This study provides a mechanistic insight into the role of Ets-1 regulated EGF induced collagen loss involving p38-MAPK and JNK signaling pathways in human carotid plaques with carotid stenosis. Selective blockade of Ets-1 and EGF receptor may be a novel strategy and promising target for treating unstable and vulnerable plaques.
Keywords: carotid plaque, collagen types I and III, epidermal growth factor, matrix metalloproteinases, v-ets erythroblastosis virus E26 oncogene homologue 1, vascular smooth muscle cells
Abstract
Although degradation of extracellular matrix by matrix metalloproteinases (MMPs) is thought to be involved in symptomatic (S) carotid plaques in atherosclerosis, the mechanisms of MMP expression are poorly understood. Here, we demonstrate that collagen loss in vascular smooth vessel cells (VSMCs) isolated from S plaques was induced by epidermal growth factor (EGF) through the activation of p38-MAPK and JNK-MAPK pathways. Inhibitors of p38-MAPK and JNK-MAPK signaling pathways downregulated the expression of MMP-1 and MMP-9. In addition, we examined whether v-ets erythroblastosis virus E26 oncogene homologue 1 (Ets-1), an important regulator of different genes, is involved in destabilizing S plaques in patients with carotid stenosis. We demonstrate that EGF induces Ets-1 expression and decreases interstitial and basement membrane collagen in vascular smooth muscle cells (VSMCs) from patients with carotid stenosis. Increased expression of MMP-1 and -9 and decreased collagen mRNA transcripts were also found in Ets-1-overexpressed VSMCs. Transfection with both dominant-negative form of Ets-1 and small interfering RNA blocked EGF-induced MMP-1 and -9 expressions and increased the mRNA transcripts for collagen I (α1) and collagen III (α1) in S compared with asymptomatic (AS) carotid plaques. Inhibitors of p38-MAPK (SB202190) and JNK-MAPK (SP600125) signaling pathways decreased the expression of Ets-1, MMP-1, and MMP-9 and increased collagen type I and III expression in EGF-treated VSMCs. This study provides a mechanistic insight into the role of Ets-1 in the plaque destabilization in patients with carotid stenosis involving p38-MAPK and JNK signaling pathways.
NEW & NOTEWORTHY
This study provides a mechanistic insight into the role of Ets-1 regulated EGF induced collagen loss involving p38-MAPK and JNK signaling pathways in human carotid plaques with carotid stenosis. Selective blockade of Ets-1 and EGF receptor may be a novel strategy and promising target for treating unstable and vulnerable plaques.
atherosclerosis is a complex disease with coronary thrombus leading to stroke and is the major cause of morbidity and mortality throughout the world (8, 24). The matrix accumulation and degradation in the extracellular matrix (ECM) may determine the outcome of plaque stability (1). Proteases produced by the cellular components of the plaque are thought to be mainly responsible for thinning of the plaque cap and the development of myocardial infarction and stroke. Inflammatory cytokines (tumor necrosis factor-α and interleukin-1) and growth factors [epidermal growth factor (EGF), transforming growth factor-β, and platelet-derived growth factor] secreted by the atherosclerotic plaques are involved in several diseases (25, 32). EGF receptor (EGFR) is a member of the protein tyrosine kinase family and plays an important role in various cellular processes such as proliferation, migration, and apoptosis (57). Many signaling cascades involving phosphatidylinositol 3-kinase-Akt (PI3K/Akt) pathway and mitogen-activated protein kinase (MAPK) are involved in cell migration and apoptosis in various pathophysiological processes (27). However, the involvement of p38-MAPK and JNK-MAPK in EGF-mediated collagen loss in atherosclerotic plaques is poorly understood. Recently, we reported that EGF induces collagen loss involving EGFR and matrix metalloproteinases (MMPs), especially MMP-1 and MMP-9, in isolated vascular smooth muscle cells (VSMCs) from vulnerable carotid plaques (45).
Many cells including endothelial cells, VSMCs, and fibroblasts express v-ets erythroblastosis virus E26 oncogene homologue 1 (Ets-1) and regulate several biological processes such as angiogenesis, development, and apoptosis (37, 49, 60). The Ets-1 proto-oncogene protein is a member of the Ets family of transcription factors with a highly conserved Ets domain (9). Several MMPs contain Ets binding sequence in their functional promoter region (20, 26). However, the Ets-1 regulation of MMP-1 and MMP-9 expression in EGF-treated VSMCs isolated from carotid plaques is not known.
The regulatory elements of different transcription factors, including activator protein 1, NF-κB, and Ets, are present in the promoter region of various MMP genes. In this present study, the regulatory role of Ets-1 on EGF-induced MMP-1 and MMP-9 expression was studied using inhibitors of different signaling pathways in VSMCs isolated from human carotid plaques. We report for the first time that both p38-MAPK and JNK-MAPK signaling pathways are required for the upregulation of Ets-1, MMP-1, and MMP-9 expression induced by EGF, leading to plaque instability in VSMCs derived from the carotid plaques of asymptomatic (AS) and symptomatic (S) patients.
MATERIALS AND METHODS
Human tissue collection.
The experimental research protocol, including the collection of the biopsy tissues, and all tissue acquisition procedures were approved by the Institutional Review Board of Creighton University and exempted the research protocol since the carotid endarterectomy specimens were truly anonymized. The patients in both AS and S groups were aged between 50–75 yr and included both men and women of any ethnic origin. These patients were deemed appropriate based on American Heart Association criteria that define the risk-to-benefit ratio in AS and S carotid disease. The carotid plaques from patients undergoing carotid endarterectomy were collected. The classification of the carotid plaques as unstable or stable from S and AS patients, respectively, was accordingly made to the criteria previously reported by us (8, 45). Briefly, the carotid plaques were clinically classified by the surgeon as S from the history and clinical evaluation of the patients, including frequent plaque rupture, fibrous cap thinning, and fibrous cap foam-cell infiltration compared with those in AS patients. The symptoms also included hemispheric transient ischemic attacks, amaurosis fugax, or stroke (8, 45). The information on the categorization (S and AS plaque) of carotid stenosis was provided to laboratory investigators in an anonymous manner. The specimens were collected fresh in the University of Wisconsin solution and transported to the laboratory where all procedures were carried out under sterile conditions.
Isolation of VSMCs.
An established method developed in this laboratory was used to isolate VSMCs from carotid plaques (8, 18, 45). Briefly, the medial layer was homogenized, digested with trypsin, followed by digestion with collagenase type IA from Clostridium histolyticum (C2674, Sigma, St. Louis, MO) and the pellet was suspended in smooth muscle cell medium (ScienCell, Carlsbad, CA). The cells from the second to fifth passage were used. The phenotype and the homogeneity of isolated smooth vessel cells were confirmed by positive staining for smooth muscle α-actin and caldesmon, as previously reported (8, 18, 45).
Cell culture and treatment protocol.
VSMCs at preconfluence were incubated in serum-free medium containing 10 ng/ml EGF for 24 h. The activation of EGFR was confirmed by treating VSMCs with an inhibitor of EGFR, and AG1478 (AG Scientific, San Diego, CA) at 15 μM in the presence or absence of EGF.
Immunofluorescence microscopy.
Cryosections (5 μm) from both S and AS carotid plaques were subjected to immunofluorescence microscopy, as described earlier (44, 45) using rabbit polyclonal antibodies for Ets-1, collagen (Col) I (α1), Col III (α1), and Col IV (α1) (1:100 dilution, Santa Cruz Biotechnology). Antibodies to phosphorylated (p)-p38-MAPK and p-JNK were obtained from Cell Signaling Technology (Beverly, MA) and used at 1:250 dilution. Primary antibodies were allowed to bind at room temperature for 2 h, followed by Alexa 594-conjugated secondary antibody (Invitrogen, Grand Island, NY) for 1 h (1:1,000 dilution) at room temperature. The slides were washed with PBS and stained with 4′,6-diamidino-2-phenylindole (DAPI), and the immunofluorescence was observed in an Olympus-inverted fluorescent microscope (Olympus BX51). The fluorescence intensity was quantified in the thin sections of carotid plaques from the patients using Image-pro software, and average intensity was calculated. Negative controls were stained with isotype IgG controls.
RNA isolation, cDNA synthesis, and real-time PCR.
Total RNA was isolated using TRIzol reagent (Sigma) from tissues and cultured VSMCs according to the manufacturer's instructions. The yield of RNA was quantified using Nanodrop (Thermo Scientific, Rockford, IL). The cDNA was synthesized using Improm II reverse transcription kit (Promega, Madison, WI) following the manufacturer's instructions. Quantitative (q)RT-PCR was performed using SYBR Green Master Mix and a real-time PCR system (CFX96, Bio-Rad, Hercules, CA). The primers for different genes were obtained from Integrated DNA Technologies (Coralville, IA). The PCR-cycling conditions included 5 min at 95°C for initial denaturation, 40 cycles of 30 s at 95°C, 30 s at 55–60°C (depending on the primer annealing temperatures), and 30 s at 72°C, followed by melting curve analysis. Fold expression of mRNA transcripts relative to controls was determined after normalizing to GAPDH. The oligonucleotide primer sequences for MMP-1, MMP-9, Col I (α1), Col III (α1), Ets-1, Ets-2, and polyoma enhancer activator-3 genes are given in Table 1. Fold expression relative to controls was determined after normalizing to GAPDH expression.
Table 1.
Primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MMP-1 | 5′-TGC AAC TCT GAC GTT GAT CCC AGA-3′ | 5′-ACT GCA CAT GTG TTC TTG AGC TGC-3′ |
| MMP-9 | 5′-ATT TCT GCC AGG ACC GCT TCT ACT-3′ | 5′-CAG TTT GTA TCC GGC AAA CTG GCT-3′ |
| EGF receptor | 5′-AGG AAG AAG CTT GCT GGT AGC ACT-3′ | 5′-TTT GCA GTG GAA GCC TTG AAG CAG-3′ |
| Col I (α1) | 5′-CAA TGC TGC CCT TTC TGC TCC TTT-3′ | 5′-CAC TTG GGT GTT TGA GCA TGG CCT-3′ |
| Col III (α1) | 5′-TAT CGA ACA CGC AAG GCT GTG AGA-3′ | 5′-GGC CAA CGT CCA CAC CAA ATT CTT-3′ |
| Ets-1 | 5′-GGA GGA CCA GTC GTG GTA AA-3′ | 5′-AAC TGC CAT AGC TGG ATT GG-3′ |
| Ets-2 | 5′-CAG CCA CCG TCC CGA CCA AG-3′ | 5′-GCT GGC TGG CGC TTG AGT GT-3′ |
| PEA3 | 5-′-CTG GAC ATT TGC CAT CCT T-3′ | 5′-AAC TGC CAT AGC TGG ATT TGG-3′ |
| GAPDH | 5′-GGT GAA GGT CGG AGT CAA CGG ATT TGG TCG-3′ | 5′-GGA TCT CGC TCC TGG AAG ATG GTG ATG GG-3′ |
MMP, matrix metalloproteinase; Col, collagen; Ets, v-ets erythroblastosis virus E26 oncogene homologue 1; PEA3, polyoma enhancer activator-3.
Analysis of signaling pathways in EGF-induced Ets-1, MMP-9, and MMP-1 expression.
Preconfluent VSMCs were treated in serum-free medium for 1 h with inhibitors of ERK1/2 (U0126), p38-MAPK (SB203580), JNK (SP600125), and PI3K (LY294002), followed by treatment with EGF at 10 ng/ml for 24 h. The mRNA expression of Ets-1, MMP-1, and MMP-9 was analyzed by qPCR.
Transient transfection experiments with Ets-1.
A full-length human Ets-1 and dominant-negative (DN) Ets-1 constructs cloned into pCDNA3.1 (−) neovector were kind gifts from Dr. Stephen Lee (Department of Cellular and Molecular Medicine, University of Ottawa, Ottawa, Ontario, Canada). VSMCs isolated from both AS and S plaques were grown to 60–80% confluence without antibiotics. For transient transfection, the cells were transfected using Lipofectamine 2000 (Life Technologies, Carlsbad, CA) according to the manufacturer's protocol with either mammalian expression constructs for Ets-1 or DN Ets-1 (17). For small interfering RNA (siRNA) experiments, cells were transfected with siRNA-targeting Ets-1 or with a scrambled control siRNA using Lipofectamine 2000 (Invitrogen). After 24 h of transfection, the cells were stimulated with or without EGF (10 ng/ml) and used for qPCR to quantify mRNA expression of Col I (α1), Col III (α1), MMP-1, and MMP-9 and GAPDH genes using the primers previously described (45).
Statistical analysis.
All data are presented as means ± SD from three independent experiments using carotid endarterectomy tissues from individual patients (N = 3–5 in each group). Statistical analysis was performed using Student's t-test between the tissues or VSMCs from AS and S plaques. Multiple group comparison was performed using ANOVA. A P value of <0.05 was considered significant.
RESULTS
Increased expression of Ets-1 in S human carotid plaques and VSMCs.
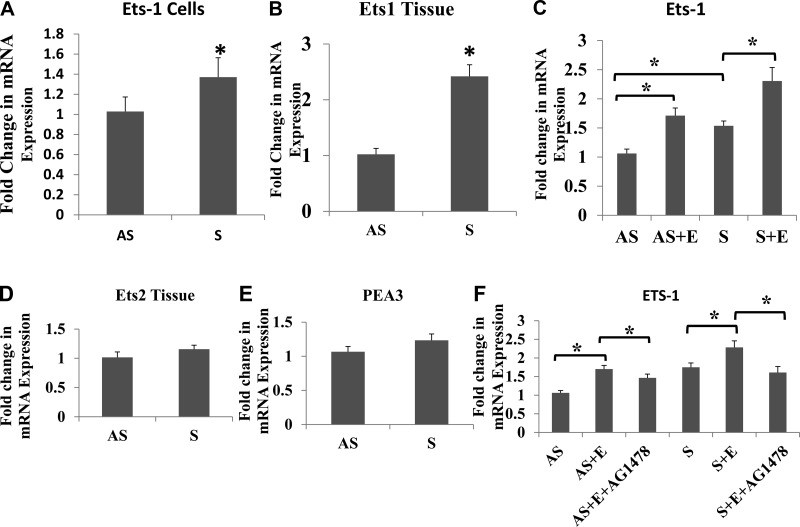
We analyzed the expression of Ets-1 in both tissue extracts and isolated VSMCs. The mRNA expression of Ets-1 was significantly increased in both isolated VSMCs and tissue carotid plaques from S patients (Fig. 1, A and B). The Ets-1 mRNA expression was increased more in tissues than in VSMCs. However, the expression of Ets-2 (Fig. 1D) and polyomavirus enhancer activator-3 (Fig. 1E) did not significantly change between AS and S groups. The Ets-1 immunofluorescence was greater in tissue sections (Fig. 2D) and VSMCs (Fig. 3M) of S compared with AS plaques. Quantification of immunofluorescence in carotid plaque tissue sections (Fig. 2G) and VMSCs (Fig. 3S) demonstrated increased Ets-1 immunoreactivity in S compared with AS plaques, and EGF treatment further enhanced the intensity of immunoreactivity of Ets-1 in VSMCs (Fig. 3P). These results confirm increased expression of Ets-1 mRNA transcripts in both tissues and VSMCs.
Fig. 1.
EGF induces the expression of v-ets erythroblastosis virus E26 oncogene homologue 1 (Ets-1) mRNA transcripts in vascular smooth vessel cells (VSMCs) (A, N = 5) and tissues (B, N = 5). Treatment with EGF (10 ng/ml) increased the Ets-1 mRNA expression in both asymptomatic (AS) and symptomatic (S) groups (C, N = 3). The mRNA expression of Ets-2 and polyoma enhancer activator-3 (PEA3) in VSMCs are given in D and E. AG1478, an inhibitor of EGF receptor (EGFR), downregulated the Ets-1 mRNA expression (F, N = 3). Results were expressed as fold change in S compared with AS. Data are shown as means ± SD. *P < 0.05. N = 5 for A, B, D, and E. S + E, symptomatic + EGF.
Fig. 2.
Immunofluorescence staining of Ets-1 in carotid plaques. The immunoreactivity of Ets-1 was greater in S (D) compared with AS (A) plaques and are in agreement with mRNA transcripts of Ets-1 in VSMCs (Fig. 1, A and B). B and E: nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI). C and F: merged images of Alexa 596 with DAPI. Quantification of average immunofluorescence intensity in carotid plaque tissue sections (G) demonstrated increased Ets-1 immunoreactivity in S compared with AS. This is a representative image from 5 individual tissues in each group. Data are shown as means ± SD. *P < 0.05; N = 5.
Fig. 3.
Immunofluorescence staining of Ets-1 in VSMCs. The immunoreactivity of Ets-1 was greater in S (M) compared with AS (D) VSMCs. EGF treatment further increased the immunostaining of Ets-1 in both the groups (G and P). Results from immunofluorescence are in agreement with mRNA transcripts of Ets-1 in VSMCs (Fig. 1, A and B). A and J: isotype negative controls (−ve). B, E, H, K, N, and Q: nuclear staining with DAPI. C, F, I, L, O, and R: merged images of Alexa 596 and DAPI. Quantification of average immunofluorescence intensity of Ets-1 (S) also confirmed increased Ets-1 expression (Fig. 1, A and C). This is a representative image from 5 individual tissues in each group. Data are shown as means ± SD. *P < 0.05; N = 5.
EGF-induced expression of Ets-1 mRNA expression is mediated by EGFR in VSMCs.
Changes in the mRNA transcripts of Ets-1 were examined in VSMCs isolated from both AS and S plaques with or without EGF treatment (10 ng/ml) for 24 h. The mRNA transcripts for Ets-1 were significantly increased in response to EGF treatment in VSMCs from both AS and S groups (Fig. 1, C and F).
EGFR mediates the expression of MMP-1 and -9 and Ets-1 in VSMCs.
We studied whether changes induced by EGF acts through its receptor (EGFR). To demonstrate the involvement of EGFR, the VSMCs isolated from both AS and S plaques were treated with EGF alone or in combination with AG1478, an inhibitor of EGFR, at 15 μM for 24 h in serum-free medium. There was no effect of AG1478 by itself on the mRNA expression of Ets-1 in either AS or S VSMCs. However, the increased mRNA expression of Ets-1 in both AS and S VSMCs treated with EGF was significantly decreased with AG1478 (Fig. 1F).
EGF induces Ets-1 expression through p38-MAPK and JNK-MAPK pathways.
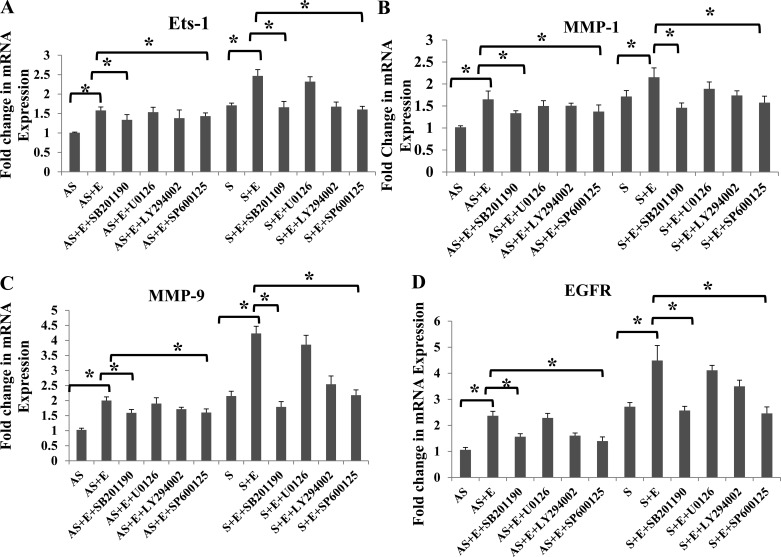
We have demonstrated (45) increased expression of MMP-1 and MMP-9 in EGF-treated VSMCs isolated from S carotid plaques compared with AS; however, the signaling pathways involved are not investigated. Therefore, we looked at the specific roles of p38-MAPK, PI3K/Akt, JNK, and ERK1/2 signaling pathways in the regulation of MMP-1, MMP-9, and Ets-1 mRNA expression using specific inhibitors (Fig. 4, A–D). VSMCs isolated from carotid plaques from AS and S were treated with selective inhibitors (SB202190 to inhibit p38-MAPK, LY294002 to inhibit PI3K/Akt, SP600125 to inhibit JNK, and U0126 to inhibit ERK1/2) with or without EGF (10 ng/ml) for 24 h. In the untreated VSMCs, there was increased expression of EGFR in S-plaque VSMCs compared with AS-plaque VSMCs (Fig. 4D). Inhibitors of p38-MAPK and JNK-MAPK significantly attenuated the EGF-stimulated mRNA transcripts for Ets-1 (Fig. 4A), MMP-1 (Fig. 4B), MMP-9 (Fig. 4C), and EGFR (Fig. 4D) in both AS and S. The PI3K inhibitor LY294002 induced a partial decrease against the changes induced by EGF. However, blockade of ERK with U0126 had no effect on the EGF-induced changes in either Ets-1 or MMPs (Fig. 4).
Fig. 4.
Ets-1 expression using p38-MAPK and JNK-MAPK signaling inhibitors. VSMCs from either AS or S were treated with different inhibitors of p38 (SB203580), phosphatidylinositol 3-kinase (LY2944002), ERK (U0126), and JNK (SB600125) and incubated with 10 ng/ml EGF for 24 h. The cDNA prepared from RNA was subjected to quantitative (q)PCR with gene-specific primers [Ets-1 (A), matrix metalloproteinase-1 (MMP-1; B), MMP-9 (C), and EGF receptor (EGFR; D)], and the fold change relative to GAPDH are shown. Results were expressed as fold change in S compared with AS. Data are shown as means ± SD; *P < 0.05. N = 3.
Increased p-p38-MAPK and p-JNK-MAPK immunostaining in tissue sections from S plaques.
Activation of p38-MAPK and JNK pathways in EGF-treated VSMCs was also demonstrated by immunofluorescence staining (Fig. 5). The immunoreactivity of p-p38–MAPK (Fig. 5D) and p-JNK–MAPK (Fig. 5J) was greater in tissue sections of S compared with AS plaques. In addition, quantification of the fluorescence intensity from the images revealed a significant increase in p38-MAPK (Fig. 5M) and JNK-MAPK (Fig. 5N) in S compared with AS plaques. These immunofluorescence studies further support the findings on the expression of EGF-induced expression of Ets-1, MMP-1, and MMP-9 in VSMCs (Fig. 4).
Fig. 5.
Immunofluorescence staining of phosphorylated (p)-p38 and p-JNK in tissue sections of S and AS carotid plaques. The immunoreactivity of p-p38 and p-JNK were greater in S tissue sections (D) and (J) than in AS carotid plaques (A and G). B, E, H, and K: nuclear staining with DAPI. C, F, I, and L: merged images of Alexa 488 and DAPI. Quantification of average immunofluorescence intensity confirmed increased p-p38 (M) and p-JNK (N) in S compared with AS plaques. This is a representative image from 5 individual tissues in each group. Data are shown as means ± SD. *P < 0.05; N = 5.
Ets-1 regulates EGF-mediated expression of MMP-1, MMP-9, Col I (α1), and Col III (α1) gene expression.
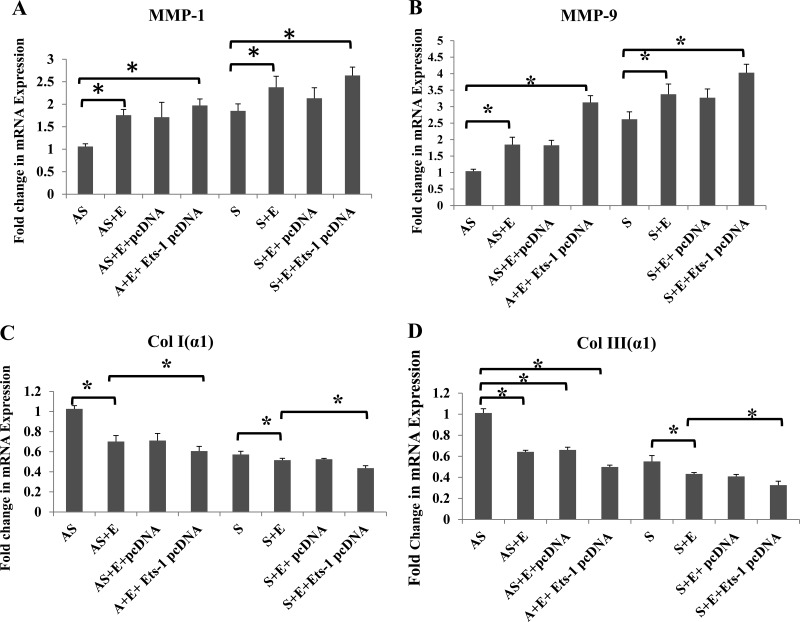
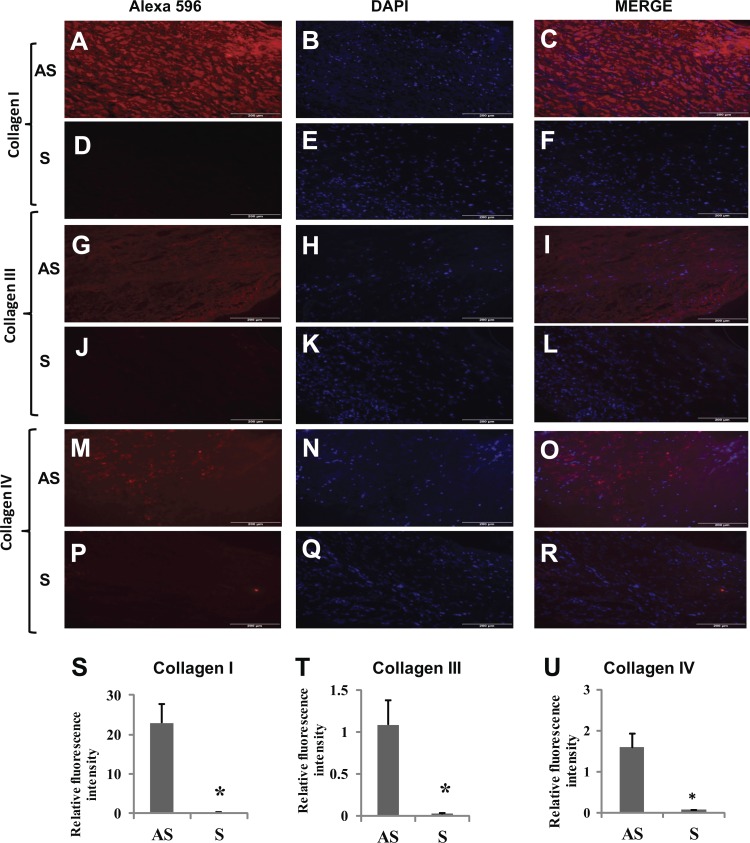
The promoter region of different MMPs and collagens is regulated by a number of transcription factors including Ets-1. We have demonstrated that EGF induces the expression of MMP-1, MMP-9, and type I and III collagen genes that are associated with the instability of atherosclerotic plaques (45). To evaluate the exact mechanism, VSMCs were transfected either with control pcDNA or Ets-1 pcDNA and then treated with EGF (10 ng/ml) for 24 h. The mRNA transcripts for MMP-1 (Fig. 6A), MMP-9 (Fig. 6B), Col I (α1) (Fig. 6C), and Col III (α1) (Fig. 6D) were significantly increased in VSMCs isolated from S compared with AS patients, and mRNA transcripts for these molecules were further increased with EGF treatment. The immunofluorescence staining and quantification of the sections from plaques further demonstrated increased protein expression of collagen types I, III, and IV in AS compared with S (Fig. 7, A, G, M, and S–U).
Fig. 6.
Ets-1 transfection upregulates the mRNA expression of MMP-1, MMP-9, collagen (Col) I (α1), and Col III (α1) in EGF-treated VSMCs from AS and S patients. The VSMCs were transfected with either pcDNA or Ets-1 pcDNA, followed by treatment with EGF (10 ng/ml) for 24 h. The RNA isolated was subjected to qPCR with gene-specific primers: MMP-1 (A), MMP-9 (B), Col I (α1) (C), and Col III (α1) (D), and the results were expressed as fold change compared with AS group. Data are shown as means ± SD. *P < 0.05; N = 3.
Fig. 7.
Immunofluorescence staining for Col I, III, and IV in tissue sections from S and AS carotid plaques. The immunofluorescence of Col I, III, and IV was greater in AS tissue sections (A, G, and M) than in S carotid plaques (D, J, and P). B, E, H, K, N, and Q: nuclear staining with DAPI. C, F, I, L, O, and R: merged images of Alexa 596 and DAPI. Quantification of average immunofluorescence intensity also showed decreased content of collagen I (S), Col III (T) and Col IV (U). This is a representative image from 5 individual tissues in each group. Data are shown as means ± SD. *P < 0.05; N = 5.
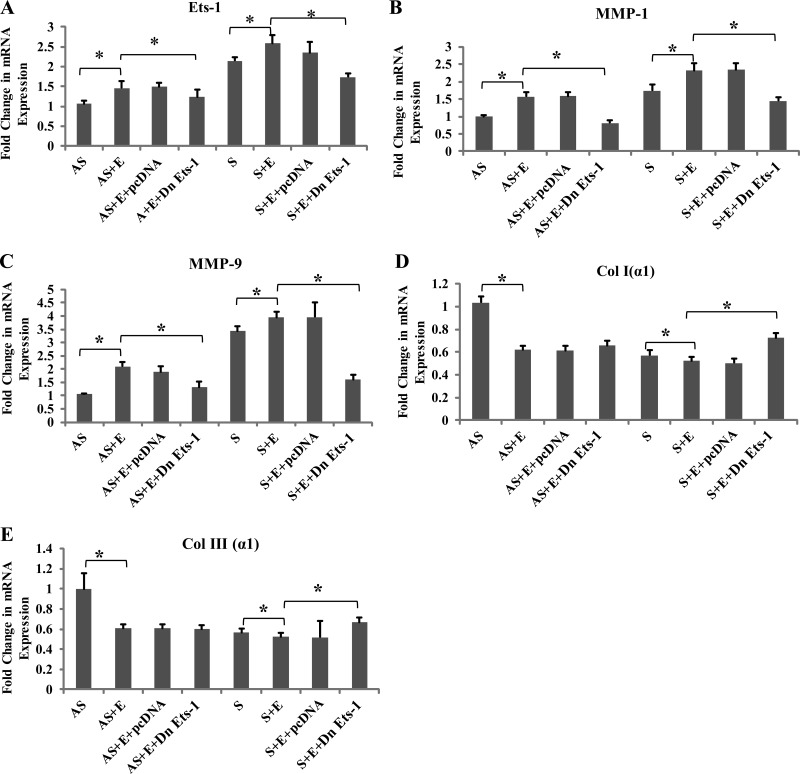
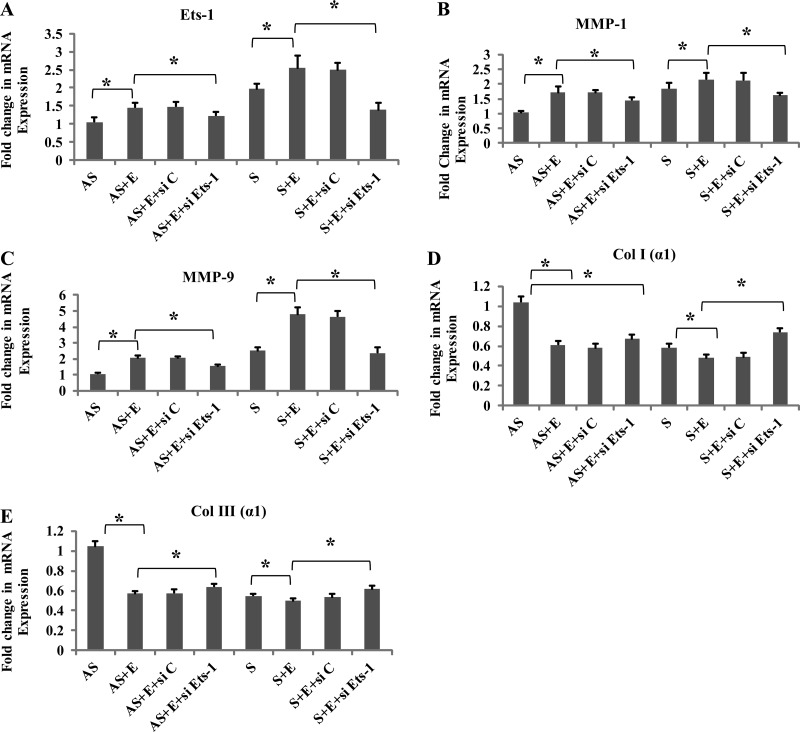
DN Ets-1 pcDNA and siRNA downregulate the expression of Col I (α1), and Col III (α1) mRNA transcripts in EGF-treated VSMCs.
Treatment with EGF significantly increased the MMP-1 and MMP-9 mRNA transcripts (Fig. 8, B and C; and Fig. 9, B and C) and decreased the expression of both Col I (α1) and Col III (α1) mRNA transcripts in VSMCs from S (Fig. 8, D and E; and Fig. 9, D and E). Transfection with DN Ets-1 or siRNA Ets-1 significantly attenuated the changes induced by EGF (Fig. 8, A–E; and Fig. 9, A–E), whereas transfection with control pcDNA did not influence either the MMP or the collagen genes. These results further confirm the role of Ets-1 in the stabilization of collagen in human carotid plaques.
Fig. 8.
Dominant negative (DN) Ets-1 transfection inhibits the mRNA expression of Ets-1, MMP-1, MMP-9, Col I (α1), and Col III (α1) in EGF. VSMCs were transfected with either pcDNA or DN-Ets-1 pcDNA, followed by treatment with EGF for 24 h. The isolated RNA was subjected to qPCR with gene-specific primers: Ets-1 (A), MMP-1 (B), MMP-9 (C), Col I (α1) (D), and Col III (α1) (E), and the results were expressed as fold change compared with AS group. Data are shown as means ± SD. *P < 0.05; N = 3.
Fig. 9.
Knockdown of Ets-1 gene on the expression of Ets-1, MMP-1, MMP-9, Col I (α1), and Col III (α1) in VSMC-treated EGF. VSMCs were transfected with either Ets-1 small interfering RNA (siRNA) or nonspecific control, followed by treatment with EGF for 24 h. The RNA was subjected to qPCR: Ets-1 (A), MMP-1 (B), MMP-9 (C), Col I (α1) (D), and Col III (α1) (E), and the results were expressed as fold change compared with AS group. Data are shown as means ± SD. *P < 0.05; N = 3.
DISCUSSION
Atherosclerosis is a progressive disease of the arterial wall and represents a major growing cause of death and disability worldwide. It is suggested that increased matrix is associated with plaque stability, whereas matrix degradation weakens the fibrous cap and increases its susceptibility to rupture.(4, 29, 55) The S carotid plaques are usually associated with ruptured atherosclerotic plaques with thin fibrous cap (23, 24, 42). The risk for atherosclerotic plaque rupture in S plaques is partially mediated by degradation of ECM by MMPs (10, 21, 33, 39). Recently, we have reported increased expression of MMP-1 and MMP-9 and decreased expression of collagen type I and III in VSMCs isolated from S plaques compared with AS plaques, supporting the role of collagen in plaque stability. These results are in agreement with similar studies (45, 50, 53). However, the molecular mechanism of MMP activation in S plaques is poorly understood. Therefore, we investigated the molecular mechanisms of signaling pathways involved in the EGF-mediated activation of MMP-1 and MMP-9, which may be closely related to collagen instability in S plaques.
The ability of EGF to stimulate MMPs depends on the specific signal transduction pathways. MAPKs play a crucial role in cell growth, differentiation, survival, and death (46). ERK-type MAPKs are activated by mitogenic growth factors and phorbol esters during cell proliferation and differentiation, whereas JNK and p38-MAPK are activated in response to inflammatory cytokines and stress stimuli (11, 46). In various cell types, different signaling pathways (Raf/MEK/ERK, p38-MAPK, and PI3K) were found to upregulate the expression of MMP-1 and MMP-9 (2, 12, 13, 38, 41, 56). In the present study we demonstrate that inhibitors of p38-MAPK and JNK-MAPK downregulated EGF-induced MMP-1 and MMP-9 and increased Col I (α1) and Col III (α1) mRNA transcripts in EGF-treated VSMCs, suggesting that two or more signaling pathways are involved in the regulation of MMPs in the atherosclerotic plaques. Although ERK-type MAPK activation plays an important role in MMP regulation in different cell types, our results suggest that it is not involved in the regulation of MMPs or collagens in EGF-treated VSMCs.
The promoter regions of MMPs and TIMPs contain activator protein 1 and Ets binding sites and are activated by EGF (22, 36, 41, 58). Ets-1 is one of the mediators of ECM remodeling associated with the regulation of MMPs and hence connected to ECM degradation (43). In response to stimuli, transcription factors containing the Ets binding motif have been implicated in the regulation of MMP genes (3, 5, 6, 15, 19, 34, 52, 59). Ets-1 is activated in VSMCs by a variety of stimuli including platelet-derived growth factor-BB, interleukin-1, and tumor necrosis factor-α (14, 30, 48, 60). Increased expression of various proteases that degrade the ECM was reported in Ets-1-overexpressing cells (7, 16, 20, 51, 54). However, the involvement of Ets-1 in EGF-induced MMP-1 and -9 expressions in carotid plaque VSMCs are not known. In this study we demonstrate that the expression of Ets-1 was significantly increased in VSMCs isolated from S compared with AS carotid plaques (Fig. 4, A and B). The EGFR inhibitor AG1478 significantly inhibited Ets-1 expression in EGF-treated VSMCs, demonstrating the involvement of EGFR. Transfection of an Ets-1 DNA plasmid further upregulates mRNA transcripts for MMP-1 and MMP-9 in VSMCs isolated from both AS and S carotid plaques, suggesting that Ets-1 regulates both MMP-1 and -9 expressions.
Members of the Ets family that are located in the collagen promoter region are believed to be associated with collagen gene expression (35, 40). Several ECM genes, including type I collagen, are regulated by Ets-1 (61). It has been shown that elevated expression of recombinant Ets-1 has the potential to suppress the transcription of type I collagen and increase matrix degradation in transforming growth factor-β-treated cells (28, 31, 47). In the present study, the finding of decreased mRNA expression of collagen type I and III in EGF-treated VSMCs suggests that Ets-1 regulates the expression of MMP-1 and MMP-9, leading to collagen loss in S compared with AS. Overexpression of either DN Ets-1 or Ets-1 siRNA suppressed the MMP transcripts and increased both collagen type I and III genes (Fig. 5, B–E), demonstrating the role of Ets-1 in the stability of plaques in VSMCs. Similar results were found with transfection of Ets-1 siRNA.
In summary, our results provide a mechanistic insight that EGF-induced Ets-1 regulates MMP-1, MMP-9, and collagen I and III expression through p38-MAPK and JNK signaling pathways (Fig. 10). The effect of the EGF on downstream signaling molecules is greater in S-plaque VSMCs than the AS-plaque VSMCs. This could be due to either increased EGFR density or increased transmembrane signaling pathways or both in S-plaque VSMCs than in AS-plaque VSMCs. This results in decreased interstitial collagens leading to plaque instability in patients with carotid stenosis. This study also provides the biochemical and molecular evidence that a selective blockade of both Ets-1 and EGFR may be a novel strategy and a promising target for stabilizing the vulnerable atherosclerotic carotid plaques.
Fig. 10.
A schematic diagram showing the role of EGF-regulated MMP-1 and -9 expressions through Ets-1 activation involving p38 and JNK-MAPK signaling pathways. The upregulated MMPs degrade the fibrillar collagens and basement membrane and destabilize the carotid plaques.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant RO1-HL-073349 (to D. K. Agrawal). The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.H.R. and D.K.A. conception and design of research; V.H.R., V.R., and S.S. performed experiments; V.H.R., V.R., and S.S. analyzed data; V.H.R., V.R., S.S., and D.K.A. interpreted results of experiments; V.H.R., V.R., and S.S. prepared figures; V.H.R., V.R., and D.K.A. drafted manuscript; V.H.R., V.R., S.S., and D.K.A. approved final version of manuscript; V.H.R., V.R., and D.K.A. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dane Marvin for assistance with the editing of this manuscript. We are also grateful to Dr. Stephen Lee (Department of Cellular and Molecular Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada) for providing a full-length human Ets-1 and dominant-negative Ets-1 constructs cloned into pCDNA3.1 (−) neovector.
REFERENCES
- 1.Adiguzel E, Ahmad PJ, Franco C, Bendeck MP. Collagens in the progression and complications of atherosclerosis. Vasc Med 14: 73–89, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft CC, Chen Z, Yeh J, Sunwoo JB, Yeh NT, Jackson S, Jackson C, Van Waes C. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-κB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer 99: 538–548, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-κB reduces matrix metalloproteinase-1,–3 and-9 production by vascular smooth muscle cells. Cardiovasc Res 50: 556–565, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Buja LM, Willerson JT. Role of inflammation in coronary plaque disruption. Circulation 89: 503–505, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269–285, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein 49: 20–37, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Czuwara-Ladykowska J, Shirasaki F, Jackers P, Watson DK, Trojanowska M. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem 276: 20839–20848, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Dhume AS, Agrawal DK. Inability of vascular smooth muscle cells to proceed beyond S phase of cell cycle, and increased apoptosis in symptomatic carotid artery disease. J Vasc Surg 38: 155–161, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Dittmer Jr. The biology of the Ets1 proto-oncogene. Mol Cancer 2: 29, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldrup N, Grønholdt ML, Sillesen H, Nordestgaard BG. Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation 114: 1847–1854, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Márquez A, Yagüe J, Cid MC. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood 94: 2754–2766, 1999. [PubMed] [Google Scholar]
- 12.Estève PO, Robledo O, Potworowski EF, St-Pierre Y. Induced expression of MMP-9 in C6 glioma cells is inhibited by PDGF via a PI 3-kinase-dependent pathway. Biochem Biophys Res Commun 296: 864–869, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Genersch E, Hayess K, Neuenfeld Y, Haller H. Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: involvement of Ras-dependent and-independent pathways. J Cell Sci 113: 4319–4330, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Goetze S, Kintscher U, Kaneshiro K, Meehan WP, Collins A, Fleck E, Hsueh WA, Law RE. TNFα induces expression of transcription factors c-fos, Egr-1, and Ets-1 in vascular lesions through extracellular signal-regulated kinases 1/2. Atherosclerosis 159: 93–101, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem 271: 10672–10680, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Hahne JC, Okuducu AF, Fuchs T, Florin A, Wernert N. Identification of ETS-1 target genes in human fibroblasts. Int J Oncol 38: 1645–1652, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Holterman CE, Franovic A, Payette J, Lee S. ETS-1 oncogenic activity mediated by transforming growth factor-β. Cancer Res 70: 730–740, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Jia G, Aggarwal A, Tyndall SH, Agrawal DK. Tumor necrosis factor-α regulates p27kip expression and apoptosis in smooth muscle cells of human carotid plaques via forkhead transcription factor O1. Exp Mol Pathol 90: 1–8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res 59: 812–823, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kita D, Takino T, Nakada M, Takahashi T, Yamashita J, Sato H. Expression of dominant-negative form of Ets-1 suppresses fibronectin-stimulated cell adhesion and migration through down-regulation of integrin α5 expression in U251 glioma cell line. Cancer Res 61: 7985–7991, 2001. [PubMed] [Google Scholar]
- 21.Kunte H, Amberger N, Busch MA, Rückert RI, Meiners S, Harms L. Markers of instability in high-risk carotid plaques are reduced by statins. J Vasc Surg 47: 513–522, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Ma C, Huang Y, Luo J, Huang C. Differential requirement of EGF receptor and its tyrosine kinase for AP-1 transactivation induced by EGF and TPA. Oncogene 22: 211–219, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol 91: 4–8, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med 8: 1257–1262, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol 23: 769–775, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mancini A, Di Battista JA. Transcriptional regulation of matrix metalloprotease gene expression in health and disease. Front Biosci 11: 423–446, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka H, Tsubaki M, Yamazoe Y, Ogaki M, Satou T, Itoh T, Kusunoki T, Nishida S. Tamoxifen inhibits tumor cell invasion and metastasis in mouse melanoma through suppression of PKC/MEK/ERK and PKC/PI3K/Akt pathways. Exp Cell Res 315: 2022–2032, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Mizui M, Isaka Y, Takabatake Y, Sato Y, Kawachi H, Shimizu F, Takahara S, Ito T, Imai E. Transcription factor Ets-1 is essential for mesangial matrix remodeling. Kidney Int 70: 298–305, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Molloy KJ, Thompson MM, Jones JL, Schwalbe EC, Bell PR, Naylor AR, Loftus IM. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation 110: 337–343, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Naito S, Shimizu S, Maeda S, Wang J, Paul R, Fagin JA. Ets-1 is an early response gene activated by ET-1 and PDGF-BB in vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C472–C480, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Nakano T, Abe M, Tanaka K, Shineha R, Satomi S, Sato Y. Angiogenesis inhibition by transdominant mutant Ets-1. J Cell Physiol 184: 255–262, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Newby AC, George SJ, Ismail Y, Johnson JL, Sala-Newby GB, Thomas AC. Vulnerable atherosclerotic plaque metalloproteinases and foam cell phenotypes. Thromb Haemost 101: 1006–1011, 2009. [PMC free article] [PubMed] [Google Scholar]
- 33.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 190: 300–309, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Oda N, Abe M, Sato Y. ETS-1 converts endothelial cells to the angiogenic phenotype by inducing the expression of matrix metalloproteinases and integrin β3. J Cell Physiol 178: 121–132, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Okano K, Hibi A, Miyaoka T, Inoue T, Sugimoto H, Tsuchiya K, Akiba T, Nitta K. Inhibitory effects of the transcription factor Ets-1 on the expression of type I collagen in TGF-β1-stimulated renal epithelial cells. Mol Cell Biochem 369: 247–254, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Okuducu AF, Zils U, Michaelis SAM, Mawrin C, von Deimling A. Increased expression of avian erythroblastosis virus E26 oncogene homolog 1 in World Health Organization grade 1 meningiomas is associated with an elevated risk of recurrence and is correlated with the expression of its target genes matrix metalloproteinase-2 and MMP-9. Cancer 107: 1365–1372, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2: 657–672, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Park MJ, Park IC, Lee HC, Woo SH, Lee JY, Hong YJ, Rhee CH, Lee YS, Lee SH, Shim BS. Protein kinase Ca activation by phorbol ester induces secretion of gelatinase B/MMP-9 through ERK 1/2 pathway in capillary endothelial cells. Int J Oncol 22: 137–143, 2003. [PubMed] [Google Scholar]
- 39.Peeters W, Hellings WE, De Kleijn DPV, de Vries J, Moll FL, Vink A, Pasterkamp G. Carotid atherosclerotic plaques stabilize after stroke insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol 29: 128–133, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Peng H, Tan L, Osaki M, Zhan Y, Ijiri K, Tsuchimochi K, Otero M, Wang H, Choy BK, Grall FT. ESE-1 is a potent repressor of type II collagen gene (COL2A1) transcription in human chondrocytes. J Cell Physiol 215: 562–573, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction 128: 355–363, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, Sukhova GK, Aikawa E, Aikawa M, Libby P. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler Thromb Vasc Biol 31: 2464–2472, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15: 237–254, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Rao VH, Kandel A, Lynch D, Pena Z, Marwaha N, Deng C, Watson P, Hansen LA. A positive feedback loop between HER2 and ADAM12 in human head and neck cancer cells increases migration and invasion. Oncogene 31: 2888–2898, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao VH, Kansal V, Stoupa S, Agrawal DK. MMP-1 and MMP-9 regulate epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells. Physiol Rep 2: 2014, e00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9: 180–186, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Sahin A, Vercamer C, Kaminski A, Fuchs T, Florin A, Hahne JC, Mattot V, Pourtier-Manzanedo A, Pietsch T, Fafeur V, Wernert N. Dominant-negative inhibition of Ets 1 suppresses tumor growth, invasion and migration in rat C6 glioma cells and reveals differentially expressed Ets 1 target genes. Int J Oncol 34: 377–389, 2009. [PubMed] [Google Scholar]
- 48.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 8: 395–405, 1993. [PubMed] [Google Scholar]
- 49.Sementchenko VI, Watson DK. Ets target genes: past, present and future. Oncogene 19: 6533–6548, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Shah PK, Galis ZS. Matrix metalloproteinase hypothesis of plaque rupture players keep piling up but questions remain. Circulation 104: 1878–1880, 2001. [PubMed] [Google Scholar]
- 51.Sherriff-Tadano R, Ohta A, Morito F, Mitamura M, Haruta Y, Koarada S, Tada Y, Nagasawa K, Ozaki I. Antifibrotic effects of hepatocyte growth factor on scleroderma fibroblasts and analysis of its mechanism. Mod Rheumatol 16: 364–371, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Shirasaki F, Makhluf HA, LeRoy C, Watson DK, Trojanowska M. Ets transcription factors cooperate with Sp1 to activate the human tenascin-C promoter. Oncogene 18: 7755–7764, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Sluijter JP, Pulskens WP, Schoneveld AH, Velema E, Strijder CF, Moll F, de Vries JP, Verheijen J, Hanemaaijer R, de Kleijn DP. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke 37: 235–239, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Suarez-Cuervo C, Merrell MA, Watson L, Harris KW, Rosenthal EL, Väänänen HK, Selander KS. Breast cancer cells with inhibition of p38α have decreased MMP-9 activity and exhibit decreased bone metastasis in mice. Clin Exp Metastasis 21: 525–533, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Svensson PA, Olson FJ, Hägg DA, Ryndel M, Wiklund O, Karlström L, Hulthe J, Carlsson LM, Fagerberg B. Urokinase-type plasminogen activator receptor is associated with macrophages and plaque rupture in symptomatic carotid atherosclerosis. Int J Mol Med 22: 459–464, 2008. [PubMed] [Google Scholar]
- 56.Thant AA, Nawa A, Kikkawa F, Ichigotani Y, Zhang Y, Sein TT, Amin AR, Hamaguchi M. Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin Exp Metastasis 18: 423–428, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Tsuchiya T, Tsuno NH, Asakage M, Yamada J, Yoneyama S, Okaji Y, Sasaki S, Kitayama J, Osada T, Takahashi K. Apoptosis induction by p38 MAPK inhibitor in human colon cancer cells. Hepatogastroenterology 55: 930–935, 2008. [PubMed] [Google Scholar]
- 58.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol 213: 355–364, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Watabe T, Yoshida K, Shindoh M, Kaya M, Fujikawa K, Sato H, Seiki M, Ishii S, Fujinaga K. The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int J Cancer 77: 128–137, 1998. [DOI] [PubMed] [Google Scholar]
- 60.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest 115: 2508–2516, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Znoyko I, Trojanowska M, Reuben A. Collagen binding α2β1 and α1β1 integrins play contrasting roles in regulation of Ets-1 expression in human liver myofibroblasts. Mol Cell Biochem 282: 89–99, 2006. [DOI] [PubMed] [Google Scholar]