Abstract
Objectives
The incidence of ischemic stroke has increased and that of hemorrhagic stroke has decreased in urban China; however, the trends in rural areas are unknown. We aimed to explore the secular trends in incidence and transition of stroke subtypes among rural Chinese.
Methods
This was a population-based stroke surveillance through the Tianjin Brain Study. A total of 14,538 residents in a township of Ji County in Tianjin, China participated in the study since 1985. We investigated the age-standardized stroke incidence (sex-specific, type-specific, and age-specific), the annual proportion of change in the incidence of stroke, and the proportion of intracerebral hemorrhage in the periods 1992–1998, 1999–2005, and 2006–2012, because the neuroimaging technique was available since 1992 in this area.
Results
The age-standardized incidence per 100,000 person-years increased significantly for both intracerebral hemorrhage (37.8 in 1992–1998, 46.5 in 1999–2005, and 76.5 in 2006–2012) and ischemic stroke (83.9 in 1992–1998, 135.3 in 1999–2005, and 238.0 in 2006–2012). The age-standardized incidence of first-ever stroke increased annually by 4.9% for intracerebral hemorrhage and by 7.3% for ischemic stroke. The greatest increase was observed in men aged 45–64 years for both stroke types (P < 0.001). The proportion of intracerebral hemorrhage was stable overall, increased among men aged 45–64 years, and decreased among men aged ≥65 years. The average age of intracerebral hemorrhage in men reduced by 7.5 years from 1992 to 2012.
Conclusion
The age-standardized incidence of main stroke subtypes increased significantly in rural China over the past 21 years; the overall proportion of intracerebral hemorrhage was stable, but the incidence increased significantly among middle-aged men. These findings imply that it is crucial to control stroke risk factors in middle-aged men for stroke prevention in future decades.
Introduction
Stroke is the leading cause of death and disability in both developed and developing countries worldwide [1–3]. The age-standardized incidence of stroke has declined over the last 30 years in developed countries [4–6]. Meanwhile, stroke incidence and the proportion of pathologic subtypes have changed significantly with aging of the worldwide population and the prevalence of stroke risk factors. In developed countries and certain developing countries, there has been an increase in the proportion of ischemic strokes (IS) and a decrease in the proportion of intracerebral hemorrhage (ICH) [7–9].
In China, stroke is the leading cause of death in rural areas but is the third most common cause of death in urban areas [10]. A study from an urban population in Beijing from 1984 to 2004 indicated that the incidence of ICH decreased while that of IS increased annually [11]. Identical trends were observed in another study from 3 cities in China [8]. However, there are currently no reports describing the long-term trends in the incidence of stroke subtypes among rural residents in China. The rural Chinese account for one-tenth of the world’s total population and have poor medical insurance, low education level, and low income. The previous studies have indicated that the incidence of first-ever stroke in rural Chinese increased rapidly in past twenty years [12,13]. However, the transition of stroke subtypes following increased incidence of stroke is unknown in rural China; therefore, the prevention of stroke in rural China is crucial to reduce stroke incidence in China.
The aim of this study was to explore the secular trends and epidemiological transition in incidence of first-ever stroke subtypes from 1992 to 2012 in a large rural population in Tianjin, northern China.
Methods
Study population
The study population was recruited to the Tianjin Brain Study, a population-based study on stroke incidence and mortality in a township of Ji County, Tianjin, China. The details of the study population were described in a previous publication [13–14]. Briefly, This study was conducted from a township of Ji County in Tianjin, China since 1985, which was chosen as the representative sample of rural residents in northern China to participate the national projects of stroke surveillence. The total population was 15,438 persons distributed within 18 administrative villages, and 95% of residents were low-income farmers. This population had a low income and low education level, and few participants were covered by national medical insurance before 2008 [12,15]; the population characteristics remained stable over the study period.
Stroke events and stroke-related deaths were monitored in this population since 1985. In this study, we analyzed the events of first-ever stroke from 1992, the year at which new diagnostic imaging techniques were first available.
The ethics committee of Tianjin Medical University General Hospital (TMUGH) approved the study, and written informed consent was obtained from each resident.
Definition of stroke events
First-ever stroke was defined as the first occurrence of rapidly developing signs of focal neurologic disturbance of presumed vascular etiology lasting for >24 hours [16]. Main pathologic types of stroke included ICH and IS, defined as thrombotic brain infarction, cardioembolic stroke, or lacunar infarct. All patients with documented stroke experienced significant clinical symptoms and signs; silent strokes (diagnosed by imaging only) were excluded. Because of the low use of diagnostic imaging from 1992 to 1998, the neurologist from TMUGH identified stroke subtypes by using imaging information or clinical examination, depending on imaging availability.
Network of stroke surveillance and quality control
Stroke events were reported as follows: local licensed village doctors reported initial stroke events to the community hospital within 24 hours of onset; doctors in the community hospital then visited patients’ homes to confirm stroke events within 72 hours. They reported confirmed stroke events (diagnosis by imaging) to TMUGH monthly, and the neurologist identified suspected cases (non-imaging) by interview as soon as possible.
To ensure accurate recording of stroke events, all participating doctors were annually trained on the predefined study protocol. The Quality Control Group consisted of senior epidemiologists and neurologists from the Department of Neurology, TMUGH. They conducted an omission survey annually by comparing multiple overlapping sources (hospital admissions register, local death register, and by interviewing patients’ relatives).
Cardiovascular risk factors survey
The cardiovascular risk factors were examined in 1991 and 2011. The method of population sampling has been published [14]. Briefly, there were 2,196 (73%) participants in 1991 and 1939 (78%) participants in 2011, which aged 35–74 years without previous history of coronary heart disease or stroke, were involved in the survey. Detailed information included demographical information, and risk factors. The physical examinations included measurement of blood pressure, body height, and weight. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or receiving medications for hypertension. Diabetes was defined as self-reported previous diabetes history. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters, and obesity was defined as BMI ≥ 28 kg/m2. Current smoking was dined as smoking at least 1 cigarette/daily more than 1 year, and alcohol consumption was defined as drinking alcohol at least weekly more than 1 year. Fasting glucose (FG), total cholesterol (TC), triglycerides (TG) in the serum were tested in the central laboratory of the Tianjin Neurological Institute. Because of limited funding, there were 1092 participants aged 35–64 years in 1991 and 1939 individuals aged 35–74 years in 2011.
Statistical analysis
In this study, the incidence of ICH and IS were analyzed separately for 1992–1998, 1999–2005, and 2006–2012; computed tomography (CT) has been widely used since 1999, and magnetic resonance imaging (MRI) became available in 2006 in this area. The age-standardized incidences were calculated directly using the world standard population in 10 age-groups: <35, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and ≥75 years [17]. The age-specific incidence of first-ever stroke was assessed by three group: < 45 years, 45–64 years, and ≥65 years. Trends in age-standardized incidence of stroke were expressed as the annual percentage of change, using the regression model log (rt) = a + bt, where log denotes the natural logarithm and t is the year. The trend b was estimated from ordinary regression [11], and 100b represented the estimated annual percentage of change of incidence. In addition, we performed a chi-square test to analysis the changes in levels of cardiovascular disease risk factors between 1991 and 2011. Analyses included all patients, with or without available imaging, because there were no significant differences in demographic characteristics between these patients (Table 1). Statistical significance was defined as P < 0.05. SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for the analyses [18].
Table 1. The demographic characteristics and proportion of related risk factors between patients diagnosis with and without imaging in the Tianjin Brain Study.
| Characteristics | 1992–1998 | 1999–2005 | 2006–2012 | |||
|---|---|---|---|---|---|---|
| Imaging | Clinical | Imaging | Clinical | Imaging | Clinical | |
| Male, n (%) | 51 (63.0) | 62 (63.9) | 114 (59.7) | 43 (63.2) | 233 (58.5) | 39 (53.4) |
| Age of onset, years, means(SE) | 60.7 (1.1) | 71.8 (1.0)* | 63.0 (0.8) | 71.7 (1.4)* | 63.3 (0.6) | 76.3 (1.1)* |
| Age group, n (%) | ||||||
| <45 | 7 (8.6) | 1 (1.0)* | 12 (6.3) | 1 (1.5)* | 25 (6.3) | 0* |
| 45–64 | 44 (54.3) | 19 (19.6)* | 79 (41.4) | 11 (16.2)* | 208 (52.3) | 11 (15.1)* |
| ≥65 | 30 (37.0) | 77 (79.4)* | 100 (52.4) | 56 (82.4)* | 165 (41.5) | 62 (84.9)* |
| Subtype, n (%) | ||||||
| Intracerebral hemorrhage | 26 (49.1) | 27 (50.9) | 52 (27.2) | 11 (16.2)* | 101 (25.4) | 7 (9.6)* |
| Ischemic stroke | 55 (45.5) | 66 (54.5)* | 138 (72.8) | 52 (83.8)* | 292 (74.6) | 61 (90.4)* |
| Death within 30 days, n (%) | 15 (23.1) | 25 (28.1) | 24 (21.2) | 13 (23.6) | 41 (35.7) | 13 (26.5) |
| Hypertension, n (%) | 67 (82.7) | 74 (76.3) | 176 (92.1) | 54 (79.4)* | 371 (93.2) | 63 (86.3)* |
| Diabetes, n (%) | 2 (2.5) | 1 (1.0) | 15 (7.9) | 2 (2.9) | 39 (9.8) | 8 (11.0) |
| Smoking, n (%) | 39 (48.1) | 43 (44.3) | 88 (46.1) | 33 (48.5) | 185 (46.5) | 26 (35.6) |
| Alcohol consumption, n (%) | 19 (23.5) | 22 (22.3) | 45 (23.6) | 13 (19.1) | 129 (32.4) | 12 (16.4)* |
* indicated P<0.05 in Chi-Square test for trend between the study periods.
Results
Characteristics of first-ever stroke patients
During 304,260 person-years of follow-up, we identified 908 patients with first-ever stroke, 224 (24.7%) patients with ICH, and 664 (73.1%) patients with IS, 7 (0.8%) with SAH, and 13 (1.4%) with USD. The age of first-ever stroke (ICH) in men decreased by 7.5 years (69.2 vs. 61.7; P = 0.003) over 21 years, but this trend was not observed in women. The proportion of diagnoses by imaging (CT/MRI) improved from 46.6% to 85.4% during the study period (P < 0.001) (Table 2).
Table 2. The descriptive characteristics of patients with first-ever stroke by gender and period in Tianjin Brain Study.
| Characteristics | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1992–1998 | 1999–2005 | 2006–2012 | 1992–1998 | 1999–2005 | 2006–2012 | 1992–1998 | 1999–2005 | 2006–2012 | |
| Person-year: | 53644 | 52073 | 52344 | 50162 | 48224 | 47813 | 103806 | 100297 | 100157 |
| Education level: year, mean(SE) | |||||||||
| 1.0(0.2) | 3.1(0.3) | 4.4(0.2)* | 0.9(0.2) | 1.5(0.2) | 2.8(0.1)* | 1.0(0.2) | 2.5(0.2) | 3.8(0.2)* | |
| Age of onset, years, means(SE) | |||||||||
| ICH † | 69.2(1.9) | 62.9(1.9) | 61.7(1.5)* | 60.9(2.2) | 63.6(2.3) | 62.4(1.9) | 66.4(1.5) | 63.2(1.5) | 62.0(1.2) |
| IS † | 67.3(1.2) | 66.4(1.0) | 65.6(0.8) | 65.6(1.8) | 65.0(1.5) | 67.0(1.0) | 66.6(1.0) | 65.9(0.9) | 66.2(0.6) |
| Total | 67.9(1.0) | 65.6(0.9) | 64.6(0.7)* | 64.3(1.5) | 64.6(1.3) | 65.9(0.7) | 66.6(0.8) | 65.2(0.7) | 65.2(0.6) |
| Diagnosis by CT/MRI, n(%) | |||||||||
| ICH † | 17(48.6) | 30(85.7) | 61(91.0)* | 9 (50.0) | 23(76.7) | 45(97.8)* | 26(49.1) | 53(81.5) | 106(93.8)* |
| IS † | 34(45.3) | 84(70.6) | 172(84.7)* | 21(45.7) | 54(76.1) | 120(80.0)* | 55(45.5) | 138(72.6) | 292(82.7)* |
| Total | 51(46.4) | 114(74.0) | 233(86.3)* | 30(46.9) | 77(76.2) | 165(84.2)* | 81(46.6) | 191(74.9) | 398(85.4)* |
*indicated P<0.05 in Chi-Square test between the study periods.
†ICH = intracerebral hemorrhage, IS = ischemic stroke.
Age-standardized incidence and relative risk of first-ever stroke
The age-standardized incidence of ICH and IS increased steadily over the past 21 years in both sexes (Table 3). The relative risk (RR) of first-ever stroke, both ICH and IS, increased with time in both sexes compared to 1992–1998; the RR for ICH in 2006–2012 was 1.9 (95% confidence interval [CI] 1.3–2.9) overall (P = 0.001), 1.7 (95% CI 1.0–2.6) in men (P = 0.030), and 2.7 (95% CI 1.3–5.4) in women (P = 0.004). The corresponding RR for IS was 2.8 (95% CI 2.2–3.6) overall, 2.6 (95% CI 2.0–3.6) in men, and 3.2 (95% CI 2.1–4.8) in women (P < 0.0001,Table 4). The age-specific incidence of ICH and IS increased significantly among people aged 45–64 years, with ICH increasing by 2.9 times overall, 6.8 times in men, and 1.4 times in women, and IS increasing by 2.5 times overall, 2.4 times in men, and 2.7 times in women (P < 0.0001). Nevertheless, the incidence of first-ever stroke among participants aged ≥65 years increased significantly for IS, but appeared unvaried for ICH in all patients (Table 3), increased significantly for ICH in patients with neuroimaging diagnosis (S1 Table).
Table 3. The age-standardized age-specific and sex-specific incidence of intracerebral hemorrhage and ischemic stroke in the Tianjin Brain Study (1/100000 person-year).
| Age group(years) | Intracerebral hemorrhage | Ischemic stroke | ||||
|---|---|---|---|---|---|---|
| 1992–1998 | 1999–2005 | 2006–2012 | 1992–1998 | 1999–2005 | 2006–2012 | |
| Men(SE) | ||||||
| Total | 53.6(34.0, 73.2) | 48.6(29.2, 68.0) | 89.6(63.9, 109.4)* | 107.0(79.4, 632.1) | 173.4(137.7, 209.1) | 280.2(234.9, 325.5)* |
| <45 | 6.2(0, 14.2) | 3.2(0, 9.5) | 22.5(5.8, 39.2)* | 6.2(0, 14.8) | 15.9(2.0, 29.8) | 22.5(5.8, 39.2) |
| 45–64 | 30.9(3.9, 57.9) | 112.2(58.9, 165.5) | 235.1(159.4, 310.8)* | 173.3(109.2, 237.4) | 250.7(171.1, 330.3) | 591.0(471.2, 710.8)* |
| ≥65 | 533.5(336.3, 730.7) | 311.5(163.7, 459.3) | 381.3(218.4, 544.2) | 857.5(608.0, 1107.0) | 1392.7(1081.8, 1703.6) | 1870.3(1512.4, 2228.2)* |
| Women(SE) | ||||||
| Total | 22.9(9.6, 36.2) | 41.0(23.0, 59.0) | 57.9(36.3, 79.5)* | 62.1(40.3, 83.9) | 103.0(74.4, 131.6) | 197.9(158.1, 237.7)* |
| <45 | 0 | 11.3(0, 24.0) | 15.6(0, 30.9)* | 14.6(0, 28.9) | 15.1(0, 29.8) | 23.5(4.7, 42.3) |
| 45–64 | 63.5(26.1, 100.9) | 50.0(15.3, 84.7) | 138.0(81.6, 194.4)* | 103.9(55.9, 151.9) | 150.1(90.1, 210.1) | 384.0(290.1, 477.9)* |
| ≥65 | 127.7(33.2, 222.1) | 297.0(156.1, 437.9) | 286.4(146.3, 426.5) | 437.8(263.0, 612.6) | 751.4(527.6, 975.2) | 1432.2(1120.6, 1743.8)* |
| Overall(SE) | ||||||
| Total | 37.8(26.0, 49.6) | 44.7(31.6, 57.8) | 72.8(56.1, 89.5)* | 83.9(66.3, 101.5) | 135.3(112.6, 158.0) | 238.0(207.8, 268.2)* |
| <45 | 3.4(0, 8.1) | 6.9(0.3, 13.6) | 19.4(7.8, 31.0)* | 10.1(2.1, 18.1) | 15.5(5.3, 25.7) | 22.9(10.4, 35.4) |
| 45–64 | 47.8(24.5, 71.1) | 80.3(48.9, 111.7) | 185.2(138.4, 232.0)* | 137.4(97.8, 177.0) | 199.1(149.5, 248.7) | 484.6(408.9, 560.3)* |
| ≥65 | 326.2(218.4, 434.0) | 301.4(200.3, 402.5) | 333.5(226.3, 440.7) | 643.1(491.8, 794.4) | 1055.0(866.4,1243.6) | 1649.7(1412.7, 1886.7)* |
* P<0.05 in Chi-Square test for trend between the study periods.
Table 4. The related risk (95%CI) of main pathological stroke types incidence by sex and age in Tianjin Brain Study.
| Age Group | Intracerebral hemorrhage | Ischemic Stroke | ||||
|---|---|---|---|---|---|---|
| 1992–1998 | 1999–2005 | 2006–2012 | 1992–1998 | 1999–2005 | 2006–2012 | |
| Men: | ||||||
| <45 | 1.0 | 0.5 (0.1,5.6) | 3.6 (0.7,17.5) | 1.0 | 2.6 (0.1,13.2) | 3.6 (0.7,17.5) |
| 45–64 | 1.0 | 3.6 (1.3,9.8)* | 7.8 (3.1,19.9)* | 1.0 | 1.5 (0.9,2.4) | 3.4 (2.2,5.2)* |
| ≥65 | 1.0 | 0.6 (0.3,1.5) | 0.7 (0.4,1.3) | 1.0 | 1.6 (1.1,2.4)* | 2.2 (1.5,5.2)* |
| Total | 1.0 | 0.9 (0.5,1.5) | 1.7 (1.0,2.6)* | 1.0 | 1.6 (1.2,2.3)* | 2.6 (2.0,3.6)* |
| Women: | ||||||
| <45 | — | 1.0 | 1.4 (0.3,6.2) | 1.0 | 0.7 (0.2,3.0) | 1.6 (0.5,5.7) |
| 45–64 | 1.0 | 0.9 (0.3,2.2) | 2.4 (1.2,5.0)* | 1.0 | 1.4 (0.8,2.7) | 3.7 (2.2,6.3)* |
| ≥65 | 1.0 | 2.3 (0.9,5.6) | 2.2 (0.9,5.5) | 1.0 | 1.7 (1.0,2.8)* | 3.3 (2.1,5.2)* |
| Total | 1.0 | 1.9 (0.9,3.9) | 2.7 (1.3,5.4)* | 1.0 | 1.7 (1.1,2.6)* | 3.2 (2.1,4.8)* |
| overall: | ||||||
| <45 | 1.0 | 2.1 (0.4,11.2) | 5.8 (1.3,26.1)* | 1.0 | 1.5 (0.5,4.3) | 2.3 (0.9,6.0) |
| 45–64 | 1.0 | 1.7 (0.9,3.1) | 3.9 (2.2,6.7)* | 1.0 | 1.4 (1.0,2.1)* | 3.5 (2.5,4.9)* |
| ≥65 | 1.0 | 0.9 (0.6,1.5) | 1.0 (0.6,1.6) | 1.0 | 1.6 (1.2,2.2)* | 2.6 (2.0,3.4)* |
| Total | 1.0 | 1.2 (0.8,1.8) | 1.9 (1.3,2.9)* | 1.0 | 1.6 (1.2,2.1)* | 2.8 (2.2,3.6)* |
* indicated P<0.05 comparing with the study period of 1992–1998.
Proportion of ICH and IS by gender and age
The proportion of ICH among all stroke events remained stable overall: 29.8%, 24.3%, and 22.9% in 1992–1998, 1999–2005, and 2006–2012, respectively. However, the proportion of ICH by age group increased significantly among those aged 45–64 years: 34.0% in 1992–1998, 39.7% in 1999–2005, and 55.6% in 2006–2012 (P = 0.002). The corresponding proportions among men were 14.3%, 48.6%, and 56.9%, respectively (P < 0.0001). In contrast, the proportion of ICH decreased among those aged ≥65 years: 66.0% in 1992–1998, 54.0% in 1999–2005, and 34.3% in 2006–2012 in both sexes. The corresponding proportions among men were 80.0%, 48.6%, and 32.3%, respectively (P < 0.0001). No corresponding changes were found in women (Table 5 and S2 Table).
Table 5. The proportion of main stroke type by sex and age (95% CI).
| Group | 1992–1998 | 1999–2005 | 2006–2012 |
|---|---|---|---|
| Men: | |||
| Total | 31.0(22.6, 39.4) | 22.3(15.8, 28.8) | 23.9(18.8, 29.0) |
| <45 | 50.0 (0, 99) | 16.7 (0, 46.5) | 50.0 (23.8, 76.2) |
| 45–64 | 15.2 (2.9, 27.4) | 29.8 (17.9, 41.7) | 28.2 (20.5, 35.9)* |
| ≥65 | 36.8 (26.0, 47.7) | 18.1 (10.3, 25.9) | 16.5 (10.1, 23.0)* |
| Women: | |||
| Total | 27.7(16.7, 38.7) | 27.5(18.9, 36.1) | 21.1(15.4, 26.8) |
| <45 | 0 | 42.9 (6.2, 79.5) | 36.4 (7.9, 64.8) |
| 45–64 | 36.7 (19.4, 53.9) | 24.2 (9.6, 38.9) | 26.1 (17.0, 35.3) |
| ≥65 | 22.6 (7.9, 37.3) | 27.4 (16.3, 38.5) | 16.0 (8.8, 23.2) |
| Overall: | |||
| Total | 29.8(23.1, 36.5) | 24.3(19.0, 29.6) | 22.9(19.2, 26.6) |
| <45 | 25.0 (0, 55.0) | 30.8 (5.7, 55.9) | 44.0 (24.5, 63.5) |
| 45–64 | 25.4 (14.7, 36.1) | 27.8 (18.5, 37.0) | 27.4 (21.5, 33.3)* |
| ≥65 | 32.7 (23.8, 41.6) | 21.8 (15.3, 28.3) | 16.3 (11.5, 21.1)* |
* indicated P<0.05 in Chi-Square test for trend between the study periods.
Annual changes in incidence of ICH and IS
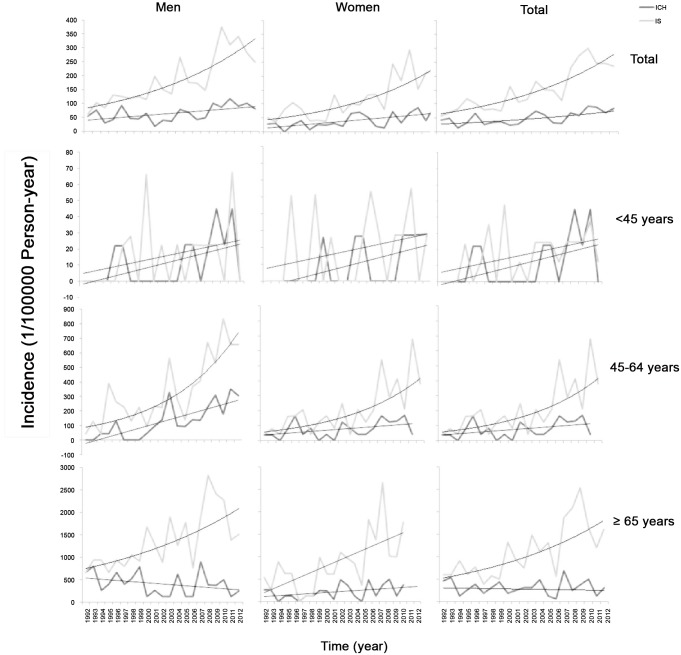
Fig 1 depicts the age-standardized incidence of ICH and IS by gender and age. The age-standardized incidence of ICH increased annually by 4.9% (3.7% in men, 5.1% in women), and IS increased annually by 7.3% (6.8% in men, 8.3% in women). The incidence of ICH among those aged 45–64 years increased by 11.8% annually overall and by 9.8% in men; the incidence of IS in those aged 45–64 years increased annually by 10.5% overall, by 10.7% in men, and by 9.9% in women (P < 0.001). The incidence of first-ever stroke among those aged ≥65 years remained stable for ICH, but increased for IS (6.2% overall, 5.1% in men, 7.4% in women annually).
Fig 1. Age-standardized incidence of intracerebral hemorrhage and ischemic stroke by gender and age groups from 1992 to 2012 (1/100,000 person-years).
The age-standardized incidence increased annually by 4.9% for intracerebral hemorrhage (ICH) and by 7.3% for ischemic stroke (IS). The incidence in those aged 45–64 years increased annually by 11.8% for ICH and by 10.5% for IS (P < 0.001). The incidence of first-ever stroke in those aged ≥65 years remained stable for ICH but increased by 6.2% in IS.
Differences in cardiovascular risk factors between 1991 and 2011
The prevalence of hypertension, diabetes, obesity, and alcohol consumption was significantly higher in 2011 than in 1991, all P<0.05; the prevalence of smoking, in contrast, decreased from 23.6% in 1991 to 19.0% in 2011. The prevalence of obesity significantly increased in all age groups. The prevalence of risk factors was obvious elevated in young group, except for diabetes; but among mid-life, the prevalence of all risk factors significantly increased, current smoking was decreased. The dramatic different trend was found in old group, the prevalence of hypertension and alcohol consumption stained unchanged, but the rate of current smoking significantly decreased (Table 6).
Table 6. The prevalence of cardiovascular risk factors in study population between 1991 and 2011.
| Risk factors | Men | Women | Total | |||
|---|---|---|---|---|---|---|
| 1991 | 2011 | 1991 | 2011 | 1991 | 2011 | |
| n (%) | 1032 | 865 | 1164 | 1074 | 2196 | 1939 |
| Hypertension, %(SE) | 38.7 (1.1) | 50.3 (1.7)* | 41.1 (1.4) | 53.6 (1.5)* | 39.9 (1.1) | 51.7 (1.1)* |
| <45 years | 23.5(2.1) | 31.2 (3.2)* | 23.7(1.8) | 39.9 (3.9)* | 23.6(1.4) | 34.9 (2.4)* |
| 45~64 years | 42.0 (2.3) | 58.9 (2.3)* | 45.4 (2.2) | 57.6 (1.8)* | 43.7 (1.6) | 58.1 (1.4)* |
| ≥65 years | 66.1(3.9) | 68.4 (3.3) | 71.4(3.6) | 74.1 (3.1) | 68.6(2.6) | 71.2 (2.2) |
| Obesity, %(SE) | 2.6 (3.2) | 17.8 (1.3)* | 8.4 (2.8) | 20.9 (2.7)* | 5.7 (2.1) | 19.5 (2.0)* |
| <45 years | 1.7 (0.7) | 16.5 (2.6)* | 7.2 (1.1) | 18.1 (3.1)* | 4.8 (0.7) | 17.2 (1.9)* |
| 45~64 years | 3.1 (2.8) | 18.4 (1.8)* | 9.8 (1.3) | 22.3 (1.6)* | 6.6 (0.8) | 20.8 (1.2)* |
| ≥65 years | 3.5 (1.4) | 18.5 (2.7)* | 6.7 (2.0) | 21.2 (2.9)* | 5.0 (1.2) | 20.2 (2.0)* |
| Diabetes*, % (SE) | 1.4 (3.2) | 3.5 (3.2)* | 3.5 (2.9) | 3.9 (2.5)* | 2.5 (2.2) | 3.7 (2.0)* |
| <45 years | 1.0(0.5) | 0.9(0.7) | 2.7(0.7) | 0.6(0.6) | 2.0(0.05) | 0.8(0.05) |
| 45~64 years | 2.0(0.7) | 4.9(1.0)* | 4.9(1.0) | 5.8(0.9) | 3.5(0.6) | 5.4(0.7)* |
| ≥65 years | 0.6(0.6) | 4.4(1.4)* | 0.6(0.6) | 8.3(1.9)* | 0.6(0.4) | 6.3(1.2)* |
| Smoking*, %(SE) | 46.0 (2.2) | 36.8 (2.7)* | 3.7 (2.9) | 4.8 (3.0)* | 23.6 (1.9) | 19.0 (2.0)* |
| <45 years | 50.9(2.5) | 63.5(3.3)* | 1.3(0.5) | 0.6(0.6) | 23.0(1.4) | 36.3(2.5)* |
| 45~64 years | 45.9(2.3) | 37.6(6.9)* | 5.9(1.1) | 4.5(0.8) | 25.3(1.4) | 17.4(1.1)* |
| ≥65 years | 34.9(3.7) | 7.3(1.8)* | 4.5(1.7) | 8.7(2.0) | 20.3(2.2) | 8.0(1.3)* |
| Alcohol drinking*, %(SE) | 18.9 (2.8) | 31.6 (2.8)* | 0.3 (1.5) | 4.5 (3.0)* | 9.1 (2.0) | 16.6 (2.1)* |
| <45 years | 21.1(2.0) | 47.4(3.4)* | 0.2(0.2) | 0.6(0.6) | 9.3(1.0) | 30.1(2.4)* |
| 45~64 years | 18.0(1.8) | 31.4(2.2)* | 0.4(0.3) | 4.1(0.7)* | 9.0(0.9) | 14.7(1.0)* |
| ≥65 years | 16.0(2.8) | 10.2(2.1) | 0.6(0.6) | 8.7(2.0)* | 8.6(1.6) | 9.5(1.4) |
| FG mmol/L, mean(SD) | 4.8(1.5) | 5.2(1.7)* | 4.6(0.9) | 5.3(1.8)* | 4.7(1.2) | 5.3(1.8)* |
| TC mmol/L, mean(SD) | 4.3(0.9) | 4.6(1.1)* | 4.3(1.0) | 4.8(1.2)* | 4.3(1.0) | 4.7(1.2)* |
| TG mmol/L, mean(SD) | 1.3(0.3) | 1.4 (1.0)* | 1.3(0.3) | 1.6(1.0)* | 1.3(0.3) | 1.5(1.03)* |
* indicated P<0.05 in Chi-Square test for comparing between two study periods.
Discussion
This is the first up-to-date, long-term report of epidemiological transition in the incidence and proportion of main pathologic stroke subtypes among rural residents in China. While the incidence of stroke has decreased in developed countries [7, 19–21], it has rapidly increased in developing countries, especially China [8,11].
The incidence of ICH and IS decreased in Japan from 1991 to 2005 [18]. From 1983 to 1997, the incidence of IS in Finland decreased, but the incidence of ICH remained stable [22]. Similarly, in the Greater Cincinnati/Northern Kentucky Stroke Study, the incidence of IS decreased in the white population, and ICH remained unchanged in the white and black population between 1993 and 2005 [23]. The Oxford Vascular Study reported a decrease of more than 50% in incidence of ICH between 1981 and 2004 [7]. Nonetheless, different trends have emerged in China. Some studies have reported an upward trend in the incidence of IS and a downward trend in the incidence of ICH in urban China [8,11]. However, the incidence of IS increased significantly and that of ICH was unchanged in Hong Kong and Changsha [24,25]. Previous studies reported that patterns of stroke subtypes in Chinese populations were rapidly adopting a Western pattern [8,26,27]. In the last century, the proportion of ICH was higher in Chinese than in white populations [23,28–31].
In this study, we observed upward trends in the incidence of both ICH and IS from 1992 to 2012. The age-standardized incidence increased annually by 4.9% for ICH and by 7.3% for IS. Simultaneously, the prevalence of hypertension, obesity, diabetes, high fasting glucose, and alcohol consumption in this study population increased significantly by 29.6%, 253.6%, 95.8%, 524.1%, and 82.4%, respectively, from 1991 to 2011. These may explain the increase in overall and age-specific incidence of both ICH and IS. Moreover, lower awareness of risk factors and poor medical sources may be the causes of the conversed trends in the incidence of ICH and IS between urban and rural in China.
The proportion of ICH in this study tended to remain stable overall, but increased significantly in participants aged 45–64 years, especially in men. In contrast, the proportion of ICH decreased in those aged ≥65 years.
The previous studies have suggested that hypertension, diabetes, smoking, alcohol, and obesity have been evidenced to be the risk factor for ICH [32–39]. The increased prevalence of hypertension in those aged 35–64 years and the increased prevalence of alcohol consumption in men over the past 21 years may partly explain the greater proportion of ICH in participants aged 45–64 years. The stable prevalence of hypertension and alcohol consumption, and the decreased rate of current smoking in old group may partly explain the decreased proportion of ICH in elders. Moreover, the lower control rate of hypertension in this low-income population (0.7% in 1991, 12% in 2011) may explain the predominance of ICH in this study [14].
Rapid economic development in China may be a potential explanation for the upward trend in prevalence rates of CRFs in our study. Over the past 21 years, there has been an extensive shift in the level of agricultural mechanization, resulting in a significant decrease in the need for rural laborers. Urbanization should also be considered as a rational reason to explain higher prevalence of CVD and its risk factors in rural population [40]. Though China has the largest population in developing countries with an unbalanced development throughout the whole country, the westernization of lifestyles is accelerated not only among urban residents but also among rural population. Documents from the Ministry of Health of the People's Republic of China showed that the energy ratio obtained from cereal decreased 15.3%, but the energy ratio obtained from animals and fat increased by 87.1% and 48.9%, respectively, among rural residents from 1992 to 2002 [41].
An increased incidence of stroke has been reported among those of lower SES in the previous studies [42–44]. A meta-analysis of 17 studies published between 1980 and 2008 demonstrated an increased incidence of stroke in those of lower SES (pooled hazard ratio, 1.67 [1.46–1.91]) [45]. Higher rates of both ischemic and hemorrhagic strokes were found in men and women from lower SES (using area-based deprivation index) in a study conducted in Italy [42]. Consistent with these studies, we found the increased incidence both in ICH and in IS in this low-income, low-education population.
The limitation of this study is, first, the lower proportion of diagnoses verified by CT/MRI (46% during 1992–1998). However, there was no significant difference in percentage of diagnosis by neuroimaging between ICH and IS (P = 0.661), and all stroke events without imaging data were verified by senior neurologists from TMUGH. Second, this study population is from a township in northern China, which does not represent the national population. The prospective study design and the long follow-up period overcome these limitations. At least 100,000 person-years of observations during all 3 study periods fulfill the criteria for a high-quality stroke incidence study [46].
Conclusions
Our study is the first to provide up-to-date secular trends in the incidence of pathological stroke subtypes in a low-income population during a 21-year period in rural China. The incidence of ICH and IS increased, while the proportion of ICH increased in young and middle aged men and decreased in the elderly population. The increased prevalence of alcohol consumption and hypertension, along with a low hypertension control rate, may explain the findings of the present study. Thus, it is crucial to control these risk factors in middle-aged men to prevent stroke in future decades in China.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
We thank all participants of the Tianjin Brain Study and all local medical care professionals for their valuable contribution.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was in part funded by the Ministry of Science and Technology of the People’s Republic of China (contracts 75-62-02-21, 85-915-01-01, 2006BAI01A01), and Tianjin Medical University General Hospital, China.
References
- 1. Carter AM, Catto AJ, Mansfield MW, Bamford JM, Grant PJ. Predictive variables for mortality after acute ischemic stroke. Stroke. 2007;38:1873–1880. [DOI] [PubMed] [Google Scholar]
- 2. Zhang XH, Guan T, Mao J, Liu L. Disparity and its time trends in stroke mortality between urban and rural populations in China 1987 to 2001: changing patterns and their implications for public health policy. Stroke. 2007;38:3139–3144. [DOI] [PubMed] [Google Scholar]
- 3. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. [DOI] [PubMed] [Google Scholar]
- 4. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 5. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. 10.1161/CIRCULATIONAHA.108.191259 [DOI] [PubMed] [Google Scholar]
- 6. Redon J, Olsen MH, Cooper RS, Zurriaga O, Martinez-Beneito MA, Laurent S, et al. Stroke mortality and trends from 1990 to 2006 in 39 countries from Europe and Central Asia: implications for control of high blood pressure. Eur Heart J. 2011; 32:1424–1431. 10.1093/eurheartj/ehr045 [DOI] [PubMed] [Google Scholar]
- 7. Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004;363:1925–1933. [DOI] [PubMed] [Google Scholar]
- 8. Jiang B, Wang WZ, Chen H, Hong Z, Yang QD, Wu SP, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. 2006;37: 63–68. [DOI] [PubMed] [Google Scholar]
- 9. Zhang LF, Yang J, Hong Z, Yuan GG, Zhou BF, Zhao LC, et al. Proportion of different subtypes of stroke in China. Stroke. 2003;34:2091–2096. [DOI] [PubMed] [Google Scholar]
- 10. The Ministry of Health of the People's Republic of China. China health statistics yearbook 2011. 1st ed Beijing: China Union Medical University Press; 2011. [Google Scholar]
- 11. Zhao D, Liu J, Wang W, Zeng Z, Cheng J, Liu J, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39:1668–1674. 10.1161/STROKEAHA.107.502807 [DOI] [PubMed] [Google Scholar]
- 12. Wang J, An Z, Li B, Yang L, Tu J, Gu H, et al. Increasing Stroke Incidence and Prevalence of Risk Factors in a Low-Income Chinese Population. Neurology. 2015; 84:374–381. 10.1212/WNL.0000000000001175 [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Ning X, Yang L, Tu J, Gu H, Zhan C, et al. Sex Differences in Trends of Incidence and Mortality of First-Ever Stroke in Rural Tianjin, China from 1992 to 2012. Stroke. 2014; 45:1626–1631. 10.1161/STROKEAHA.113.003899 [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Ning X, Yang L, Lu H, Tu J, Jin W, et al. Trends of hypertension prevalence, awareness, treatment and control in rural areas of northern China during 1991–2011. J Hum Hypertens. 2014;28:25–31. 10.1038/jhh.2013.44 [DOI] [PubMed] [Google Scholar]
- 15. Wang LD, Kong LZ, Wu F, Bai Y, Burton R. Preventing chronic diseases in China. Lancet. 2005; 366: 1821–1824. [DOI] [PubMed] [Google Scholar]
- 16. Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980; 58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: a new who world standard GPE Discussion Paper Series, No 31. Geneva, EIP/GPE/EBD, WHO; 2001. [Google Scholar]
- 18. The SPSS Inc. SPSS Version 15.0 for Windows. Chicago, IL, USA; 2006. [Google Scholar]
- 19. Kim RB, Kim BG, Kim YM, Seo JW, Lim YS, Kim HS, et al. Trends in the incidence of hospitalized acute myocardial infarction and stroke in Korea, 2006–2010. J Korean Med Sci. 2013;28:16–24. 10.3346/jkms.2013.28.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27:287–295. 10.1007/s10654-012-9673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugama C, Isa K, Okumura K, Iseki K, Kinjo K, Ohya Y. Trends in the incidence of stroke and cardiovascular risk factors on the isolated island of Okinawa: the Miyakojima study. J Stroke Cerebrovasc Dis. 2013;22:e118–123. 10.1016/j.jstrokecerebrovasdis.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 22. Sivenius J, Tuomilehto J, Immonen-Räihä P, Kaarisalo M, Sarti C, Torppa J, et al. Continuous 15-year decrease in incidence and mortality of stroke in Finland: the FINSTROKE study. Stroke. 2004; 35: 420–425. [DOI] [PubMed] [Google Scholar]
- 23. Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. 10.1161/STROKEAHA.109.575043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chau PH, Woo J, Goggins WB, Tse YK, Chan KC, Lo SV, et al. Trends in stroke incidence in Hong Kong differ by stroke subtype. Cerebrovasc Dis. 2011;31:138–146. 10.1159/000321734 [DOI] [PubMed] [Google Scholar]
- 25. Sun XG, Wang YL, Zhang N, Wang T, Liu YH, Jin X, et al. Incidence and trends of stroke and its subtypes in Changsha, China from 2005 to 2011. J Clin Neurosci. 2013;21:436–440. 10.1016/j.jocn.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 26. Kay R, Woo J, Kreel L, Wong HY, Teoh R, Nicholls MG. Stroke subtypes among Chinese living in Hong Kong: the Shatin Stroke Registry. Neurology. 1992;42: 985–987. [DOI] [PubMed] [Google Scholar]
- 27. Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541–1545. [DOI] [PubMed] [Google Scholar]
- 28. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264–272. 10.1212/WNL.0b013e31829bfde3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fuh JL, Wang SJ, Liu HC, Shyu HY. Incidence of stroke on Kinmen, Taiwan. Neuroepidemiology. 2000;19: 258–264. [DOI] [PubMed] [Google Scholar]
- 30. Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. [DOI] [PubMed] [Google Scholar]
- 31. Jeng JS, Su TC. Epidemiological studies of cerebrovascular diseases and carotid atherosclerosis in Taiwan. Acta Neurol Taiwan. 2007;16:190–202. [PubMed] [Google Scholar]
- 32. Zia E, Hedblad B, Pessah-Rasmussen H, Berglund G, Janzon L, Engstrom G. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke. 2007;38:2681–2685. [DOI] [PubMed] [Google Scholar]
- 33. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case—control study. Lancet. 2010;376:112–123. 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- 34. Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–588. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Lifestyle factors on the risks of ischemic and hemorrhagic stroke. Arch Intern Med. 2011; 171(20):1811–1818. 10.1001/archinternmed.2011.443 [DOI] [PubMed] [Google Scholar]
- 36. Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009; 40(6):2068–2072. 10.1161/STROKEAHA.108.540112 [DOI] [PubMed] [Google Scholar]
- 37. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010; 375(9733):2215–2222. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012. August;14(4):300–306. 10.1007/s11883-012-0252-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pezzini A, Grassi M, Paciaroni M, Zini A, Silvestrelli G, Iacoviello L, et al. Obesity and the risk of intracerebral hemorrhage: the multicenter study on cerebral hemorrhage in Italy. Stroke. 2013;44(6):1584–1589. 10.1161/STROKEAHA.111.000069 [DOI] [PubMed] [Google Scholar]
- 40. Zhang L, Qin L, Cui H, Liu A, Wang P. Prevalence of cardiovascular risk factors clustering among suburban residents in Beijing, China. International Journal of Cardiology. 2011;151: 46–49. 10.1016/j.ijcard.2010.04.056 [DOI] [PubMed] [Google Scholar]
- 41. The Ministry of Health of the People's Republic of China. China Health Statistics Yearbook 2011. Beijing: China Union Medical University Press; 2011. [Google Scholar]
- 42. Cesaroni G, Agabiti N, Forastiere F, Perucci CA. Socioeconomic differences in stroke incidence and prognosis under a universal healthcare system. Stroke. 2009; 40:2812–2819. 10.1161/STROKEAHA.108.542944 [DOI] [PubMed] [Google Scholar]
- 43. Li C, Hedblad B, Rosvall M, Buchwald F, Khan FA, Engstrom G. Stroke incidence, recurrence, and case-fatality in relation to socioeconomic position: a population-based study of middle-aged Swedish men and women. Stroke. 2008;39: 2191–2196. 10.1161/STROKEAHA.107.507756 [DOI] [PubMed] [Google Scholar]
- 44. Grimaud O, Bejot Y, Heritage Z, Vallee J, Durier J, Cadot E, et al. Incidence of stroke and socioeconomic neighborhood characteristics: an ecological analysis of Dijon stroke registry. Stroke. 2011;42: 1201–1206. 10.1161/STROKEAHA.110.596429 [DOI] [PubMed] [Google Scholar]
- 45. Kerr GD, Slavin H, Clark D, Coupar F, Langhorne P, Stott DJ. Do vascular risk factors explain the association between socioeconomic status and stroke incidence: a meta-analysis. Cerebrovasc Dis. 2011;31: 57–63. 10.1159/000320855 [DOI] [PubMed] [Google Scholar]
- 46. Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM, Oxford Vascular (OXVASC) Study. Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke. 2004;35:2041–2045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.