This is the first report that demonstrates that Axl promotes STAT1-dependent signaling via inhibition of suppressor of cytokine signaling 1 in immune activation and mechanical activation of smooth muscle cells in vein graft remodeling.
Keywords: Axl, smooth muscle cell, vascular remodeling, inflammation
Abstract
The pathophysiological mechanisms of the immune activation of smooth muscle cells are not well understood. Increased expression of Axl, a receptor tyrosine kinase, was recently found in arteries from patients after coronary bypass grafts. In the present study, we hypothesized that Axl-dependent immune activation of smooth muscle cells regulates vein graft remodeling. We observed a twofold decrease in intimal thickening after vascular and systemic depletion of Axl in vein grafts. Local depletion of Axl had the greatest effect on immune activation, whereas systemic deletion of Axl reduced intima due to an increase in apoptosis in vein grafts. Primary smooth muscle cells isolated from Axl knockout mice had reduced proinflammatory responses by prevention of the STAT1 pathway. The absence of Axl increased suppressor of cytokine signaling (SOCS)1 expression in smooth muscle cells, a major inhibitory protein for STAT1. Ultrasound imaging suggested that vascular depletion of Axl reduced vein graft stiffness. Axl expression determined the STAT1-SOCS1 balance in vein graft intima and progression of the remodeling. The results of this investigation demonstrate that Axl promotes STAT1 signaling via inhibition of SOCS1 in activated smooth muscle cells in vein graft remodeling.
NEW & NOTEWORTHY
This is the first report that demonstrates that Axl promotes STAT1-dependent signaling via inhibition of suppressor of cytokine signaling 1 in immune activation and mechanical activation of smooth muscle cells in vein graft remodeling.
vascular remodeling is a pathophysiological response that can be maladaptive (26). Inflammation, proliferation, and migration of smooth muscle cells (SMCs) govern the remodeling of blood vessels. Several markers of immune activation are upregulated, such as cell surface adhesion molecules (including VCAM-1 and ICAM-1) and major histocompatibility complex class II (MHC II), during the late stage of vascular remodeling (31). This secondary immune activation of SMCs is not well understood in the pathogenesis of intima formation.
Axl belongs to the Tyro 3, Axl, Mertk (TAM) receptor tyrosine kinase family, which has been implicated in cancer and immune and cardiovascular diseases (14). We previously demonstrated that survival of SMCs via activation of phosphoinositide 3-kinase (PI3K) and PKB/Akt is the major cellular mechanism that is regulated by Axl (15, 22). Axl expression was upregulated during later phases of the remodeling of rat carotid arteries (22), as was found for MHC II expression (31). However, our recent findings in Axl chimeras indicated that Axl not only contributes to the vascular wall thickening by preventing apoptosis but also that Axl stimulates immune activation of SMCs (8).
The proinflammatory role of Axl in vascular remodeling is opposite to the documented inhibition of the innate immune response by the TAM receptor family (27). However, the impact of Axl-dependent signaling on the immune response in vascular disorders remains unclear. For example, higher expression of growth arrest-specific (Gas) 6 and Axl was found in the left internal mammary artery in patients after coronary artery bypass grafts (18). This suggests that Axl might be important for allograft remodeling.
The goal of the present study was to investigate mechanisms by which Axl regulates immune activation of SMCs in vein graft vascular remodeling.
MATERIALS AND METHODS
Animals.
Experiments were performed on male and female Axl wild-type (Axl+/+) and Axl knockout (Axl−/−) mice at ages of 6–8 wk old. Mice were housed individually under a 12:12-h light-dark cycle (lights on from 6 AM to 6 PM) with free access to chow and water. The University of Rochester Animal Care Committee approved all procedures on animals that were conducted under guidelines of the National Institutes of Health (NIH) for the use of laboratory animals.
Primary SMCs from Axl mice.
Mouse aortic SMCs (MASMCs) were isolated from Axl+/+ and Axl−/− mice as previously described (8). Harvested cells were maintained in DMEM containing 10% FBS at 37°C in a humidified atmosphere of 5% CO2-95% air. MASMCs at 70–80% confluence from passages 3 to 5 were used in experiments. We stimulated cells with mouse interferon (IFN)-γ (1,000 U/ml). Control MASMCs were treated with PBS for 30 min and 24 h.
Proliferation and viability assays in Axl cells.
Proliferation of MASMCs was assessed by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, cells were seeded in 96-well plates at 10,000 cells/well and incubated at 37°C for 24 h. MASMCs were then treated with PBS or IFN-γ for 24 h. MTT reagent was added directly into the well, and, after 3 h, the reaction was stopped by the addition of the solubilizing agent. Changes in the optical density between the groups were measured at 570 nm using a microtitter plate reader (Wallace). MASMC viability was determined by measuring cleaved caspase-3 expression after treatment with PBS or IFN-γ for 24 h. Rat aortic SMCs (RASMCs) were treated with H2O2 (0.6 μM) for 16 h and used as positive controls.
Gene expression profiles in SMCs.
Gene expression profiles in MASMCs were conducted as we have previously reported in carotid arteries from Axl mice (8). Triplicates of four groups of MASMCs were studied: 1) Axl+/+ MASMCs with PBS, 2) Axl−/− MASMCs with PBS, 3) Axl+/+ MASMCs with IFN-γ; and 4) Axl−/− MASMCs with IFN-γ for 24 h. Briefly, mRNA was isolated from Axl MASMCs using a Qiagen RNAeasy Micro kit. RNA integrity was examined by an Agilent 2100 Bioanalyser using a RNA6000 NanoAssay (Agilent Technology). Quantitative RT-PCR analyses were performed using an ABI Prism 7900HT sequence detection system. Amplified cDNAs were assayed for inflammatory cytokines, chemokines, and their receptors using a PCR array (PAMM-011, Qiagen, Valencia, CA). Three housekeeping genes were chosen for normalization: β-glucuronidase, hypoxanthine guanine phosphoribosyltransferase 1, and β-actin. Data analyses were done using a web-based suite at SABiosciences (Qiagen). We used the curated InnateDB resource for pathway analyses as we have previously described (1, 8).
Protein expression in SMCs.
Treated cells were washed with ice-cold PBS and lysed by the addition of 1× lysis buffer (Cell Signaling) containing 0.1% protease and phosphatase inhibitor cocktail for 5 min on ice. Cells were scraped, and lysates were homogenized. In a separate experiment, supernatant was collected after centrifugation of the cell lysate at 10,000 rpm at 4°C for 20 min. We used the BCA protein assay kit (Thermo Scientific) to measure protein concentrations. Concentrations of primary antibodies were used as follows: MHC II (1:500, eBioscience), cleaved caspase-3 (1:1,000, Cell Signaling), STAT1 (1:1,000, Cell Signaling), phosphorylated (p-)STAT1 (1:1,000, Cell Signaling), suppressor of cytokine signaling (SOCS)1 (1:500, Santa Cruz Biotechnology), β-actin (1:5,000, Cell Signaling), and α-tubulin (1:5,000, Sigma-Aldrich). Equal amounts of proteins were separated on 10% SDS polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Bio-Rad). Immunoblots were probed with phosphorylated antibody and incubated with horseradish peroxidase-conjugated secondary antibody. Protein expressions were detected with an ECL kit (Thermo Scientific). Individual membranes were stripped with stripping buffer (Thermo Scientific), reblocked with 5% skim milk in Tris-buffered saline with Tween 20, and reprobed with total STAT1 antibody. Quantifications of protein levels were assessed through densitometry analyses with ImageJ software (NIH) and expressed as the ratio of the target proteins to the loading control.
Vein graft surgery.
Vein graft transplantation was done as previously reported with minor modifications (37). Briefly, a 1-cm segment of the inferior vena cava was harvested from a donor male mouse and anastomosed to a female recipient's carotid artery under isoflurane anesthesia. We performed reciprocal vein graft transplantations between Axl genotypes to compare 1) Axl wild types (Axl+/+ → Axl+/+) to 2) vascular (Axl−/− → Axl+/+), 3) systemic (Axl+/+ → Axl−/−), and 4) global (Axl−/− → Axl−/−) depletion of Axl in vein graft remodeling. The isolated vena cava segment was excised and washed with lactated Ringer solution containing 50 U/ml heparin, which was stored in the heparinized solution at 25°C until transplantation. A segment of the right common carotid artery (∼15 mm) was isolated in the graft recipient mouse. This segment was then gently occluded proximally and distally with 6-0 silk sutures. End-to-side anastomosis between the vena cava and carotid was performed with 9-0 nylon (Ethicon). We used two fixed sutures at the proximal and distal corners of each arteriotomy. The 6-0 silk ligatures were then removed from the proximal and distal common carotids, and patency of the transplanted vein graft was confirmed by observing blood flow through the wall of the turgid graft. The incision was then closed in two layers with running 6-0 silk sutures. Animals received one-two doses of analgesics [flunixin meglumine (120 mg/kg ip)], housed individually, and allowed to recover for 4 wk after the surgery.
Ultrasound measurements of vascular grafts.
We used a high-resolution ultrasound system (Vevo2100, Visual Sonics) to evaluate successful engraftment of the veins (17). Initial assessments of the blood flow through the graft were done at 1 wk and confirmed at 3 wk postsurgery. All imaging was performance with a 128-element transducer operating at 40 MHz. We analyzed blood flow rates, graft thickness, and graft stiffness using the VevoVasc package. Graft stiffness was determined as follows: resistive index = peak systolic velocity − end-diastolic velocity/peak systolic velocity and pulsatility index = peak systolic velocity − end-diastolic velocity/velocity time integral of the mean velocity.
Morphometry of vascular grafts.
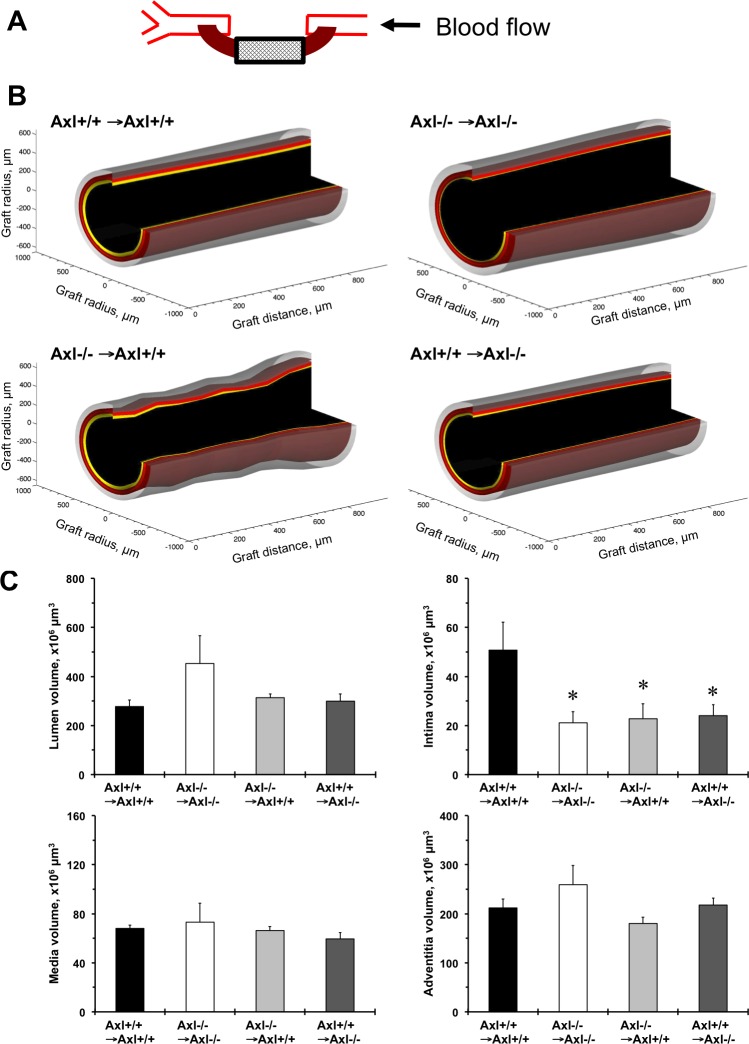
Mice were anesthetized with an intraperitoneal injection of ketamine (130 mg/kg) and xylazine (8.8 mg/kg) and perfusion fixed with 10% paraformaldehyde in sodium phosphate buffer (pH 7.0). We performed tissue processing, staining with hematoxylin and eosin (DAKO), and morphometry analyses (MCID image software) of the four sections every 200 μm from the proximal part of the graft (closer to carotid bifurcation) were performed as previously described (8). We developed custom software using the MATLAB (Matworks) programming environment to generate three-dimensional volume-rendered images from the averaged compartment area over the long axis of the grafts. Vein graft compartments were color coded: black = lumen, yellow = intima, red = media, and transparent = adventitia. We manually removed a quarter of the area to create an open-ended solid surface of each of the vessel layers.
Immunohistochemistry of vascular grafts.
Graft cross-sections were stained with MHC II (1:1,000, eBioscience), pSTAT1 (1:1,000, Cell Signaling), and SOCS1 (1:1,000, Santa Cruz Biotechnology) antibodies and hematoxylin (DAKO) counterstain. Endogenous peroxidase activity was inhibited by incubation with 3% H2O2. Antigen retrieval with Decloaker buffer (pH 6.0, Biocare) and high temperature was done in all protocols. Primary antibodies were subsequently incubated at room temperature for 60 min, which was then followed by application of the Rabbit-on-Rodent HRP kit (Biocare). We performed double staining of CD45.2 (1:100, eBiosciences) and smooth muscle α1-actin (1:1,000, DAKO) antibodies with methyl green counterstain as previously described with modifications (8, 30). Specifically, vein graft sections underwent antigen retrieval (as described above) with subsequent incubation of primary antibodies at 37°C for 60 min (CD45.2) and for 40 min (smooth muscle α1-actin) followed by incubation with Mouse-on-Mouse HRP (CD45.2) and Mouse-on-Mouse AP (smooth muscle α1-actin) kits for 20 min each. Peroxidase-binding sites (MHC II, pSTAT1, SOCS1, and CD45.2) were verified with 3,3′-diaminobenzidine (DAKO). Alkaline phosphatase-binding sites (smooth muscle α1-actin) were recognized by fast red (Vulcan Red, Biocare). We used an Apoptosis Detection Kit (Chemicon) for the detection of apoptotic cells in vein grafts as previously described (15). Images of stained graft sections (3 mice/group) were taken by a SPOT INSIGHT FireWire camera (Diagnostic Instruments) at ×60 magnification. We similarly adjusted sizes and contrasts of the images using Adobe Photoshop CS3 (version 10.0.1). We analyzed positively stained vein grafts in three mice (2–3 sections/mouse) using ImagePro software as previously described (8).
Statistical analyses.
Data are reported as means ± SE. We used JMP Pro9 (SAS) for statistical tests. Differences between two groups were determined by Student's t-test. Differences between more than two groups were determined by one-way ANOVA with post hoc comparisons of all means by Student's t-test. Immunohistochemical staining was analyzed using nonparametric Wilcoxon/Kruskal-Wallis tests, which were followed by each pair comparisons using a Wilcoxon method. P values of <0.05 were considered as significant.
RESULTS
Axl is important for vein graft remodeling.
Our previous data in Axl chimeric mice suggested a central role for Axl in nonbone marrow cells in carotid remodeling in response to low blood flow (8). Therefore, we performed vein graft transplantations between Axl genotypes to investigate global, systemic, and vascular depletion of Axl in vein graft remodeling. Mechanical stretch is an important stimulus in addition to immune activation in vein grafts (4). We and others have shown that Axl is important in shear stress-mediated vascular remodeling (5, 15). Therefore, we assessed vein graft transplantations between Axl genotypes by ultrasound imaging (Table 1). We found that Axl−/− → Axl−/− mice had higher flow velocity profiles compared with the other groups by 3 wk after the graft procedures. Only mice with donor Axl−/− veins (Axl−/− → Axl+/+) had reduced vein graft pulsatility and resistive indexes (Table 1). Depletion of Axl resulted in a reduction of vein graft thicknesses compared with Axl+/+ → Axl+/+ mice (data not shown). Our data suggest that expression of Axl in veins is required for vein graft stiffness.
Table 1.
Time course of blood flow profiles in vein grafts across experimental groups
| Group | Velocity Time Integral, mm | Mean Velocity, mm/s | Mean Gradient, mmHg | Resistive Index | Pulsatility Index |
|---|---|---|---|---|---|
| Axl+/+ → Axl+/+ | 49 ± 12* | 123 ± 26* | 0.07 ± 0.03* | 0.83 ± 0.02 | 1.52 ± 0.04 |
| Axl−/− → Axl+/+ | 46 ± 8* | 112 ± 17* | 0.06 ± 0.02* | 0.77 ± 0.02†‡ | 1.36 ± 0.04†‡ |
| Axl+/+ → Axl−/− | 52 ± 6* | 146 ± 18* | 0.10 ± 0.02* | 0.84 ± 0.03 | 1.53 ± 0.04 |
| Axl−/− → Axl−/− | 105 ± 19 | 229 ± 38 | 0.26 ± 0.08 | 0.78 ± 0.02 | 1.48 ± 0.05 |
Values are means ± SE; n = 4 mice/group.
P < 0.05 compared with the Axl−/− → Axl−/− group;
P < 0.05 compared with the Axl+/+ → Axl+/+ group;
P < 0.05 compared with the Axl+/+ → Axl−/− group (by ANOVA).
Histological evaluation revealed a reduction in vein graft thickening among the groups (Fig. 1). Serial cross-sections over 800 μm from the proximal anastomosis of the vein grafts showed consistent graft remodeling (Fig. 1A). There was a significant variation in vein graft remodeling across experimental groups based on the three-dimensional reconstruction of histological data (Fig. 1B). The largest and uniform intimal compartment (yellow color) was found in Axl+/+ → Axl+/+ mice (Fig. 1B). We calculated graft compartmental volumes based on measurements of a series of cross-sections (Fig. 1C). The intima volume significantly reduced after global, vascular, and systemic deletion of Axl compared with Axl+/+ → Axl+/+ mice (Fig. 1C). Taken together, these data show that Axl is necessary for vein graft remodeling via intima formation by vascular and nonvascular cells.
Fig. 1.
Axl contributes to vein graft thickening in mice. A: schematic representation of the vein-to-carotid grafting. Only the boxed area was used for morphometric analyses of a series of cross-sections marked with the cross-hatched pattern. Arrow shows the direction of blood flow. B: three-dimensional reconstruction of vein graft vessel compartments based on a series of histological sectioning over 800-μm length. Graft compartments are color coded: black = lumen area, yellow = intima area, red = media area, and transparent = adventitia area. C: graft vessel component volumes (lumen, intima, media, and adventitia). Solid bars, Axl+/+ → Axl+/+ mice; open bars, Axl−/− → Axl−/− mice; light shaded bars, Axl−/− → Axl+/+ mice; dark gray bars, Axl+/+ → Axl−/− mice. Values are means ± SE; n = 5–7 mice/group. *P < 0.05 compared with Axl+/+ → Axl+/+ mice.
Role of the donor Axl genotype in vein graft cells.
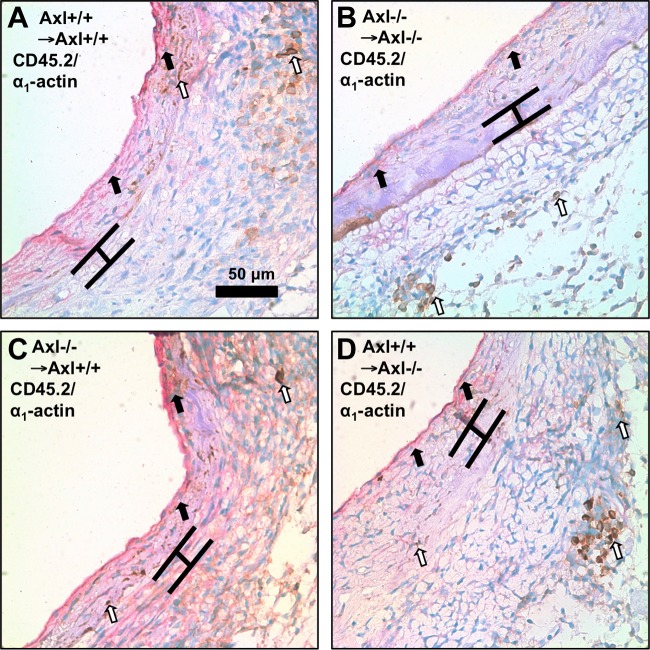
We previously reported that Axl−/− mice had reduced intimal-medial thickening in response to low blood flow via an increase in apoptosis and alteration in inflammatory responses (8, 15). We found that the majority of cells in intima and media vein graft compartments were positive for smooth muscle α1-actin (pink color) in Axl vein grafts (Fig. 2). No differences were noticed between Axl genotypes in smooth muscle α1-actin staining in donor veins or aortas (data not shown). However, we observed more CD45.2-positive (brown color) cells in adventitia of double-stained grafts from Axl+/+ → Axl−/− mice (Fig. 2D). These results suggest that SMCs are the primary cell type involved in vein graft remodeling. Systemic deletion of Axl in nonvascular cells (Axl+/+ → Axl−/−) significantly increased graft apoptosis and lead to a reduction of intimal thickening.
Fig. 2.
Immunohistochemical evaluation of leukocytes and smooth muscle cells (SMCs) in grafts from Axl mice. Representative CD45.2/smooth muscle α1-actin double staining of vein grafts is shown. A: Axl+/+ → Axl+/+; B: Axl−/− → Axl−/−; C: Axl−/− → Axl+/+; D, Axl+/+ → Axl−/−. Smooth muscle α1-actin-positive cells are pink (solid arrows). CD45.2-positive cells are dark brown (open arrows). Brackets show the area in between internal and external elastic lamina. Scale bar = 50 μm.
Axl is critical for immune activation of SMCs.
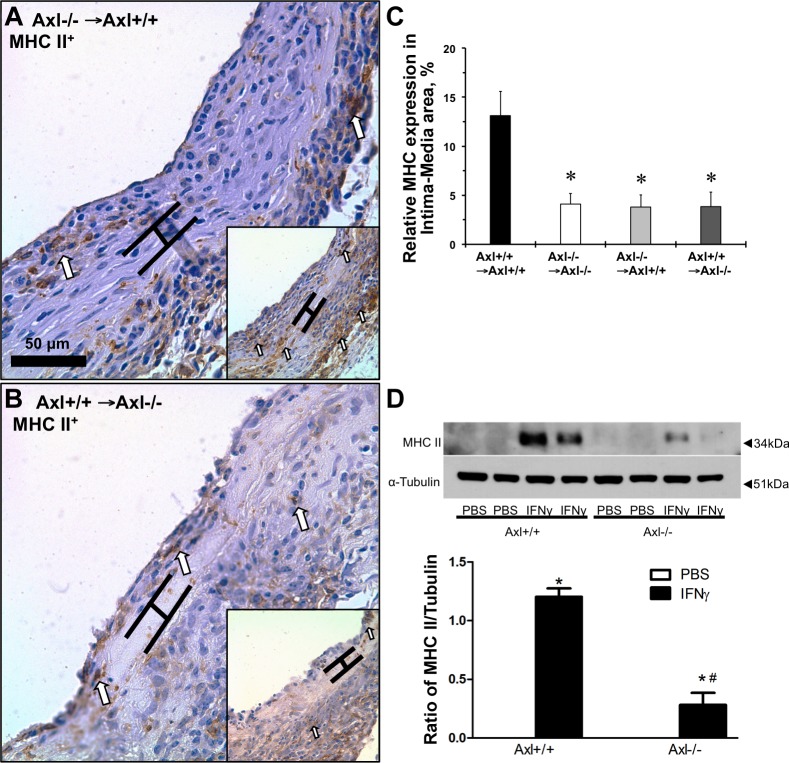
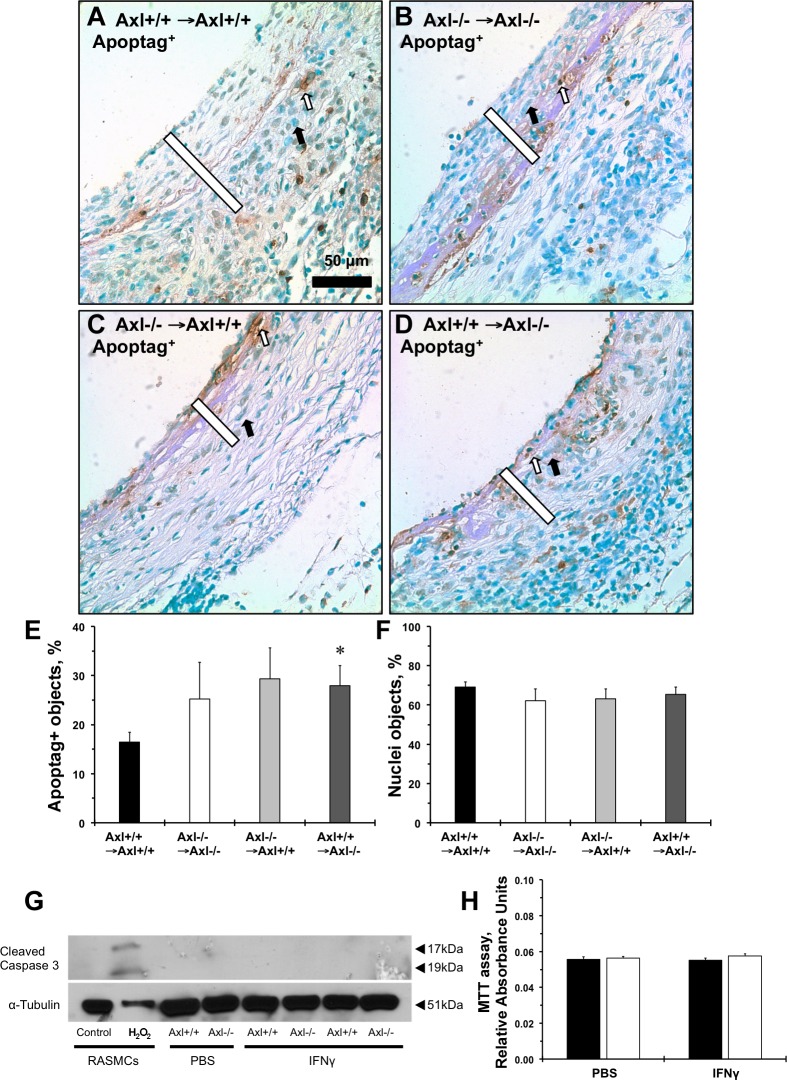
We previously showed that deletion of Axl in cultured MASMCs reduced immune activation (as measured by the MHC II gene, H2-AB1) after IFN-γ stimulation (8). Immunoreactivity to anti-MHC II antibody in the intima-media compartment was significantly reduced in grafts with vascular, systemic, and global depletion of Axl (Fig. 3, A–C). Similar to in vivo findings in vein grafts, lack of Axl significantly reduced MHC II protein expression after stimulation of the cells with IFN-γ (Fig. 3D). Apoptotic cells were located mostly within the medial part of systemic Axl−/− grafts (Fig. 4B); vascular Axl deletion resulted in more staining in the intima (Fig. 4C), whereas Axl+/+ → Axl−/− grafts exhibited positive cells in the intima and media of the vein grafts (Fig. 4D). Quantitative analyses of apoptotic cells in the intima and media showed a slight increase in apoptosis in Axl−/− → Axl−/− versus Axl+/+ → Axl+/+ mice (Fig. 4E). There was a significant increase in apoptotic cells in Axl+/+ → Axl−/− mice compared with Axl+/+ → Axl+/+ mice (Fig. 4E). Relative numbers of nonpositive nuclei were similar across the groups (Fig. 4F). In cultured SMCs from Axl+/+ or Axl−/− mice, application of IFN-γ had no effect on apoptosis, as shown by the absence of cleaved caspase-3 expression across the groups (Fig. 4G). We also observed no change in proliferation of MASMCs under the studied conditions (Fig. 4H). Our findings support the importance of Axl in immune activation of SMCs in response to IFN-γ. This is independent of Axl effects on SMC survival or proliferation.
Fig. 3.
Deletion of Axl reduces immune activation of SMCs in vivo and in vitro. Representative photomicrographs of major histocompatibility complex class II (MHC II) staining of vein graft cross-sections from mice. A: Axl−/− → Axl+/+; B: Axl+/+ → Axl−/−. Controls are shown in insets [Axl+/+ → Axl+/+ (A) and Axl−/− → Axl−/− (B)]. Open arrows point to positive staining (dark brown). Brackets show the area in between internal and external elastic lamina. Scale bar = 50 μm. Values are means ± SE; n = 4 mice/group. *P < 0.05 vs. the Axl+/+ → Axl+/+ group. C: relative expression of MHC II in the intima-media area of vein grafts (in %). D: MHC II expression after interferon (IFN)-γ stimulation for 24 h in mouse aortic SMCs (MASMCs). Representative Western blots are shown on the top; quantification of MHC II expression is shown on the bottom. n = 3 mice/group. *P < 0.01 vs. PBS; #P < 0.01 vs. IFN-γ in Axl+/+ mice.
Fig. 4.
Role of Axl on vein graft apoptosis and after immune stimulation of cultured SMCs. Representative Apoptag staining of grafts from Axl+/+ → Axl+/+ mice (A), Axl−/− → Axl−/− mice (B) Axl−/− → Axl+/+ mice (C), and Axl+/+ → Axl−/− (D) is shown. Apoptotic cells are stained in dark brown (open arrows). Counterstained cells are green (solid arrows). Open bars show the intima + media graft areas. Scale bar = 50 μm. E: quantification of Apoptag-positive cells in vein grafts (in %). F: relative number of counterstained nuclei in vein grafts (in %). Values are means ± SE; n = 3–4 mice/group. *P < 0.05 compared with the Axl+/+ → Axl+/+ group. G: representative immunoblots of cleaved caspase-3 after IFN-γ stimulation for 24 h in MASMCs. Rat aortic SMCs (RASMCs) were treated with H2O2 and used as a positive control. α-Tubulin was used as loading control. H: MTT assay of SMCs treated with PBS or IFN-γ for 24 h. Solid bars are Axl+/+ MASMCs; open bars are Axl−/− MASMCs. Values are means ± SE; n = 3 mice/group.
Axl regulates the STAT1 pathway via inhibition of SOCS1 in SMCs.
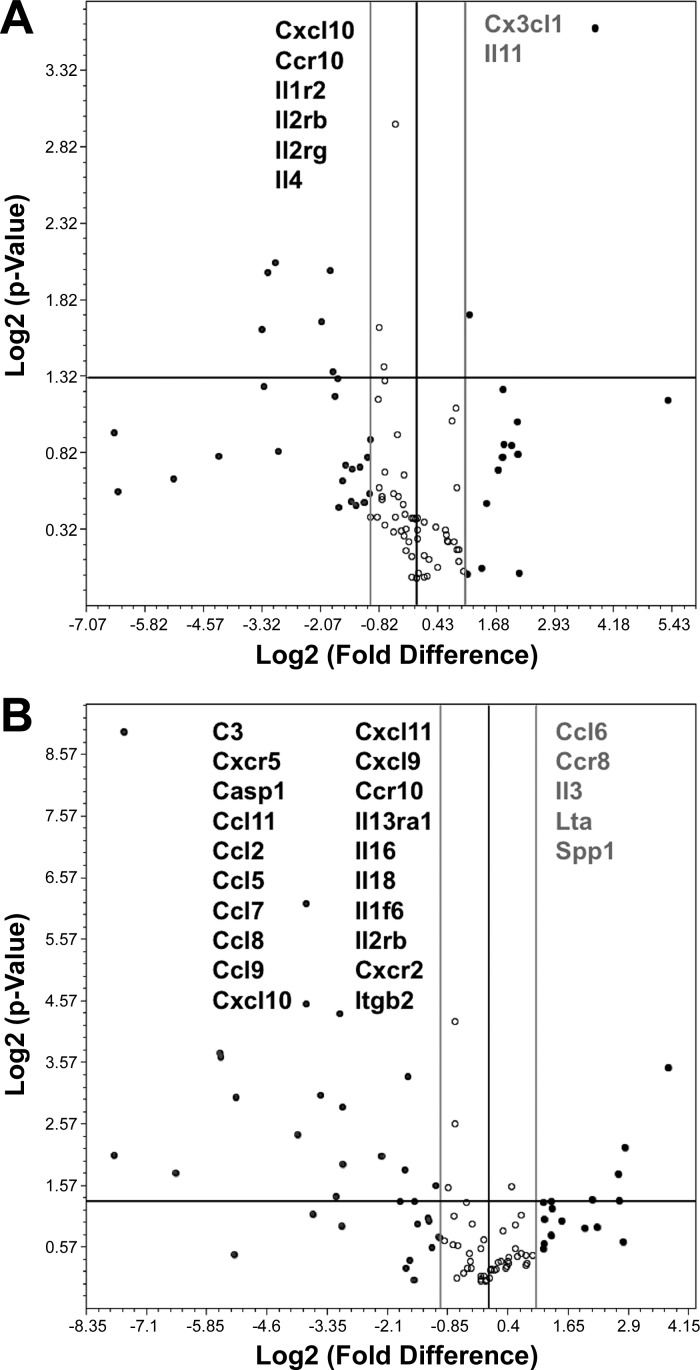
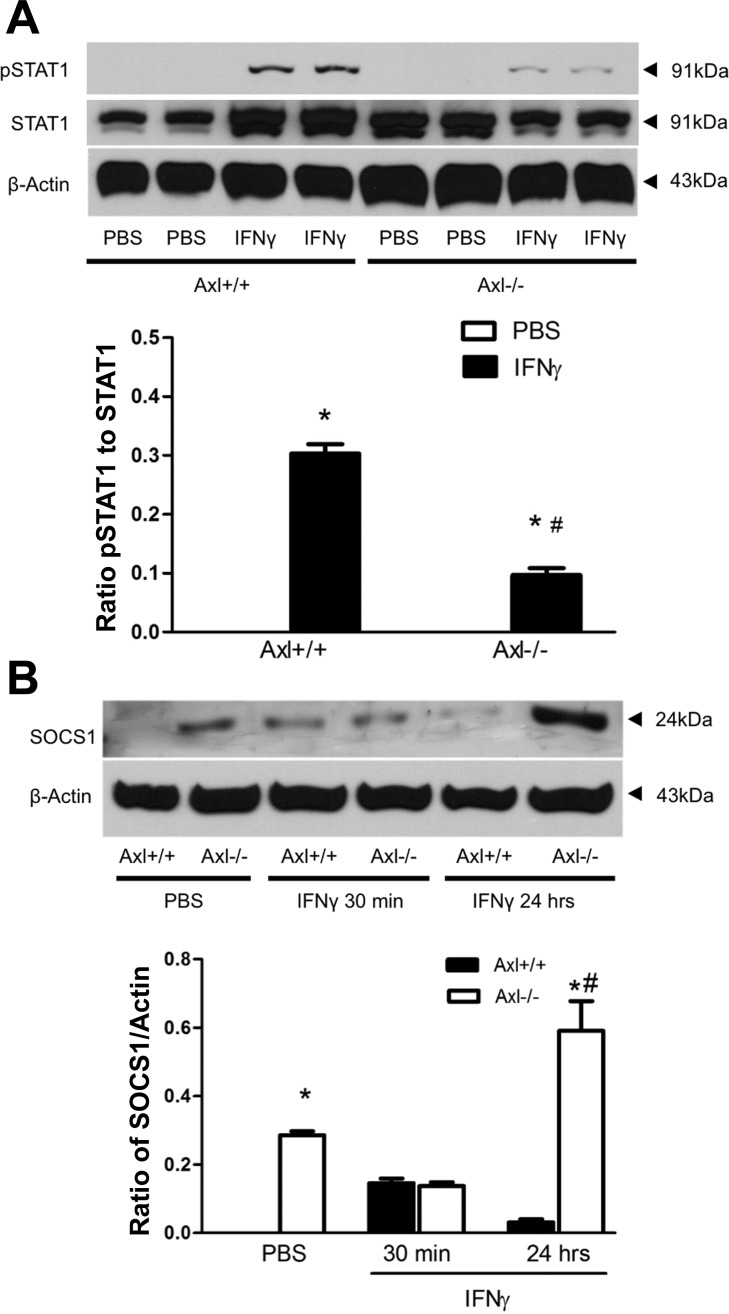
We compared gene expression profiles in SMCs isolated from two Axl genotypes with or without IFN-γ stimulation (Fig. 5). We observed that total mRNA levels of chemokine (C-X-C motif) ligand 10, chemokine (C-C motif) receptor 10, IL-1 receptor type II, IL-2 receptor-β, IL-2 receptor-γ, and IL-4 were significantly lower, whereas expression of chemokine (C-X3-C motif) ligand 1 and IL-11 were higher in Axl−/− compared with Axl+/+ MASMCs treated with PBS (Fig. 5A). This pattern of cytokine/chemokine expression in Axl+/+ versus Axl−/− MASMCs was even more robust upon stimulation with IFN-γ (Fig. 5B). Pathway analyses of the differentially expressed genes supported the central role of Axl in immune activation of MASMCs (Tables 2–4). Among the various immune pathways in these analyses, the JAK/STAT1 pathway was downregulated the most in Axl−/− versus Axl+/+ MASMCs (Tables 2 and 4). Our findings indicate that regulation of IFN-γ-induced proinflammatory responses by Axl could be mediated through activation of the STAT1 pathway in SMCs. We confirmed a significant reduction in the phosphorylation of STAT1 (pSTAT1) in Axl−/− versus Axl+/+ MASMCs upon stimulation with IFN-γ (Fig. 6A). The ratio of pSTAT1 and STAT1 expression was significantly lower in Axl−/− MASMCs (Fig. 6A). The inhibitory protein SOCS1 is the primary regulator of STAT1 signaling in SMCs (29). SOCS1 is also an important mediator of the TAM family in inhibition of STAT1/STAT3-dependent cytokine production in innate immune cells (27). In contrast to immune cells, SOCS1 expression levels were much higher in SMCs from Axl−/− mice (Fig. 6B). A time course of IFN-γ stimulation showed that SOCS1 protein was transiently increased in Axl+/+ MASMCs (Fig. 6B). However, SOCS1 levels were further increased in Axl−/− MASMCs by 24 h after IFN-γ (Fig. 6B). Our results suggest that Axl regulates STAT1 signaling via a reduction of SOCS1 expression in SMCs.
Fig. 5.
Modulation of cytokine and chemokine profiles in Axl SMCs with or without IFN-γ. A: expression profiles between PBS-treated Axl SMCs. B: differences in expression between Axl−/− and Axl+/+ SMCs after IFN-γ for 24 h. Individual genes are shown as dots on volcano plots. The x-axis shows log2-transformed fold changes in gene expression; negative values are downregulated genes and positive values are upregulated genes. The y-axis shows P values. The middle vertical line crosses at zero, and the two thinner vertical lines are positive/negative twofold cutoffs. The horizontal line represents a level of significance at P = 0.05. n = 3 per group.
Table 2.
Differences in inflammatory pathways in Axl−/− MASMCs after PBS
| Pathway ID | Pathway Name | Gene Ratio, % | Pathway P Value | Gene Symbols |
|---|---|---|---|---|
| Upregulated pathways | ||||
| 604 | Cytokine-cytokine receptor interaction | 1 | 0.00116 | Cx3cl1; Il11 |
| Downregulated pathways | ||||
| 604 | Cytokine-cytokine receptor interaction | 3 | 5.05e−11 | Ccr10; Cxcl10; Il1r2; Il2rb; Il2rg; Il4; Il6ra |
| 9938 | Cytokine receptor degradation signaling (JAK-STAT pathway and regulation pathway) | 12 | 1.16e−8 | Il1r2; Il2rb; Il2rg; Il6ra |
| 9526 | Negative regulation of (phosphorylation of the cytokine receptor) in the JAK-STAT pathway (JAK-STAT pathway and regulation pathway) | 12 | 1.16e−8 | Il1r2; Il2rb; Il2rg; Il6ra |
| 9644 | Negative feedback regulation of the JAK-STAT pathway by cytokine receptor degradation signaling (JAK-STAT pathway and regulation pathway) | 10 | 2.32e−8 | Il1r2; Il2rb; Il2rg; Il6ra |
| 10320 | JAK-STAT pathway and regulation pathway | 2 | 5.54e−7 | Il1r2; Il2rb; Il2rg; Il4;Il6ra |
| 651 | JAK-STAT signaling pathway | 3 | 5.51e−6 | Il2rb; Il2rg; Il4; Il6ra |
| 9571 | IL-4 signaling pathway (JAK1, JAK3, and STAT6) and IL-4 signaling (JAK1, JAK3, and STAT6) | 33 | 1.16e−5 | Il2rg; Il4 |
| 10050 | IL-2 signaling pathway (JAK1, JAK3, and STAT5) and IL-2 signaling (JAK1, JAK3, and STAT5) | 25 | 2.16e−5 | Il2rb; Il2rg |
| 682 | Hematopoietic cell lineage | 4 | 4.34e−5 | Il1r2; Il4; Il6ra |
| 8107 | Intestinal immune network for IgA production | 5 | 0.00059 | Ccr10; Il4 |
| 4351 | Chemokine signaling pathway | 1 | 0.01065 | Ccr10; Cxcl10 |
| 4385 | Endocytosis | 1 | 0.01572 | Il2rb; Il2rg |
MASMCs, mouse aortic smooth muscle cells.
Table 3.
Upregulated pathways in Axl−/− MASMCs after interferon-γ
| Pathway ID | Pathway Name | Gene Ratio, % | Pathway P Value | Gene Symbols |
|---|---|---|---|---|
| 604 | Cytokine-cytokine receptor interaction | 2 | 4.50e−8 | Ccl6; Ccr8; Ifng; Il3; Lta |
| 10166 | Heterotrimeric GPCR signaling pathway (through Gαi and pertussis toxin) and GPCR signaling (pertussis toxin) | 1 | 0.00025 | Ifng; Il3; Lta |
| 9794 | Heterotrimeric GTP-binding protein-coupled receptor signaling pathway (through Gαi, adenylate cyclase, and cAMP) and GPCR signaling (Gαi) | 1 | 0.00025 | Ifng; Il3; Lta |
| 9789 | Heterotrimeric GTP-binding protein-coupled receptor signaling pathway (through Gαs, cholera toxin, adenylate cyclase, and cAMP) and GPCR signaling (cholera toxin) | 1 | 0.00026 | Ifng; Il3; Lta |
| 9827 | Heterotrimeric GPCR signaling pathway (through Gαs, adenylate cyclase, Epac, BRaf, and ERK cascade) and GPCR signaling (Gαs, Epac, and ERK) | 1 | 0.00028 | Ifng; Il3; Lta |
| 10320 | JAK-STAT pathway and regulation pathway | 1 | 0.00028 | Ifng; Il3; Lta |
| 9890 | Heterotrimeric GPCR signaling pathway (through Gαq, phospholipase C-β, and ERK cascade) and GPCR signaling (Gαq) | 1 | 0.00029 | Ifng; Il3; Lta |
| 9754 | Heterotrimeric GPCR signaling pathway (through Gαs, adenylate cyclase, PKA, BRaf, and ERK cascade) and canonical GPCR signaling (Gαs, PKA, and ERK) | 1 | 0.00030 | Ifng; Il3; Lta |
| 637 | Type I diabetes mellitus | 4 | 0.00053 | Ifng; Lta |
| 651 | JAK-STAT signaling pathway | 1 | 0.00395 | Ifng; Il3 |
| 4351 | Chemokine signaling pathway | 1 | 0.00523 | Ccl6; Ccr8 |
GPCR, G protein-coupled receptor.
Table 4.
Downregulated pathways in Axl−/− MASMCs after interferon-γ
| Pathway ID | Pathway Name | Gene Ratio, % | Pathway P Value | Gene Symbols |
|---|---|---|---|---|
| 604 | Cytokine-cytokine receptor interaction | 6 | 1.29e−18 | Ccl11; Ccl2; Ccl5; Ccl7; Ccl8; Ccl9; Ccr10; Cxcl10; Cxcl9; Cxcr2; Cxcr5; Il13ra1; Il18; Il2rb |
| 4351 | Chemokine signaling pathway | 6 | 9.54e−15 | Ccl11; Ccl2; Ccl5; Ccl7; Ccl8; Ccl9; Ccr10; Cxcl10; Cxcl9; Cxcr2; Cxcr5 |
| 8111 | NOD-like receptor signaling pathway | 11 | 3.41e−11 | Casp1; Ccl11; Ccl2; Ccl5; Ccl7; Ccl8; Il18 |
| 8116 | Cytosolic DNA-sensing pathway | 7 | 6.15e−6 | Casp1; Ccl5; Cxcl10; Il18 |
| 11853 | Chemokine receptors bind chemokines | 14 | 1.53e−5 | Ccl5; Ccr10; Cxcr2 |
| 9938 | Cytokine receptor degradation signaling (JAK-STAT pathway and regulation pathway) | 9 | 5.33e−5 | Cxcr2; Il13ra1; Il2rb |
| 9526 | Negative regulation of phosphorylation of the cytokine receptor in the JAK-STAT pathway (JAK-STAT pathway and regulation pathway) | 9 | 5.33e−5 | Cxcr2; Il13ra1; Il2rb |
| 9644 | Negative feedback regulation of the JAK-STAT pathway by cytokine receptor degradation signaling (JAK-STAT pathway and regulation pathway) | 8 | 8.86e−5 | Cxcr2; Il13ra1; Il2rb |
| 10373 | Malaria | 6 | 0.00018 | Ccl2; Il18; Itgb2 |
| 699 | Toll-like receptor signaling pathway | 3 | 0.00143 | Ccl5; Cxcl10; Cxcl9 |
| 10401 | Chagas disease (American trypanosomiasis) | 3 | 0.00152 | C3; Ccl2; Ccl5 |
| 10320 | JAK-STAT pathway and regulation pathway | 2 | 0.00157 | Cxcr2; Il13ra1; Il18; Il2rb |
| 10352 | Staphylococcus aureus infection | 4 | 0.00575 | C3; Itgb2 |
| 11557 | Immunoregulatory interactions between a lymphoid cell and a nonlymphoid cell | 3 | 0.00767 | C3; Itgb2 |
| 10360 | Leishmaniasis | 3 | 0.00928 | C3; Itgb2 |
| 651 | JAK-STAT signaling pathway | 1 | 0.04584 | Il13ra1; Il2rb |
Fig. 6.
Role of Axl in suppressor of cytokine signaling (SOCS1)-dependent inhibition of STAT1 signaling in SMCs. A: representative Western blots of phosphorylated (p)STAT1 and STAT1 in Axl MASMCs after IFN-γ stimulation for 30 min. Values are means ± SE. *P < 0.01 vs. PBS; #P < 0.01 vs. IFN-γ in Axl+/+ MASMCs. B: representative Western blots of SOCS1 after IFN-γ stimulation of Axl MASMCs. β-Actin was used as a loading control. Values are means ± SE; n = 3 per group. *P < 0.01 vs. PBS in Axl+/+; #P < 0.01 vs. IFN-γ in Axl+/+.
Role of the donor Axl genotype on proinflammatory signals in vein grafts.
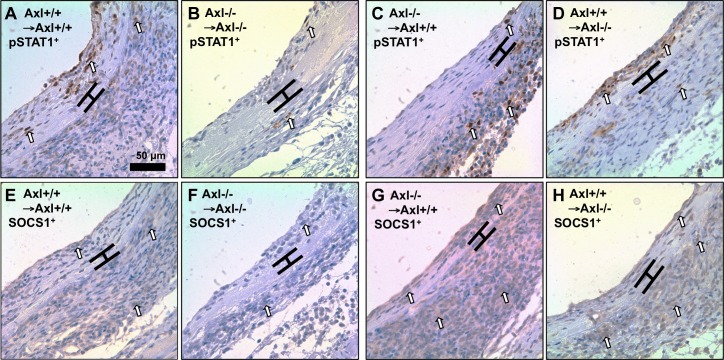
Next, we evaluated expression of proinflammatory (pSTAT1) versus anti-inflammatory (SOCS1) proteins in vein grafts with global, systemic, and vascular depletion of Axl (Fig. 7). Similar to IFN-γ-stimulated MASMCs, pSTAT1-positive cells were localized in the intima of vein grafts in Axl+/+ → Axl+/+ mice (Fig. 7A). Vascular depletion of Axl in Axl−/− → Axl+/+ vein grafts prevented pSTAT1 expression in the intima but augmented pSTAT1-positive cells in the adventitia (Fig. 7C). In contrast, systemic deletion of Axl in Axl+/+ → Axl−/− vein grafts had no effect on intimal pSTAT1 (Fig. 7D), whereas global deletion of Axl prevented pSTAT1 increases throughout vein grafts (Fig. 7B). To our surprise, SOCS1 immunoreactivity was similar throughout the graft wall between Axl−/− → Axl−/− and Axl+/+ → Axl+/+ mice (Fig. 7, E and F). However, vascular depletion of Axl in Axl−/− → Axl+/+ vein grafts exaggerated SOCS1 expression throughout the vein graft (Fig. 7G). Systemic deletion of Axl resulted in greater adventitial expression of SOCS1 (Fig. 7H). Thus, Axl is required for intimal thickening via modulation of the STAT1 pathway by SOCS1 expression in vein graft remodeling.
Fig. 7.
Role of Axl expression on the SOCS1-STAT1 balance in vein graft remodeling. A–D: representative photomicrographs of pSTAT1-positive staining in the nuclei. A: Axl+/+ → Axl+/+; B: Axl−/− → Axl−/−; C: Axl−/− → Axl+/+; D: Axl+/+ → Axl−/−. E–H: representative photomicrographs of SOCS1-positive stainingin the cytosol. E: Axl+/+ → Axl+/+; F: Axl−/− → Axl−/−; G: Axl−/− → Axl+/+; H: Axl+/+ → Axl−/−. Positive cells are dark brown (open arrows). Brackets show the area in between internal and external elastic lamina. Scale bar = 50 μm.
DISCUSSION
The present study provides new insights into immune mechanisms by which Axl contributes to vascular remodeling. To our knowledge, this is first reported study that demonstrates that vein graft remodeling is significantly reduced after vascular and systemic depletion of Axl. Additionally, local expression of Axl is critical for vascular stiffness during vein graft remodeling. Immunohistochemical evaluation of the grafts suggested that SMCs are the primary cells in vein grafts. Local depletion of Axl had the greatest effect on immune activation (as measured by the MHC II expression) throughout the vein grafts. Induction of MHC II and proinflammatory cytokines and chemokines after IFN-γ was significantly attenuated in SMCs isolated from Axl−/− mice. A decrease in SOCS1, a major STAT1 inhibitory protein, is the primary mechanisms by which Axl is involved in immune activation of SMCs. Vascular expression of Axl is critical for the pSTAT1-SOCS1 balance and determines intimal thickening in vein graft remodeling. Yet, systemic deletion of Axl reduced intima thickening by increasing vein graft apoptosis. Taken together, our data suggest that Axl promotes STAT1-dependent signaling via inhibition of SOCS1 in immunologically activated SMCs. This is opposite to the inhibitory effects of the TAM receptor family in innate immune cells (27).
Immune activation of SMCs (increase in MHC II, VCAM1, etc.) has been described during the late phase of vascular remodeling (31). Animal and cell culture experiments suggested that the secretion of the proinflammatory cytokine IFN-γ by leukocytes is required for MHC II expression in SMCs (11, 33). Elevation of systemic levels of IFN-γ potentiates intimal thickening (16, 34), and neutralization of IFN-γ using either antibodies or by genetic depletion of IFN-γ or its receptor inhibited intimal expansion (9, 23, 24, 28). However, Wang et al. (32) reported that IFN-γ induced intima thickening of human coronary arteries via SMC proliferation in the absence of leukocytes using immune-deficient (SCID) mice (32). We have previously shown that deletion of Axl in nonbone marrow-derived cells abolished MHC II vascular expression and prevented carotid remodeling (8). We also found that the survival of bone marrow cells in the intima relies on Axl. However, in the present study, systemic deletion of Axl reduced intima thickness due to an increase in apoptosis in vein graft remodeling. These data suggest that the survival of immune cells in a more severe immunological model of vein graft remodeling is crucial compared with responses to low blood flow. Our new findings show that local expression of Axl is more important for immune activation of intimal cells. This was supported by reduced proinflammatory cytokines and chemokines after IFN-γ stimulation of Axl−/− SMCs. We cannot completely exclude contribution of SMCs from the neighboring carotid artery to vein graft remodeling, as has been recently found by lineage tracing experiments (20). It has been previously reported that higher doses of IFN-γ could induce SMC death (7, 25). However, we observed no changes in apoptosis or proliferation of mouse cells upon treatment with IFN-γ, which is similar to stimulation of human cells (33). Thus, IFN-γ is the key mediator responsible for the intima formation by promoting immune activation of SMCs.
The majority of mechanistic studies on the IFN-γ/MHC II axis in SMCs have been focused on modulation of the extracellular matrix (3). Two regulatory mechanisms have been described in controlling MHC II transactivator (CIITA) activity, involving peroxisome proliferator-activated receptor-γ and histone deacetylase 2 (12, 13). The major downstream target for IFN-γ in vascular diseases is STAT1 (21, 29). To date, only one study (35) in mesangial cells has suggested a connection between Axl and STAT1 signaling. The authors described activation of the Gas6/Axl pathway, leading to mesangial cell proliferation via the latent transcription factor STAT3. Axl signaling likely depends on the cell type and experimental conditions, since application of IFN-γ had no effect on SMC proliferation in this study. Phosphorylation of Akt was not affected by Axl expression in IFN-γ-treated SMCs (data not shown). More information on the relationship between the TAM receptor family and STAT signaling exists in innate immune cells (19). Specifically, IFN type I (IFN-α or IFN-β) induces the Axl pathway and further inhibits receptor signaling via elevation of the expression of suppressors of proinflammatory signals, such as Twist1, SOCS1, and SOCS3 (27). In contrast, Axl acts as a positive regulator of proinflammatory signaling via IFN type II (IFN-γ) in SMCs by inhibiting SOCS1 expression. Our findings are supported by a previously reported yeast two-hybrid screen, which identified direct interactions between the cytoplasmic domain of Axl and SOCS1 protein (10).
Another possibility is related to specific inhibition of SOCS1 (and not SOCS2–SOCS4) in response to mechanical stretching of human SMCs, which was independent of the STAT1 pathway (6). The authors suggested that this inhibitory effect of stretching is dependent on an integrin interaction with the urokinase receptor. D'Arcangelo et al. (5) found that exposure of endothelial cells to laminar shear stress lead to the formation of the Axl/β3-integrin complex and survival of the endothelium. Stretch-dependent activation of receptor tyrosine kinases has been implicated in vascular remodeling (2). Specifically, stretch activation of the IFG-1 receptor contributed to intimal thickening of vein grafts (4). Only vein grafts with local deletion of Axl exhibited a significant decrease in graft stiffness, which was complemented by the greatest increase in intimal SOCS1 expression. Taken together, we showed that Axl inhibits SOCS1 and promotes STAT1 signaling for immune activation of SMCs and vascular remodeling.
In conclusion, our study introduces a new mechanism by which Axl promotes immune activation of SMCs in transplant disorders. These effects of Axl are opposite to the inhibitory functions of the TAM receptor family in activated innate immune cells (27). It was shown that Gas6 and TAM receptors are implicated in the pathogenesis of chronic rejection after kidney transplantation in rats (36). A recent clinical study (18) on 19 patients after coronary artery bypass graft showed higher expression of Gas6 and Axl in the left internal mammary artery compared with the aorta. Therefore, inhibition of Axl signaling could be beneficial in the prevention of transplant vascular remodeling.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-105623 (to V. A. Korshunov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.N.B. and V.A.K. conception and design of research; S.N.B., J.X., K.A.K., and V.A.K. performed experiments; S.N.B., J.X., K.A.K., M.M.D., and V.A.K. analyzed data; S.N.B. and J.X. drafted manuscript; S.N.B., J.X., M.M.D., J.-I.A., C.N.M., and V.A.K. edited and revised manuscript; S.N.B., J.X., K.A.K., M.M.D., J.-I.A., C.N.M., and V.A.K. approved final version of manuscript; S.N.B., J.X., and V.A.K. prepared figures; J.-I.A. and C.N.M. interpreted results of experiments.
ACKNOWLEDGMENTS
The authors thank Michelle Zanche (Functional Genomics Core) for assistance with gene expression assays, Janice Gerloff for help with in vitro experiments, and Kathy Donlon and Deanne Mickelsen for help with animal experiments.
Present address of S. N. Batchu: St. Michaels Hospital, University of Toronto, Toronto, ON, Canada.
REFERENCES
- 1.Batchu SN, Hughson A, Gerloff J, Fowell DJ, Korshunov VA. Role of Axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension 62: 302–309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batchu SN, Korshunov VA. Novel tyrosine kinase signaling pathways: implications in vascular remodeling. Curr Opin Nephrol Hypertens 21: 122–127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buttice G, Miller J, Wang L, Smith BD. Interferon-γ induces major histocompatibility class II transactivator (CIITA), which mediates collagen repression and major histocompatibility class II activation by human aortic smooth muscle cells. Circ Res 98: 472–479, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol 27: 1744–1751, 2007. [DOI] [PubMed] [Google Scholar]
- 5.D'Arcangelo D, Ambrosino V, Giannuzzo M, Gaetano C, Capogrossi MC. Axl receptor activation mediates laminar shear stress anti-apoptotic effects in human endothelial cells. Cardiovasc Res 71: 754–763, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Dangers M, Kiyan J, Grote K, Schieffer B, Haller H, Dumler I. Mechanical stress modulates SOCS-1 expression in human vascular smooth muscle cells. J Vasc Res 47: 432–440, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Geng YJ, Wu Q, Muszynski M, Hansson GK, Libby P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-γ, tumor necrosis factor-α, and interleukin-1β. Arterioscler Thromb Vasc Biol 16: 19–27, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Gerloff J, Korshunov VA. Immune modulation of vascular resident cells by Axl orchestrates carotid intima-media thickening. Am J Pathol 180: 2134–2143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 99: 2752–2761, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafizi S, Alindri F, Karlsson R, Dahlback B. Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem Biophys Res Commun 299: 793–800, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Jonasson L, Holm J, Hansson GK. Smooth muscle cells express Ia antigens during arterial response to injury. Lab Invest 58: 310–315, 1988. [PubMed] [Google Scholar]
- 12.Kong X, Fang M, Fang F, Li P, Xu Y. PPARγ enhances IFNγ-mediated transcription and rescues the TGFβ antagonism by stimulating CIITA in vascular smooth muscle cells. J Mol Cell Cardiol 46: 748–757, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Kong X, Fang M, Li P, Fang F, Xu Y. HDAC2 deacetylates class II transactivator and suppresses its activity in macrophages and smooth muscle cells. J Mol Cell Cardiol 46: 292–299, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Korshunov VA. Axl-dependent signalling: a clinical update. Clin Sci (Lond) 122: 361–368, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res 98: 1446–1452, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kusaba K, Kai H, Koga M, Takayama N, Ikeda A, Yasukawa H, Seki Y, Egashira K, Imaizumi T. Inhibition of intrinsic interferon-gamma function prevents neointima formation after balloon injury. Hypertension 49: 909–915, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Le NT, Takei Y, Shishido T, Woo CH, Chang E, Heo KS, Lee H, Lu Y, Morrell C, Oikawa M, McClain C, Wang X, Tournier C, Molina CA, Taunton J, Yan C, Fujiwara K, Patterson C, Yang J, Abe J. p90RSK targets the ERK5-CHIP ubiquitin E3 ligase activity in diabetic hearts and promotes cardiac apoptosis and dysfunction. Circ Res 110: 536–550, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CH, Shieh YS, Tsai CS, Hung YJ, Tsai YT, Lin CY. Expression of growth arrest-specific protein 6 and Axl molecules in the left internal mammary artery of patients undergoing coronary artery bypass grafting. J Clin Pathol 67: 506–511, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol 8: 327–336, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang M, Liang A, Wang Y, Jiang J, Cheng J. Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol 109: 431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrero MB. Introduction to JAK/STAT signaling and the vasculature. Vasc Pharmacol 43: 307–309, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Melaragno MG, Wuthrich DA, Poppa V, Gill D, Lindner V, Berk BC, Corson MA. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res 83: 697–704, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-γ deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest 100: 550–557, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raisanen-Sokolowski A, Glysing-Jensen T, Koglin J, Russell ME. Reduced transplant arteriosclerosis in murine cardiac allografts placed in interferon-γ knockout recipients. Am J Pathol 152: 359–365, 1998. [PMC free article] [PubMed] [Google Scholar]
- 25.Rosner D, Stoneman V, Littlewood T, McCarthy N, Figg N, Wang Y, Tellides G, Bennett M. Interferon-gamma induces Fas trafficking and sensitization to apoptosis in vascular smooth muscle cells via a PI3K- and Akt-dependent mechanism. Am J Pathol 168: 2054–2063, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross R, Glomset J, Harker L. Response to injury and atherogenesis. Am J Pathol 86: 675–684, 1977. [PMC free article] [PubMed] [Google Scholar]
- 27.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131: 1124–1136, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. III. Effects of recipient treatment with a monoclonal antibody to interferon-γ. Transplantation 57: 1367–1371, 1994. [PubMed] [Google Scholar]
- 29.Sikorski K, Czerwoniec A, Bujnicki JM, Wesoly J, Bluyssen HA. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNγ, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev 22: 211–219, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Smolock EM, Machleder DE, Korshunov VA, Berk BC. Identification of a genetic locus on chromosome 11 that regulates leukocyte infiltration in mouse carotid artery. Arterioscler Thromb Vasc Biol 33: 1014–1019, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation 88: 1788–1803, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res 101: 560–569, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Warner SJ, Friedman GB, Libby P. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis 9: 279–288, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-γ enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol 157: 1819–1824, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanagita M, Arai H, Nakano T, Ohashi K, Mizuno K, Fukatsu A, Doi T, Kita T. Gas6 induces mesangial cell proliferation via latent transcription factor STAT3. J Biol Chem 276: 42364–42369, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Yin JL, Pilmore HL, Yan YQ, McCaughan GW, Bishop GA, Hambly BD, Eris JM. Expression of growth arrest-specific gene 6 and its receptors in a rat model of chronic renal transplant rejection. Transplantation 73: 657–660, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Hagen PO, Kisslo J, Peppel K, Freedman NJ. Neointimal hyperplasia rapidly reaches steady state in a novel murine vein graft model. J Vasc Surg 36: 824–832, 2002. [PubMed] [Google Scholar]