neuropsychological testing battery consisted of multiple measures related to memory functioning including the California Verbal Learning Test (CVLT) (Delis et al., 2000), Visual Reproduction, Symbol Span, Digit Span and Letter-Number Sequencing from the Wechsler Memory Scale-IV (Wechsler, 2009). In addition, Vocabulary and Matrix Reasoning from the Wechsler Adult Intelligence Scale-IV (Wechsler, 2008), and the Color Trails Test (D'Elia et al., 1996) were included as estimates of verbal intelligence, nonverbal reasoning and processing speed/cognitive switching, respectively.
During the cognitive testing session subjects also performed computer-based tasks, programmed and presented in E-prime®, designed to probe working and episodic memory functioning. Remember-Know and Scene Recognition tasks were designed to test verbal and visual episodic memory, respectively. Verbal and spatial working memory capacity, maintenance and manipulation were also tested. Detailed task descriptions are available in the appendix (SA1).
2.3 Statistical Analysis
Statistical analyses were conducted using R (Team, 2015). Group differences on cognitive performance were tested using MANOVA followed by univariate ANOVA and Tukey’s HSD to clarify patterns of performance in each domain. Effect size (Hedge’s g) was calculated to compare the relative size of group effects and linear discriminant analysis with leave-one-out cross validation was used to determine how well tasks differentiated between groups.
To evaluate whether the same subjects showed impairment across memory domains, we examined correlations between the primary performance measures for each task, both in terms of their original continuous scaling and in terms of consistency interclass correlations of measures transformed into quintile rankings based on the full sample distribution. The consistency with which an individual falls in the lowest performance range is of particular interest as they are more likely to suffer associated functional impediments. Kappa coefficient of agreement was to measure the consistency of individuals in this lowest quintile of performance, where each measure was included as a separate ‘rater’ of performance. These measures of consistency were conducted across all tasks as well as within a priori defined domains of memory (i.e. working vs. episodic memory and verbal vs. spatial memory. See SA2 for a list of tasks in each domain).
Principal component analysis (PCA) was then used to further examine the data. The first principal component was determined to represent a general cognitive factor and tested for group differences. The residual variance was then tested using the above methods to determine how the structure of memory impairments among the subject groups is affected by generalized versus task specific effects.
3. Results
3.1 Sample Characteristics
CON and SCZ/BP differed significantly on age and there was a significant difference in the proportion of males in SCZ compared with the other groups. Thus, age and gender were included as covariates in the group-contrast analyses. There were also significant group differences on ethnicity with CON and SCZ having higher percentages of Hispanic participants than BP and ADHD. As expected, patient groups differed on ratings of clinical variables, with more severe overall psychopathology, positive and negative symptoms in SCZ and both SCZ and BP experiencing more depression symptoms than ADHD (Table 1).
Table 1.
Demographic, clinical and performance characteristics of study participants
| Control Subjects (CON) |
Schizophrenia Patients (SCZ) |
Bipolar Patients (BP) |
ADHD Patients (ADHD) |
Statistic | Patterna | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | p | |||
| Demographic | |||||||||||
| Age (yrs) | 31.34 | 8.47 | 35.84 | 8.61 | 35.29 | 9.03 | 32.91 | 10.67 | F(3,1250) = 8.25 | p<.001 | C<B**; C<S*** |
| Gender (%male) | 46.7% | 75.9% | 57.1% | 52.2% | X2(3)= 20.537 | p<.001 | S>A; S>B; S>C | ||||
| Education | 15.02 | 2.01 | 12.66 | 1.84 | 14.55 | 1.96 | 14.61 | 1.73 | F(3,1250) = 26.58 | p<.001 | S<A,B,C*** |
| Maternal Education | 13.09 | 3.88 | 12.33 | 2.87 | 13.67 | 3.44 | 14.50 | 2.49 | F(3,1229) = 3.12 | p=.025 | S<A* |
| Paternal Education | 13.72 | 4.26 | 13.04 | 3.74 | 15.13 | 3.64 | 14.67 | 2.80 | F(3.1194) = 2.85 | p=.036 | S<B(ns) |
| Ethnicity (%hispanic) | 40.7% | 58.6% | 26.5% | 15.2% | X2(3)= 24.067 | p<.001 | S>C>A,B | ||||
| Clinical Rating Scales | |||||||||||
| Hamilton Depressionb | n/a | 15.45 | 11.5 | 18.11 | 13.01 | 10.78 | 7.25 | F(2,144)=5.28 | p=.006 | A<B** | |
| BPRS | n/a | 52.74 | 14.98 | 44.81 | 11.05 | 38.02 | 7.31 | F(2,147)=19.90 | p<.001 | S>A***,S>B**, B>A* | |
| SAPS | n/a | 32.16 | 22.32 | 7.17 | 8.41 | n/a | F(1,82)=44.36 | p<.001 | |||
| SANS | n/a | 38.24 | 24.09 | 20.05 | 13.12 | n/a | F(1,85)=18.76 | p<.001 | |||
| Neuropsychological Performance (Raw score) | |||||||||||
| CVLT | 55.62 | 9.23 | 40.62 | 10.07 | 51.49 | 11.17 | 54.74 | 8.74 | F(3, 1249)=38.11 | p<.001 | S<A,B,C***, B<C* |
| Visual Reproduction I | 37.53 | 4.79 | 31.98 | 8.22 | 35.86 | 5.03 | 36.96 | 5.59 | F(3,1249)=19.24 | p<.001 | S<A,B,C*** |
| Visual Reproduction II | 31.40 | 8.21 | 22.95 | 10.98 | 27.31 | 10.17 | 29.63 | 9.04 | F(3,1249)=15.22 | p<.001 | S<A,C***, S<B*, B<C** |
| Symbol Spanc | 24.31 | 6.36 | 17.00 | 6.14 | 21.96 | 6.46 | 22.37 | 6.94 | F(3,1247)=19.62 | p<.001 | S<A,B,C*** |
| Digit Span | 29.47 | 6.02 | 22.93 | 5.17 | 28.37 | 6.11 | 29.13 | 4.70 | F(3,1249)=20.93 | p<.001 | S<A,B,C*** |
| Letter-Number Sequencing | 20.47 | 3.17 | 17.47 | 3.24 | 19.80 | 2.69 | 19.93 | 2.61 | F(3,1249)=15.02 | p<.001 | S<A,B,C*** |
| Vocabulary | 41.16 | 9.83 | 31.16 | 10.46 | 42.59 | 9.85 | 43.07 | 9.72 | F(3,1249)=19.84 | p<.001 | S<A,B,C*** |
| Matrix Reasoning | 19.59 | 4.27 | 15.26 | 5.22 | 19.22 | 4.41 | 20.39 | 3.76 | F(3,1249)=16.70 | p<.001 | S<A,B,C*** |
| Color Trails 1 | 34.24 | 12.75 | 43.31 | 19.47 | 35.16 | 11.05 | 35.41 | 12.24 | F(3,1248)=6.93 | p<.001 | S<C***,S<B**, S<A* |
| Color Trail 2 | 66.88 | 20.41 | 88.17 | 27.54 | 70.86 | 17.97 | 69.63 | 20.92 | F(3,1249)=15.47 | p<.001 | S<A,B,C*** |
| Memory Performance (d-prime) | |||||||||||
| Remember-Know | 1.77 | .70 | 1.19 | .83 | 1.47 | .77 | 1.70 | .71 | F(3,1127)=10.59 | p<.001 | S<A,B,C***, B<C* |
| Scene Recognition | 2.37 | .72 | 1.72 | .78 | 2.29 | .69 | 2.43 | .72 | F(3,1247)=13.91 | p<.001 | S<A,B,C*** |
| Spatial Capacity | 2.60 | .69 | 1.92 | .89 | 2.42 | .70 | 2.35 | .84 | F(3,1130)=16.59 | p<.001 | S<C***, S<B**, S<A* |
| Verbal Capacity | 2.41 | .67 | 1.80 | .88 | 2.06 | .59 | 2.36 | .73 | F(3,1131)=12.58 | p<.001 | S<A,C***, B<C** |
| Spatial Maintenance | 2.43 | 1.08 | 1.52 | 1.32 | 2.16 | 1.05 | 2.21 | 1.29 | F(3,1246)=12.26 | p<.001 | S<A,B,C*** |
| Spatial Manipulation | 1.54 | 1.01 | 1.19 | .98 | 1.42 | 1.13 | 1.43 | 1.05 | F(3,1247)=2.17 | p=.090 | |
| Verbal Maintenance | 3.28 | 1.25 | 1.81 | 1.32 | 3.02 | 1.35 | 3.02 | 1.33 | F(3,1246)=20.29 | p<.001 | S<A,B,C*** |
| Verbal Manipulationd | 2.41 | 1.41 | 1.14 | 1.22 | 1.78 | 1.40 | 2.19 | 1.29 | F(3,1246)=13.16 | p<.001 | S<A,B,C***, B<C* |
Mean and standard deviation for each group on demographic, clinical and behavioral testing measures. Statistical tests reported are those with age and gender as a covariate. For the neuropsychological test battery the primary metric used was the total raw score for each measure. True positive, false positive, true negative and false negative scores were calculated for the computer based memory performance measures and the primary metric used for this study was d-prime.
For tests where there was a main effect of group, post-hoc contrasts using Tukey’s HSD were performed. A=ADHD, B=BP, C=CON, S=SCZ. Only significant differences are reported (*** p<.001;** p<.01; *p<.05).
There was a main effect of group on Hamilton Depression score. Tukey’s HSD indicate lower depression scores for ADHD compared to BP (p=.005) and a trend towards lower depression scores for ADHD compared to SCZ (p=.087)
There was a main effect of group on SSP performance. Tukey’s HSD indicate worse performance for SCZ than CON, ADHD, and BP (all p<.001). There were no other significant group differences though there was a trend towards worse performance for BP than CON (p=.056)
There was a main effect of group on VMNM manipulation performance. Tukey’s HSD indicate worse performance for SCZ than CON and ADHD (p<.001). BP also demonstrated worse performance than CON (p=.012). There was a trend towards worse performance for SCZ than BP (p=.092).
3.2 Cognitive Testing Performance
Groups differed significantly on cognitive performance [F(3,989)=4.16, p<.001], age [F(1,989)=18.95, p<.001] and gender [F(1,989)=6.93, p<.001], with no significant interactions. The group effect was also significant for each measure of test performance at the univariate level (Table 1). Moreover, effect sizes revealed a moderate to large deficit for SCZ compared to CON, BP and ADHD on all measures, with g > .5 on 14 of the 18 measures (Table 2).
Table 2.
Effect sizes (hedge’s g) for differences between all groups on each memory measure and average over all the tasks. Also provides the proportion of each group that falls in the lowest quintile of the overall distribution on each memory measure.
| Effect Sizes | Lowest Quintile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test | SCZvsCON | SCZvsBP | SCZvsADHD | BPvsCON | ADHDvsCON | BPvsADHD | CON 1stQ | SCZ 1stQ | BP 1stQ | ADHD 1stQ |
| CVLT | −1.62 | −1.02 | −1.47 | −0.44 | −0.10 | −0.32 | 0.18 | 0.71 | 0.35 | 0.18 |
| Visual Reproduction I | −1.10 | −0.55 | −0.69 | −0.35 | −0.12 | −0.21 | 0.20 | 0.51 | 0.37 | 0.21 |
| Visual Reproduction II | −1.01 | −0.41 | −0.65 | −0.49 | −0.21 | −0.24 | 0.21 | 0.42 | 0.37 | 0.26 |
| Symbol Span | −1.15 | −0.78 | −0.82 | −0.37 | −0.30 | −0.06 | 0.19 | 0.56 | 0.28 | 0.31 |
| Digit Span | −1.09 | −0.96 | −1.24 | −0.18 | −0.06 | −0.14 | 0.22 | 0.58 | 0.26 | 0.15 |
| Letter Number Sequencing | −0.95 | −0.77 | −0.82 | −0.21 | −0.17 | −0.05 | 0.27 | 0.51 | 0.33 | 0.31 |
| Vocabulary | −1.01 | −1.11 | −1.17 | 0.15 | 0.19 | −0.05 | 0.21 | 0.56 | 0.19 | 0.21 |
| Matrix Reasoning | −1.00 | −0.81 | −1.10 | −0.09 | 0.19 | −0.28 | 0.19 | 0.53 | 0.21 | 0.13 |
| Color Trails I | −0.69 | −0.50 | −0.47 | −0.07 | −0.09 | 0.02 | 0.21 | 0.42 | 0.19 | 0.23 |
| Color Trails II | −1.02 | −0.73 | −0.74 | −0.20 | −0.13 | −0.06 | 0.18 | 0.56 | 0.21 | 0.26 |
| Remember-Know | −0.83 | −0.35 | −0.66 | −0.43 | −0.10 | −0.30 | 0.18 | 0.51 | 0.42 | 0.18 |
| Scene Recognition | −0.90 | −0.78 | −0.94 | −0.11 | 0.08 | −0.20 | 0.18 | 0.56 | 0.26 | 0.18 |
| Spatial Capacity | −0.98 | −0.61 | −0.49 | −0.27 | −0.37 | 0.09 | 0.18 | 0.62 | 0.26 | 0.33 |
| Verbal Capacity | −0.89 | −0.34 | −0.68 | −0.52 | −0.07 | −0.45 | 0.19 | 0.49 | 0.26 | 0.18 |
| Spatial Maintenance | −0.84 | −0.53 | −0.52 | −0.25 | −0.21 | −0.04 | 0.20 | 0.42 | 0.33 | 0.28 |
| Spatial Manipulation | −0.34 | −0.21 | −0.23 | −0.11 | −0.11 | −0.01 | 0.19 | 0.24 | 0.28 | 0.23 |
| Verbal Maintenance | −1.18 | −0.90 | −0.91 | −0.21 | −0.20 | 0.00 | 0.18 | 0.53 | 0.23 | 0.33 |
| Verbal Manipulation | −0.91 | −0.49 | −0.83 | −0.45 | −0.16 | −0.30 | 0.18 | 0.56 | 0.28 | 0.23 |
| Average | −0.97 | −0.66 | −0.80 | −0.26 | −0.11 | −0.14 | 0.20 | 0.52 | 0.28 | 0.23 |
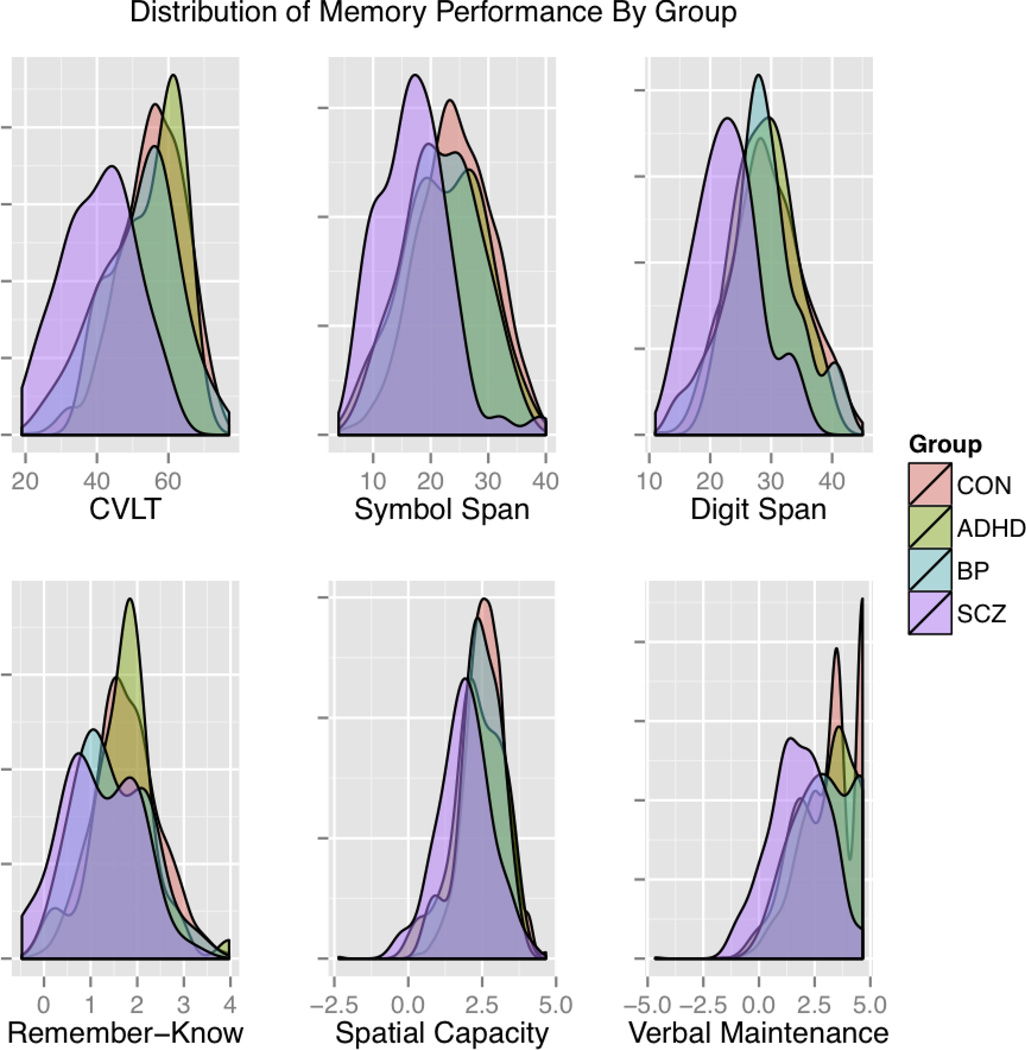
Frequency distributions (Figure 1) show that the performance of the patient groups generally falls within the range of the CON sample. Mean differences appear as an overall downward shift of the distributions for the SCZ group and, to a lesser extent, the BP and ADHD groups. Furthermore, discriminant analysis was unable to accurately identify patient groups when compared to the large reference sample of controls utilized in this study (SA3).
Figure 1.
Density plots of the distribution of memory performance for each group on a representative subset of the measures. Distributions are represented as the proportion of each sample falling in a given range using a Gaussian kernel density curve to facilitate comparisons across groups despite sample size differences. Three neuropsychological and three cognitive/experimental measures are shown covering verbal episodic memory and spatial and verbal working memory. The primary measures for comparison were the total raw score for the CVLT, Symbol Span and Digit Span and d-prime for the Remember-Know, Spatial Capacity (SCAP), and Verbal Maintenance (VMNM – Maintenance) measures. Performance on each task was scaled (M=0, SD=1) across all participants.
Group differences in memory performance were independent of clinical symptom severity (SA4). Few BP participants were currently experiencing psychotic symptoms, but 27 (55%) reported a history of positive psychotic symptoms (i.e., hallucinations or delusions on the SCID). However, contrary to our hypotheses, there were no significant differences between BP subjects with and without history of psychosis on any measure (all p’s > .11).
3.3 Consistency of Impairments
As identification of individuals with clinically relevant cognitive impairments is most pertinent for allocating resources such as remediation, the consistency with which an individual falls in the lowest performance range across tasks is of particular interest. Across tasks, a large percentage of patients with schizophrenia were in the lowest quintile of the overall distribution (Table 2). However, analyses of cross-test agreement in classification of subjects in the lowest quintile indicated only modest overlap (Fleiss’ Kappa for m “Raters” (tests): Overall = .209; CON=.208; SCZ=.259; BP=.213; ADHD=.215). Restricting analyses to a priori classifications of memory domains (SA2) did not produce appreciably higher consistency metrics. Overall, kappa for lowest quintile of performance within measures of working memory was .221 [CON=.190, SCZ=.275, BP=.311, ADHD=.198], while kappa for lowest quintile of performance between measures of working memory and episodic memory was .177 [CON=.140, SCZ=.257, BP=.157, ADHD=.224] and between measures of working memory and general cognition was .221 [CON=.201, SCZ=.234, BP=.191, ADHD=.185]. Similarly, kappa for agreement on lowest quintile of performance within measures of episodic memory was .248 [CON=.205, SCZ=.291, BP=.311, ADHD=.256] and between measures of episodic memory and general cognition was .189 [CON=.156, SCZ=.253,BP=.174, ADHD=.177]. Similar results were found when measures were classified as either verbal memory or visual memory. While evaluating the consistency with which tests identify individuals in the lowest quintile most closely parallels the identification of individuals considered to be clinically impaired, this does necessitate collapsing across the remaining range of performance. However, test by test consistency statistics, analyses of correlations and ICCs for continuous measures of performance across tasks produced highly parallel results, with similarly modest agreement and weak support for consistency within proposed domains (ST1–5,SA5).
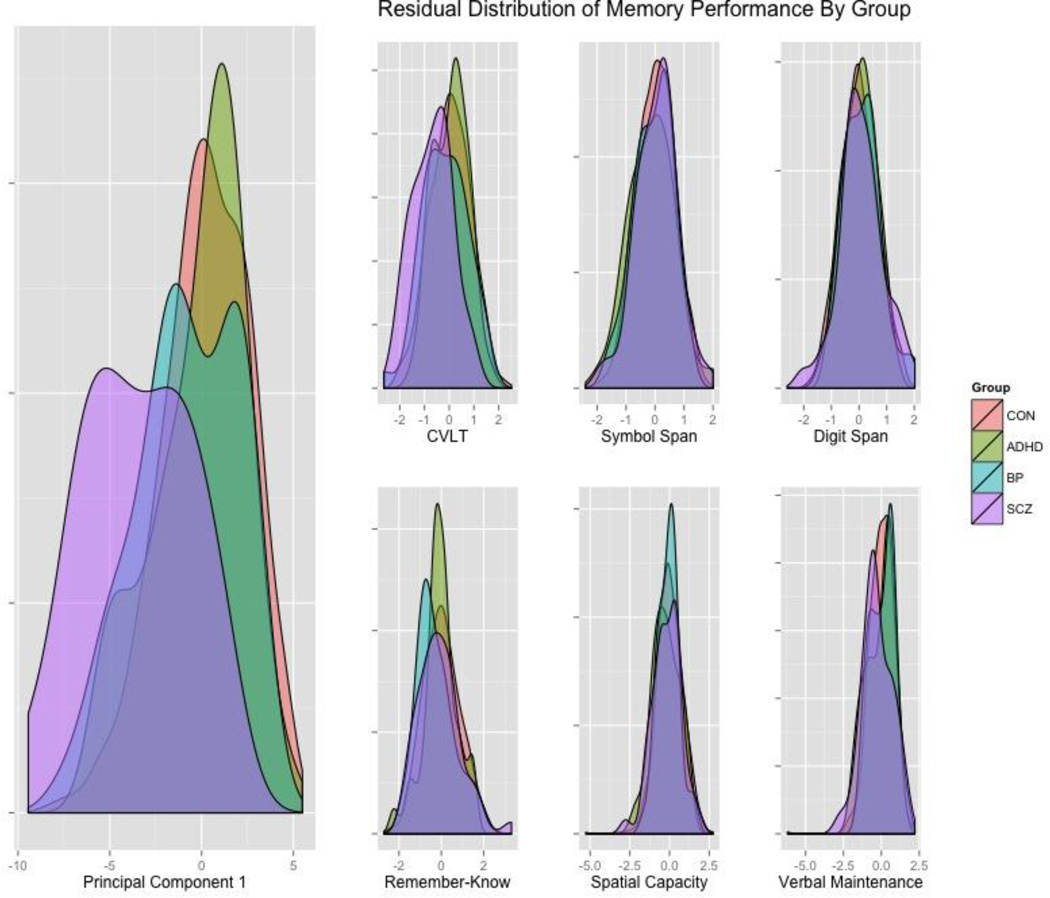
3.4 Principal Component Analysis
Principal component analysis revealed one primary component (PC1) that accounted for 36.6% of the total variance. All tasks loaded on to this first component, with coefficients ranging from .16 for Spatial Manipulation to .29 for Symbol Span (Table 3). There were significant group differences on PC1 [F(3,1001)=34.79, p<.001], with SCZ scoring lower than CON (g=− 1.53), BP (g=-.95) and ADHD (g=−1.20) and BP scoring lower than CON (g=−.42) (Figure 2). Group differences in performance on individual tasks were then reassessed using the residuals after regressing out the contribution of PC1 score. Residual variance demonstrated a greatly reduced effect size for group differences across tasks (Table 3), and only CVLT maintained a significant group difference [F(3,1001)=11.45, p<.001]. Regressing out PC1 also eliminated the modest levels of agreement for the originally scaled, quintile-rescaled, and lowest-quintile kappa metrics across the tasks (ST5).
| Test | PC1 Loading | SCZvsCON | SCZvsBP | SCZvsADHD | BPvsCON | ADHDvsCON | BPvsADHD |
|---|---|---|---|---|---|---|---|
| CVLT Residual | 0.21 | −0.86 | −0.58 | −0.96 | −0.26 | 0.02 | −0.29 |
| Visual Reproduction I Residual | 0.26 | 0.14 | 0.16 | 0.01 | −0.06 | 0.13 | −0.19 |
| Visual Reproduction II Residual | 0.25 | 0.22 | 0.40 | 0.20 | −0.27 | 0.01 | −0.25 |
| Symbol Span Residual | 0.29 | 0.06 | 0.13 | 0.21 | −0.07 | −0.17 | 0.09 |
| Digit Span Residual | 0.28 | 0.14 | −0.03 | 0.04 | 0.17 | 0.09 | 0.08 |
| Letter Number Sequencing Residual | 0.27 | 0.17 | 0.08 | 0.25 | 0.08 | −0.11 | 0.23 |
| Vocabulary Residual | 0.22 | −0.25 | −0.69 | −0.50 | 0.51 | 0.30 | 0.21 |
| Matrix Reasoning Residual | 0.28 | 0.30 | −0.05 | −0.19 | 0.36 | 0.53 | −0.16 |
| Color Trails I Residual | 0.22 | 0.26 | 0.11 | 0.28 | 0.14 | −0.09 | 0.24 |
| Color Trails II Residual | 0.27 | 0.02 | −0.12 | 0.07 | 0.18 | −0.08 | 0.27 |
| Remember-Know Residual | 0.18 | −0.12 | 0.19 | −0.03 | −0.33 | −0.08 | −0.25 |
| Scene Recognition Residual | 0.18 | −0.13 | −0.19 | −0.32 | 0.05 | 0.19 | −0.14 |
| Spatial Capacity Residual | 0.21 | −0.23 | −0.12 | 0.08 | −0.11 | −0.31 | 0.22 |
| Verbal Capacity Residual | 0.21 | 0.00 | 0.32 | −0.00 | −0.36 | 0.00 | −0.35 |
| Spatial Maintenance Residual | 0.18 | −0.04 | −0.01 | 0.06 | −0.03 | −0.12 | 0.09 |
| Spatial Manipulation Residual | 0.16 | 0.41 | 0.27 | 0.35 | 0.10 | 0.04 | 0.05 |
| Verbal Maintenance Residual | 0.23 | −0.36 | −0.39 | −0.16 | 0.07 | −0.17 | 0.23 |
| Verbal Manipulation Residual | 0.27 | 0.21 | 0.44 | 0.42 | −0.19 | −0.18 | −0.02 |
| PC1 | −1.53 | −0.95 | −1.20 | −0.42 | −0.16 | −0.24 |
Figure 2.
Density plots showing the scores of the groups on the first principal component and residual performance variance within groups after removing that component on a representative subset of the measures. Three neuropsychological and three cognitive/experimental measures are shown covering verbal episodic memory and spatial and verbal working memory. The primary measures for comparison were the total raw score for the CVLT, Symbol Span and Digit Span and d-prime for the Remember-Know, Spatial Capacity (SCAP), and Verbal Maintenance (VMNM – Maintenance) measures.
Despite including primarily memory tests, the general factor identified appears to be representative of broader cognitive ability. When non-memory tasks were excluded from the PCA, the first principal component among memory measures still correlated highly with Vocabulary [r(1003)=.476, p<.001], which is a good approximation of general cognitive functioning. However, the memory derived principal component measure continued to show group differences even controlling for Vocabulary performance [F(3,1000)=26.01; p<.001].
The effects reported above include age and gender as covariates of non-interest in the statistical models, which may not fully account for demographic effects (Miller and J. P. Chapman, 2001). Thus, a subset of 430 controls nearest-neighbor matched to the patient group with no demographic group differences (all p > .06) was tested as well and this produced results comparable to results including the full sample (SA6).
4. Discussion
Despite moderate to large differences in group means across numerous verbal and spatial working and episodic memory tasks, memory performance is limited in its ability to separate SCZ from controls or to classify subjects with SCZ, bipolar disorder, and ADHD into separate groups. Performance distributions were unimodal, with only modest consistency in the membership of the lowest performing quintile of subjects across tasks. Further, variation in a general factor common to all tasks accounted for nearly all group differences in performance and the inter-correlation among tasks. Together, these results suggest that the pathophysiological processes involved in schizophrenia act primarily on general abilities required in most tasks rather than on specific abilities within different specific memory domains and that these effects represent a shift in the overall distribution (i.e., with each case functioning at a lower level than they would have if not for the illness), rather than presence of a low-performing subgroup of patients or of multiple subgroups performing poorly on different tasks.
That performance on tests tapping into different memory domains (working memory, episodic memory) and modalities (verbal, visuospatial) were only modestly correlated (and not correlated after controlling for general ability) supports the functional dissociations among these divisions within memory systems. Intercorrelations among the different tasks were the same among healthy subjects and each of the three patient groups, indicating that this modularity is preserved in each of the neuropsychiatric conditions examined.
These results suggest that efforts to isolate discrete subcomponents of memory systems may have limited utility in research on the pathophysiology of schizophrenia (Carter and Barch, 2007). Rather, these results are more consistent with the argument that schizophrenia is closely tied to a general underlying deficit (Dickinson et al., 2011; 2008). Similarly, controlling for general cognition has been found to account for most deficits in spatial working memory across schizophrenia and bipolar probands and relatives (Hill et al., 2015) This general deficit may impact cognitive abilities that contribute to most or all memory performance parameters, such as the ability to encode and organize information. Variations in the level of impairment across particular tasks or components of tasks may be related to their reliance on this generalized ability. For example, recollection-based episodic memory is relatively more impaired than familiarity-based episodic memory in schizophrenia (Haut et al., 2014; van Erp et al., 2008). These findings were replicated in this sample, with SCZ showing impairment on R responses [F(3,1127)=3.99, p=.008] and not K responses [F(3,1127)=.2750, p=.84] on the R-K task. However, R loaded more strongly on to PC1 than K (.15 versus .05) and there were no group differences in the residual variance. Furthermore, the effect size of impairment in SCZ trended towards a correlation with the degree that each task loaded on to the generalized factor (r=.42, p=.08). Recent neuroanatomical findings also suggest systemic abnormalities that are more parsimonious with a general cognitive factor explanation (Dickinson and Harvey, 2009).
One exception to this conclusion may relate to self-generated verbal declarative memory, as schizophrenia patients continued to show deficits on the CVLT when controlling for general ability. This may be related to task requirements, as the CVLT requires the test taker to self-generate word list items from memory, whereas most of the other tasks in this study used a recognition format in which previously studied items were presented mixed with foils. Similarly, this effect may be artifactual due to differential discriminating abilities of the tasks (e.g. specific psychometric properties of the CVLT such as difficulty) (L. J. Chapman and J. P. Chapman, 1973) and controlling statistically for general ability may not adequately address these confounds. On the other hand, self-generated verbal declarative memory retrieval may also represent a specific deficit in schizophrenia, over and above the generalized deficit.
Controlling for the first principal component of the neuropsychological tasks potentially extracted much of the reliable proportions of variance within each performance measure. In this case, the group differences would disappear because the variance remaining after controlling for the reliable component of variance is the random component. Arguing against this interpretation, the first principal component accounted for only 37% of the variance in test performance overall, and the loadings of individual test on the general ability factor were all below 0.30. Given that the reliabilities of the measures from each of the tests used are generally 0.75 or above, the first principal component would appear to account for less than half of the reliable variance in each measure.
Memory-related dysfunction does not appear to represent an area of substantial overlap between schizophrenia and bipolar disorder or ADHD, though both can present with memory-related impairments. BP did demonstrate impairment across the memory tasks; however, these were at most intermediate to the deficits found in SCZ. BP showed moderate impairments relative to controls on some tasks; however, similar to SCZ, there did not appear to be specific subgroups of BP that accounted for most of the impairments. Contrary to our hypothesis and some previous findings (Glahn et al., 2006), a history of psychotic features did not reliably differentiate BP on any task or on the general memory component. However, given the overall sample size and the limited number of BP subjects with current psychotic symptoms, this study was likely underpowered to detect group differences. Furthermore, this study’s reliance on lifetime history and self-report may have reduced sensitivity to effects associated with psychosis in BP as more recent findings show that greater cognitive impairment tends to be associated with more significant psychosis and fewer affective symptoms (Hill et al., 2013). Adults with a history of ADHD may show some relative problems with memory, especially visuospatial memory capacity and maintenance (Martinussen et al., 2005), though these may be more specifically related to goal-directed activity (Castel et al., 2011). These tasks did not contain specific reward components and so may not be as sensitive to that aspect of cognitive impairment in ADHD.
This study was not designed to determine the sources of cognitive deficits in schizophrenia; other research suggests that memory deficits are present early in the illness (Bilder et al., 2000) and during the prodromal phase (Seidman et al., 2010), suggesting that they correlate with factors involved in pathophysiology rather than secondary phenomena. The inclusion of a very large community reference sample provides a strong basis with which to represent the normal distribution of performance on these tasks. This and the similarity to published norms (Norman et al., 2000) suggests it is unlikely that the group differences detected in this study are due to over-sampling of high functioning healthy individuals. The smaller sizes of the patient groups means that we may not have sampled the full performance distribution in the patients and may not be able to detect subtle differences in the underlying cognitive structure (Lam et al., 2014). However, our results did not differ when factor loadings were derived from controls alone. Furthermore, the effect sizes in this study generally match those in the literature (Heinrichs and Zakzanis, 1998) and a previous study utilizing a very large sample of schizophrenia patients reported similar PCA results (Keefe et al., 2006).
In conclusion, schizophrenia appears to involve a deficit in general ability that manifests across multiple tasks, rather than deficits within specific memory domains and modalities per se. Further, the overall distribution of general ability is shifted lower among patients with schizophrenia (i.e., with each case functioning at a lower level than they would have if not for the illness), rather than group differences being driven by a subgroup of patients. As the focus of this study was on memory systems, we did not include tasks designed to isolate subcomponents of other domains of cognition of relevance to schizophrenia. Further research is needed to determine whether patient deficits in these other domains, including perception, attention, executive function, and affective processing are also accounted for by the generalized deficit.
Supplementary Material
Acknowledgements
This work was supported by the Consortium for Neuropsychiatric Phenomics (NIH Roadmap for Medical Research grants UL1- DE019580 (Bilder, PI), RL1MH083269 (Cannon, PI) and PL1MH083271 (Bilder, PI)).
Role of Funding Agencies
None of the funding agencies had a role in collection, management, analysis or interpretation of the data or in preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RB, TC, NF, EL, and FS developed the study concept and study design. KK and EC contributed to neurocognitive task design, programming, and scoring, and JV contributed to recruitment and clinical assessment. KH and TC performed the data analysis and interpretation and drafted the paper and RB, EC, KK, EL, FS provided critical revisions. All authors approved the final version of the paper for submission.
Conflict of Interest
The authors report no conflicts of interest.
References
- Agnew-Blais J, Seidman LJ. Neurocognition in youth and young adults under age 30 at familial risk for schizophrenia: A quantitative and qualitative review. Cognitive Neuropsychiatry. 2012;18:44–82. doi: 10.1080/13546805.2012.676309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Technical Report. Iowa City: University of Iowa; 1983a. The Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- Andreasen NC. Technical Report. Iowa City: University of Iowa; 1983b. The Scale for the Assessment of Negative Symptoms (SANS) [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulus E, Willson DF, Alvir JMJ, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. American Journal of Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive Neuroscience-Based Approaches to Measuring and Improving Treatment Effects on Cognition in Schizophrenia: The CNTRICS Initiative. Schizophrenia Bulletin. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, Geyer M, Green M, Nuechterlein KH, Robbins T, Silverstein S, Smith EE, Strauss M, Wykes T, Heinssen R. Identifying Cognitive Mechanisms Targeted for Treatment Development in Schizophrenia: An Overview of the First Meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biological Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel AD, Lee SS, Humphreys KL, Moore AN. Memory capacity, selective control, and value-directed remembering in children with and without attention-deficit/ hyperactivity disorder (ADHD) Neuropsychology. 2011;25:15–24. doi: 10.1037/a0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychological Bulletin. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward New Approaches to Psychotic Disorders: The NIMH Research Domain Criteria Project. Schizophrenia Bulletin. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia LF, Satz P, Uchiyama CL, White T. Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996. Color Trails Test. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – second edition. Adult version. Manual. 2000 [Google Scholar]
- Dickinson D, Goldberg TE, Gold JM, Elvevåg B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. 2011 doi: 10.1093/schbul/sbq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Harvey PD. Systemic Hypotheses for Generalized Cognitive Deficits in Schizophrenia: A New Take on An Old Problem. Schizophrenia Bulletin. 2009;35:403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and Specific Cognitive Deficits in Schizophrenia: Goliath Defeats David? Biological Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, McGue M. The origins of individual differences in memory among the elderly: a behavior genetic analysis. Psychol Aging. 1993;8:527–537. doi: 10.1037//0882-7974.8.4.527. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (SCID-I/P) Version 2.0. New York: Biometrics Research, New York State Psychiatric Institute; 1995. Structured Clinical Interview for DSM-IV Axis I Disorders, patient edition. [Google Scholar]
- Forbes NF, Carrick LA, McIntosh AM, Lawria SM. Working memory in schizophrenia: a meta-analysis. Psychological Medicine. 2009;39:889–905. doi: 10.1017/S0033291708004558. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, Meyenberg N, Castro MP, Barrett J, Nicolini H, Raventós H, Escamilla MA. Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet. 2007;144B:242–249. doi: 10.1002/ajmg.b.30446. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disorders. 2006;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lönnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gold JM, Dickinson D. “Generalized Cognitive Deficit” in Schizophrenia: Overused or Underappreciated? Schizophrenia Bulletin. 2013;39:263–265. doi: 10.1093/schbul/sbs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut KM, van Erp TGM, Knowlton B, Bearden CE, Subotnik K, Ventura J, Nuechterlein KH, Cannon TD. Contributions of Feature Binding During Encoding and Functional Connectivity of the Medial Temporal Lobe Structures to Episodic Memory Deficits Across the Prodromal and First-Episode Phases of Schizophrenia. Clinical Psychological Science. 2014 doi: 10.1177/2167702614533949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hill SK, Buchholz A, Amsbaugh H, Reilly JL, Rubin LH, Gold JM, Keefe RSE, Pearlson GD, Keshavan MS, Tamminga CA, Sweeney JA. Working memory impairment in probands with schizoaffective disorder and first degree relatives of schizophrenia probands extend beyond deficits predicted by generalized neuropsychological impairment. Schizophr Res. 2015;166:310–315. doi: 10.1016/j.schres.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. American Journal of Psychiatry. 2013;170:1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Bachman P, Winkler AM, Bearden CE, Glahn DC. Genetic influence on the working memory circuitry: behavior, structure, function and extensions to illness. Behav Brain Res. 2011;225:610–622. doi: 10.1016/j.bbr.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, Miller DD, Canive JM, Adler LW, Manschreck TC, Swartz M, Rosenheck R, Perkins DO, Walker TM, Stroup TS, McEvoy JP, Lieberman JA. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RSE, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR. The MCCB impairment profile for schizophrenia outpatients: Results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126:124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness: Profile and effects of clinical state. Neuropsychology. 2009;23:551–562. doi: 10.1037/a0016277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Collinson SL, Eng GK, Rapisarda A, Kraus M, Lee J, Chong SA, Keefe RSE. Refining the latent structure of neuropsychological performance in schizophrenia. Psychological Medicine. 2014;44:3557–3570. doi: 10.1017/S0033291714001020. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- McDermid Vaz SA, Heinrichs RW. Schizophrenia and memory impairment: evidence for a neurocognitive subtype. Psychiatry Research. 2002;113:93–105. doi: 10.1016/s0165-1781(02)00246-9. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Norman MA, Evans JD, Miller WS, Heaton RK. Demographically corrected norms for the California verbal learning test. J Clin Exp Neuropsychol. 2000;22:80–94. doi: 10.1076/1380-3395(200002)22:1;1-8;FT080. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Owens SF, Picchioni MM, Rijsdijk FV, Stahl D, Vassos E, Rodger AK, Collier DA, Murray RM, Toulopoulou T. Genetic overlap between episodic memory deficits and schizophrenia: results from the Maudsley Twin Study. Psychological Medicine. 2011;41:521–532. doi: 10.1017/S0033291710000942. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RSE, Heinssen R, Cornblatt BA North American Prodrome Longitudinal Study (NAPLS) Group. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. R: A language and environment for statistical computing. 2015 [Google Scholar]
- Trandafir A, Méary A, Schürhoff F, Leboyer M, Szöke A. Memory tests in first-degree adult relatives of schizophrenic patients: a meta-analysis. Schizophr Res. 2006;81:217–226. doi: 10.1016/j.schres.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TGM, Lesh TA, Knowlton BJ, Bearden CE, Hardt M, Karlsgodt KH, Shirinyan D, Rao V, Green MF, Subotnik KL, Nuechterlein K, Cannon TD. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100:181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein K, Subotnik K, Gilbert E. Symptom dimensions in recentonset schizophrenia: The 24-item Expanded BPRS. Presented at the Paper presented at the International Congress on Schizophrenia Research; Warm Springs, VA. 1995. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - IV. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-IV. San Antonio, TX: The Psychological Corporation; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.