Abstract
Persistent neuroadaptations following chronic psychostimulant exposure include reduced striatal dopamine D2 receptor (D2R) levels. The signaling of D2Rs is initiated by Gαi/o proteins and terminated by regulator of G protein signaling (RGS) proteins. The purpose of this study is to examine the association of the drug taking behavior and gene expression profile of D2/D3Rs, and their associated signaling proteins in the ventral tegmental area (VTA) and nucleus accumbens (NAc) using a rodent model of amphetamine (AMPH) self-administration. Rats were allowed to self-administer AMPH (0.187 mg/kg/infusion for a maximum of 40 injections in 6 hr daily sessions) for 5 days during which rats showed an escalated rate of AMPH intake across days. AMPH self-administration induced profound brain region-dependent alterations of the targeted genes. There was a positive correlation of the mRNA levels of RGS10 between the VTA and the NAc in the control animals, which was abolished by AMPH self-administration. AMPH self-administration also produced a negative correlation of the mRNA levels of RGS7 and RGS19 between the two brain regions, which was not present in the control group. Furthermore, AMPH taking behavior was associated with changes in certain gene expression. The mRNA levels of RGS2 and RGS4 in both the VTA and NAc were positively correlated with the rate of AMPH intake. Additionally, the rate of AMPH intake was also positively correlated with RGS10 and negatively correlated with RGS17 and the short form of D2Rs mRNA level in the VTA. Although there were significant changes in the mRNA levels of RGS7 and RGS8 in the NAc, none of these measures were correlated with the rate of AMPH intake. The present study suggested that short-term AMPH self-administration produced pronounced changes in the VTA that were more associated with AMPH taking behavior than changes in the NAc.
Keywords: Dopamine D2/D3 receptors, RGS proteins, Gαi/o proteins, amphetamine, self-administration, gene expression, qPCR: real-time polymerase chain reaction, GPCR: G protein-coupled receptor
INTRODUCTION
Chronic abuse of psychostimulants causes neuroadaptations in the expression and function of dopamine D2/D3 receptors in the striatum. Reduced striatal D2/D3 receptor expression has been observed consistently in methamphetamine abusers (Volkow et al., 2001), cocaine addicts (Volkow et al., 1993) and alcoholics (Volkow et al., 1996). We previously showed that amphetamine (AMPH) self-administration also decreased the function of D2/D3 receptors as evidenced by the weakened ability of D2/D3 receptors to inhibit electrically evoked striatal dopamine release (Calipari et al., 2014). Reduced availability and function of striatal D2/D3 receptors are hypothesized to contribute to relapse in drug addicts (Wang et al., 2012). Thus, it is important to understand how D2/D3 receptors and their associated signaling proteins are modulated by psychostimulants. The main purpose of the present study was to assess the neuroadaptive changes in the gene expression of D2/D3 receptors and their associated signaling proteins in the ventral tegmental area (VTA) and nucleus accumbens (NAc) in an animal model of amphetamine (AMPH) self-administration. Altered gene expression may result from psychostimulant-induced epigenetic modifications, which could subsequently impact protein expression, synaptic plasticity and addictive behavior. We also determined whether there were any associations between AMPH taking behavior and the expression levels of targeted genes in different brain regions.
Dopamine D2/D3 receptors are coupled to inhibitory Gαi/o proteins for intracellular signaling (Beaulieu and Gainetdinov, 2011). Chronic treatment with psychostimulants may alter the activity and/or content of G proteins, compromising D2/D3 receptor signaling. It has been demonstrated that there was a significant decrease in the efficacy of stimulated [35S]GTPγS binding to striatal D2/D3 receptors in rats with a history of cocaine self-administration (Frankowska et al., 2013), indicating attenuated G protein-dependent signaling of D2/D3 receptors. We also previously reported that Gαi2 was the primary G protein for midbrain D2/D3 receptor signaling and AMPH self-administration disrupted the coupling between D2/D3 receptors and Gαi2 assessed by the antibody capture-based scintillation proximity assay (Calipari et al., 2014). Moreover, chronic cocaine treatment reduced the mRNA level of Gαi and Gαo subunit in the rat VTA and NAc without altering the level of Gαs or Gβ subunit (Nestler et al., 1990). The present study determined whether AMPH self-administration would alter the gene expression of Gαi/o subtypes that are known to couple to D2/D3 receptors.
Recent evidence indicates that the trafficking and signaling of D2/D3 receptors in heterologous expression systems are modulated by regulator of G protein signaling (RGS) proteins (Celver et al., 2010, Min et al., 2012). RGS proteins are a family of proteins that negatively modulate the function of heterotrimeric G proteins by accelerating GTP hydrolysis. Thus, changes in the mRNA levels of RGS proteins following chronic psychostimulant exposure may lead to functional alterations of G protein coupled receptors (GPCR). There are at least 30 identified subtypes of RGS proteins in mammals. Based on the similarity in amino acid composition, RGS proteins are divided into different families which include the R4 (RGS1, 2, 3, 4, 5, 8, 13, 16, 18 and 21), R7 (RGS6, 7, 9 and 11), R12 (RGS10, 12 and 14), RZ (RGS17, 19 and 20), RA (axin, conductin) and RL (P115RhoGEF, GRK2 and GRK3) family (Siderovski and Willard, 2005). Because of the lack of specific antibodies for majority of RGS subtypes, the distribution of RGS proteins in the brain is primarily based on the mRNA expression determined by in situ hybridization. RGS proteins are expressed in a brain-region dependent manner and many RGS subtypes are present in the mesolimbic and nigrostriatal dopaminergic system (Gold et al., 1997) although the functional roles of many RGS subtypes in the brain are largely unknown. Limited data indicate that several RGS subtypes are capable of modulating D2/D3 receptor trafficking and function in heterologous expression systems using gene overexpression or knockdown approaches. For example, overexpression of RGS4, a member of the R4 family, inhibited D2/D3 receptor signaling by reducing agonist-induced phosphorylation of ERK in HEK293 cells (Min et al., 2012). Overexpression of RGS9, a member of the R7 family, prevented agonist-induced internalization of D2 and D3 receptors in HEK293 cells (Celver et al., 2010, Min et al., 2012). RGS19, a member of the RZ family, was recruited to the membrane and colocalized with D2 receptors when D2 receptors were stimulated in CHO cells (Jeanneteau et al., 2004). However, it remains unknown which RGS subtypes have specificity for modulation of D2/D3 receptors in vivo. Changes in RGS proteins may alter D2/D3 receptor signaling and associated behavior. More in vivo experiments are warranted for better understanding the functional role of RGS proteins. The present study provided the gene expression profile of RGS subtypes in the VTA and NAc along with D2/D3 receptors in a rat model of AMPH self-administration as described previously (Calipari et al., 2014). In this paradigm, animals demonstrated an escalation of AMPH intake across session, which is characteristic of human drug taking behavior (Dackis and O’Brien, 2001). We used a short-term AMPH self-administration paradigm to determine whether there were any associations between the early changes in gene expression and escalated AMPH taking behavior.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (375–400 g; Harlan Laboratories, Frederick, Maryland) were used for all experiments. Rats were maintained on a 12:12 hour reverse light/dark cycle (3:00 am lights off; 3:00 pm lights on) with food and water ad libitum. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest Health Sciences.
AMPH self-administration
Rats were anesthetized with ketamine and xylazine and implanted with chronic indwelling jugular catheters as previously described (Liu et al., 2007). Following surgery, animals were singly housed, and all self-administration session took place in the home cage during the active/dark cycle. After a 7-day recovery period, animals underwent a training paradigm during which animals were given access to an AMPH-paired lever on a fixed ratio one (FR1) schedule. A lever press produced an intravenous infusion of 0.187 mg/kg AMPH over 4 seconds. This dose was chosen because it is the most reinforcing dose as measured by the peak of the progressive ratio dose-response curve for AMPH (Richardson and Roberts, 1996). After each AMPH delivery, the lever was retracted and a stimulus light was illuminated for a 20-second timeout period. Training sessions were 6 hr in length or until 20 injections had been earned. Acquisition was considered to have occurred when an animal responded for 20 injections for 3 consecutive days and a stable pattern of infusion intervals was present. Following acquisition, daily self-administration sessions continued and were terminated either at the end of 6 hr or after 40 injections of drug had been earned for 5 consecutive days. All animals achieved all available injections of AMPH over these 5 days and typically exhibited an escalation of AMPH intake across days (i.e. the 40 reinforcers were obtained in fewer minutes). Rats were sacrificed approximately 18 hr after the final self-administration session. Since the half-life of AMPH clearance is approximately 60 min for intravenous self-administration (Hutchaleelaha et al., 1994), the 18-hr withdrawal from the last session of AMPH self-administration allowed the clearance of AMPH. This ensured, therefore, that we would be able to measure changes that persist in the absence of AMPH, minimizing any potential acute responses to AMPH while still onboard. Importantly, we previously reported that the ability of D2/D3 receptors to inhibit evoked dopamine release was significantly attenuated following 18 hr withdrawal from AMPH (Calipari et al. 2014). In order to parallel the functional assay on D2/D3 receptors, we chose to examine the gene expression within the same time window. Control animals had sham surgery, were housed in the same room and sacrificed at the same time as AMPH self-administering animals.
RNA extraction
Brains were rapidly removed after sacrifice and cooled in ice-cold diethylpyrocarbonate-treated PBS. The ventral tegmental area (VTA) and nucleus accumbens (NAc) were dissected using a rat brain block. The total RNA was extracted using TRIZOL (Invitrogen, Cartsbad, CA). Contaminating genomic DNA was removed by DNase I digestion using DNA-free RNA kit (ZYMO Research, Irvine, CA). Quality and concentrations of RNA were checked and measured using NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific).
Reverse transcription
Reverse transcription was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Total RNA (100–500 ng) was converted to single stranded cDNA. Reverse transcription without reverse transcriptase was also performed to assess genomic DNA contamination.
Real-time polymerase chain reaction (qPCR)
Primers were designed using qPCR primer design software from Integrated DNA Technology and listed in Table 1. The qPCR was performed using All-in-One qPCR SYBR Green Master Mix (GeneCopoeia Inc., Rockville MD) in a 96-well format on an ABI PRISM 7500 Fast real-time PCR System (Applied Biosystems, Forester City, CA). PCR reactions contained 0.2 μM of primers and 20 ng of reverse transcribed total RNA in 20 μL. PCR was performed with an initial 3-minute denaturation at 95°C followed by 40 cycles of PCR (15 seconds at 95°C, 20 seconds at 60°C and 15 seconds at 72°C ). Under the condition of qPCR, melt curve analysis performed at the end of qPCR reproducibly showed a single peak for each gene in each sample. The relative change in the target gene expression was analyzed using 2-ΔΔCT method as described previously (Livak and Schmittgen, 2001). Samples containing no cDNA template and no reverse transcriptase were run as negative controls for contamination and amplification of genomic DNA, respectively. All samples were run in triplicate. For each gene, qPRC reactions for control and AMPH self-administration groups were run concurrently on the same 96-well plate. The mRNA levels were normalized to the average of the two housekeeping genes: actin and GAPDH.
Table 1.
Primers for quantitative PCR analyses
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| RGS2 | F: 5′-CACCACAGAAACTGTCCTCAA-3′ | R: 5′-CCACTTGTAGCCTCTTGGATATT-3′ |
| RGS3 | F: 5′-CAGAGAAGGCAGAGTGCTTATT-3′ | R: 5′-TCTTCCTCTCCGTAGGTGTT-3′ |
| RGS4 | F: 5′-ACCGTCGTTTCCTCAAGTCTCGAT-3′ | R: 5′-TTTCGGTTCTCTGCCTCTGTGTGA-3′ |
| RGS5 | F: 5′-CTGGGTTGCCTGTGAGAATTA-3′ | R: 5′-TGGTCAATGTTCACCTCTTTAGG-3′ |
| RGS7 | F: 5′-TCACACTCAAGGAT GATGGCACCT-3′ | R: 5′-TGCTAGCTCTAGCCTTGCCTTGTT-3′ |
| RGS8 | F: 5′-ACGAGGTGGGCAGATTCCTTTGAT-3′ | R: 5′-TAGCTTTGCAGTCGACCTGGTCTT-3′ |
| RGS9 | F: 5′-AGCGCCTTTGGATCTCTAAC-3′ | R: 5′-GTACGGCGTCTGAAATCGATAG-3′ |
| RGS10 | F: 5′-CTACATGACCTTCCTGTCCAATAA-3′ | R: 5′-CATCAGAGGGTGTGGTTCTTC-3′ |
| RGS12 | F: 5′-GCTTCAGTTCATCTCCGTTCT-3′ | R: 5′-GTTGCTGCTGGTGCTATTATTC-3′ |
| RGS17 | F: 5′-GAACAGAATACAGC GAGGAGAA-3′ | R: 5′-GACCTCTTTCGGTGACAGTATAG-3′ |
| RGS19 | F: 5′-TTCCTGCGCACAGAATACA-3′ | R: 5′-TGGACACGTAGTCCTCATAGA-3′ |
| RGS20 | F: 5′-ACGAACTCAGAACAGACATTCC-3′ | R: 5′-CTGGAGTGACCATTAGGTTGTC-3′ |
| GαO | F: 5′-CGGATCCAGCCTCGACTCCTATTT-3′ | R: 5′-CTCTATCAAGATTCCAGCGGCGAAGA-3′ |
| Gαi1 | F: 5′-AGAAGGACCTTTC GAGAAGGCGAA-3′ | R: 5′-TCGCACTAGGACCATAAG TTGCCA-3′ |
| Gαi2 | F: 5′-CTCCCCACTACCTGTGAGGAAGAT-3′ | R: 5′-GGCACCTCAGAC AAAGGTGGGTTT-3′ |
| Gαi3 | F: 5′-GTCTCTTGGGTCAGCGACAAGCAT-3′ | R: 5′-CAGCACCTCGACTTCAATATGCCC-3′ |
| D2S | F: 5′-ACTCAAGGATGCTGCCCGCCGA-3′ | R: 5′-TCTTCTCTGGTTTGGCAGGACTGT-3′ |
| D2L | F: 5′-TTCTACGTGCCCTTCATCGTCACT-3′ | R: 5′-TGCAGAGTTTCATGTCCTCAGGGT-3′ |
| D3R | F: 5′-GGTCATTGTGCTTGGAGCCTTCAT-3′ | R: 5′-GGCTTTGCGGAACTCCACATTGAA-3′ |
| Actin | F: 5′-TGAGAGGGAAATCGTG CGTGACAT-3′ | R: 5′-ACCGCTCATTGCCGATAGTGATGA-3′ |
| GAPDH | F: 5′-GAATGGGAAGCTGGTCATCAA-3′ | R: 5′-CCAGTAGACTCCACGACATACT-3′ |
Note: D2S: the short form of D2 receptors; D2L: the long form of D2 receptors; D3R: dopamine D3 receptors
Data analyses
Graph Pad Prism (version 6, La Jolla, CA, USA) was used for statistical analyses. Data were analyzed using a two-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons tests to study the effects of AMPH self-administration on the targeted gene expression with main factors of brain region and drug treatment. Pearson’s correlation analyses were carried out to measure the correlation coefficients. Statistical significance was set at p<0.05.
RESULTS
Escalation of AMPH self-administration across sessions
Once acquired, rats were allowed to self-administer AMPH (0.187 m/kg/infusion) for 5 days in a daily 6-hr sessions with a maximum of 40 injections. Using linear regression analysis of the rate of AMPH intake versus the self-administration session, the best-fit line (y=0.08573X+0.7513) significantly deviated from zero (F1,38=9.659, P<0.01). Under this behavioral paradigm, therefore, animals exhibited an escalation in the rate of AMPH intake across days (Fig. 1).
Fig. 1.
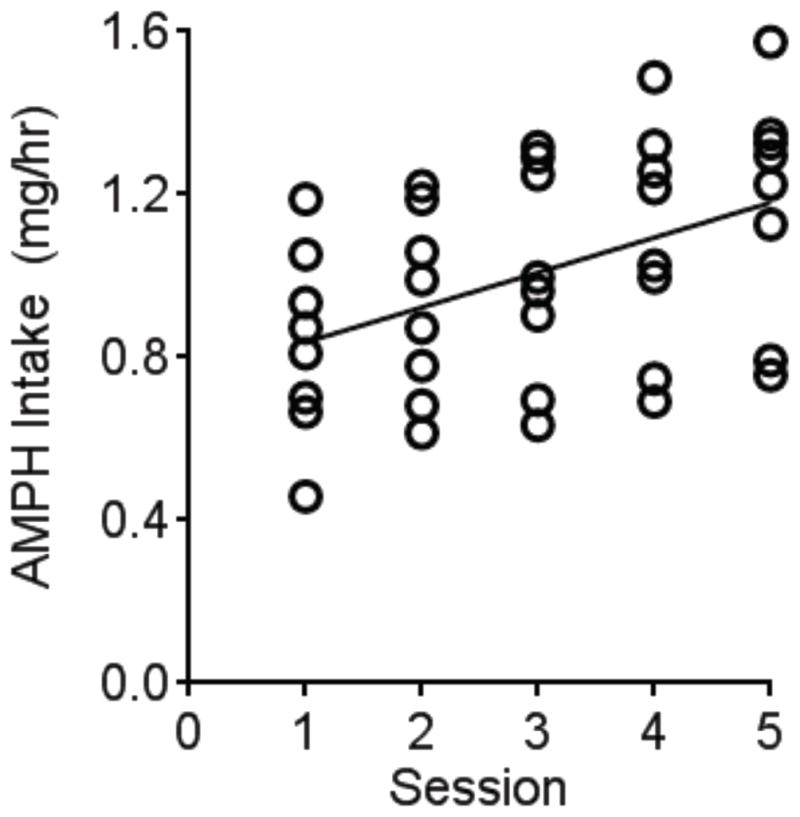
An escalation of AMPH self-administration rate across sessions. Rats were allowed to self-administer AMPH (0.187 mg/kg/infusion) on a fixed-ratio one schedule of reinforcement for 5 consecutive days with a maximum of 40 infusions in 6 hours. The scatter plot indicates the rate of AMPH intake (mg/hr) for each individual animal (N=8). Rats significantly escalated their rate of intake over sessions as shown by the linear regression.
Differential gene regulation of D2/D3 receptors by AMPH self-administration
Animals were sacrificed 18 hr after the last AMPH infusion. We examined the effect of AMPH self-administration on the mRNA level of D2/D3 receptors in the VTA (Fig. 2A) and the NAc (Fig. 2B). Dopamine D2 receptors have two isoforms derived from alternative splicing, which are referred to as long vs short isoforms (D2L vs D2S) (Picetti et al., 1997). These two variants differ by the presence or absence of 29 amino acids in the third intracellular loop. A two-way (brain region × drug treatment) ANOVA analysis of the mRNA levels of for each dopamine receptor subtype was performed. Overall, AMPH self-administration significantly decreased the mRNA levels of D2S (F1,28=14.32, P<0.01) and D2L (F1,28=12.91, P<0.01) and increased D3R (F1,26=34.25, P<0.01) in the brain. There was a significant main interaction effect for D2S (F1,28=4.485, P<0.05) and D2L (F1,28=5.082, P<0.05), indicating the mRNA levels of these genes are brain region dependent. Tukey’s multiple comparisons tests revealed that the mRNA levels of D2S and D2L were significantly lower in the VTA following AMPH self-administration compared to the control group.
Fig. 2.
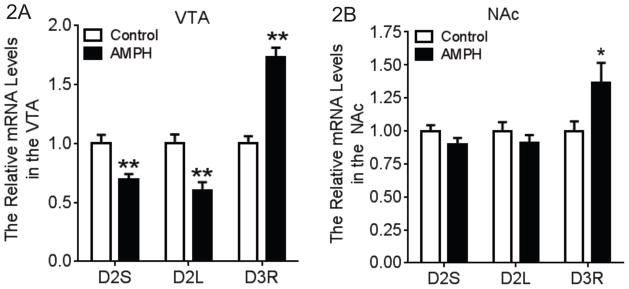
Gene expression profile of dopamine D2 and D3 receptors in the VTA and NAc following AMPH self-administration. A two-way (brain region × drug treatment) ANOVA with posthoc analysis was conducted for each subtype of dopamine receptors. (A) In the VTA, the mRNA levels of D2S and D2L were significantly decreased following AMPH self-administration when compared to the control group. In contrast, the mRNA level of D3 receptors was significantly increased (N=6–8). (B) In the NAc, AMPH self-administration significantly increased the mRNA level of D3 receptors without any effect on the D2L and D2S (N=6–8). *p<0.05, **p<0.01 vs. the control group
Differential gene regulation of G protein subtypes by AMPH self-administration
We examined the gene expression of subtypes of Gαi/o proteins which are coupled to D2/D3 receptors in both the VTA (Fig. 3A) and the NAc (Fig. 3B). A two-way ANOVA (brain region × drug treatment) was performed for each subtype of Gαi/o proteins. There was a significant main interaction effect for Gαi1 (F1,26=20.85, P<0.01) and Gαi2 (F1,26=6.500, P<0.05). Tukey’s multiple comparisons tests showed that there was a significant increase in the mRNA levels of Gαi1 (P<0.01) and Gαi2 (P<0.01) in the VTA following AMPH self-administration compared to the control group.
Fig. 3.
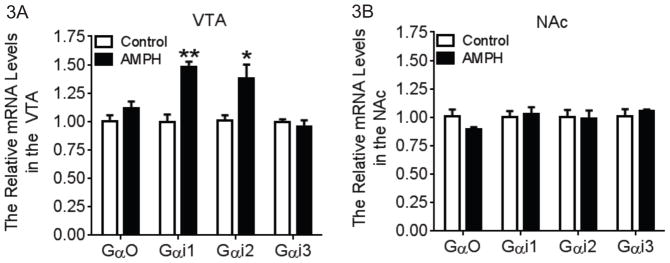
Gene expression profile of subtypes of Gαi/o proteins in the VTA and NAc following AMPH self-administration. A two-way (brain region × drug treatment) ANOVA with posthoc analysis was conducted for each subtype of Gαi/o proteins. (A) In the VTA, the mRNA levels of Gαi1 and Gαi2 were significantly increased following AMPH self-administration. There was no change in the mRNA levels of Gαi3 and Gαo (N=7–8). (B) In the NAc, AMPH self-administration did not alter the mRNA levels of all the subtypes of Gαi/o (N=7–8). *p<0.05, **p<0.01 vs. the control group
Differential gene regulation of subtypes of RGS proteins by AMPH self-administration
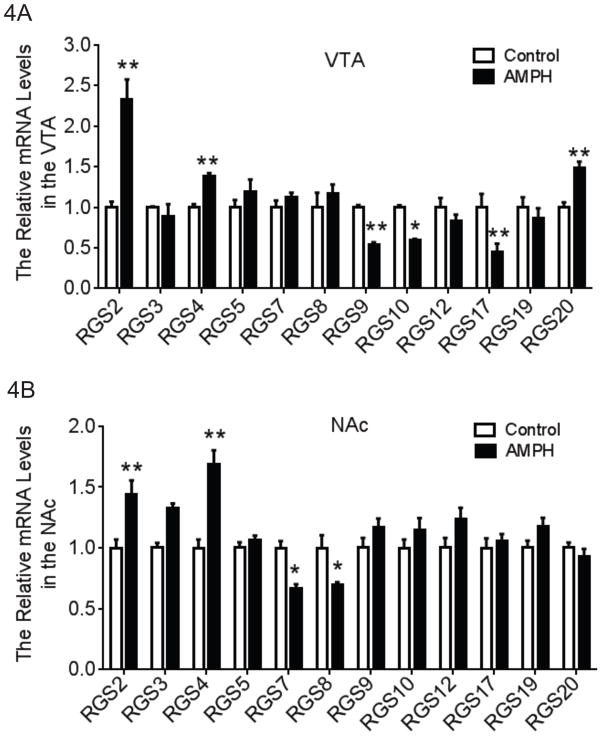
The mRNA levels of RGS subtypes in the VTA and the NAc were shown in Figs. 4A &4B. Changes in RGS mRNA levels following AMPH self-administration were RGS subtype- and brain region-dependent. A two-way ANOVA (brain region × drug treatment) was performed for each RGS subtype. There was a significant main interaction effect for RGS2 (F1,25=14.98, P<0.01), RGS4 (F1,28=9.157, P<0.01), RGS7 (F1,20=15.14, P<0.01), RGS8 (F1,22=4.807, P<0.05), RGS9 (F1,26=26.03, P<0.01), RGS10 (F1,22=22.43, P<0.01), RGS17 (F1,24=22.81, P<0.01) and RGS20 (F1,22=14.38, P<0.01). Tukey’s multiple comparisons tests indicated that AMPH self-administration significantly increased the mRNA levels of RGS2 and RGS4 in both brain regions although the increase in RGS2 was more pronounced in the VTA and the increase in RGS4 was more substantial in the NAc. AMPH self-administration also significantly decreased RGS9, RGS10 and RGS17 and increased RGS20 mRNA levels in the VTA, though there was no effect on these genes in the NAc. Additionally, AMPH self-administration resulted in a notable reduction in the mRNA levels for RGS7 and RGS8 in the NAc.
Fig. 4.
Gene expression profile of RGS subtypes in the VTA and NAc following AMPH self-administration. A two-way (brain region × drug treatment) ANOVA with posthoc analysis was conducted for each subtype of RGS proteins. (A) In the VTA, AMPH self-administration significantly increased the mRNA levels of RGS2, RGS4 and RGS20 when compared to the control group. In contrast, the mRNA levels of RGS9, RGS10 and RGS17 were significantly decreased in the AMPH self-administration group (N=6–8). (B) In the NAc, AMPH self-administration significantly increased the mRNA levels of RGS2 and RGS4 and significantly decreased the mRNA levels of RGS7 and RGS8 (N=6–8). *p<0.05, **p<0.01 vs. the control group.
Correlation of gene expression between the brain regions
Pearson’s correlations analyses were performed to investigate 1) whether there was a relationship in gene expression between the VTA and NAc in sham controls, and 2) whether these relationships were affected by AMPH self-administration. These analyses revealed a significant positive relationship for RGS2 (R=0.716, P<0.05), RGS4 (R=0.787, P<0.05) and RGS10 (R=0.887, P<0.05) mRNA levels between the VTA and the NAc in the drug naïve group. In the AMPH self-administration group, there was also a significant positive correlation between the brain regions for RGS2 (R=0.820, P<0.05) and RGS4 (R=0.877, P<0.01) mRNA levels. Thus, AMPH self-administration proportionally increased the mRNA levels of RGS2 and RGS4 in both brain regions. However, AMPH self-administration abolished the correlation of RGS10 mRNA levels between the VTA and NAc. Moreover, there were significant negative correlations between the brain regions for RGS7 (R=−0.907, P<0.001) and RGS19 (R=−0.869, P<0.01) mRNA levels in the AMPH self-administration group, genes that were not correlated in the control group (Table 2). These data suggest that the effect of AMPH self-administration on certain genes is brain region-dependent.
Table 2.
Correlations between the mRNA levels of genes in the VTA and the NAc for the control and AMPH self-administration groups
| Gene | Control | AMPH SA |
|---|---|---|
| RGS2 | 0.716* (7) | 0.820* (7) |
| RGS3 | 0.590 (8) | 0.184 (8) |
| RGS4 | 0.787* (8) | 0.877** (8) |
| RGS5 | 0.590 (8) | 0.758 (6) |
| RGS7 | −0.186 (6) | −0.907** (6) |
| RGS8 | −0.027 (6) | −0.694 (7) |
| RGS9 | −0.346 (8) | −0.646 (7) |
| RGS10 | 0.887* (6) | −0.376 (6) |
| RGS12 | 0.561 (6) | 0.596 (7) |
| RGS17 | 0.639 (6) | −0.436 (8) |
| RGS19 | 0.375 (6) | −0.869** (6) |
| RGS20 | 0.200 (7) | 0.329 (6) |
| D2S | 0.253 (8) | 0.521 (8) |
| D2L | 0.423 (8) | 0.586 (8) |
| D3R | 0.378 (8) | 0.654 (6) |
| Gαi1 | −0.552 (8) | 0.224 (7) |
| Gαi2 | −0.438 (7) | −0.073 (7) |
| Gαi3 | −0.471 (7) | −0.063 (7) |
| GαO | −0.296 (7) | −0.721 (7) |
Note: Values are the Pearson correlation coefficients. The samples sizes are indicated in parentheses.
Correlation between the rate of AMPH intake and the mRNA levels
Pearson’s correlation analyses were then performed to determine whether gene expression in the VTA and/ or NAc was correlated with AMPH self-administration behavior. Because the total intake for AMPH per session for each animal was fixed (40 injections), the rate of AMPH intake in the final self-administration session was used as a measure AMPH-related behavior. These analyses revealed that the rate of AMPH intake was positively correlated with the mRNA level of RGS2 (VTA: R=0.828, P<0.05; NAc: R=0.803, P<0.05) and RGS4 (VTA: R=0.716, P<0.05; NAc: R=0.742, P<0.05) in both brain regions. Additionally, the rate of AMPH intake was positively correlated with RGS10 (R=0.864, P<0.05) and RGS20 (R=0.822, P<0.05) mRNA levels, and negatively correlated with RGS17 (R=−0.858, P<0.01) and D2S (R=−0.941, P<0.01) mRNA levels in the VTA. None of these correlations were observed in the NAc (Table 3).
Table 3.
Correlation between the rate of AMPH intake and the mRNA levels of the target gene in the VTA and the NAc
| Gene | Behavior vs. VTA mRNA | Behavior vs. NAc mRNA |
|---|---|---|
| RGS2 | 0.828* (7) | 0.803* (7) |
| RGS3 | 0.1587 (8) | 0.5583 (8) |
| RGS4 | 0.716* (8) | 0.742* (8) |
| RGS5 | −0.093 (8) | 0.037 (6) |
| RGS7 | 0.577 (6) | −0.679 (6) |
| RGS8 | 0.584 (6) | −0.340 (7) |
| RGS9 | 0.243 (8) | −0.437 (7) |
| RGS10 | 0.864* (6) | −0.419 (6) |
| RGS12 | −0.199 (6) | −0.084 (7) |
| RGS17 | −0.858**(6) | 0.145 (8) |
| RGS19 | −0.063 (6) | 0.003 (6) |
| RGS20 | 0.822* (7) | 0.475 (6) |
| D2S | −0.941**(8) | 0.429 (8) |
| D2L | −0.611 (8) | −0.398 (8) |
| D3R | 0.551 (8) | 0.265 (6) |
| Gαi1 | 0.734 (8) | −0.245 (7) |
| Gαi2 | 0.694 (7) | −0.393 (7) |
| Gαi3 | −0.404 (7) | 0.570 (7) |
| GαO | 0.018 (7) | −0.505 (7) |
Note: Values are the Pearson correlation coefficients. The samples sizes are indicated in parentheses.
DISCUSSION
In the present study, we investigated the effect of AMPH self-administration on gene expression of D2/D3 receptors, Gαi/o and RGS proteins in the rat VTA and NAc and their association with AMPH taking behavior. Our data demonstrate that there were profound brain-region dependent changes in the mRNA levels of these genes in a rodent model of AMPH self-administration. It is important to model the effects of AMPH using a contingent model since non-contingent models have been shown to produce differential changes in gene and protein expression from self-administration models (Hemby et al., 1997, Stefanski et al., 2007, Fumagalli et al., 2013). This is the first study to concurrently examine the mRNA levels of D2/D3 receptors and their associated signaling proteins in the animal models of AMPH self-administration and correlate the changes in the targeted genes with AMPH self-administration behavior.
One interesting finding from the present study was that AMPH self-administration modulated the mRNA level of D2 and D3 receptors in the VTA in an opposite manner. Most studies in the literature have focused primarily on changes of D2/D3 receptor mRNA levels in the striatum following psychostimulant treatments. The effect of psychostimulants on the gene expression of D2 and D3 receptors in the VTA has yet to be investigated. Recent evidence indicates that the midbrain availability of D2/D3 receptors is associated with impulsivity and the subjective effect of AMPH in human (Buckholtz et al., 2010). Moreover, midbrain D2 receptor knockout mice showed increased sensitivity to cocaine treatment (Bello et al., 2011). Thus, it is important to examine the gene expression profile of D2/D3 receptors in the VTA-NAc circuitry, which is known to be involved in drug addiction. The present study demonstrated that AMPH self-administration decreased D2 receptor (both D2S and D2L) and increased D3 receptor mRNA level in the VTA. In contrast to the observation in the VTA, AMPH self-administration only increased the mRNA level of D3 receptors in the NAc without any effect on either D2S or D2L receptors. This result is consistent with reports of increased mRNA levels of D3 receptors in the NAc of human cocaine overdose victims (Segal et al., 1997) and no change in the NAc mRNA level of D2 receptors in the postmortem brains from cocaine addicts (Meador-Woodruff et al., 1993). It appears that D2 receptors in the VTA are more vulnerable to changes induced by AMPH self-administration than in the NAc. It is possible that more significant changes of gene expression in the NAc may require lengthy exposure of AMPH. Most importantly, there was a negative correlation between the rate of AMPH intake and the mRNA level of D2S in the VTA. Given that the D2S receptor is thought to have a predominantly autoreceptor role (Usiello et al., 2000, Wang et al., 2000), lower levels of expression may result in enhanced dopamine signaling, particularly in the terminal fields of the dopaminergic neurons (the NAc). Thus, reduced levels of theD2S gene in the VTA may precede drug-induced neuroadaptations in the NAc.
The effect of AMPH self-administration on the gene expression of Gαi/o proteins was limited to the VTA. There was a significant increase in the mRNA levels of Gαi1 and Gαi2 in the VTA and no effect was observed in the NAc, which paralleled the gene expression pattern for D2/D3 receptors. These data are consistent with our hypothesis that the VTA is more susceptible to AMPH-induced neuroadaptations than the NAc. We showed previously that Gαi2 is the primary G protein for D2/D3 receptor signaling (Calipari et al., 2014). Thus, the increase in the mRNA levels of Gαi2 may compensate for the reduction in function and gene expression of D2/D3 receptors. Although the protein expression of midbrain Gαi1 and Gαi2 was not increased in our previous report following the same regimen of AMPH self-administration (Calipari et al., 2014), the increased mRNA levels of Gαi1 or Gαi2 may lead to a delayed increase in the protein expression which requires further confirmation. It is unknown which subtype of Gαi/o proteins is primarily coupled to D2 and D3 receptors in vivo respectively. However, experiments using heterologous expression systems indicate that there is a functional coupling of D2 receptors with all subtypes of Gαi/o proteins in Sf9 cells (Gazi et al., 2003), whereas there is a high-affinity coupling between D3 receptors and Gαo in HEK293 cells (Lane et al., 2008). Whether the increased mRNA level of Gαi1 or Gαi2 is associated with changes in D2/D3 receptors in the VTA remains to be determined. Despite the significant increase in the mRNA levels of Gαi1 and Gαi2 in the VTA, there was no correlation between the rate of AMPH intake and these two gene levels.
RGS proteins are known to be involved in drug addiction. It has been shown that the mRNA levels of several RGS proteins (e.g. RGS2, RGS4 and RGS9) in the striatum are modulated by acute and chronic psychostimulant treatments including cocaine and AMPH [see review in (Traynor, 2010)]. However, current knowledge of the impact of chronic psychostimulant exposure on RGS mRNA levels is mostly limited to RGS2 and RGS4 subtype in the striatum. This study expanded the targets to include 12 subtypes of RGS proteins, some of which (e.g. RGS17, RGS19 and RGS20) are expressed in the mesolimbic dopamine system (Larminie et al., 2004) and are yet to be investigated in response to psychostimulant treatments. This study also examined the gene expression pattern of RGS subtypes in the VTA, a brain region that has not been fully examined. Our results demonstrated that AMPH self-administration produced RGS subtype-dependent upregulation and downregulation of gene expression not only in the NAc but also in the VTA. Among all the RGS subtypes, the mRNA levels of RGS2 and RGS4 in the VTA were positively correlated with their mRNA levels in the NAc for both control and AMPH self-administering animals. AMPH self-administration increased the mRNA levels of RGS2 and RGS4 in both regions in a similar magnitude. This observation is consistent with our previous report on increased RGS2 mRNA in the rat midbrain following the same regimen of AMPH self-administration (Calipari et al., 2014). These data are also in agreement with a report that chronic AMPH treatment causes an increase in striatal RGS2 mRNA levels (Seeman et al., 2007). However, in contrast, Burchett et al. (1999) reported a lack of change in RGS2 mRNA following AMPH treatment in rats (Burchett et al., 1999). These discrepancies likely result from variations in the schedule of the AMPH treatment and the length of AMPH withdrawal. RGS4, in addition to RGS2 gene, was also upregulated by AMPH self-administration in both the VTA and NAc, in accordance with the increased striatal mRNA level of RGS4 following chronic AMPH treatment (Burchett et al., 1999). The mRNA levels of RGS2 and RGS4 in both the VTA and NAc were positively correlated with the rate of AMPH intake, indicating that an increase in RGS2 and RGS4 gene expression may contribute to the development of compulsive drug taking behavior.
This study also identified a few novel genes of RGS subtypes that were modulated by AMPH self-administration. There were negative correlations between the mRNA levels of the VTA and the NAc for RGS7 and RGS19, which were not observed in the control animals. Moreover, there was a positive correlation between the mRNA levels of RGS10 in the two brain regions of the control animals, which was abolished in the AMPH self-administration group. These data suggest that AMPH self-administration may have exerted opposite effects on the expression of the targeted genes in these two brain regions. Alternatively, AMPH self-administration may have a preferential effect on one brain region over the other for specific genes. Among all the RGS subtypes, AMPH self-administration significantly decreased RGS9, RGS10 and RGS17 and increased RGS20 mRNA levels in the VTA. There was also a significant decrease in RGS7 and RGS8 mRNA levels in the NAc. RGS10 was previously shown to be significantly decreased in the prefrontal cortex following short-term abuse of opiate in humans (White and Wang, 1984), which is consistent with the reduced RGS10 mRNA level induced by AMPH self-administration. Moreover, a RGS17 polymorphism (SNP rs596359), which produces lower RGS17 mRNA levels in human, is associated with dependence of alcohol, cocaine, opioid and marijuana (Zhang et al., 2012). The reduced mRNA level of RGS17 in the VTA following AMPH self-administration in rats, therefore, supports these observations in human drug addicts. Our study also revealed that the mRNA level of RGS20 was increased in the VTA. RGS20 is a brain specific RGS protein (Wang et al., 1998) and has selectivity for both Gαi/o and Gαz protein (Wang et al., 2002). This is the first report indicating that RGS20 is subject to modulation by AMPH self-administration. Additionally, there was a discrepancy in the change of RGS9 mRNA level between our study and the literature. Reduced RGS9 mRNA was observed in the striatum of rats treated non-contingently with AMPH following 1 month of abstinence (Seeman et al., 2007). The difference in AMPH treatment regimen and the length of withdrawal probably contributes to the discrepancy. Among all the changes in these RGS genes, the rate of AMPH intake was positively correlated with RGS10 and RGS20 mRNA levels in the VTA, and negatively correlated with RGS17 mRNA level in the VTA. Although the mRNA levels of RGS7 and RGS8 in the NAc were significantly lower, there was no correlation between their gene expressions and the rate of AMPH intake. To summarize, there were more RGS genes in the VTA that were associated with AMPH taking behavior than in the NAc. Future experiments using extended access to AMPH self-administration would help elucidate whether these early changes in gene expression would promote adaptations in the NAc. Because the neurons in the VTA and NAc are interconnected and play an important role in drug reward, the differential effects of AMPH self-administration on gene expressions in these two brain regions may alter homeostasis of neurotransmitter transmission and subsequently lead to development of drug abuse behavior.
Because of technical limitations, we should be cautious in some of our interpretations of the data. First, our control animals received sham surgeries, instead of receiving yoked-saline injections. It is plausible that changes in gene expression could result from responses to the learning environment or act of level pressing. Since self-administration occurred in the animal’s home cage, the environment alone should not be a confounding factor in this experimental design. However, AMPH self-administering animals learned the association of lever pressing with AMPH delivery (reward) whereas control animals rarely level pressed for saline because saline is not considered reinforcing. Whether the learning process altered the gene expression, therefore, requires future investigation. Second, samples were collected from homogenized brain tissues containing a heterogeneous cell population; thus, these changes in RGS and Gi/o proteins may not be solely related to D2/D3 receptors. Third, the current study investigating gene expressions do not necessarily translate to functional alterations in the proteins themselves. Thus, future experiments will examine potential causal relationships between the level of RGS subtypes and the function of D2/D3 receptors in vivo following AMPH self-administration. Identification of specific subtypes of RGS proteins involved in D2/D3 receptor signaling will provide novel targets for the development of future therapeutic drugs that may normalize aberrant dopamine transmission and treat drug addiction.
CONCLUSION
The present study revealed that AMPH self-administration induced a differential brain-region dependent gene expression profile of D2/D3 receptors and their associated signaling proteins Gαi/o and RGS proteins from control animals. AMPH self-administration differentially altered the correlations of the gene expressions between the VTA and the NAc for RGS7, RGS10 and RGS19. Among all the changes in gene expression profiles that occurred in response to AMPH self-administration, AMPH taking behavior was correlated with D2S, RGS2, RGS4, RGS10, RGS17 and RGS20 mRNA levels in the VTA, and RGS2 and RGS4 mRNA levels in the NAc. These data indicate that the early-onset changes of the gene expression in the VTA may importantly contribute to the development of AMPH abuse.
Highlight.
AMPH reduced D2R gene
AMPH disrupted correlations of RGS gene between brain regions
AMPH taking behavior was correlated with RGS and D2R gene
Abbreviations
- AMPH
amphetamine
- RGS
regulator of G protein signaling
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- mRNA
messenger ribonucleic acid
- D2S
the short form of dopamine D2 receptors
- D2L
the long form of dopamine D2 receptors
- D3R
dopamine D3 receptors
Footnotes
DISCLOSURES
This work was supported by NIH grants DA030161 (SRJ), DA014030 (SRJ), DA006634 (TJB, SRJ and RC), T32 DA007246 and F31 DA031533 (ESC). The authors would like to thank Dr. Linda Porrino for her helpful discussion of data analysis methods. The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nature neuroscience. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett SA, Bannon MJ, Granneman JG. RGS mRNA expression in rat striatum: modulation by dopamine receptors and effects of repeated amphetamine administration. J Neurochem. 1999;72:1529–1533. doi: 10.1046/j.1471-4159.1999.721529.x. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC, Jones SR, Chen R. Amphetamine Self-Administration Attenuates Dopamine D2 Autoreceptor Function. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celver J, Sharma M, Kovoor A. RGS9-2 mediates specific inhibition of agonist-induced internalization of D2-dopamine receptors. J Neurochem. 2010;114:739–749. doi: 10.1111/j.1471-4159.2010.06805.x. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. Journal of substance abuse treatment. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Frankowska M, Marcellino D, Adamczyk P, Filip M, Fuxe K. Effects of cocaine self-administration and extinction on D2-like and A2A receptor recognition and D2-like/Gi protein coupling in rat striatum. Addiction biology. 2013;18:455–466. doi: 10.1111/j.1369-1600.2012.00452.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Moro F, Caffino L, Orru A, Cassina C, Giannotti G, Di Clemente A, Racagni G, Riva MA, Cervo L. Region-specific effects on BDNF expression after contingent or non-contingent cocaine i.v. self-administration in rats. Int J Neuropsychopharmacol. 2013;16:913–918. doi: 10.1017/S146114571200096X. [DOI] [PubMed] [Google Scholar]
- Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. British journal of pharmacology. 2003;138:775–786. doi: 10.1038/sj.bjp.0705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hutchaleelaha A, Sukbuntherng J, Chow HH, Mayersohn M. Disposition kinetics of d- and l-amphetamine following intravenous administration of racemic amphetamine to rats. Drug metabolism and disposition: the biological fate of chemicals. 1994;22:406–411. [PubMed] [Google Scholar]
- Jeanneteau F, Guillin O, Diaz J, Griffon N, Sokoloff P. GIPC recruits GAIP (RGS19) to attenuate dopamine D2 receptor signaling. Molecular biology of the cell. 2004;15:4926–4937. doi: 10.1091/mbc.E04-04-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JR, Powney B, Wise A, Rees S, Milligan G. G protein coupling and ligand selectivity of the D2L and D3 dopamine receptors. J Pharmacol Exp Ther. 2008;325:319–330. doi: 10.1124/jpet.107.134296. [DOI] [PubMed] [Google Scholar]
- Larminie C, Murdock P, Walhin JP, Duckworth M, Blumer KJ, Scheideler MA, Garnier M. Selective expression of regulators of G-protein signaling (RGS) in the human central nervous system. Brain Res Mol Brain Res. 2004;122:24–34. doi: 10.1016/j.molbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Morgan D, Roberts DC. Cross-sensitization of the reinforcing effects of cocaine and amphetamine in rats. Psychopharmacology (Berl) 2007;195:369–375. doi: 10.1007/s00213-007-0909-6. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Little KY, Damask SP, Mansour A, Watson SJ. Effects of cocaine on dopamine receptor gene expression: a study in the postmortem human brain. Biol Psychiatry. 1993;34:348–355. doi: 10.1016/0006-3223(93)90178-g. [DOI] [PubMed] [Google Scholar]
- Min C, Cheong SY, Cheong SJ, Kim M, Cho DI, Kim KM. RGS4 exerts inhibitory activities on the signaling of dopamine D2 receptor and D3 receptor through the N-terminal region. Pharmacological research : the official journal of the Italian Pharmacological Society. 2012;65:213–220. doi: 10.1016/j.phrs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Walker JR, Sevarino KA, Duman RS. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Picetti R, Saiardi A, Abdel Samad T, Bozzi Y, Baik JH, Borrelli E. Dopamine D2 receptors in signal transduction and behavior. Critical reviews in neurobiology. 1997;11:121–142. doi: 10.1615/critrevneurobiol.v11.i2-3.20. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Seeman P, Ko F, Jack E, Greenstein R, Dean B. Consistent with dopamine supersensitivity, RGS9 expression is diminished in the amphetamine-treated animal model of schizophrenia and in postmortem schizophrenia brain. Synapse. 2007;61:303–309. doi: 10.1002/syn.20368. [DOI] [PubMed] [Google Scholar]
- Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. International journal of biological sciences. 2005;1:51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanski R, Ziolkowska B, Kusmider M, Mierzejewski P, Wyszogrodzka E, Kolomanska P, Dziedzicka-Wasylewska M, Przewlocki R, Kostowski W. Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system. Brain research. 2007;1157:1–10. doi: 10.1016/j.brainres.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Traynor J. Regulator of G protein-signaling proteins and addictive drugs. Ann N Y Acad Sci. 2010;1187:341–352. doi: 10.1111/j.1749-6632.2009.05150.x. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, clinical and experimental research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Molecular psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ducret A, Tu Y, Kozasa T, Aebersold R, Ross EM. RGSZ1, a Gz-selective RGS protein in brain. Structure, membrane association, regulation by Galphaz phosphorylation, and relationship to a Gz gtpase-activating protein subfamily. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho G, Zhang JJ, Nieuwenhuijsen B, Edris W, Chanda PK, Young KH. Regulator of G protein signaling Z1 (RGSZ1) interacts with Galpha i subunits and regulates Galpha i-mediated cell signaling. J Biol Chem. 2002;277:48325–48332. doi: 10.1074/jbc.M206116200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu R, Sasaoka T, Tonegawa S, Kung MP, Sankoorikal EB. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984;231:275–280. [PubMed] [Google Scholar]
- Zhang H, Wang F, Kranzler HR, Anton RF, Gelernter J. Variation in regulator of G-protein signaling 17 gene (RGS17) is associated with multiple substance dependence diagnoses. Behavioral and brain functions : BBF. 2012;8:23. doi: 10.1186/1744-9081-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]