Abstract
Aims
In this study, the efficacy of proanthocyanidins (PCs) against oxidative damage was systematically reviewed to facilitate their use in various applications.
Methods
A meta-analysis was performed by two researchers. Each investigator independently searched electronic databases, including Cochrane, PubMed, Springer, Web of Science, China National Knowledge Infrastructure (CKNI), China Science and Technology Journal Database (CSTJ), and WanFang Data, and analyzed published data from 29 studies on the effects of PCs against oxidative damage. Oxidative stress indexes included superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), glutathione (GSH), glutathione peroxidase (GPx), and total antioxidative capacity (T-AOC).
Results
Compared with the oxidative damage model group, PCs effectively improved the T-AOC, SOD, GSH, GPx, and CAT levels, and reduced the MDA levels; these differences were statistically significant (P < 0.05). In studies that used the gavage method, SOD (95% CI, 2.33–4.00) and GPx (95% CI, 2.10–4.05) were 3.16-fold and 3.08-fold higher in the PC group than in the control group, respectively. In studies that used the feeding method, SOD (95% CI, 0.32–1.74) and GPx (95% CI, -0.31 to 1.65) were 1.03-fold and 0.67-fold higher in the PC group than in the control group, respectively. Statistically significant differences in the effects of PCs (P < 0.00001) were observed between these two methods. MDA estimated from tissue samples (95% CI, -5.82 to -2.60) was 4.32-fold lower in the PC group than in the control group. In contrast, MDA estimated using serum samples (95% CI, -4.07 to -2.06) was 3.06-fold lower in the PC group than in the control group. The effect of PCs on MDA was significantly greater in tissue samples than in serum samples (P = 0.02).
Conclusion
PCs effectively antagonize oxidative damage and enhance antioxidant capacity. The antagonistic effect may be related to intervention time, intervention method, and the source from which the indexes are estimated.
Introduction
Oxidative stress is caused by an imbalance between the production of reactive oxygen species (ROS) and the ability of a biological system to eliminate ROS or repair the resulting damage [1]. Thus, oxidative stress may result in an increased number of free radicals and cause lipid peroxidation, eventually leading to apoptosis and many diseases [2]. Increasing evidence has shown that oxidative stress plays a particularly important role in the development of cardiovascular diseases such as atherosclerosis, hypertension, atrial fibrillation, and cardiomyopathy [3]. Many reactive substances, such as arsenic [4] and hydrogen peroxide (H2O2) [5], can result in organismal damage via ROS and oxidative stress. Therefore, it is important to repair damage using antioxidant agents.
The effects of antioxidant substances such as vitamin C [6], E [7], and luteins [8] have been extensively studied owing to their health benefits. Additionally, the relative antioxidant efficacy of these substances has been previously examined. In particular, proanthocyanidins (PCs) have gained recent attention. These polyphenols are abundant in grape, haw, and gingko [9]. PCs have high antioxidant capacities and are efficient free radical scavengers. They are highly water soluble, easy to extract, rich in various plants, and can be absorbed naturally [10]. The antioxidative effects of PCs have not been systematically reviewed; additionally, the reported antioxidant efficacy of these compounds differs among studies [11–13], and their antioxidative ability is still unclear. Therefore, we performed a systematic review and meta-analysis based on a literature search to comprehensively analyze relevant data regarding the efficacy of PCs against oxidative damage. This work provides a scientific basis for the development and utilization of PC-based resources. According to the PICOS framework, the subjects, intervention, controls, and outcomes considered in this analysis were mice, PCs, an oxidative damage model, and enzyme levels with respect to oxidative stress, respectively. Randomized controlled mouse experiments were considered.
Materials and Methods
Eligibility criteria
The eligibility criteria were as follows. Randomized controlled mouse experiments and studies published in either Chinese or English were included. All strains and mouse genders were included in the present study. Oxidative damage model groups induced by any substance were used as the controls. The experimental groups included interventions with PCs only. If various doses of PCs were used in a study, the highest dose was chosen for this analysis. Valid outcome measures included the levels of enzymes related to oxidative stress measured by a microplate reader. These indicators of oxidative stress included superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), glutathione (GSH), glutathione peroxidase (GPx), and total antioxidative capacity (T-AOC).
Exclusion criteria
The exclusion criteria were as follows: (1) repeat publications, (2) incomplete information, (3) insufficient or insignificant statistical data, (4) unrelated to the study objectives, (5) lack of appropriate controls, and (6) reviews.
Search strategy
Searches were performed using the electronic databases Cochrane, PubMed, Springer, Web of Science, China Science and Technology Journal Database (CSTJ), WanFang Data, and China National Knowledge Infrastructure (CKNI) (last search updated on April 30, 2015) using PICOS. The key search string was (mice OR rat) AND (procyanidins OR proanthocyanidins) AND (antioxygenation OR antioxidant OR antioxidation) and the language was restricted to English and Chinese. We read both of the title and abstract first to make a decision whether the study is suitable for our study.
Data extraction
Two reviewers (SGL and MCX) independently screened full-length articles. The following information was extracted from the complete manuscripts of each qualified study: publication characteristics (title of the study, first author, publication date, and journal/magazine), basal data (n, mean ± SD) for the experimental and control groups, PC intervention modes, period of PC treatment, outcome indicators, and the source of indicator estimates (i.e., serum or tissue samples) (S1 File). If the two reviewers hold different opinions, then we invited the Prof. GSX, who is teaching meta-analysis subject in university, to make a final decision of the results.
Data analysis
The mean values for each outcome indicator differed between the experimental and control groups. Significant heterogeneity was detected (P < 0.05, I 2 > 75%); therefore, a random-effects model was applied for the meta-analysis. A multivariate meta-regression analysis was performed to determine the source of heterogeneity. Continuous variables were estimated as standardized mean differences (SMDs) with 95% confidence intervals (CI) between the PC-treated animals and control animals. All reported P-values are two-sided and a significance level of 0.05 was used. For additional insight, subgroup analyses were performed based on intervention mode (feed or gavage), length of PC treatment (<30 d or ≥30 d), and sample source (serum or tissue samples) to determine the factors associated with differences among study results in the outcome indicators. Publication bias was explored using funnel plots. All analyses were implemented in Review Manager Version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) and Stata 12.0.
Results
Study characteristics
Using the search strategy, 462 articles were identified (Fig 1), of which 29 were valid for the meta-analysis according to the eligibility and exclusion criterias. [14–42] (Table 1). Mice were used as animal models in these studies, and each study investigated the effect of PCs on oxidative damage. The oxidative damage models were primarily mice induced by various substances (e.g., arsenite, H2O2, and fluorine), and the antioxidative damage models were provided various PCs as interventions. PCs were administered by feeding (n = 3) or gavage (n = 26). The PC intervention time varied among studies, and was categorized as <30 d (n = 17) or ≥30 d (n = 12). Oxidative stress indexes (i.e., MDA, SOD, GPx, T-AOC, GSH, and CAT) were examined using serum (n = 19) and tissue samples (n = 10).
Fig 1. Flowchart of search strategy.
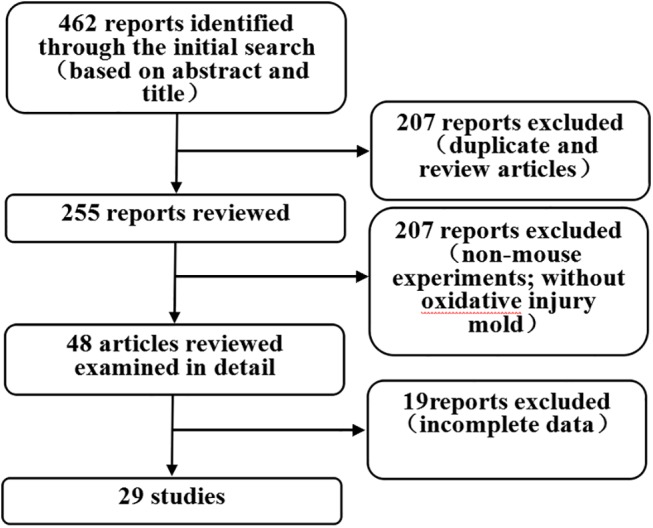
The meta-analysis included animal studies that investigated the antioxidant effect of proanthocyanidins (PCs).
Table 1. Characteristics of the animal studies included in the meta-analysis.
| First author (year) | Language | n | Mode of intervention | Period of PC(day) | Source of indicators | Outcome indicators |
|---|---|---|---|---|---|---|
| Su-Jin2009 [14] | English | 10 | Feed | <30d | Tissue | 2.3.6 |
| Mi-Ok Shin2010 [15] | English | 6 | Feed | <30d | Tissue | 1 |
| Xiayuan2010 [16] | Chinese | 8 | Gavage | <30d | Serum | 1.2 |
| Adem Guler2011 [17] | English | 8 | Gavage | <30d | Tissue | 1.2.3.4 |
| HUANG Qi-liang2011 [18] | English | 10 | Gavage | ≥30d | Serum | 1.2 |
| Lijianling2011 [19] | Chinese | 10 | Gavage | <30d | Tissue | 1.2 |
| Osama M.Ashour2011 [20] | English | 12 | Gavage | <30d | Tissue | 1.2.5.6 |
| Xielimei2011 [21] | Chinese | 10 | Gavage | ≥30d | Tissue | 1.2 |
| Soo-Kyong Choi2012 [22] | English | 8 | Feed | <30d | Tissue | 2.3.5 |
| Vijayakumar2012 [23] | English | 6 | Gavage | <30d | Tissue | 2.3.5.6 |
| Wangweifen2012 [24] | Chinese | 10 | Gavage | <30d | Tissue | 1.2 |
| Xiao GENG2012 [25] | English | 8 | Gavage | <30d | Tissue | 1.2 |
| Yu Deng2012 [26] | English | 8 | Gavage | <30d | Tissue | 1.2.3.5 |
| zhangxuan2012 [27] | English | 16 | Gavage | <30d | Tissue | 1.2.3.6 |
| zhaopeng2012 [28] | Chinese | 12 | Gavage | ≥30d | Serum | 1.2.3 |
| Bailijun2013 [29] | Chinese | 5 | Gavage | <30d | Serum | 1.2.3.6 |
| Dingyusong2013 [30] | Chinese | 10 | Gavage | ≥30d | Tissue | 1.2 |
| Hanaa A2013 [31] | English | 6 | Gavage | ≥30d | Tissue | 1.2.3.4 |
| Jiangyanfei2013 [32] | Chinese | 12 | Gavage | ≥30d | Serum | 1.2 |
| Miaozhiru2013 [33] | Chinese | 10 | Gavage | ≥30d | Serum | 1.2.3.5 |
| E Bakar2014 [34] | English | 7 | Gavage | <30d | Tissue | 1.2.4.5 |
| Gaolu2014 [35] | Chinese | 10 | Gavage | ≥30d | Serum | 1.2.3.6 |
| Hua Zhang2014 [36] | English | 10 | Gavage | ≥30d | Tissue | 1.2.5.6 |
| Juan Xiao2014 [37] | English | 10 | Gavage | <30d | Serum | 1.2.3 |
| Noorah2014 [38] | English | 10 | Gavage | <30d | Tissue | 2.6 |
| Tingting Ren2014 [39] | English | 8 | Gavage | ≥30d | Serum | 2.5 |
| Wangcheng2014 [40] | Chinese | 10 | Gavage | ≥30d | Tissue | 1.2.4.5 |
| Ying GAO2014 [41] | English | 10 | Gavage | ≥30d | Tissue | 1.2.3 |
| Esrafil Mansouri2015 [42] | English | 10 | Gavage | <30d | Serum | 1.2.3.6 |
Note: n = number of experimental animals; 1 = malondialdehyde, 2 = superoxide dismutase, 3 = glutathione peroxidase, 4 = total antioxidative capacity, 5 = glutathione, and 6 = catalase.
Meta-analyses
Effect of PC on SOD
A total of 28 studies estimated SOD levels. A pooled analysis showed that the SOD level was 2.91-fold higher in the experimental group than in the control group (95% CI, 2.16–3.67; Z = 7.53; P < 0.00001) with significant heterogeneity (P < 0.0001; I 2 = 88%; Fig 2).
Fig 2. Effect of PC on superoxide dismutase (SOD).
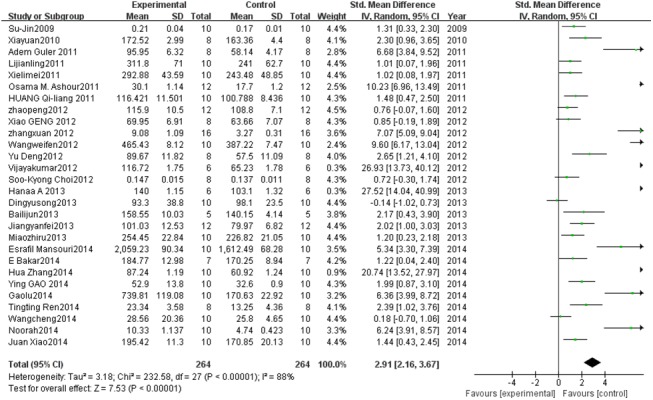
Forest plot showing the impact of PC treatment on SOD compared with controls. Abbreviations: SMD = standardized mean difference, IV = independent variable, 95% CI = 95% confidence interval.
Effect of PC on T-AOC
A total of 4 studies estimated T-AOC levels. A pooled analysis showed that the T-AOC level was 3.79-fold higher in the experimental group than in the control group (95% CI, 0.69–6.88; Z = 2.40; P = 0.02) with significant heterogeneity (P < 0.0001; I 2 = 94%; Fig 3).
Fig 3. Effect of PC on total antioxidative capacity (T-AOC).
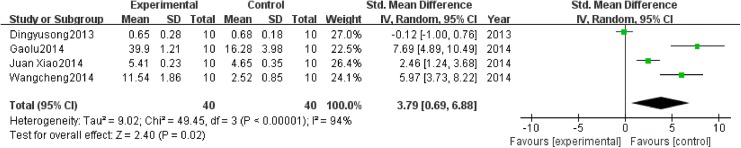
Forest plot showing the impact of PC treatment on T-AOC, compared with controls. Abbreviations: SMD = standardized mean difference, IV = independent variable, 95% CI = 95% confidence interval.
Effect of PC on GSH
A total of 9 studies estimated GSH levels. A pooled analysis showed that the GSH level was 4.53-fold higher in the experimental group than in the control group (95% CI, 2.30–6.76; Z = 3.97; P < 0.0001) with significant heterogeneity (P < 0.00001; I 2 = 93%; Fig 4).
Fig 4. Effect of PC on glutathione (GSH).
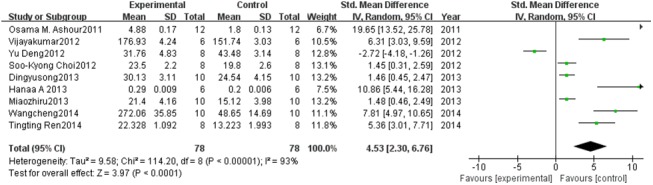
Forest plot showing the impact of PC treatment on GSH, compared with controls. Abbreviations: SMD = standardized mean difference, IV = independent variable, 95% CI = 95% confidence interval.
Effect of PC on GPx
Fourteen studies described GPx levels. A pooled analysis showed that the GPx level was 2.68-fold higher in the experimental group than in the control group (95% CI, 1.80–3.56; Z = 5.96; P< 0.00001) with significant heterogeneity (P < 0.00001; I 2 = 85%; Fig 5).
Fig 5. Effect of PC on glutathione peroxidase (GPx).
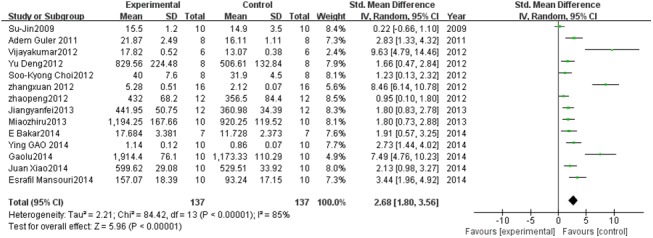
Forest plot showing the impact of PC treatment on GPx, compared with controls. Abbreviations: SMD = standardized mean difference, IV = independent variable, 95% CI = 95% confidence interval.
Effect of PC on CAT
Nine studies estimated CAT levels. A pooled analysis showed that the CAT level was 4.95-fold higher in the experimental group than in the control group (95% CI, 2.99–6.90; Z = 4.96; P < 0.00001) with significant heterogeneity (P < 0.00001; I 2 = 92%; Fig 6).
Fig 6. Effect of PC on catalase (CAT).
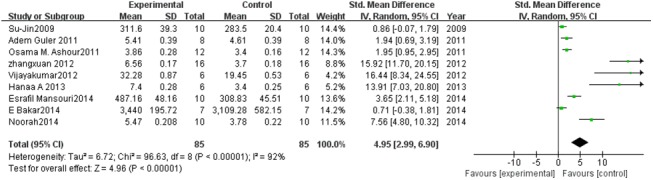
Forest plot showing the impact of PC treatment on CAT, compared with controls. Abbreviations: SMD = standardized mean difference, IV = independent variable, 95% CI = 95% confidence interval.
Effect of PC on MDA
Twenty-five studies described MDA levels. A pooled analysis showed that the MDA level was 3.06-fold lower in the experimental group than in the control group (95% CI, 4.07–2.06; Z = 5.99; P < 0.00001) with significant heterogeneity (P < 0.00001; I 2 = 92%; Fig 7).
Fig 7. Effect of PC on malondialdehyde (MDA).
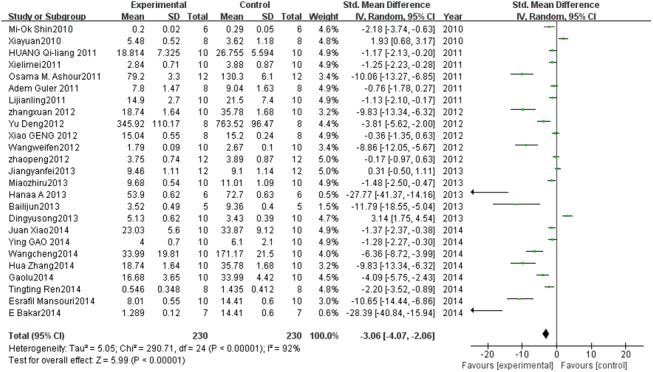
Forest plot showing the impact of PC treatment on MDA, compared with controls. Abbreviations: SMD = standardized mean difference, IV = independent variable, 95% CI = 95% confidence interval.
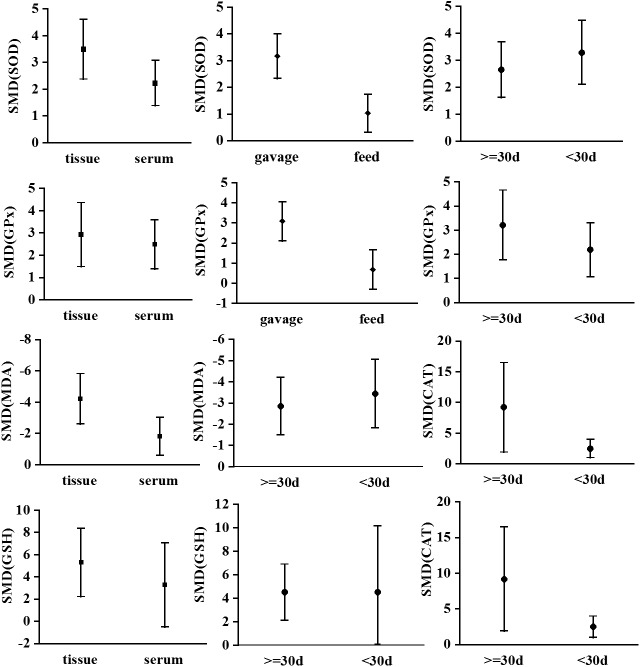
Subgroup analyses
We conducted a subgroup analysis considering the mode of intervention (gavage vs. feed), intervention period (<30 d vs. ≥30 d), and source of samples (tissue vs. serum). The SMD between PCs and control groups for SOD and GPx of tissue samples, gavage, and ≥30-d interventions were higher than those for serum samples, feeding, and <30-d interventions (P < 0.05, see Fig 8A1, 8A2, 8B1, 8B2 and 8B3). Furthermore, the SMD of MDA between the PC and control groups was significantly higher for tissue samples than for serum samples (P < 0.05, see Fig 8C1). The SMD of CAT between the PC and control groups was also higher for interventions of ≥30 d than for those of <30 d (P < 0.05, see Fig 8D3). We did not detect statistically significant differences in GSH or T-AOC (see Fig 8C3, 8D1 and 8D2).
Fig 8. Subgroup analyses to determine the effect of PC on oxidative damage.
Based on a subgroup analysis, the effect of PC using the gavage mode was stronger than that observed using the feeding mode (P < 0.00001; A2, B2). The effect of PC on MDA measured in tissue samples was significantly stronger than that measured in serum samples (P = 0.02; C1). Abbreviations: SMD = standardized mean difference.
Sensitivity analysis
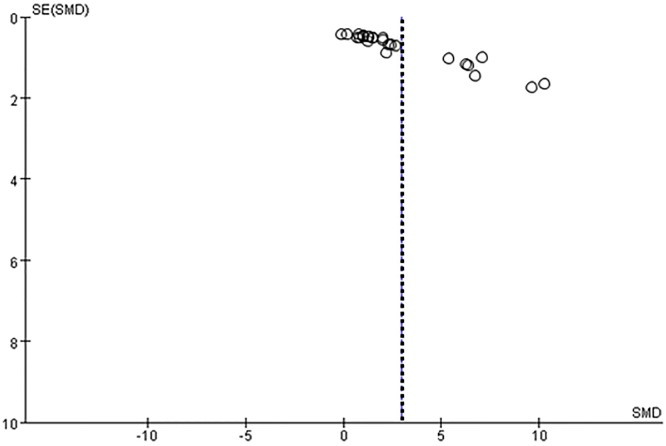
A sensitivity analysis was performed to evaluate the robustness of the study results. Specifically, we conducted a sensitivity analysis for SOD because it was estimated in 28 studies. Fig 9 shows the stability results for all studies; these results indicated that no individual study influenced the combined results. The method of intervention and the source of the outcome indicators (i.e., serum or tissue samples) significantly influenced the outcome indicators. A trend toward greater improvements was observed (Fig 8) when PC treatments were applied using the gavage method and when parameter estimates were based on tissue samples. The funnel plot for the studies that include estimates of SOD suggests that values were approximately evenly distributed around the overall mean estimate (Fig 10). Based on a multivariate meta-regression analysis, the source of outcome indicators (P = 0.040) and intervention method (P = 0.038) were significantly associated with differences in SOD.
Fig 9. Sensitivity analysis for SOD.
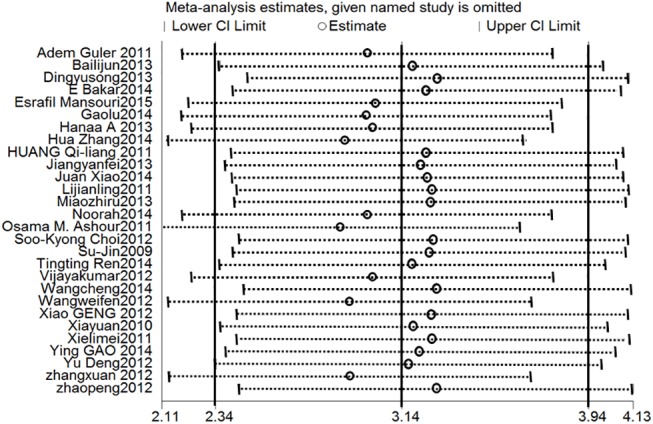
Stable results were observed for all studies, indicating that no individual study influenced the combined results. Abbreviations: SMD = standard mean difference, SE = standard error.
Fig 10. Funnel plot for the studies that estimated SOD.
Dotted line shows the overall estimated standard mean difference.The figure showed that the studies distributed symmetrically around the overall mean estimate.
Discussion
Our results showed that PC intervention increases the levels of the antioxidative indicators SOD, CAT, GSH, GPx, and T-AOC, and decreases the concentration of MDA in oxidative damage mouse models. The reported effects of PCs were also influenced by other factors, such as the mode of intervention, treatment period, and sample source. Based on this meta-analysis of published papers, PCs have an obvious antioxidative effect.
PCs, a type of polyphenol, were first extracted from haw in Germany [43]. These compounds contain various amounts of catechin and epicatechin [44]. Depending on the degree of polymerization, dipolymer–tetramers are usually called oligomeric procyanidins, and others are usually called procyanidolic polymers [45]. The widely distributed dipolymers are the focus of research and are among the most important PCs [46].
PCs are excellent antioxidants and free-radical scavengers; their antioxidative ability exceeds that of vitamins C and E [47]. The results of this meta-analysis also indicated that PCs, which can effectively improve the activity of antioxidative enzymes and reduce lipid peroxidation products, have an obvious antioxidant effect. The influence of PCs on SOD, GPx, and CAT can be maximized by applying the gavage mode instead of the normal feeding mode. This may be attributed to the precise control of PC intake by the investigator when using the gavage method. PCs can more effectively enhance antioxidant enzyme activity in tissues than in serum. We speculated that the indicators in serum samples reflect the whole-body oxidation-antioxidation levels, rather than that of a specific organ or tissue. In addition, the effect of PCs on the T-AOC index was not significant, probably owing to the small sample size (i.e., 4 studies). The results of the subgroup analysis will facilitate the selection of detection indexes in future studies regarding the antioxidative effect of PCs.
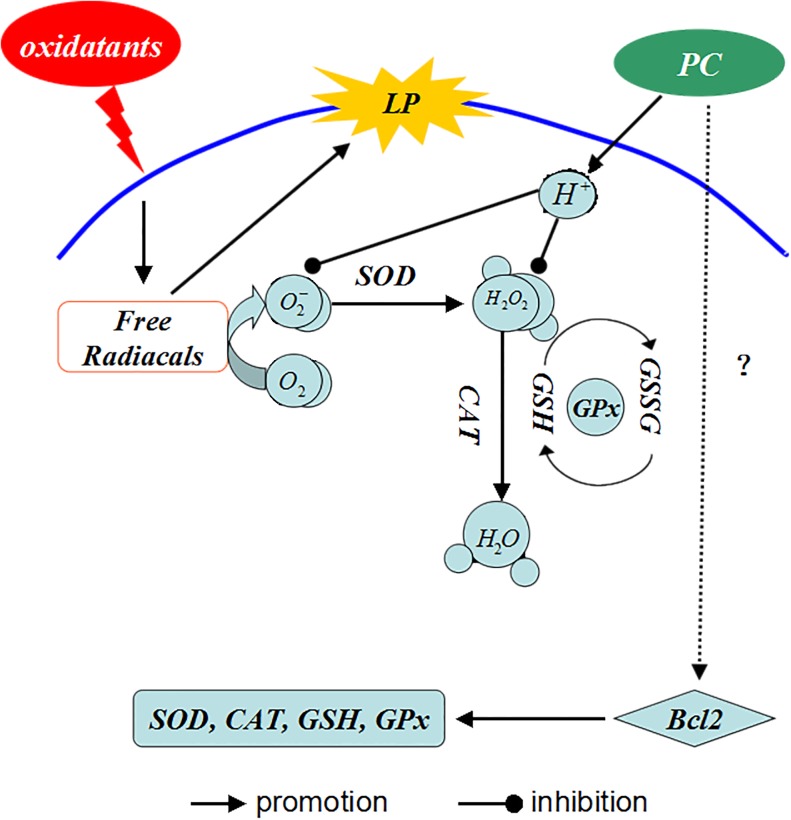
The antioxidative role of PCs is complex (Fig 11). Some harmful substances (such as H202, ethanol, galactose, and so on) induce oxidative stress and ROS production, and ultimately cause lipid peroxidation. The antioxidant defense system is activated and antioxidants (such as GSH, SOD, CAT, and GPx) remove excess free radicals and peroxides. If the degree of oxidation is beyond the capacity of antioxidant molecules, the levels of GSH, SOD, CAT, GPx, etc., will be reduced. PCs contain many phenolic hydroxyl groups and release H+ when they are oxidized, which can bind active oxygen radicals competitively to block the reaction chains of free radicals [48]. This reduces the consumption of antioxidants, increases the activity of antioxidative enzymes, improves antioxidative ability, and increases the T-AOC levels. In addition, it may be connected with increased expression of B-cell lymphoma-2 (Bcl-2), which can enhance antioxidation in cells. Liming [49], Yujie [50], and others have found that PC significantly increases the expression of Bcl-2, which increases the activity of antioxidant enzymes based on in vivo and vitro experiments.
Fig 11. Antioxidant mechanism of PCs.
PCs contain many phenolic hydroxyl groups and release H+ when they are oxidized, which can bind active oxygen radicals and competitively block the reaction chains of free radicals, reducing the consumption of antioxidants and increasing the activity of antioxidant enzymes. Abbreviations: LP represents lipid peroxidation, GSSG represents oxidized glutathione.
This meta-analysis included 29 published papers. The quality of these studies was sufficient to analyze the combined effects of PCs. The sensitivity analysis demonstrated the robustness of the overall results. Similarly, the symmetric distribution of the studies in a funnel plot demonstrated the lack of a publication bias. Although there was heterogeneity among studies, the randomized effect model was used to integrate the results and a subgroup analysis and meta-regression were used to evaluate the heterogeneity. All of the above analyses support the validity of using the combined results of the 29 studies to determine the effect of PCs.
In summary, the results of the present study support the strong antioxidative effect of PCs as evidenced by the levels of oxidative stress indicators in a systematic review of relevant published papers. PCs are able to block the free radical chain reaction by eliminating radicals. They may also regulate the signaling pathway related to oxidative stress, thereby improving antioxidative activity. These results provide a scientific basis for the development and utilization of PC-based resources.
Outlook
PCs have important antioxidative and antitumor effects and have protective effects with respect to the cardiovascular system and other biological activities [51]. They are widely used in medicine, health care products, and cosmetics [52]. Despite their wide use and increasing data related to their effects, the mechanisms that mediate the antioxidative effect of PCs are unclear. To improve product development and the utilization of PCs, it is necessary to determine the enzymes, receptor genes, and signaling pathways involved in the antioxidation process [53]. Additional examinations of the molecular mechanisms of PCs are needed to maximize their benefits with respect to human health.
Limitations
A limitation of the present study was the obvious heterogeneity in the data. Heterogeneity was observed with respect to subgroup factors, animal strains, reagents, PC dosages, and many other factors. Using a funnel plot analysis, we detected some evidence for a publication bias. We only considered manuscripts published in English and Chinese in this study and were not able to retrieve negative results.
Supporting Information
All data in the present study were extracted from 29 papers (references 14–42) and we display the data in the table as a supporting information to show the data availability.
(XLS)
Acknowledgments
The authors would like to thank the Department of Public Health, Shihezi University School of Medicine for assistance with this work, as well as funding from the Key Areas of Science and Technology Research Project of Xinjiang Production and Construction Corps (No. 2014BA039, No. 2015AG014), the High-tech Intellectual Project of Shi Hezi University (No. RCZX201112), and the National Natural Science Foundation of China (No. 81560517).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Key Areas of Science and Technology Research Project of Xinjiang Production and Construction Corps (No. 2014BA039, No. 2015AG014); High-tech Intellectual Project of Shi Hezi University (No. RCZX201112); and the National Natural Science Foundation of China (No. 81560517).
References
- 1. Shugang L, Yusong D, Qiang N, Shangzhi X, Lijuan P, Rulin M, et al. Lutein Has a Protective Effect on Hepatotoxicity Induced by Arsenic via Nrf2 Signaling. BioMed Research International, 2015: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qingli G, Xue L, Yan L. Update of Mitochondrial Mechanism Under the Diseases Related to Oxidative Stress. Chinese Journal of Cell Biology, 2013, 35(10): 1540–1545. [Google Scholar]
- 3. Khoubnasab Jafari M, Ansarin K, Jouyban A. Comments on "Use of Malondialdehyde as a Biomarker for Assesing Oxidative Stress in Different Disease Pathologies: A Review". Iran J Public Health, 2015, 44(5): 714–715. [PMC free article] [PubMed] [Google Scholar]
- 4. ShuGang L, ShangZhi X, Qiang N, YuSong D, LiJuan P, RuLin M, et al. Lutein alleviates arsenic-induced reproductive toxicity in male mice via Nrf2 signaling. Human and Experimental Toxicology, 2015, 1(10): 1–10. [DOI] [PubMed] [Google Scholar]
- 5. Tao J, Chen BD, Ma YT, Yang YN, Li XM, Ma X, et al. FrzA gene protects cardiomyocytes from H2O2-induced oxidative stress through restraining the Wnt/Frizzled pathway. Lipids Health Dis, 2015, 14(1): 90 10.1186/s12944-015-0088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valacchi G, Sticozzi C, Belmonte G, Cervellati F, Demaude J, Chen N, et al. Vitamin C Compound Mixtures Prevent Ozone-Induced Oxidative Damage in Human Keratinocytes as Initial Assessment of Pollution Protection. PLoS One, 2015, 10(8): e0131097 10.1371/journal.pone.0131097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saita E, Kondo K, Momiyama Y. Anti-Inflammatory Diet for Atherosclerosis and Coronary Artery Disease: Antioxidant Foods. Clin Med Insights Cardiol, 2015, 8(Suppl 3): 61–65. 10.4137/CMC.S17071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng F, Zhang Q, Yan FF, Wan JF, Lin CS. Lutein protects against ischemia/reperfusion injury in rat skeletal muscle by modulating oxidative stress and inflammation. Immunopharmacol Immunotoxicol, 2015, 37(4): 329–334. [DOI] [PubMed] [Google Scholar]
- 9. Bingzheng Z, Shugang L, Qiang N, Yusong D, Shuxia G, Shangzhi X, et al. Intervention of grape seed procyanidin extract on reproductive toxicity induced by arsenic in male mice. Journal of Toxicology, 2014, 05: 397–400. [Google Scholar]
- 10. Aruoma OI, Sun B, Fujii H, Neergheen VS, Bahorun T, Kang KS, et al. Low molecular proanthocyanidin dietary biofactor Oligonol: Its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors, 2006, 27(1–4): 245–265. [DOI] [PubMed] [Google Scholar]
- 11. Shu Gang L, Yu Song D, Qiang N, Shang Zhi X, Li Juan P, Ru Lin M, et al. Grape Seed Proanthocyanidin Extract Alleviates Arsenic-Induced Oxidative Reproductive Toxicity in Male Mice. Biomendical and Environmental Sciences, 2015, 28(4): 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansouri E, Khorsandi L, Abdollahzade Fard A. Protective role of grape seed proanthocyanidin antioxidant properties on heart of streptozotocin-induced diabetic rats. Vet Res Forum, 2015, 6(2): 119–124. [PMC free article] [PubMed] [Google Scholar]
- 13. Gao M, Zhao Z, Lv P, Li Y, Gao J, Zhang M, et al. Quantitative combination of natural anti-oxidants prevents metabolic syndrome by reducing oxidative stress. Redox Biol, 2015, 6: 206–217. 10.1016/j.redox.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su-Jin L, Soo-Kyong C, Jung-Sook S. Grape skin improves antioxidant capacity in rats fed a high fat diet. Nutrition Research and Practice, 2009, 3(4): 279–285. 10.4162/nrp.2009.3.4.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mi-Ok S, Jeon-Ok M. Effect of dietary supplementation of grape skin and seeds on liver fibrosis inducedby dimethylnitrosamine in rats. Nutrition Research and Practice(Nutr Res Pract), 2010, 4(5):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan X, Zhifang Z, Min L, Boling L. Procyanidins’ Impact on SOD Activity, MDA Content in Rats Exposed to Cadmium in Serum and Liver. Modern Chinese Doctor, 2010, 48(23): 8–10. [Google Scholar]
- 17. Adem G, Mehmet Ali S, Orhan Y, Mehmet Y, Mehmet G, Ertugrul O, et al. Proanthocyanidin prevents myocardial ischemic injury in adult rats. Med Sci Monit, 2011, 17(11): BR326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiliang H, Shan J, Xuejun W, Lishan X. Effects of Proanthocyanidin from Pine Needles onSOD Activity, MDA Content in Serum and VisceraIndex of Mice. Medicinal Plant, 2011, 2(6): 43–44, 46. [Google Scholar]
- 19. Jianling L, Bin H, Linlin L. Protective effects of procyanidins on rats brain after cerebral ischemia-reperfusion. Chinese Journal of Biochemical Pharmaceutics, 2011, 32(6):463–466. [Google Scholar]
- 20. Osama MA, Ahmed AE, Abdulrahman MA, Ameen MM, Ayman AN, Ashraf BA, et al. Protective effect of bilberry(Vaccinium myrtillus)against doxorubicin-induced oxidative cardiotoxicity in rats. Med Sci Monit, 2011, 17(4): BR110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Limei X, Shue W, Hongmei J, Dianjuan F, Xiaosi R. Grape seed extract’ s impact on oxidative damage in mice induced by radiation. Journal of Environment and Health, 2011, 28(8):725–726. [Google Scholar]
- 22. Soo-Kyong C, Xian-Hua Z, Jung-Sook S. Suppression of oxidative stress by grape seed supplementation in rats. Nutrition Research and Practice (Nutr Res Pract), 2012, 6(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vijayakumar S, Subramaniam R, Koikaramparambil Robert N, Kaliyamoorthy P. Investigation of in vivo antioxidant property of Abelmoschus esculentus (L) moench. fruit seed and peel powders in streptozotocin-induced diabetic rats. J Ayurveda Integr Med, 2012, 3(4): 188–193. 10.4103/0975-9476.104432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weifen W, Youliang W, Beibei Z, Lejiu Z, Jieying H, Peiyi L, et al. Effect of procyanidins on liver toxicity induced by semicarbazide in male mice. Studies of Trace Elements and Health, 2012, 29(4): 1–3. [Google Scholar]
- 25.Xiao G, Zhu M, Quan-sheng S. Effects of Procyanidins on Free Radical Metabolism and Apoptosis of Myocardial Tissue in Rat after Exercise. Proceedings of the Xi'an 2012 International Conference of Sport Science and Physical Education, 2012: pp394-399.
- 26. Yu D, Zhaofa X, Wei L, Haibo Y, Bin X, Yangang W. Effects of Lycopene and Proanthocyanidins on HepatotoxicityInduced by Mercuric Chloride in Rats. Biol Trace Elem Res, 2012, 146:213–22. 10.1007/s12011-011-9242-3 [DOI] [PubMed] [Google Scholar]
- 27. Xuan Z, Yizhen H. Inhibitory Effects of Grape Seed Proanthocyanidin Extract on Selenite-induced Cataract Formation and Possible Mechanism. J Huazhong Univ Sci Technol, 2012,32(4):613–619. [DOI] [PubMed] [Google Scholar]
- 28. Peng Z, Weizhong F, Bin L, Jiehong Z, Liang P, Huiyan T, et al. The antioxidant effect of grape seed powder in aging rats. Journal of Toxicology, 2012, 26(6): 434–436. [Google Scholar]
- 29. Lijun B, Ying L, Huazhi Z, Ruiping J. Effect of Proanthocyanidin on retina ischemia-reperfusion of ra. Journal of New Chinese Medicine, 2013, 45(6): 169–171. [Google Scholar]
- 30. Yusong D, Shugang L, Qiang N, Rulin M, Shuxia G, et al. The study of the antagonism of proanthocyanidin on arsenic trioxide-induced the oxidative damage of the testis tissue in male mice. Journal of Toxicology, 2013, 27 (6): 423–426. [Google Scholar]
- 31. Hanaa A, Hassan Ahmed M, Isa Wafaa M, El-Kholy Samar E, Nour. Testicular disorders induced by plant growth regulators: cellular protection with proanthocyanidins grape seedsextract. Cytotechnology, 2013, 65: 851–862. 10.1007/s10616-012-9525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yanfei J, Zhaofeng Z, Lei B, Lulu J, Ye D, Xiaoqian D, et al. Effect of Long-Term Intervention with Proanthocyanidin on Oxidative Stress in Type Ⅱ Diabetes. Food Science, 2013, 34(7): 262–265. [Google Scholar]
- 33. Zhiru M, Xia Y, Lina L, Zhonghai H, Guojun J. Experimental study on anti-oxidative effect of proanthocyanidins phospholipid complex in grape seed. Journal of Pharmaceutical Practice, 2013, 31(5): 362–365. [Google Scholar]
- 34. Bakar E, Ulucam E, Cerkezkayabekir A. Protective effects of proanthocyanidin and vitamin E against toxic effects of formaldehyde in kidney tissue. Biotechnic & Histochemistry, 2015, 90(1): 69–78. 10.3109/10520295.2014.954620 [DOI] [PubMed] [Google Scholar]
- 35. Lu G, Ying W, Shengqi R, Zhenquan Y, Agen H, Boran H. Antioxidant Activity of Grape Seed Proanthocyanidin Extract in Aging Mouse Model. Food Science, 2014, 35(23): 253–256. [Google Scholar]
- 36. Hua Z, XiaoQin S, Jian-Min C, HaiTao Z, Xian G, Yi W. Protective effect of epimedium combined with oligomeric proanthocyanidins on exercise-induced renal ischemia-reperfusion injury of rats. Int J Clin Exp Med, 2014, 7(12): 5730–5736. [PMC free article] [PubMed] [Google Scholar]
- 37. Juan X, Shuyi L, Yong S, Qian W, Xiaopeng L, Bijun X, et al. Lactobacillus casei-01 Facilitates the AmeliorativeEffects of Proanthocyanidins Extracted from LotusSeedpod on Learning and Memory Impairment in Scopolamine-Induced Amnesia Mice. PLOS ONE, 2014, 9 (11): e112773 10.1371/journal.pone.0112773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noorah Saleh A, Nadia H. M. The Protective Effect of Grape Seed Extract on Cardiotoxicity Induced by Doxorubicin Drug in Male Rats. Advances in Bioscience and Biotechnology, 2014, 5: 1078–1089. [Google Scholar]
- 39. Tingting R, Chao H, Mingliang C. Dietary Blueberry and Bifidobacteria Attenuate Nonalcoholic Fatty Liver Disease in Rats by Affecting SIRT1-Mediated Signaling Pathway. Oxidative Medicine and Cellular Longevity, 2014: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng W, Shugang L, Yusong D, Qiang N, Shangzhi X, Rulin M, et al. Intervention of Grape Seed Procyanidin Extract (GSPE) onLiver Toxicity of Mice Induced by Arsenic. Journal of Environmental Hygiene, 2014, 4(5): 430–433. [Google Scholar]
- 41. Ying G, Hongwei H, Xinyu L, Min L, Weilin W. The Antioxidation of Proanthocyanidins from in Rhodiola rosea L. Medicinal Plant, 2014, 5(8): 8–11. [Google Scholar]
- 42. Esrafil M, Layasadat K, Maasoumeh Zare M. Grape Seed Proanthocyanidin Extract Improved some of Biochemical Parameters and Antioxidant Disturbances of Red Blood Cells in Diabetic Rats. IJPR, 2015, 14 (1): 329–334. [PMC free article] [PubMed] [Google Scholar]
- 43. Yan Z, Xiuxiang W. Research progress on procyanidins. Pharmacology and Clinics of Chinese Materia Medica, 2011, 27(6): 112–116. [Google Scholar]
- 44. Jinjing W, Yan S, Chunfeng L, Qi L. Study on the anti-oxidative capability of proanthocyanidins from hops. Science and Technology of Food Industry, 2013, 34(8): 118–122, 126. [Google Scholar]
- 45. Wiesneth S, Petereit F, Jürgenliemk G. Salix daphnoides: A Screening for Oligomeric and Polymeric Proanthocyanidins. Molecules, 2015, 20(8): 13764–79. 10.3390/molecules200813764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. BagchiI D, Garg A, Kmhn R L. Oxygen freeradicalscavenging abilities of vitamin C and vitamin E, and a grape seed proanthocyanidins extract in vitro. Res Commun Mol Pathol Pharmacol, 1997, 95(2): 179–189. [PubMed] [Google Scholar]
- 47. Feng G, Kun Z, Xintian S, Xiaogang W, Jingying Z. Study on Anti-Oxidation Function of Extraction from Grape Seed to Human. Chinese Journal of Public Health Engineering, 2010, 9(2): 99–100. [Google Scholar]
- 48. Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology, 2000, 148 (2–3): 187–197. [DOI] [PubMed] [Google Scholar]
- 49. Liming D, Xiaomin H, Wei Z, Rufeng L. Inhibition of Procyanidins on the Apoptosis of Human Umbilical Vein Endothelial Cell through the Regulating Caspase-3 and Bcl-xl and Bax. Chinese Archives of Traditional Chinese Medicine, 2013, 31(3): 582–584. [Google Scholar]
- 50. Yujie J, Lianqiu M, Zhansheng J, Chang L. Influence of procyanidin on Bcl-2 and Bax after cerebral ischemia. Chinese Journal of Clinical Rehabilitation, 2006, 10(23): 65–66. [Google Scholar]
- 51. Schroeter H, Heiss C, Spencer JP, Keen CL, Lupton JR, Schmitz HH. Recommending flavanols and procyanidins for cardiovascular health: current knowledge and future needs. Molecular Aspects Of Medicine, 2010, 31(6): 546–557. 10.1016/j.mam.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 52. Anunciato TP, Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. Journal Of Cosmetic Dermatology, 2012, 11(1): 51–54. 10.1111/j.1473-2165.2011.00600.x [DOI] [PubMed] [Google Scholar]
- 53. Neus Martinez M, Noemi Gonzalez A, Anna A, Montserrat P, Maria Teresa B. Procyanidins and inflammation: Molecular targets and health implications. Official Publication Of The Federation Of American Societies For Experimental Biology, 2005, 19(3): 479–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All data in the present study were extracted from 29 papers (references 14–42) and we display the data in the table as a supporting information to show the data availability.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.