Abstract
Global warming and ozone depletion, and the resulting increase of ultraviolet radiation (UVR), have far-reaching impacts on biota, especially affecting the algae that form the basis of the food webs in aquatic ecosystems. The aim of the present study was to investigate the interactive effects of temperature and UVR by comparing the photosynthetic responses of similar taxa of Chlorella from Antarctic (Chlorella UMACC 237), temperate (Chlorella vulgaris UMACC 248) and tropical (Chlorella vulgaris UMACC 001) environments. The cultures were exposed to three different treatments: photosynthetically active radiation (PAR; 400–700 nm), PAR plus ultraviolet-A (320–400 nm) radiation (PAR + UV-A) and PAR plus UV-A and ultraviolet-B (280–320 nm) radiation (PAR + UV-A + UV-B) for one hour in incubators set at different temperatures. The Antarctic Chlorella was exposed to 4, 14 and 20°C. The temperate Chlorella was exposed to 11, 18 and 25°C while the tropical Chlorella was exposed to 24, 28 and 30°C. A pulse-amplitude modulated (PAM) fluorometer was used to assess the photosynthetic response of microalgae. Parameters such as the photoadaptive index (Ek) and light harvesting efficiency (α) were determined from rapid light curves. The damage (k) and repair (r) rates were calculated from the decrease in ΦPSIIeff over time during exposure response curves where cells were exposed to the various combinations of PAR and UVR, and fitting the data to the Kok model. The results showed that UV-A caused much lower inhibition than UV-B in photosynthesis in all Chlorella isolates. The three isolates of Chlorella from different regions showed different trends in their photosynthesis responses under the combined effects of UVR (PAR + UV-A + UV-B) and temperature. In accordance with the noted strain-specific characteristics, we can conclude that the repair (r) mechanisms at higher temperatures were not sufficient to overcome damage caused by UVR in the Antarctic Chlorella strain, suggesting negative effects of global climate change on microalgae inhabiting (circum-) polar regions. For temperate and tropical strains of Chlorella, damage from UVR was independent of temperature but the repair constant increased with increasing temperature, implying an improved ability of these strains to recover from UVR stress under global warming.
Introduction
The anthropogenic release of chlorofluorocarbons (CFCs) and other active compounds into the atmosphere causes the breakdown of ozone in the stratosphere and this leads to a rise in the flux of ultraviolet-B radiation (UV-B, 280–320 nm) transmitted to the Earth’s surface. This is most marked at (but is not exclusive to) high latitudes [1]. Increased global warming, leading to enhanced cooling in the stratosphere, influences the extent of ozone depletion and the consequent increases in UV-B incident on the surface of the planet [2]. Although this is expected to show a gradual recovery in years to come [3, 4], ozone depletion, exacerbated by global warming [5], is still a significant problem for many organisms.
Photoautotrophic organisms, including algae (which contribute 50% of the planet’s primary productivity [6, 7]), use solar radiation as a principal energy source to drive physiological processes such as photosynthesis and growth. However, high levels of solar ultraviolet radiation (UVR), and especially UV-B, are considered to be a stressor for many physiological processes and will cause damage to DNA [8, 9], inhibit photosynthetic rate [10, 11] and inactivate enzymes [11]. These biologically harmful effects of UVR can negatively affect the diversity and species richness of algal communities [12].
Photosystem II (PSII) is one of the main molecular targets of UV-B-induced photoinhibition in algae [13–15] through the effects of UVB on D1 and D2 proteins in the PSII reaction centre, though UV-B also affects the C-fixing enzyme Rubisco. A number of studies have revealed that the maximum quantum yield (ΦPSIImax, = Fv/Fm) and the electron transport rate (ETR) of microalgae are negatively affected by UV-B radiation [16–20]. These impacts, however, are modulated by interactions between UVR and other factors such as light availability, nutrient limitation and levels of dissolved inorganic carbon, all of which are features of the environment that are likely to alter as a consequence of global change predicted for the next century and beyond. Interactions between UV-B and nutrient levels [21, 22], PAR intensity [23] and CO2 [24] have all been reported recently [25].
Changing atmospheric and surface sea temperatures will potentially enhance water column stratification and thereby have an impact on the nutrient status of phytoplankton, at least in the tropics and mid-latitudes [26], which in turn will exert an influence on UV-B sensitivity (see above). Extensive studies have been carried out on the independent effects of UVR and temperature on the physiology of algae [27–35]. Our study adds to the growing body of work showing interactions between temperature and UVR sensitivity by specifically examining effects of temperature on the damage and repair cycle under UVR [36–41].
The net effect of UVR on photosynthesis (P), and particularly on PSII activity, can be explained by a number of models, the simplest being the Kok model [42] in which the net impact is a balance between damage (k), which is a function of the number of remaining targets, and repair (r) at time t, where Pinitial is the rate of photosynthesis before exposure commences [43] (Eq 1).
| (1) |
Since damage to PSII reaction centres is driven by photochemistry, and photochemical events are largely temperature insensitive, values of k should be relatively insensitive to temperature. In contrast, repair processes such as turnover and re-synthesis of D1 protein are enzymically driven, so the repair constant r should be strongly temperature sensitive within physiological limits.
Algae live in environments with multiple abiotic factors that change simultaneously, act in combination or are inter-dependent. An increase in UVR has been reported over the past 20 years as a result of decreased stratospheric ozone [44–46] while the average global surface temperature will increase by around 4–5°C over the next century because of global warming [47]. It is therefore important to understand possible interactions between temperature and UVR due to global warming and to estimate the possible ecological implications of such environmental changes.
The small coccoid chlorophyte, Chlorella, which is cosmopolitan in occurrence, is one of the best-studied phototrophic eukaryotes. It can be found in a diverse range of habitats such as in soil, freshwater lakes, ponds, marine and brackish waters, and snow as well as in hot springs [48–49]. Studies have reported that Chlorella strains isolated from Antarctic and Arctic are eurythermal, able to grow at temperatures ranging from 4–30°C and 3–27°C, respectively [35, 50]. Chlorella is an ideal experimental organism for investigating various research questions and has potential applications in biotechnology [48]. Due to the wide occurrence and applications of Chlorella, and the availability of strains from various thermal habitats, this alga was chosen for the present study, which seeks to explore further the question of how microalgae, especially Chlorella, can cope with exposure to UVR.
Here we report on studies of the interactive effects of temperature and UVR by comparing the responses of an isolate of Chlorella from the Antarctic to those of temperate and tropical isolates of the same genus. We test the hypothesis that r should exhibit a greater temperature sensitivity than k and that therefore higher temperatures should promote repair more than damage, with the consequence that net UVR damage should be less at higher temperatures. To this end we exposed cultures of 3 strains of Chlorella spp., isolates from polar, temperate and tropical freshwaters, to a range of temperatures and used PAM fluorometry to measure photosynthetic characteristics during exposure to photosynthetically active radiation (PAR, 400–700 nm), PAR plus UV-A (320–400 nm) radiation (PAR + UV-A) and PAR plus UV-A and UV-B (280–320 nm) radiation (PAR + UV-A + UV-B).
Materials and Methods
Ethics statement
Chlorella isolates were obtained from existing holdings in the University of Malaya Algae Culture Collection (UMACC). They are not endangered or protected species. No permits are required to study these algae.
Algae cultures
Three strains of Chlorella, namely Chlorella UMACC 237, Chlorella vulgaris UMACC 248 and C. vulgaris UMACC 001 were used in the present study. The Antarctic Chlorella UMACC 237 was isolated in 2002 from a soil sample collected near a wastewater pond at Casey Station, Antarctica. The temperate Chlorella UMACC 248 was obtained from the Culture Collection of Algae and Protozoa (CCAP) and was originally isolated from a freshwater lake in the Netherlands in 1892, while the tropical Chlorella UMACC 001 was isolated from a fish pond at the University of Malaya in 1987 [32]. Although the Chlorella isolates used in the present study were isolated at different times, each was maintained at or close to its temperature optimum as it is known that long-term cultivation may cause accumulation of mutations and in vitro selection [51]. However, some microalgal strains may show high genetic stability despite long-term cultivation [52]. The cultures were grown in Bold’s basal medium (BBM) [53] and maintained in a controlled-environment incubator at 4, 18 or 28°C for the Antarctic, temperate and tropical Chlorella, respectively, illuminated with cool white fluorescent lamps (Philips, TLD 18W/54-765) providing 42 μmol m-2 s-2 PAR on a 12 h:12 h light:dark cycle. The identification of the Chlorella spp. from the Antarctic and tropics was based on morphological studies (unpublished data).
Experimental design
The cultures were exposed in the incubator at different temperatures for one hour, to three light treatments: PAR + UV-A, PAR + UV-A + UV-B and PAR alone. They were irradiated with a combination of three types of lamps: two tubes of daylight fluorescent lamps (Philips, TLD 18W/54-765, Thailand) providing 42 μmol m-2 s-1 of photosynthetically active radiation (PAR), one UV-B lamp (Philips, TL 20W/12RS, Holland) providing an irradiance of 1.17 W m-2 and UV-A lamps (Philips, TLK 40W/10R, Holland) providing an irradiance of 8.54 W m-2. The emission range for the UV-B lamp is 290 to 320 nm with a peak at 302 nm while the UV-A lamp has a wavelength of between 315 to 380 nm with an emission peak at 350 nm. Details of spectra for these lamps are available at http://www.lighting.philips.com/main/prof/lamps/fluorescent-lamps. The irradiances were measured using a SpectroSense2 4-channel display meter fitted with UV-A, UV-B and PAR Quantum sensors. The UV-B dose applied was higher, and the UV-A lower, than is found in many habitats but were used to provide an acute UV stress to determine how temperature influenced the damage and repair processes [43]. Various cut-off filters were used to obtain the different UVR treatments. For the PAR alone, the cultures were grown in quartz tubes covered with polycarbonate sheet to eliminate UV-A and UV-B radiation. To obtain the PAR + UV-A treatment, Mylar sheet was used to cut off the UV-B radiation. The cultures receiving PAR + UV-A + UV-B were covered with a Whirl-PackR bag to allow the light spectrum above 280 nm to pass through. It has been shown that these bags are biologically inert and transparent to ecologically relevant UVR wavelengths [54]. The Antarctic Chlorella was exposed to 4, 14 and 20°C. The temperate Chlorella was exposed to 11, 18 and 25°C while the tropical Chlorella was exposed to 24, 28 and 30°C. These temperatures were chosen to represent sub-optimal, optimal and, in the case of the Antarctic isolate at 20°C, supra-optimal temperatures for the different strains [34, 35].
An Underwater Fluorometer Diving-PAM (Heinz Walz GmbH, Effeltrich, Germany) was used to measure the photosynthetic properties of the microalgae studied [55]. A rapid light curve (RLC) curve was measured at 0 h (before exposure to UVR) and after 1 h exposure to the appropriate UVR and temperature treatment. RLCs were run with actinic light intensities up to 1560 μmol m-2 s-1, with each actinic light exposure lasting 10 s. A dark adaptation period of 15 minutes before the RLC allowed determination of maximum quantum yield (ΦPSIImax). Photosynthetic parameters such as the photoadaptive index (Ek; a measure of the light intensity for saturation of electron transport), maximum rates of electron transport (rETRmax), and light harvesting efficiency (α) were determined according to [55], and are related through the equation Ek = rETRmax/alpha. Exposure response curves examining the effects of UVR and temperature on effective quantum yield (ΦPSIIeff) were carried out by applying a saturation pulse every 5 minutes for 1 h for cells exposed to PAR + UV-A + UV-B, at different temperatures. The effect of temperature on damage (k) and repair (r) rates was calculated from the decrease in ΦPSIIeff over time by fitting the data to the Kok model (Eq 1), using GraphPad Prism.
Statistical analysis
The means and standard deviation values of the triplicate cultures under each treatment were calculated. One-way analysis of variance (ANOVA) was used to determine whether there was any significant difference (p<0.05) between the treatments used in all the experiments, followed by comparison of means using Duncan’s test. All statistical analyses were performed using SPSS software.
Results
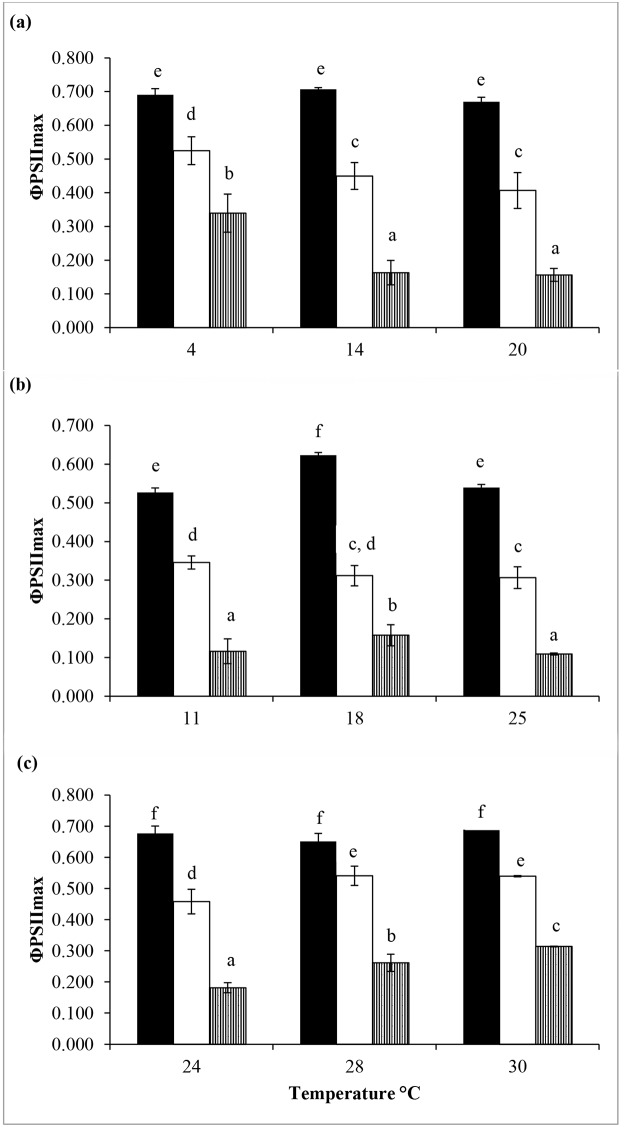
A general trend was observed in the effect of UVR on maximum quantum yield (ΦPSIImax) of the three isolates of Chlorella. A significant reduction in ΦPSIImax was observed when the cultures were exposed to both PAR + UV-A and PAR + UV-A + UV-B compared to PAR alone (P<0.05) (Fig 1), with exposure to PAR+UVA+UVB causing maximum inhibition. However, isolates showed different trends in ΦPSIImax in their responses to increasing temperature. The effect of UVR on ΦPSIImax of both Antarctic and tropical Chlorella was temperature-dependent. The Antarctic Chlorella showed decreasing ΦPSIImax with increasing temperature under UVR exposure while the reverse trend was observed in the tropical Chlorella (Fig 1a). The ΦPSIImax values of the Antarctic Chlorella were 0.339, 0.163 and 0.156 when exposed to PAR + UV-A + UV-B at 4, 14 and 20°C, respectively. In contrast, the corresponding values of the tropical Chlorella were 0.181, 0.262, 0.314 when exposed PAR + UV-A + UV-B at 24, 28 and 30°C, respectively (Fig 1c). However, the reduction of ΦPSIImax in the temperate Chlorella under exposure to UVR was temperature-independent, whereby ΦPSIImax values at 11 and 25°C were significantly lower than that at 18°C (P<0.05) (ΦPSIImax = 0.116, 0.158 and 0.109 for 11, 18 and 25°C, respectively, under PAR + UV-A + UV-B) (Fig 1b).
Fig 1. Effect of temperature and UVR on the maximum quantum yield of fluorescence (ΦPSIImax) of (a) polar, (b) temperate and (c) tropical isolates of Chlorella.
Vertical bars denote standard deviations from triplicate samples. Different letters indicate significant differences at p<0.05. PAR (filled), PAR + UV-A (open), PAR + UV-A + UV-B (hatched).
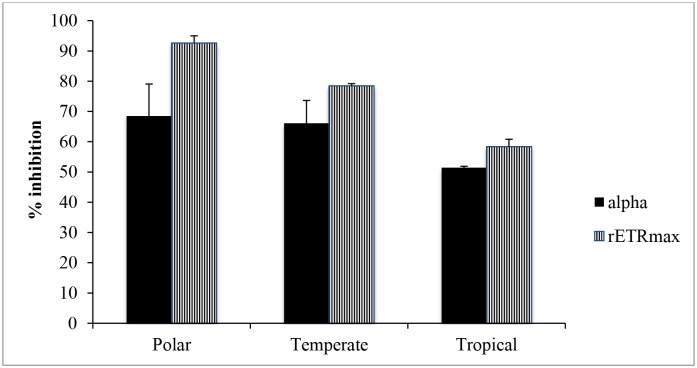
Growth rates of Antarctic and temperate Chlorella have been shown to be optimal between 18 and 20°C, whereas the tropical isolate showed fastest growth at the maximum temperature (30°C) used in this study [35]. At these optimum temperatures, the photosynthetic characteristics, as represented by photosynthetic efficiency (alpha) and maximal electron transport rate (rETRmax), of the tropical strain were less affected by UVR compared to the temperate and Antarctic strains (Fig 2).
Fig 2. Inhibition of light harvesting efficiency (alpha) and maximal electron transport rate (rETRmax) of polar, temperate and tropical Chlorella grown at their optimum temperatures (18, 20 and 30°C, respectively) then exposed to PAR + UV-A + UV-B for 60 min.
Vertical bars denote standard deviations from triplicate samples.
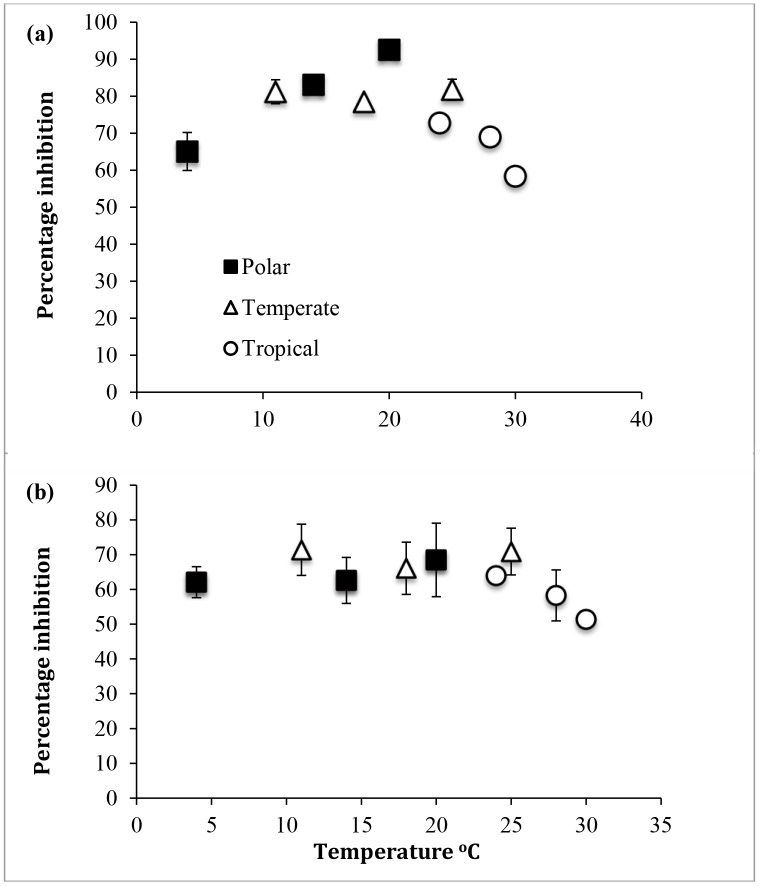
The three isolates of Chlorella grown at different temperatures showed different trends in the inhibition of rETRmax and alpha by UVR (PAR + UV-A + UV-B treatment) (Fig 3). Inhibition by UVR of rETRmax for the Antarctic Chlorella showed a significant increase with increasing temperatures (P<0.05). The reverse trend was found in tropical Chlorella, and the temperate Chlorella showed no effect of temperature on UV-B inhibition of rETRmax (P>0.05). Inhibition of light harvesting efficiency (α) was essentially temperature independent except for the tropical strain at the higher temperatures used, which showed lower inhibition values (P<0.05).
Fig 3. Percentage inhibition, by PAR + UV-A + UV-B, of (a) rETRmax and (b) alpha for all three isolates of Chlorella.
Vertical bars denote standard deviations from triplicate samples.
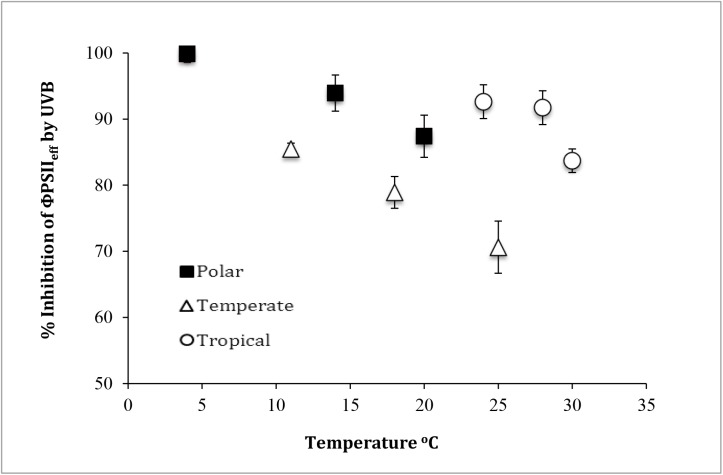
Cultures exposed to PAR + UV-A + UV-B showed a decline in ΦPSIIeff, which fitted the Kok equation (Eq 1) well (data not shown). This allowed calculation of rate constants for damage (k) and repair (r) as well as the overall inhibition of ΦPSIIeff following 60 minutes of exposure to UVB, at which point all cultures had reached an asymptote and equilibrium levels of inhibition. Values for UVR inhibition of ΦPSIIeff after 60 min exposure were temperature dependent (Fig 4) with higher temperatures showing the least inhibition. This effect was most marked in the temperate strain and least marked in the tropical strain of Chlorella.
Fig 4. Inhibition of ΦPSIIeff of polar, temperate and tropical Chlorella after 60 min exposure to PAR + UV-A + UV-B.
Vertical bars denote standard deviations from triplicate samples.
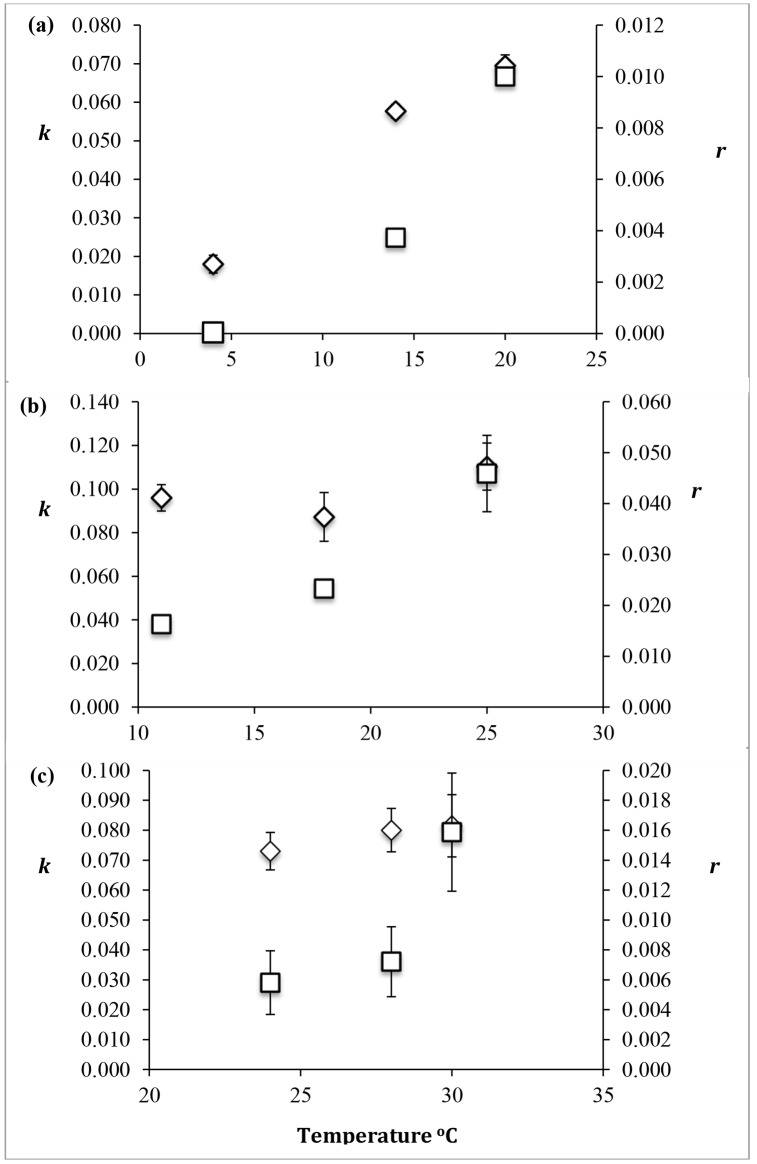
Although the rate constant for damage by UVR (k) was observed to increase with temperature in the Antarctic Chlorella, the repair constant (r) also showed an increase with temperature (Fig 5a). This indicated that the damage caused by UVR was being repaired faster at higher temperatures. For temperate Chlorella, damage from UVR was independent of temperature but the repair constant increased with increasing temperature (Fig 5b). In the tropical Chlorella, damage from UVR was unaffected by increasing temperature but repair strongly increased under higher temperatures (Fig 5c).
Fig 5. Damage (k, diamonds) and repair (r, squares) of (a) polar, (b) temperate and (c) tropical Chlorella strains as a function of temperature.
Units for k and r are min-1. Vertical bars denote standard deviations from triplicate samples.
Discussion
This study demonstrated the possibility of important interactions, between increased temperature associated with global change and the sensitivity of algae to UVR exposure, on the photosynthetic response of microalgae from different geographical regions. In general, there have been limited studies on the interactive effects of temperature and UVR on algae [38, 56, 57]. The interaction between these two factors can lead to variable responses. We hypothesized that UVR repair mechanisms are temperature dependent, while UVR-induced damage is less affected. Thus increasing global temperature might offset the negative effects of UVR due to increased repair or photoprotection at higher temperature.
In the present study, UV-A caused much lower inhibition than UV-B in photosynthesis in all Chlorella isolates, as shown by the value of maximum quantum yield, ΦPSIImax. This is consistent with previous work that reported UV-A does not cause any adverse effect on the growth of Antarctic, tropical and temperate microalgae [31–33]. The low sensitivity of Chlorella isolates to UV-A could be due to the low UV-A radiation (and high UV-B:UV-A ratio) applied in the study. Nevertheless, UV-A has been shown to benefit photosynthesis of Gracilaria lemaneiformis [58] as well as that of phytoplankton species [59].
It is well known that exposure to high UV-B can cause detrimental effects on photosynthesis of algae [10, 11]. However, the three isolates of Chlorella examined here showed different trends in their photosynthesis responses under the combined effects of UVR (PAR + UV-A + UV-B treatment) and temperature. Of the three Chlorella isolates, the Antarctic strain showed a distinct decrease in ΦPSIImax when the culture was exposed to temperatures of 14°C and 20°C compared to 4°C. In addition, the percentage inhibition of rETRmax was highest for the cultures exposed to higher temperatures. This indicated that the UVR exposure resulted in photosynthesis stress, and that this stress increased with increasing temperatures, counter to our initial hypothesis that higher temperature would decrease UVR-sensitivity. This observation is, though, in accordance with the data on two sub-Antarctic brown algae, whereby elevated temperatures from 15 to 20°C exacerbated the detrimental effects of UVR on photochemical parameters such as ΦPSIImax and ETR [41]. However, the findings from this experiment are in contrast to those of van de Poll et al. [57] and Rautenberger & Bischof [38]. Both these studies reported that the photosynthetic efficiency of the Antarctic green algae Ulva clathrata and U. bulbosa, as well as the Arctic cold-temperate red algae Palmaria palmata, Coccotylus truncatus and Phycodrys rubens, was less affected by UVR at warmer temperatures. The increasing value of the damage constant (k) at higher temperature observed in the current study indicates that photosynthesis was badly affected under the combination of UVR exposure and increased temperature. This may be due to chronic inhibition of the photosynthetic apparatus or damage to key enzymes involved in photosynthetic production [60, 61]. The repair constant (r) increased with increasing temperature under UVR exposure and it may be postulated that the repair mechanism was temperature dependent. However, our results showed that the ΦPSIImax and rETRmax were negatively affected (more inhibited) by increasing temperature under UVR exposure. Therefore, it may be concluded that the repair (r) mechanisms at higher temperature are not sufficient to repair the damage caused by the UVR in the Antarctic Chlorella strain examined.
The photosynthesis response of the tropical Chlorella strain showed the reverse trend compared to the Antarctic strain. There was a negative effect of UVR on ΦPSIImax (decreased ΦPSIImax under UVR exposure compared to under PAR alone), however, the ΦPSIImax value increased significantly with increasing temperature. This was again supported by the observation that the lowest percent inhibition of rETRmax and alpha occurred when the temperature was increased to 30°C. Moreover, the repair constant (r) increased with the increasing temperature while the damage (k) was not affected by temperature. This is consistent with the concept that elevated temperatures in tropical environments are conducive to high rates of UV-B tolerance and is in agreement with the finding of Gao et al. [39] who reported that the inhibition of photosynthesis (decrease in effective quantum yield) due to both PAR and UVR decreased in Arthrospira platensis when the temperature was increased from 15 to 30°C. The increasing value of the repair constant (r) at higher temperature is consistent with the faster turnover of the D1 protein that led to recovery, after UVR treatment, of desmid strains from different geographical regions as demonstrated by Stamenković & Hanelt [52]. These authors speculated that the tropical species Cosmarium beatum displayed exceedingly high rates of de novo protein synthesis after UVR treatment under a higher temperature, which was in accordance with the fact that plant and algal species adapted to high-light intensities possess exceedingly high rate of D1 turnover and de novo protein synthesis [52, 62, 63]. In addition, UVR-induced photoinhibition of Photosystem II was less pronounced in sporophytes of the red alga Gelidium pulchellum at 20°C compared to 15°C [56]. These authors speculated that repair and photoprotective mechanisms under UVR were equally stimulated by increasing temperature, results supported in part by direct measurements of r and k in the present study. Hoffman et al. [36] reported that the inhibitory effect of UVR on spores and gametophytes of Alaria marginata as well on zygotes and germlings of Fucus gardneri was less strong at higher temperatures.
The optimum growth temperature for the temperate Chlorella strain is 18°C [35]. Increasing or decreasing the temperature, in combination with UVR exposure, relative to this optimum caused a slight decrease in ΦPSIImax, while the percent inhibition of rETRmax and alpha by UV-B was not significantly different to those at its optimum temperature. As for the other strains, damage was unaffected by temperature but repair was stimulated.
Although the photosynthetic apparatus of the Chlorella strains examined here was severely inhibited by the acute UVR dose applied in this study, the damage constant was less affected by temperature than repair implying that algae would be advantaged, in terms of UV-B sensitivity, by elevated temperatures resulting from global change. This is consistent with observations that higher temperatures decrease sensitivity of some algae to UVR ([64] and references therein)
Tolerance to UVR can be due to various mechanisms in addition to repair processes; these include ROS quenching, increase in antioxidative stress compounds as well as production of photoprotective compounds such as mycosporine amino acids (MAAs) and carotenoids. Although our data suggest that phototrophic organisms living in cold environments may be especially prone to the damaging effects of UVR because of the limited repair capabilities at low temperatures [65] and that higher temperatures may ameliorate the damaging impact of UV-B, effects of temperature on these other processes need to be taken into account. Furthermore, increased surface temperatures in water bodies (oceans, lakes) will, as a consequence of enhanced stratification lead to decreased nutrient availability and higher PAR as well as UV-B fluxes, which also impact on the sensitivity of algae to UV-B [25]. The interplay of these factors is likely to be complex, so a clear picture of how algae will respond will require considerable future study of these interactions.
Acknowledgments
We thank the staff of Casey Station, Antarctica, for their field assistance in sample collection.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors would like to thank the following funding agency in providing research grants for the implementation of the research project: Antarctic Biodiversity Grants (#8123204) from the Ministry of Science, Technology and Innovation (MOSTI), Malaysia, coordinated by the Academy of Sciences Malaysia (ASM): S-MP; Antarctic Flagship Project (FP0712E012), Ministry of Science, Technology & Innovation (MOSTI), Malaysia: C-YW; Knowledge Management (2015: RU009K): S-MP; University of Malaya Research Grant Programme (RP002C-13SUS): P-EL; Ministry of Education, Malaysia, HiCOE research grant (IOES-2014): S-MP, P-EL.
References
- 1. Hegglin MI, Shepherd TG. Large climate-induced changes in ultraviolet index and stratosphere-to-troposphere ozone flux. Nature Geoscience. 2009; 2: 687–691. [Google Scholar]
- 2. Hartmann DL, Wallace JM, Limpasuvan V, Thompson DW, Holton JR. Can ozone depletion and global warming interact to produce rapid climate change? Proceedings of the National Academy of Sciences. 2000; 97: 1412–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, Madronich S. Ozone depletion and climate change: impacts on UV radiation. Photochemical and Photobiological Sciences. 2011; 10: 182–198. doi: 10.1039/c0pp90034f [DOI] [PubMed] [Google Scholar]
- 4. Williamson CE, Zepp RG, Lucas RM, Madronich S, Austin AT, Ballare CL et al. Solar ultraviolet radiation in a changing climate. Nature Climate Change. 2014; 4(June): 434–441. [Google Scholar]
- 5. Watanabe S, Sudo K, Nagashima T, Takemura T, Kawase H, Nozawa T. Future projections of surface UV-B in a changing climate. Journal of Geophysical Research. 2011; 116: 5249–5257. [Google Scholar]
- 6. Woodwell GM, Whittaker RH. Primary production in terrestrial ecosystems. American Zoologist. 1968; 8: 19–30. [Google Scholar]
- 7. Siegenthaler U, Sarmiento JL. Atmosphreric carbon dioxide and the ocean. Nature. 1993; 365: 119–259. [Google Scholar]
- 8. Buma AGJ, de Boer MK, Boelen P. Depth distributions of DNA damage in Antarctic marine phyto- and bacterioplankton exposed to summertime UV radiation. Journal of Phycology. 2001; 37: 200–8. [Google Scholar]
- 9. Häder DP, Sinha RP. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact. Mutation Research: Fundamental and Molecular Mechanisms of Mutagenesis. 2005; 571: 221–33. [DOI] [PubMed] [Google Scholar]
- 10. Holm-Hansen O, Helbling EW, Lubin D. Ultraviolet radiation in Antarctica: inhibition of primary production. Photochemistry and Photobiology. 1993; 58: 567–70. [Google Scholar]
- 11. Villafañe VE, Sundbäck K, Figueroa FL, Helbling EW. Photosynthesis in the aquatic environment as affected by UVR In: Helbling EW, Zagarese HE, editors. UV Effects in Aquatic Organisms and Ecosystems. Cambridge: The Royal Society of Chemistry; 2003. pp. 357–98. [Google Scholar]
- 12. Dobretsov SV, Qian P-Y, Wahl M. Effects of solar ultraviolet radiation on the formation of shallow, early successional biofouling communities in Hong Kong. Marine Ecology Progress Series. 2005; 290: 55–65. [Google Scholar]
- 13. Grzymski J, Orrico C, Schofield OM. Monochromatic ultraviolet light induced damage to Photosystem II efficiency and carbon fixation in the marine diatom Thalassiosira pseudonana (3H). Photosynthesis Research. 2001; 68: 181–192. [DOI] [PubMed] [Google Scholar]
- 14. Häder DP, Porst M, Lebert M. Photoinhibition in common Atlantic macroalgae measured on site in Gran Canaria. Helgoland Marine Research. 2001; 55: 67–76. [Google Scholar]
- 15. White AL, Jahnke LS. Contrasting effects of UV-A and UV-B on photosynthesis and photoprotection of ß-carotene in two Dunaliella spp. Plant and Cell Physiology. 2002; 43: 877–884. [DOI] [PubMed] [Google Scholar]
- 16. Forster RM, Schubert H. Wavelength dependence of photoinhibition in the green algae Chlorella vulgaris . Photosynthetica. 1997; 33: 541–552. [Google Scholar]
- 17. Hermann H, Häder DP, Ghetti F. Inhibition of photosynthesis by solar radiation in Dunaliella salina: relative efficiencies of UVB, UVA & PAR. Plant, Cell and Environment. 1997; 20: 359–365. [Google Scholar]
- 18. Nilawati J, Greenberg BM, Smith REH. Influence of ultraviolet radiation on growth and photosynthesis of two cold ocean diatom. Journal of Phycology. 1997; 33: 215–224. [Google Scholar]
- 19. Liang Y, Beardall J, Heraud P. Effects of nitrogen source and UV radiation on the growth, chlorophyll fluorescence and fatty acid composition of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Journal of Photochemistry and Photobiology B: Biology. 2006a; 82: 161–172. [DOI] [PubMed] [Google Scholar]
- 20. Liang Y, Beardall J, Heraud P. Effects UV radiation on growth, chlorophyll fluorescence and fatty acid composition of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Phycologia. 2006b; 45(6): 605–615. [DOI] [PubMed] [Google Scholar]
- 21. Shelly K, Heraud P, Beardall J. Nitrogen limitation in Dunaliella tertiolecta Butcher (Chlorophyceae) leads to increased susceptibility to damage by ultraviolet-B radiation but also increased repair capacity. Journal of Phycology. 2002; 38: 1–8. [Google Scholar]
- 22. Heraud P, Roberts S, Shelly K, Beardall J. Interactions between UVB exposure and phosphorus nutrition: II. Effects on rates of damage and repair. Journal of Phycology. 2005; 41: 1212–1218. [Google Scholar]
- 23. Shelly K, Heraud P, Beardall J. Interactive effects of PAR and UV-B radiation on PSII electron transport in the marine alga Dunaliella tertiolecta (Chlorophyceae). Journal of Phycology. 2003; 39: 509–512. [Google Scholar]
- 24. Sobrino C, Ward ML, Neale PJ. Acclimation to elevated carbon dioxide and ultraviolet radiation in the diatom Thalassiosira pseudonana: Effects on growth, photosynthesis, and spectral sensitivity of photoinhibition. Limnology and Oceanography. 2008; 53: 494–505. [Google Scholar]
- 25. Beardall J, Sobrino C, Stojković S. Interactions between the impacts of ultraviolet radiation, elevated CO2 and nutrient limitation on marine primary producers. Photochemical & Photobiological Sciences. 2009; 8: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 26. Doney SC. Plankton in a warmer world. Nature. 2006; 444: 695–696. [DOI] [PubMed] [Google Scholar]
- 27. Franklin LA, Forster RM. The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. European Journal of Phycology. 1997; 32: 207–232. [Google Scholar]
- 28. Bischof K, Gómez I, Molis M, Hanelt D, Karsten U, Lüder U et al. Ultraviolet radiation shapes seaweed communities. Reviews in Environmental Science and Biotechnology. 2006; 5 (2): 141–166. [Google Scholar]
- 29. Roleda MY, Wiencke C, Hanelt D, Bischof K. Sensitivity of the early life stages of macroalgae from the Northern Hemisphere to ultraviolet radiation. Photochemistry and Photobiology. 2007; 83: 851–862. [DOI] [PubMed] [Google Scholar]
- 30. Wiencke C, Lüder UH, Roleda MY. Impact of ultraviolet radiation on physiology and development of the brown alga Alaria esculenta from Spitsbergen. Physiologia Plantarum. 2007; 130: 601–612. [Google Scholar]
- 31. Wong CY, Chu WL, Marchant H, Phang SM. Growth response, biochemical composition and fatty acid profiles of four Antarctic microalgae subjected to UV radiation stress. Malaysian Journal of Science. 2004; 23(2): 103–118. [Google Scholar]
- 32. Wong CY, Chu WL, Marchant H, Phang SM. Comparing the response of Antarctic, tropical and temperate microalgae to ultraviolet (UVR) stress. Journal of Applied Phycology. 2007; 19: 689–699. [Google Scholar]
- 33. Wong CY, Teoh ML, Phang SM, Chu WL. Effect of ultraviolet radiation (UVR) on the tropical microalgae Chlorella vulgaris. Malaysian Journal of Science. 2011; 30(1): 3–15. [Google Scholar]
- 34. Teoh ML, Chu WL, Marchant H, Phang SM. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. Journal of Applied Phycology. 2004; 16: 421–430. [Google Scholar]
- 35. Teoh ML, Phang SM, Chu WL. Response of Antarctic, temperate and tropical microalgae to temperature stress. Journal of Applied Phycology. 2013; 25(1): 285–297. [Google Scholar]
- 36. Hoffmann JR, Hansen LJ, Klinger T. Interactions between UV radiation and temperature limit interferences from single-factor experiments. Journal of Phycology. 2003; 39: 268–272. [Google Scholar]
- 37. Doyle SA, Saros JE, Williamson CE. Interactive effects of temperature and nutrient limitation on the response of alpine phytoplankton growth to ultraviolet radiation. Limnology and Oceanography. 2005; 50: 1362–1367. [Google Scholar]
- 38. Rautenberger R, Bischof K. Impact of temperature on UV-susceptibility of two Ulva (Chlorophyta) species from Antarctic and Sub-Antarctic regions. Polar Biology. 2006; 29: 988–996. [Google Scholar]
- 39. Gao KS, Li P, Watanabe T, Helbling EW. Combined effects of ultraviolet radiation and temperature on morphology, photosynthesis, and DNA of Arthrospira (Spirulina) platensis (Cyanophyta). Journal of Phycology. 2008; 44: 777–786. [DOI] [PubMed] [Google Scholar]
- 40. Müller R, Wiencke C, Bischof K. Interactive effects of UV radiation and temperature on microstages of Laminariales (Phaeophyceae) from the Arctic and North Sea. Climate Research. 2008; 37: 203–213. [Google Scholar]
- 41. Cruces E, Huovinen P, Gomez I. Interactive effects of UV radiation and enhanced temperature on photosynthesis, phlorotannin induction and antioxidant activities of two sub-Antarctic brown algae. Marine Biology. 2013; 160(1): 1–13. [Google Scholar]
- 42. Kok B. On the inhibition of photosynthesis by intense light. Biochimica et Biophysica Acta. 1956; 21: 234–44. [DOI] [PubMed] [Google Scholar]
- 43. Heraud P, Beardall J. Changes in chlorophyll fluorescence during exposure of Dunaliella tertiolecta to ultraviolet radiation exposure indicate a dynamic interaction between damage and repair processes. Photosynthesis Research. 2000; 63: 123–134. [DOI] [PubMed] [Google Scholar]
- 44. Shindell DT, Rind D, Lonergan P. Increased polar stratospheric ozone losses and delayed eventual recovery owing to increased greenhouse-gas concentrations. Nature. 1998; 392: 589. [Google Scholar]
- 45. McKenzie RL, Björn LO, Bais AF, Ilyas M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. Photochemical and Photobiological Sciences. 2003; 2: 5–15. [DOI] [PubMed] [Google Scholar]
- 46. Deschamps L, Roff G, Fraser P, Klekociuk A, Grainger S. Ozone and UV September 2004. BMRC Research Letters. 2004; 1: 1–4. [Google Scholar]
- 47. IPCC. Synthesis report In: Pachauri RK, Reisinger A, editors. Climate change: Contribution of working groups I, II and III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland; 2007. pp. 104. [Google Scholar]
- 48. Phang SM, Chu WL. The University of Malaya Algae Culture Collection (UMACC) and potential applications of a unique Chlorella from the collection. Japanese Journal of Phycology. 2004; 52: 221–224. [Google Scholar]
- 49. Sakai N, Sakamoto Y, Kishimoto N, Chihara M, Karube I. Chlorella strains from hot springs tolerant to high temperature and high CO2 . Energy Conversion and Management. 1995; 36:693–696. [Google Scholar]
- 50. Cao K, He M, Yang W, Chen B, Luo W, Zou S et al. The eurythermal adaptivity and temperature tolerance of a newly isolated psychrotolerant Arctic Chlorella sp. Journal of Applied Phycology. 2015. (in press). [Google Scholar]
- 51. Lakeman MB, von Dassow P, Cattolico RA. The strain concept in phytoplankton ecology. Harmful Algae. 2009; 8: 746–758. [Google Scholar]
- 52. Stamenković M, Hanelt D. Sensitivity of photosynthesis to UV radiation in several Cosmarium strains (Zygnematophyceae, Streptophyta) is related to their geographical distribution. Photochemical & Photobiological Sciences. 2014; 13(7): 1066–81. [DOI] [PubMed] [Google Scholar]
- 53. Nichols HW. Growth media—freshwater In Stein J, editor. Handbook of Phycological Methods: Culture methods and growth measurements. Cambridge: Cambridge University Press; 1973. pp. 7–24. [Google Scholar]
- 54. Prezelin BB, Boucher NP and Smith RC. Marine primary production under the influence of the ozone hole: Icecolors ‘90 In: Weiler CS and Penhale PA, editors. Ultraviolet Radiation in Antarctica: Measurements and Biological Effects, Antarctic Research Series 62. Washington, DC: American Geophysical Union; 1994. pp. 159–186. [Google Scholar]
- 55. Ralph PJ, Gademann R. Rapid light curves: a powerful tool to assess photosynthetic activity. Aquatic Botany. 2005; 82(3): 222–237. [Google Scholar]
- 56. Gómez I, Figueroa FL, Sousa-Pinto I, Viñegla B, Pérez-Rodríguez E et al. Effects of UV radiation and temperature on photosynthesis as measured by PAM fluorescence in the red alga Gelidium pulchellum (Turner) Kützing. Botanica Marina. 2001; 44: 9–16. [Google Scholar]
- 57. van de Poll WH, Eggert A, Buma AGJ, Breeman AM. Temperature dependence of UV radiation in Arctic and temperate isolates of three red macrophytes. European Journal of Phycology. 2002; 37: 59–68. [Google Scholar]
- 58. Gao K, Xu J. Effects of solar UV radiation on diurnal photosynthetic performance and growth of Gracillaria lemaneiformis (Rhodophyta). European Journal of Phycology. 2008; 43: C297–C307. [Google Scholar]
- 59. Gao K, Wu Y, Li G, Wu H, Villafañe VE, Helbling EW. Solar UV radiation drives CO2 fixation in marine phytoplankton: a double-edged sword. Plant Physiology. 2007; 144: 54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bischof K, Hanelt D, Wiencke C. Effects of ultraviolet radiation on photosynthesis and related enzyme reactions of marine macroalgae. Planta. 2000; 211: 555–62. [DOI] [PubMed] [Google Scholar]
- 61. Bischof K, Kräbs G, Wiencke C, Hanelt D. Solar ultraviolet radiation affects the activity of ribulose-1,5-bisphosphate carboxylase-oxygenase and the composition of photosynthetic and xanthophyll cycle pigments in the intertidal green alga Ulva lactuca L. Planta. 2002; 215: 502–9. [DOI] [PubMed] [Google Scholar]
- 62. Stamenković M, Hanelt D. Protection strategies of several Cosmarium strains (Zygnematophyceae, Streptophyta) isolated from various geographic regions against excessive photosynthetically active radiation. Photochemical & Photobiological Sciences. 2013; 89: 900–910. [DOI] [PubMed] [Google Scholar]
- 63. Häder DP, Lebert M, Rajeshwar PS, Barbieri ES, Helbling EW. Role of protective and repair mechanisms in the inhibition of photosynthesis in marine macroalgae. Photochemical and Photobiological Sciences. 2002; 1: 809–814. [DOI] [PubMed] [Google Scholar]
- 64. Beardall J, Stojkovic S, Gao K. Interactive effects of nutrient supply and other environmental factors on the sensitivity of marine primary producers to ultraviolet radiation: implications for the impacts of global change. Aquatic Biology. 2014; 22:5–23. [Google Scholar]
- 65. Häder DP, Kumar HD, Smith RC, Worrest RC. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochemistry and Photobiology Science. 2007; 6: 267–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.