Abstract
Cholangiocarcinoma is a heterogeneous malignant process, which is further classified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC). The poor prognosis of the disease is partly due to the lack of understanding of the disease mechanism. Multiple gene alterations identified by various molecular techniques have been described recently. As a result, multiple targeted therapies for ICC and ECC are being developed. In this study, we identified and compared somatic mutations in ICC and ECC patients using next generation sequencing (NGS) (Ampliseq Cancer Hotspot Panel v2 and Ion Torrent 318v2 chips). Eleven of 16 samples passed internal quality control established for NGS testing. ICC cases (n = 3) showed IDH1 (33.3%) and NRAS (33.3%) mutations. Meanwhile, TP53 (75%), KRAS (50%), and BRAF (12.5%) mutations were identified in ECC cases (n = 8). Our study confirmed the molecular heterogeneity of ICC and ECC using NGS. This information will be important for individual patients as targeted therapies for ICC and ECC become available in the future.
Keywords: Intrahepatic cholangiocarcinoma, Extrahepatic cholangiocarcinoma, Next generation sequencing, Somatic mutations, Targeted therapy
1. Background
Cholangiocarcinoma is a malignant process which arises from the bile duct epithelial cells. It may originate within the liver as an intrahepatic cholangiocarcinoma (ICC) or involve large hilar bile ducts and extrahepatic biliary tree as an extrahepatic cholangiocarcinoma (ECC) or bile duct carcinoma. Both types of cholangiocarcinoma are biologically distinctive as they have different risk factors, genetic mutations, expression profiling, and clinical outcomes (Sempoux et al., 2011). The overall incidence and mortality rates of cholangiocarcinoma have been increasing over the past few decades. It has been reported that the occurrence of ICC has increased in the United States, while ECC has declined or remained stable. A recent study revealed that the increasing rate of ICC was partly due to the misclassification of Klatskin/perihilar tumors as intrahepatic instead of extrahepatic tumors (Khan et al., 2012).
The established risk factors for cholangiocarcinoma include primary sclerosing cholangitis, parasitic biliary infection (Opisthorchis viverrini in Thailand and Laos; Clonorchis sinensis in Southwest China), choledochal cysts, Caroli’s disease, and toxins. Furthermore, patients with chronic hepatitis C infection and hepatolithiasis are at risk for ICC, while pancreaticobiliary maljunction with bile duct dilatation, cholelithiasis, and cholecystectomy are mainly risk factors for ECC (Cardinale et al., 2010). The majority of cholangiocarcinoma cases occur sporadically despite the well-established risk factors.
Surgery is the only curative option for early-stage ICC and ECC patients. ICC patients undergo either segmental or lobe resection, while pancreaticoduodenectomy is the mainstay treatment for resectable ECC cases. Unfortunately, most cholangiocarcinoma patients present with advanced and unresectable disease. Moreover, local recurrence and distant metastasis are frequently seen after the surgical resection.
The disease prognosis varies; hilar cholangiocarcinoma is associated with slightly better prognosis even in the locally advanced disease setting with liver transplantation, while suboptimal outcome is seen in ICC patients with similar treatment (Churi et al., 2014). Interestingly, distal ECC shows a similar clinical course with pancreatic adenocarcinoma. The 5-year survival rates for localized ICC and ECC are 12% and 30%, respectively. Meanwhile, ICC and ECC patients who develop metastatic disease have a 5-year survival rate of 2% (http://www.cancer.org/acs/groups/cid/documents/webcontent/003084-pdf.pdf).
Limited understanding of the pathogenesis is partly responsible for the overall low survival rates in both ICC and ECC. Available palliative chemotherapy has not improved the outcome of these patients and no molecular targeted agents have been approved for cholangiocarcinoma treatment. Recent studies have described the utility of different molecular techniques in the identification of gene alteration in this entity (Churi et al., 2014; Jiao et al., 2013; Miller et al., 2009; Ross et al., 2014; Sia et al., 2013, 2015; Turaga et al., 2013). The long-term objective of these studies is to find actionable genes which may improve the management and outcome of ICC and ECC patients.
Next generation sequencing (NGS) is an affordable technology which consolidates a broad range of molecular oncology testing into a single platform and single assay (Tsongalis et al., 2014). In the era of personalized medicine, NGS plays an important role in identifying mutations which may predict the prognosis or alter the management for cancer patients. In this study, we identified and compared somatic mutations in ICC and ECC patients using NGS. Recent advances and molecular insights on cholangiocarcinoma will also be discussed.
2. Materials and methods
2.1. Case selection
Sixteen patients with ICC (n = 8) and ECC (n = 8) who underwent biopsy and/or resection at Dartmouth-Hitchcock Medical Center (DHMC) from 2005–2013 were selected for our study. The histologic slides (hematoxylin and eosin-stained slides) were retrieved and the diagnosis for each case was confirmed by 2 pathologists (J.P. and A.A.S.). Histologic characteristics, demographic and clinical information for each patient were recorded. This study was approved by the Committee for the Protection of Human Subjects at Dartmouth College.
2.2. Sample collection and DNA extraction
The appropriate formalin-fixed paraffin-embedded (FFPE) tissue block was selected for each case. Sixteen unstained FFPE tissue sections of 5 μm each were obtained. One case was excluded because of exhausted tissue in the paraffin-block. The lesional area and the percent tumor cell content for each case were identified and assessed by a pathologist (J.P.).
Before DNA extraction, all samples were evaluated to ensure each case contained a minimum of 10% tumor content, previously established during the validation process (Reitman and Yan, 2010). Two samples were excluded from the study because of their low tumor content. Unstained slides from 13 cases were deparaffinized and rehydrated using Xylene and graded ethanol washes, followed by water. Genomic DNA (gDNA) was obtained using the Gentra Pure Gene Kit (Qiagen), and quantified using the Quant-iT ™PicoGreen® dsDNA Assay Kit (Invitrogen) according to the manufacturer’s recommendations.
2.3. Next generation sequencing and data analysis
Next generation sequencing was performed using the Ion AmpliSeq™ Cancer Hotspot Panel v2 which consists of 50 oncogenes and tumor suppressor genes (Table 1), covering approximately 2800 Catalogue of Somatic Mutations in Cancer (COSMIC) mutations. In 2013, the molecular pathology laboratory at DHMC validated (Tsongalis et al., 2014) and incorporated sequencing as a routine clinical test for somatic mutational screening in patients with metastatic carcinoma. Since 2013, the laboratory has received over 1,100 FFPE clinical samples, including non-small cell lung carcinomas (NSCLC), colon adenocarcinomas, gliomas/glioblastomas, melanomas, breast carcinomas, and samples consisting of other tumor types (sarcomas, uterus, kidney, pancreas), as well as almost 500 FFPE samples for research projects.
Table 1.
Characteristics and mutation in intrahepatic cholangiocarcinoma patients.
| No | Sex | Age (years) | Tumor grade | Mutation | Current status |
|---|---|---|---|---|---|
| 1 | Male | 85 | Poorly differentiated | NRAS (c.35G>T, p.G12V) | Deceased |
| 2 | Male | 80 | Poorly differentiated | wt* | Deceased |
| 3 | Female | 56 | Poorly differentiated | IDH-1 (c.395G>T, p.R132L) | Deceased |
wt = wild type.
Barcoded libraries were prepared using at least 10 ng of gDNA. They were quantified using the Ion Library Quantitation qPCR Kit (Life Technologies) and combined to a final concentration of 100 pM each. Two samples failed the qPCR minimum threshold due to a lack of amplification (<10 pM each). Eleven samples were sequenced on the Ion Torrent Personal Genome Machine (PGM™) using Ion 318™ chips.
Sequencing reads were aligned to hg19, and variant calling was performed using Torrent Suite (v4.0.2) and the Variant Caller Plugin (v4.0). Variant annotation and functional predictions were performed using Golden Helix’s SNP and Variation Suite Software SVS (v.8.2.1). Some variants were also manually interrogated using the Broad Institute’s Integrative Genomics Viewer (IGV).
In order to ensure the overall quality of the test, post-sequencing QC metrics were incorporated to the data analysis workflow. These metrics included chip loading efficiency, total usable sequences, percent lowquality reads, percent of aligned reads, percent of aligned bases, on target reads, and coverage uniformity. Sequencing runs and/or samples that did not pass one of the QC metrics above were not included in this study. Also, only variants detected at more than 5.0% allelic frequency, and covered at more than 500× were reported (cutoffs determined during validation process) (Tsongalis et al., 2014).
3. Results
Eleven patients were analyzed after passing the internal quality control established in-house for NGS testing. These patients included 8 patients with ECC and 3 patients with ICC. Nine of the samples used in the sequencing were derived from biopsy/resection specimens (3 ICC cases and 6 ECC cases) and the remaining 2 samples were from fine-needle aspirates (2 ECC cases).
3.1. Histological morphology of ICC and ECC
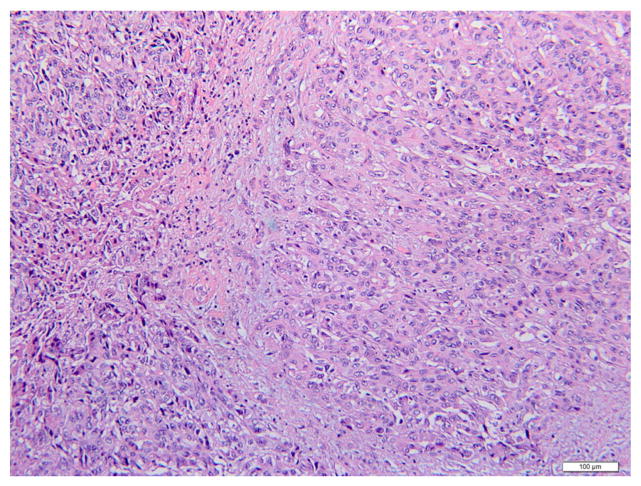
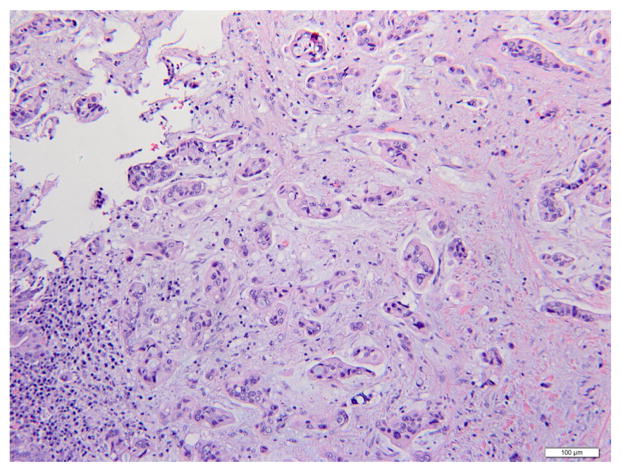
Histological examination showed poorly-differentiated lesions in all ICC (100%) and ECC cases (100%). Fig. 1 showed a hematoxylin and eosin (H&E) stained image of ICC. The lesion was comprised of dense poorly-formed glands with desmoplastic stroma in the background. The nuclei were pleomorphic and mitotic activities were noted. An example of ECC was shown in Fig. 2. Multiple angulated, mucin-producing glands were seen with desmoplastic stroma and striking irregular and hyperchromatic nuclei. Immunohistochemical studies were performed in challenging cases to distinguish these lesions from the common differential diagnosis, such as hepatocellular carcinoma and metastatic adenocarcinomas.
Fig. 1.
Intrahepatic cholangiocarcinoma (hematoxylin and eosin, 200×). High-grade tumor with poorly-formed glands and atypical nuclei. Scattered inflammatory cells are seen in the fibrous stroma.
Fig. 2.
Extrahepatic cholangiocarcinoma (hematoxylin and eosin, 200×). Multiple angulated and mucin-producing glands are seen with a background of desmoplastic stroma. Nuclear pleomorphism and hyperchromasia are also noted.
3.2. Somatic mutations in ICC patients
Two male and 1 female patients with ICC were analyzed for the study. Their average age was 73.6 ± 15.5 years. Mutated genes seen in 2 ICC patients were isocitrate dehydrogenase 1 (IDH1) (25%) and neuroblastoma RAS viral oncogene homolog (NRAS) (25%). No other somatic mutations in the 50 gene panel were identified in the other patient. Table 2 includes the demographic information, histological grade, clinical follow-up, and the mutated genes seen in ICC patients.
Table 2.
Oncogenes and tumor suppressor genes included in Ion AmpliSeq™ Cancer Hotspot Panel v2.
| Ion Ampliseq™ Cancer Hostspot Panel v2 (CHPV2) | ||||
|---|---|---|---|---|
| ABL1 | EGFR | GNAQ | KRAS | PTPN11 |
| AKT1 | ERBB2 | GNAS | MET | RB1 |
| ALK | ERBB4 | HNF1A | MLH1 | RET |
| APC | EZH2 | HRAS | MPL | SMAD4 |
| ATM | FBXW7 | IDH1 | NOTCH1 | SMARCB1 |
| BRAF | FGFR1 | IDH2 | NPM1 | SMO |
| CDH1 | FGFR2 | JAK2 | NRAS | SRC |
| CDKN2A | FGFR3 | JAK3 | PDGFRA | STK11 |
| CSF1R | FLT3 | KDR | PIK3CA | TP53 |
| CTNNB1 | GNA11 | KIT | PTEN | VHL |
3.3. Somatic mutations in ECC patients
The predominant ECC patients were male patients (M:F = 3:1) with an average age of 72.6 ± 8.5 years. Tumor protein p53 (TP53) was the most frequent mutated gene in these patients (75%). Other mutated genes identified in ECC included Kirsten rat sarcoma viral oncogene homolog (KRAS) (50%) and v-raf murine sarcoma viral oncogene homolog B (BRAF) (12.5%). Two of the eight patients (25%) were wild-type (wt) for the gene sequences present in the AmpliSeq Hotspot Cancer Panel v2. In addition, potential benign polymorphisms were identified in these patients. The demographic information, histologic grade, clinical follow-up, and the mutated genes in patients with ECC are shown in Table 3.
Table 3.
Characteristics and mutation in extrahepatic cholangiocarcinoma patients.
| No | Sex | Age (years) | Tumor grade | Mutation | Current status |
|---|---|---|---|---|---|
| 1 | Female | 74 | Poorly differentiated | KRAS (c.35G>T, p.G12V), | Deceased |
| TP53 (c.428G>A, p.C143Y and c.224C>T, p.P75L) | |||||
| 2 | Male | 57 | Poorly differentiated | KRAS (c.35G>A, p.G12D) | Deceased |
| TP53 (c.818G>A, p.R273H) | |||||
| 3 | Male | 64 | Poorly differentiated | TP53 (c.844C>T, p.R282W) | Deceased |
| 4 | Male | 78 | Poorly differentiated | KRAS (c.35G>A, p.G12D) | Unknown |
| TP53 (c.200G>T, p.G67V) | |||||
| 5 | Female | 73 | Poorly differentiated | Benign polymorphism | Deceased |
| 6 | Male | 73 | Poorly differentiated | wt* | Deceased |
| 7 | Male | 74 | Poorly differentiated | BRAF (c.1742A>C, p.N581T) | Deceased |
| TP53 (c.637C>T, p.R213) | |||||
| 8 | Male | 88 | Poorly differentiated | KRAS (c.35G>A, p.G12D) | Living |
| TP53 (c.658T>A, p.Y220N) |
wt = wild type.
3.4. Clinical follow-up
Most of the patients in the study (83.3%) were deceased at the time of writing. All 3 ICC patients (100%) were deceased. Only 1 of the ECC patients survived (12.5%), while 6 patients were deceased (75%) and 1 patient was lost to follow-up (12.5%). The causes of death and time interval between the diagnosis and death were not analyzed since most of this information was not available.
4. Discussion
Recent technical advances in molecular analysis with reduced costs of sequencing have resulted in a paradigm shift of cancer management (Churi et al., 2014). A series of studies using traditional and advanced technologies have described a variety of gene mutations and genomic alterations in cholangiocarcinoma, particularly ICC, to further explore the disease mechanism and to develop potential targeted therapies (Ross et al., 2014).
Comparative genomic hybridization and gene expression data has shown an overlap in pathogenic mechanism for all subsets of cholangiocarcinoma; however, significant diversity of mutational findings between individual patients is also apparent (Miller et al., 2009). Here we demonstrate the utility of NGS in detecting somatic mutations for both ICC and ECC patients. Using a 50-gene panel, we identified IDH1 and NRAS gene alterations in patients with ICC and mutated KRAS, TP53, and BRAF genes in ECC cases.
IDH1 is one of the most commonly altered genes in ICC patients (Ross et al., 2014). It is identified in 10–20% of ICC and the mutation is not usually seen in ECC patients (Borger et al., 2012; Wang et al., 2013; Ward et al., 2010). Using DNA sequencing of hybridization-captured libraries in 28 ICC cases, Ross et al. reported that altered IDH1/2 alteration was identified in 36% of the cases, similar prevalence to AT rich interactive domain 1 A (ARID1A) (36%), TP53 (36%), and myeloid cell leukemia 1 (MCL1) (21%) alterations (Ross et al., 2014).
Similar to one of our ICC patients, the majority (99%) of somatic mutations in IDH1 is found at codon R132, which is functionally conserved and aligns with R172 of IDH2 (Rohle et al., 2013). A heterozygous mutation at IDH1 codon R132 will cause defective IDH1 enzyme activity which results in a decrease of cellular antioxidant activities (Reitman and Yan, 2010). The mutant enzyme induces the reduction of a-ketoglutarate to 2-hydroxyglutarate, with the conversion of NADPH to NADP+ (Rohle et al., 2013). Accumulation of this potential oncometabolite, 2-hydroxyglutarate, has been demonstrated in cancers with IDH mutation. In addition, increased levels of p53 and DNA hypermethylation are seen in IDH-mutant tumors (Wang et al., 2013).
IDH1 mutations are associated with poorly differentiated tumors with clear cell changes histologically (Kipp et al., 2012). However, IDH1 genetic alteration has not shown any prognostic significance (Churi et al., 2014). Preclinical evidence suggests that targeted therapy is potentially applicable for IDH1/2-mutant cancers. In gliomas, AG1-5198, a selective IDH1 inhibitor, blocks the 2-hydroxyglutarate production in a dose-dependent manner and stunted the growth of IDH-1 mutant tumor cells sparing the IDH1 wild-type cells (Rohle et al., 2013). Currently, IDH inhibitors are being investigated in clinical trials. AG-120, a specific IDH1 inhibitor, was reported by Rizvi et al. to show a clinical benefit in 70% of phase-1 trial patients with IDH1-mutant associated solid tumors including cholangiocarcinoma (Rizvi et al., 2014).
NRAS mutation is less frequently identified in ICC cases, with a prevalence of 3% in recent studies (Jiao et al., 2013; Zhu et al., 2014). Due to the low frequency, no information on clinicopathological correlation is available for tumors with this particular gene mutation and thus it would be considered a variant of unknown clinical significance in this tumor type.
Mutation of KRAS and loss-of-function mutations of TP53 are frequently seen in both ICC and ECC (Churi et al., 2014). TP53 mutations were reported in 21% of cholangiocarcinoma cases in a review of studies with 229 patients (Khan et al., 2005). A direct DNA sequencing analysis of 69 cholangiocarcinomas identified KRAS and BRAF mutations in 22% and 45% of the tumors (Tannapfel et al., 2003). Both KRAS and BRAF are members of mitogen-activated protein kinase/extracellular-signal-regulated kinases (MAPK/ERKs) pathway which mediate cellular response to growth signals. Neither the status of KRAS, BRAF, or both alterations influenced the survival of patients with cholangiocarcinoma (Tannapfel et al., 2003). The strategy for KRAS-mutated tumor targeted therapy has focused on the downstream effector pathways of KRAS. Phase II studies of a MAPK/ERK kinase (MEK) inhibitor in biliary tract cancer cases that included cholangiocarcinomas have been promising. Furthermore, a direct KRAS inhibitor may be useful when it becomes available in the future (Rizvi et al., 2014).
Sia et al. recently proposed a molecular classification of ICC into proliferation and inflammatory-type ICC based on high-throughput genomic data (Sia et al., 2013). Proliferation-type ICC, the more common type which usually shows aggressive clinical behavior and similar genomic profile to poor-prognosis hepatocellular carcinoma, is characterized by induction of cellular signal in cell cycle progression and proliferation. Meanwhile, an enriched inflammation-related pathway, mainly interleukin (IL)-10/-6 and signal transducer and activator of transcription 3 (STAT3), is the characteristic of inflammatory-type ICC (Sia et al., 2013).
Inactivating mutations of multiple chromatin-remodeling genes have also been reported in ICC. Through exome sequencing of 32 ICCs, Jiao et al. reported 1,259 somatic mutations in 1128 genes; Frequent inactivating mutations in multiple chromatin-remodeling genes (BRCA-associated protein 1 (BAP1), ARID1A, and polybromo-1 (PBRM1)), and mutation in one of these genes occurred in 47% of the carcinomas sequenced (Jiao et al., 2013). Subjects with a mutation in any one of these genes trended toward worse survival, although no significant differences were seen in the survival time. Histone deacetylase inhibitors, which target chromatin remodeling genes, have been proposed as potential targeted therapies (Jiao et al., 2013).
A novel recurrent oncogenic fusion, fibroblast growth factor receptor 2 (FGFR2) — periphilin1 (PPHLN1), gene and damaging mutations in the V-raf murine sarcoma 3611 viral oncogene homolog (ARAF) gene were recently described in 16% and 11% of 122 ICC cases (Sia et al., 2015). The transforming and oncogenic activity of the FGFR2-PPHLN1 fusion can potentially be inhibited by a selective FGFR2 inhibitor (Sia et al., 2015).
A recent study which utilized a high throughput mass spectrometry-based platform showed different mutational patterns between ICC cases that arise in normal liver and those which are associated with preexisting chronic liver disease (Jang et al., 2014). In the study, phosphatidylinositol-4,5 biphosponate 3-kinase, catalytic subunit alpha (PIK3CA), phosphatase and tensin homolog (PTEN), cyclin-dependent kinase inhibitor 2A (CDKN2A), and TP53 mutations were harbored exclusively in ICCs with normal background liver. Meanwhile, epidermal growth factor receptor (EGFR) mutation was seen more frequently in ICCs with chronic liver disease. Most of these EGFR mutations were located at exon 19, identical to deletions identified in non-small cell lung carcinomas.
V-erb-B2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2) was reported to be one of the common genes altered in ECC patients (Churi et al., 2014). A study from Churi et al. (2014) showed that ERBB2 mutations were seen in the kinase domain (V777L) and extracellular domain (S310F). However, immunohistochemistry for ERBB2 overexpression was negative in all of these cases.
Another study which utilized immunohistochemistry showed higher ERBB2 expression in 300 ECC cases (8.5%) compared to 106 ICC cases (0.9%). They also analyzed the expression of EGFR and vascular endothelial growth factor (VEGF) between these two groups. They reported that EGFR expression was associated with tumor progression and VEGF expression was involved in hematogenic metastasis (Yoshikawa et al., 2008). Some of these molecular studies have led to the development of sorafenib (multitargeted kinase inhibitor) as a treatment option for cholangiocarcinoma patients (El-khoueiry et al., 2012).
Our study confirms the molecular heterogeneity in ICC and ECC. Currently, no effective therapy is available for patients with unresectable tumors. The majority of patients do not survive in order to assess follow-up of the disease, independent of the somatic mutations identified. This finding is consistent with the reported 5-year survival rates of 12 and 30% in localized ICC and ECC and extremely low survival rates (2%) in advanced diseases. Therefore, there is an urgent need to characterize the molecular profile and to develop targeted therapies for these patients.
5. Conclusions
Many targeted therapies are being developed for ICC and ECC based on the findings of advanced molecular technologies. Our study supports the premise that NGS is an effective diagnostic tool to identify multiple somatic mutations in different genes simultaneously. NGS testing will be an important part of patient management strategies when targeted therapies for cholangiocarcinoma become available in the near future.
Acknowledgments
The authors wish to thank the staff of the DHMC Molecular Pathology Laboratory and the Translational Research Program. The data presented in this manuscript was in part generated through the Department of Pathology Translational Research Shared Resource Laboratory of the Geisel School of Medicine at Dartmouth, the Dartmouth Hitchcock Medical Center and the Norris Cotton Cancer Center.
References
- Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010;2:407–416. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation, profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-khoueiry AB, Rankin CJ, Ben-Josef E, Lenz HJ, Gold PJ, Hamilton RD, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. [Last date accessed: 02/17/15];Invest New Drugs. 2012 30:1646–1651. doi: 10.1007/s10637-011-9719-0. http://www.cancer.org/acs/groups/cid/documents/webcontent/003084-pdf.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Chun SM, Hong SM, Sung CO, Park H, Kang HJ, et al. High throughput molecular profiling reveals differential mutation patterns n intrahepatic cholangiocarcinomas arising in chronic advanced liver diseases. Mod Pathol. 2014;27:731–739. doi: 10.1038/modpathol.2013.194. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Thomas HC, Toledano MB, Cox IJ, Taylor-Robinson SD. P53 mutations in human cholangiocarcinoma: a review. Liver Int. 2005;25:704–716. doi: 10.1111/j.1478-3231.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–1558. doi: 10.1016/j.humpath.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Miller G, Socci ND, Dhall D, D’Angelica M, DeMatteo RP, Allen PJ, et al. Genome wide analysis and clinical correlation of chromosomal and transcriptional mutations in cancers of the biliary tract. J Exp Clin Cancer Res. 2009;28:62. doi: 10.1186/1756-9966-28-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102:932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Borad MJ, Patel T, Gores GJ. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Semin Liver Dis. 2014;34:456–464. doi: 10.1055/s-0034-1394144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31:49–60. doi: 10.1055/s-0031-1272839. [DOI] [PubMed] [Google Scholar]
- Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–840. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia D, Losic B, Moeini A, Cabellos L, Hao K, Revill K, et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- Tannapfel A, Sommerer F, Benicke M, Katalinic A, Uhlmann D, Witzigmann H, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsongalis GJ, Peterson JD, De Abreu FB, Tunkey CD, Gallagher TL, Strausbaugh LD, et al. Routine use of the Ion Torrent AmpliSeq Cancer Hotspot Panel for identification of clinically actionable somatic mutations. Clin Chem Lab Med. 2014;52:707–714. doi: 10.1515/cclm-2013-0883. [DOI] [PubMed] [Google Scholar]
- Turaga KK, Tsai S, Wiebe LA, Evans DB, Gamblin TC. Novel multimodality treatment sequencing for extrahepatic (mid and distal) cholangiocarcinoma. Ann Surg Oncol. 2013;20:1230–1239. doi: 10.1245/s10434-012-2648-0. [DOI] [PubMed] [Google Scholar]
- Wang P, Dong Q, Zhang W, Kuan PF, Liu Y, Jeck WR, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Borger DR, Kim Y, Cosgrove D, Ejaz A, Alexandrescu S, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol. 2014;21:3827–3834. doi: 10.1245/s10434-014-3828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]