Abstract
It has previously been postulated that the anaerobic work capacity (W′) may be utilized during resting blood flow occlusion in the absence of mechanical work. We tested the hypothesis that W′ would not be utilized during an initial range of time following the onset of resting blood flow occlusion, after which W′ would be utilized progressively more. Seven men completed blood flow occlusion constant power severe intensity handgrip exercise to task failure following 0, 300, 600, 900, and 1,200 s of resting blood flow occlusion. The work performed above critical power (CP) was not significantly different between the 0-, 300-, and 600-s conditions and was not significantly different from the total W′ available. Significantly less work was performed above CP during the 1,200-s condition than the 900-s condition (P < 0.05), while both conditions were significantly less than the 0-, 300-, and 600-s conditions (P < 0.05). The work performed above CP during these conditions was significantly less than the total W′ available (P < 0.05). The utilization of W′ during resting blood flow occlusion did not begin until 751 ± 118 s, after which time W′ was progressively utilized. The current findings demonstrate that W′ is not utilized during the initial ∼751 s of resting blood flow occlusion, but is progressively utilized thereafter, despite no mechanical work being performed. Thus, the utilization of W′ is not exclusive to exercise, and a constant amount of work that can be performed above CP is not the determining mechanism of W′.
Keywords: W′, critical power, exercise tolerance, blood flow occlusion
severe intensity exercise tolerance is defined by a hyperbolic relationship between power and time to exhaustion (21, 22, 26), and this power-duration relationship is of paramount importance in the field of integrative physiology (25). Critical power (CP) is the power asymptote of this relationship and is the defining boundary between the heavy- and severe-exercise intensity domains (2, 6, 17, 24, 26), while the anaerobic work capacity (W′) dictates the tolerable duration of severe intensity exercise (5, 10, 13, 19–21). The mechanisms determining W′ are associated with the depletion of intramuscular energy stores [e.g., phosphocreatine (PCr), glycogen, and O2 stores] (17, 19–21) and the accumulation of fatigue-inducing metabolites [e.g., inorganic phosphate (Pi) and hydrogen ions (H+)] (5, 10, 13, 17). Thus, the magnitude of the W′ is related to factors that may constrain or arise from these alterations in the myofiber milieu, such as the breadth of the severe intensity domain, the magnitude of the oxygen uptake slow component, and the development of fatigue (3, 4, 23, 30, 31).
Several previous studies have investigated the alterations in intramuscular [PCr] and muscle oxygenation during resting blood flow occlusion. Hamaoka et al. (16) demonstrated that muscle oxygenation progressively decreased to a nadir over the initial several minutes following the onset of resting blood flow occlusion, during which time [PCr] was not altered. At approximately the same time at which the nadir in muscle oxygenation occurred, the progressive depletion of [PCr] began. Lanza et al. (18) demonstrated approximately the same magnitude of [PCr] depletion for the same duration of resting blood flow occlusion. These findings suggest that resting metabolism is maintained via aerobic energy production utilizing tissue oxygen stores over the initial several minutes of resting blood flow occlusion, after which resting metabolism must be maintained via supplemental anaerobic energy production. The depletion of intramuscular energy stores and accumulation of fatigue-inducing metabolites consequent to the increased reliance on anaerobic energy production would be expected to diminish W′ despite no mechanical work being performed (i.e., rest). Consistent with this, we have previously suggested that W′ may be utilized in its entirety if the duration of resting blood flow occlusion is sufficient, despite no mechanical work being performed (1).
The purpose of the present investigation was to empirically assess the extent to which W′ would be utilized during resting blood flow occlusion. Specifically, we tested the hypothesis that W′ would not be utilized during an initial range of time following the onset of resting blood flow occlusion, after which W′ would be progressively utilized. Furthermore, we tested the hypothesis that the time of the onset in W′ utilization would not be significantly different from the muscle oxygenation nadir during resting blood flow occlusion.
METHODS
Experimental design.
All experimental procedures were approved by the Institutional Review Board of Kansas State University and conformed to the standards set by the Declaration of Helsinki. Written informed consent was obtained after the subjects were informed of the overall protocol and potential risks of participation. Seven healthy men (age: 26.0 ± 2.2 yr; height: 179 ± 4 cm; weight: 84.2 ± 12.1 kg) volunteered to participate in the study. Subjects were free of overt cardiovascular or metabolic disease, determined via medical health history evaluation.
Testing sessions were separated by at least 24 h, and the subjects were instructed to abstain from vigorous activity during the 24 h preceding each test. Subjects were further instructed to abstain from caffeine and alcohol consumption during the 2 and 12 h preceding each test, respectively. Two-handed handgrip exercise was performed for each test on a calibrated custom-built ergometer (2). Briefly, the ergometer is fixed to a pneumatic cylinder, by which resistance was adjusted via pressurization. A fixed linear displacement of 4 cm was provided for each complete contraction, and task failure was defined as the inability to successfully complete the requisite 4-cm displacement or maintain the requisite contraction frequency for three consecutive contraction cycles. Each contraction cycle was 3 s in duration, consisting of 1.5-s contraction and 1.5-s relaxation durations (i.e., 50% duty cycle and 20 contractions per min). An audio recording with the specific timing was used in union with feedback provided by an investigator to ensure correct timing.
After familiarization to the timing of the contraction cycle, a step incremental power output test (1.0 W + 0.5 W/min) was completed for the determination of peak power output (Ppeak), defined as the greatest power output for which at least half of the stage was completed. Subsequently, four constant-power testing sessions to task failure were completed at 17, 35, 80, and 135% Ppeak (selected to elicit task failure in ∼1–15 min) in randomized order. During the constant power testing sessions, brachial artery blood flow was occluded with a vascular cuff positioned around the brachial region of each arm, which was rapidly inflated (< 0.3 s) to suprasystolic pressures (≥275 mmHg) (E20 Rapid Cuff Inflator, Hokanson, Bellevue, WA). Blood flow occlusion was verified by the absence of a radial pulse, and the cuff pressures were continuously monitored to ensure ≥275 mmHg. The power and duration data from these four constant-power tests were fit with a two-parameter hyperbolic model (21, 26) to establish CP and W′ for each subject: [W′/(P − CP)], where t is the duration in s, W′ is the curvature constant in Joules (J), P is power in W, and CP is critical power in W.
After establishing the power-duration relationship for each subject, four additional randomly ordered blood flow occlusion constant-power testing sessions were conducted at 35% Ppeak (supra-CP for occlusion) with 300, 600, 900, and 1,200 s of resting blood flow occlusion prior to the commencement of the exercise. During the resting blood flow occlusion, subjects were positioned at the ergometer in the same position required for exercise. Brachial artery blood flow was occluded as described above during rest and exercise. The duration of exercise was determined from the onset of exercise following resting blood flow occlusion until task failure.
Resting W′ utilization.
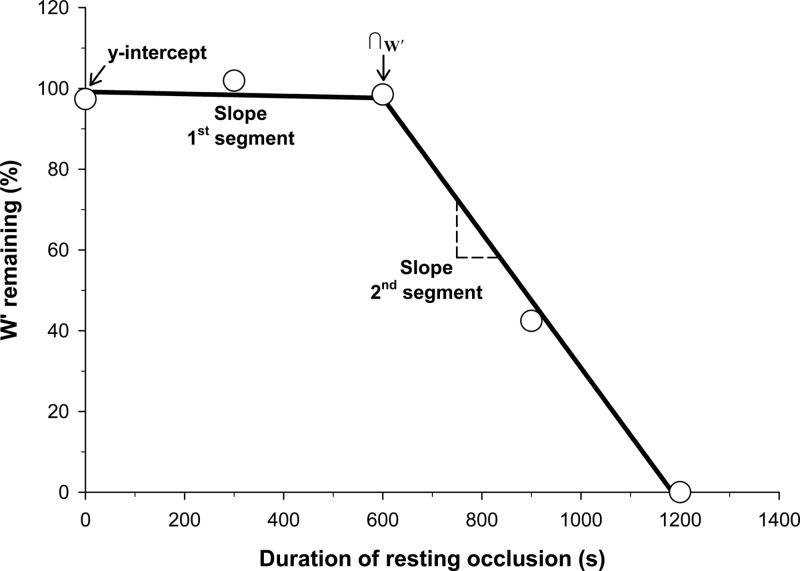
The amount of W′ utilized during each resting blood flow occlusion protocol was estimated from the total W′ available (established from the power-duration relationship) and the amount of work performed above CP during the post-resting occlusion exercise. The W′ expended during the exercise following resting blood flow occlusion was estimated by rearrangement of the two-parameter hyperbolic model: W′ = t(P − CP). Thus, any utilization of W′ during resting blood flow occlusion would decrease the amount of W′ available for supra-CP exercise, which would diminish the amount of work performed and the duration of exercise. The estimated W′ remaining after each resting blood flow occlusion condition was plotted as a function of the resting occlusion duration. A two-segment linear model was utilized to determine the y-intercept of the first linear segment, the slopes of the two linear segments, and the intersection of the two linear segments (∩W′) (Fig. 1). The ∩W′ was assumed to represent the time at which the W′ began to be utilized during resting blood flow occlusion. The slope of the second linear segment (J/min) was converted into units of oxygen uptake (ml O2/min) using the constant 0.047335 ml O2/J (derived from 0.000239006 kcal/J and 198 ml O2/kcal) (32).
Fig. 1.
Resting W′ utilization across each condition for subject 5 and a representation of the two-segment linear model for W′ utilization. W′ utilization was determined for each condition as the difference between the amount of work performed above critical power (CP) during each exercise bout following resting blood flow occlusion and the total W′ determined from the power duration relationship. The two-segment linear model was utilized to determine the y-intercept, the slope of the 1st linear segment, the slope of the 2nd linear segment, and the intersection of the two linear segments (∩W′) as a representation for the onset of W′ utilization.
Near-infrared spectroscopy and electromyography.
A frequency-domain multidistance near-infrared spectroscopy (NIRS) system (Oxiplex TS, ISS, Champaign, IL) was used to measure the oxygenation characteristics of the flexor digitorum superficialis of the right forearm during each testing session. Detailed descriptions of the principles and algorithms of the NIRS technology have previously been described (12, 15). Briefly, this device consists of one light-emitting diode (LED) detector fiber bundle and eight LEDs operating at wavelengths of 690 and 830 nm (four LEDs per wavelength). The LED detector fiber bundle separation distances are 2.0, 2.5, 3.0, and 3.5 cm. This NIRS device measures and incorporates the dynamic reduced scattering coefficients to provide absolute concentrations (μM) for deoxygenated-[hemoglobin + myoglobin] (deoxy-[Hb + Mb]), which is relatively insensitive to changes in blood volume (7, 11, 14) and has been used to reliably estimate the fractional oxygen extraction (7, 9, 11, 12, 14). Additionally, the NIRS device provides measures of total [Hb + Mb] (as oxygenated-[Hb + Mb] + deoxy-[Hb + Mb]), and %Saturation [Hb + Mb] (%Sat, as oxygenated − [Hb + Mb]/total − [Hb + Mb]). The NIRS probe was calibrated prior to each test, according to the manufacturer's recommendations. The belly of the flexor digitorum superficialis of the right arm was identified via palpation and strong electromyogram (EMG) activity when the fingers were flexed, but not with ulnar or radial deviation. The NIRS probe was secured along the belly of the flexor digitorum superficialis and was wrapped with an elastic bandage to prevent movement of the probe. The position of the NIRS probe was marked with indelible ink for reproducible placement throughout the study. The NIRS data were collected at 50 Hz and stored for post hoc analysis. The NIRS data were analyzed using 9-s time-binned mean values (covering three contraction cycles). The %Sat data from the resting occlusion portions of the 900- and 1,200-s protocols were fit using a monoexponential model: %Sat(t) = %Sat(b) − A[1 − e−(t−TD)/τ], where %Sat(t) is the %Sat at any point in time, %Sat(b) is the baseline %Sat prior to the onset of resting blood flow occlusion, A is the amplitude of the %Sat response, TD is the time delay prior to the decrease in %Sat, and τ is the time constant of the decrease in %Sat. The time at which the nadir in %Sat was attained was estimated at an interval equal to four time constants.
Surface EMG (Trigno EMG, Delsys, Boston, MA) single differential measurements were obtained during each test from the left flexor digitorum superficialis. The flexor digitorum superficialis was identified as described above. The position of the EMG sensor was marked with indelible ink for reproducible placement throughout the study. The EMG data were collected at a sampling rate of 1,000 Hz and band-pass filtered (13–400 Hz) using a fifth-order Butterworth filter. The filtered EMG activity corresponding to each muscle contraction was processed using MATLAB (MATLAB R2011a; The Mathworks, Natick, MA) to provide integrated EMG normalized to the first contraction (iEMG) and median power frequency (MedPF) for each muscle contraction. The EMG data were analyzed using 9-s time-binned mean values (covering three contraction cycles).
Statistical analysis.
All statistical analyses were performed using a commercially available software package (SigmaStat, Systat Software, Point Richmond, CA). Two-way repeated-measures ANOVAs (condition × time) were used to test for main effects for NIRS variables at baseline, the end of resting occlusion (i.e., at exercise onset), and at task failure. Two-way repeated-measure ANOVAs were used to test for main effects for the EMG variables at the first contraction and at task failure. One-way repeated-measures ANOVAs were used to test for main effects for exercise duration and W′ utilization. Student-Newman-Keuls post hoc analyses were conducted when significant main effects were detected. The %Sat exponential parameters for the 900- and 1,200-s conditions were compared using paired t-tests. Each slope of the two-segment linear model for resting W′ utilization was compared with zero using one-sample t-tests. The y-intercept of the first linear segment from the two-segment linear model for resting W′ utilization was compared with the total W′ available (established from the power-duration relationship) using paired t-tests. In addition, the y-intercept expressed as a percentage of the power-duration derived W′ was compared with 100% using a one-sample t-test. Differences were considered statistically significant when P < 0.05. All data are presented as means ± SD unless otherwise noted.
RESULTS
Resting W′ utilization.
The power-duration relationship determined from the initial four constant-power tests with blood flow occlusion yielded a CP of −0.5 ± 0.2 W and W′ of 751 ± 136 J. The subsequent 35% Ppeak constant-power tests were conducted at 2.1 ± 0.4 W. The durations of exercise following the 0, 300, 600, 900, and 1,200 s of resting blood flow were 298 ± 50 s, 302 ± 60 s, 291 ± 57 s, 235 ± 86 s, and 112 ± 66 s, respectively. Exercise duration was not significantly different between the 0-, 300-, and 600-s conditions. The duration of exercise was significantly shorter for both the 900- and 1,200-s conditions than the 0-, 300-, and 600-s conditions, and the 1,200-s condition was significantly shorter than the 900-s condition. The work performed above CP was not significantly different between the 0-, 300-, and 600-s conditions and was not significantly different from the total W′ available. Significantly less work above CP was performed for the 900-s condition than the 0- (P = 0.03), 300- (P = 0.04), and 600-s (P = 0.02) conditions and the 1,200-s condition than the 0 (P < 0.001), 300- (P < 0.001), 600- (P < 0.001), and 900-s (P < 0.001) conditions (Table 1). The work performed above CP during both the 900- and 1,200-s conditions was significantly less than the total W′ available.
Table 1.
Amount of work performed above CP following resting blood flow occlusion
| Duration of Resting Blood Flow Occlusion |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | 0 s | 300 s | 600 s | 900 s | 1200 s | |||||
| 1 | 831 | (110) | 729 | (97) | 676 | (90) | 459 | (61) | 421 | (56) |
| 2 | 807 | (99) | 787 | (97) | 858 | (106) | 763 | (94) | 603 | (74) |
| 3 | 885 | (104) | 872 | (102) | 885 | (104) | 761 | (89) | 160 | (19) |
| 4 | 725 | (102) | 757 | (107) | 706 | (100) | 569 | (80) | 214 | (30) |
| 5 | 522 | (97) | 546 | (102) | 528 | (99) | 227 | (42) | 0 | (0) |
| 6 | 684 | (106) | 724 | (112) | 655 | (101) | 590 | (91) | 449 | (69) |
| 7 | 946 | (100) | 1009 | (112) | 954 | (101) | 936 | (99) | 322 | (24) |
| Mean | 771 | (103) | 775 | (103) | 752 | (100) | 615 | (80)† | 310 | (40)‡ |
| SD | 142 | (4) | 143 | (6) | 151 | (5) | 232 | (21) | 202 | (27) |
Data are presented as joules (percentage of W′) performed above CP during constant power blood flow occlusion exercise following the specified durations of resting blood flow occlusion.
Significantly different from the 0-, 300-, and 600-s conditions (P < 0.05).
Significantly different from the 0-, 300-, 600-, and 900-s conditions (P < 0.001).
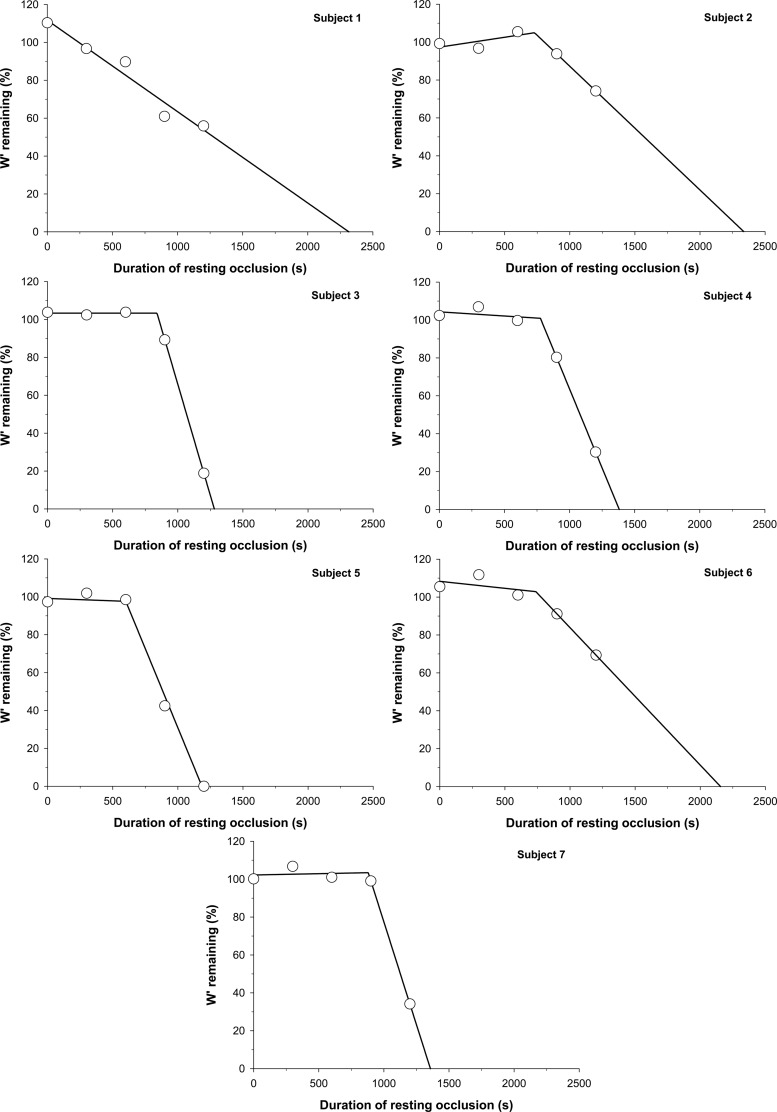
Six out of the seven subjects demonstrated an initial range of resting blood flow occlusion duration for which W′ utilization did not occur (Fig. 2). Subject 1 demonstrated a linear W′ utilization for all resting blood flow occlusion durations, with no apparent initial range of occlusion duration, where W′ was not altered. Of the six subjects who demonstrated this initial range, the amount of work performed above CP during these exercise tests ranged from 97 to 112% of the total W′, with a 3.9 ± 1.9% coefficient of variation. A two-segment linear model was fit to the data of these six subjects with an average r2 = 0.98 ± 0.02. The y-intercept value (779 ± 141 J) was not significantly different from the total W′ available (751 ± 136 J; P = 0.09). The ∩W′ (761 ± 98 s; Table 2) occurred significantly later than the attainment of the %Sat nadir (482 ± 139 s; P = 0.009). In absolute values, the slope of the first linear segment (0.002 ± 0.046 J/s) was significantly different than the slope of the second linear segment (−1.07 ± 0.71 J/s; P = 0.004). The slope of the first linear segment was not significantly different from zero (P = 0.95), while the slope of the second linear segment was significantly different from zero (P = 0.004). The estimated metabolic rate from the slope of the second linear segment was 3.0 ± 2.0 ml O2/min (Table 2).
Fig. 2.
Individual subject resting W′ utilization across each condition. W′ utilization was determined for each condition as the difference between the amount of work performed above critical power (CP) during each exercise bout following resting blood flow occlusion and the total W′ determined from the power-duration relationship.
Table 2.
Resting W′ utilization and estimated V̇o2
| Subject | CP, W | W′, J | ∩W′, s | W′ Utilization, J/min | Estimated V̇o2, ml/min |
|---|---|---|---|---|---|
| 1 | −0.8 | 753 | 21.8 | 1.0 | |
| 2 | −0.8 | 813 | 729 | 31.9 | 1.5 |
| 3 | −0.3 | 852 | 840 | 120.0 | 5.7 |
| 4 | −0.4 | 708 | 776 | 70.8 | 3.4 |
| 5 | −0.6 | 536 | 600 | 53.7 | 2.5 |
| 6 | −0.3 | 648 | 738 | 28.2 | 1.3 |
| 7 | −0.3 | 945 | 880 | 122.7 | 5.8 |
| Mean | −0.5 | 751 | 761 | 64.2 | 3.0 |
| SD | −0.2 | 136 | 98 | 42.5 | 2.1 |
CP, critical power; W′, anaerobic work capacity; ∩W′, intersection between no W′ utilization and W′ utilization; V̇o2, oxygen uptake estimated from the rate of W′ utilization.
In percent values, the y-intercept percentage of W′ from the two-segment linear model (104 ± 5%) was not significantly different from 100% of the total W′ available (P = 0.09). The slope of the first linear segment (−0.0004 ± 0.0062%/s) was significantly different than the slope of the second linear segment (−0.139 ± 0.076%/s; P = 0.002). The slope of the first linear segment was not significantly different from zero (P = 0.88), while the slope of the second linear segment was significantly different from zero (P = 0.002).
NIRS and EMG.
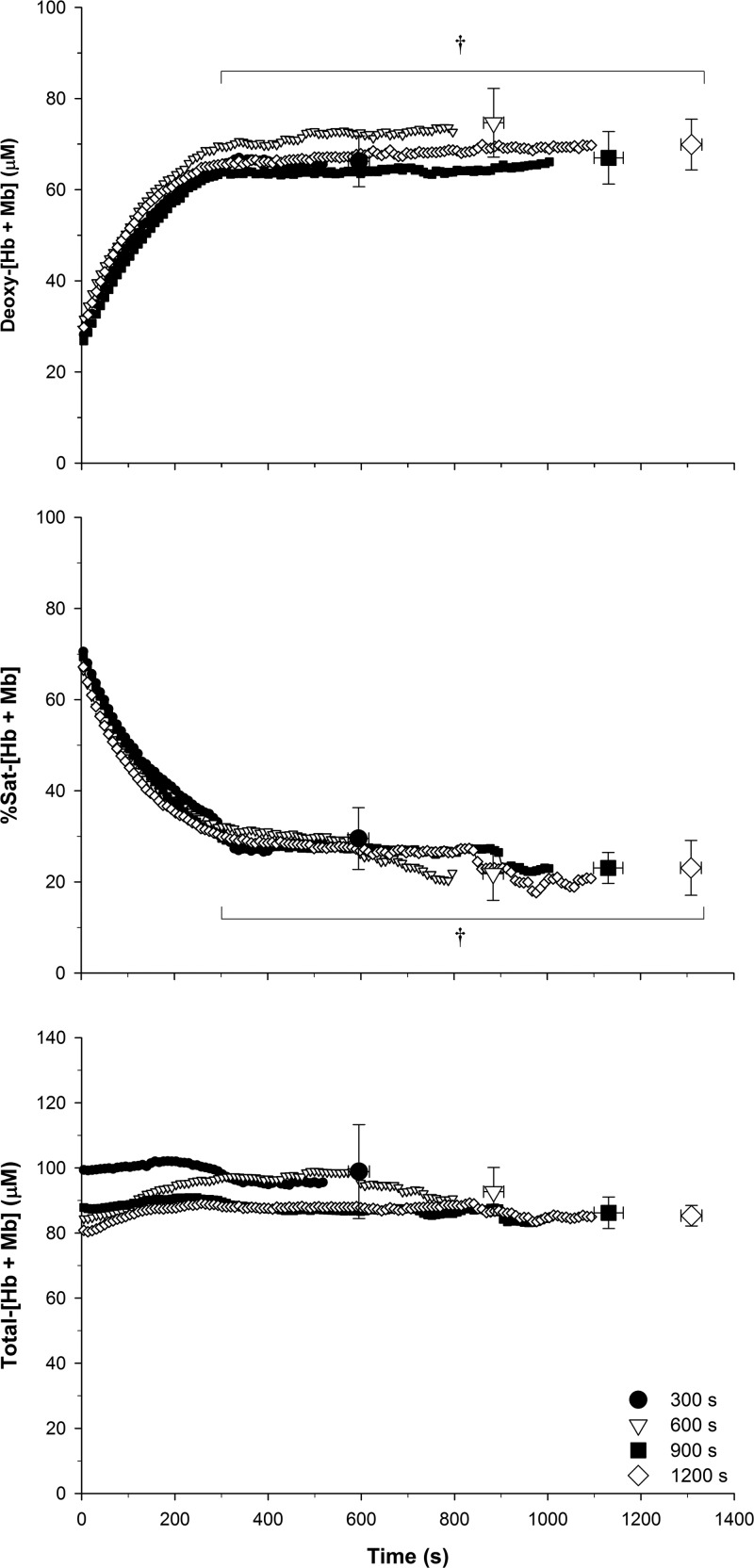
The NIRS data for each resting blood flow occlusion condition are presented in Table 3 and shown graphically in Fig. 3. Deoxy-[Hb + Mb] was not significantly different at baseline across each condition. Deoxy-[Hb + Mb] values at the end of resting occlusion (i.e., at the onset of exercise) and at task failure were significantly greater than baseline (P < 0.001), while there was no significant difference between the end of resting occlusion and task failure (P = 0.64). There were no significant differences in %Sat at baseline across each condition. %Sat was significantly below baseline at the end of resting occlusion and at task failure (P < 0.001), but there was no significant difference between the end of resting occlusion and task failure (P = 0.23). There were no significant differences in total-[Hb + Mb] between baseline, end of resting occlusion, and task failure. The %Sat exponential parameters were not significantly different between the 900- and 1,200-s conditions. Therefore, the data were analyzed in aggregate. The mean %Sat(b) was 67.8 ± 5.2%, A was 42.4 ± 9.0%, TD was 19.2 ± 19.5 s, τ was 122.3 ± 36.1 s, and r2 was 0.99 ± 0.01. The time at which the nadir %Sat was attained was 489 ± 144 s.
Table 3.
NIRS data for each duration of resting blood flow occlusion
| 0 s | 300 s | 600 s | 900 s | 1200 s | ||
|---|---|---|---|---|---|---|
| Deoxy-[Hb + Mb] (μM) | Baseline | 27.0 ± 3.8 | 27.0 ± 4.3 | 30.0 ± 6.0 | 25.9 ± 4.0 | 28.5 ± 5.3 |
| End Occ | 64.6 ± 16.7† | 72.7 ± 19.8† | 64.2 ± 16.2† | 71.2 ± 17.1† | ||
| Task failure | 68.6 ± 16.0† | 66.8 ± 15.1† | 76.0 ± 20.0† | 67.5 ± 15.4† | 70.3 ± 15.4† | |
| Total-[Hb + Mb] (μM) | Baseline | 90.0 ± 11.0 | 99.1 ± 23.7 | 85.0 ± 27.3 | 88.6 ± 9.8 | 81.8 ± 24.6 |
| End Occ | 98.9 ± 27.7 | 99.4 ± 19.5 | 87.9 ± 15.9 | 90.0 ± 10.8 | ||
| Task failure | 87.0 ± 12.8 | 99.6 ± 39.1 | 93.1 ± 19.2 | 86.6 ± 12.7 | 85.9 ± 8.6 | |
| %Sat (%) | Baseline | 69.9 ± 2.1 | 72.1 ± 5.6 | 68.8 ± 4.8 | 70.8 ± 3.3 | 69.0 ± 5.4 |
| End Occ | 33.2 ± 18.3† | 29.7 ± 14.9† | 27.6 ± 10.1† | 25.1 ± 14.5† | ||
| Task failure | 21.8 ± 13.9† | 29.1 ± 18.9† | 20.7 ± 16.3† | 22.9 ± 9.6† | 23.8 ± 13.9† |
Deoxygenated-[hemoglobin + myoglobin] (deoxy-[Hb + Mb]), total-[Hb + Mb], and %saturation-[Hb + Mb] (%Sat).
Data were obtained prior to blood flow occlusion (baseline), at the end of resting blood flow occlusion (End Occ), and at the end of exercise (task failure).
Significantly different from baseline (P < 0.001).
Fig. 3.
Mean near-infrared spectroscopy (NIRS) muscle oxygenation data throughout the 300-, 600-, 900-, and 1,200-s conditions. Deoxygenated-[hemoglobin + myoglobin] (deoxy-[Hb + Mb]), total-[Hb + Mb], and percent saturation-[Hb + Mb] (%Sat) during the resting blood flow occlusion and exercise portions of each condition. The time scale is from the onset of resting blood flow occlusion, such that the blood flow occlusion portion was the initial 300, 600, 900, and 1,200 s respectively, while the exercise portion was the duration following this time period until task failure. †End of resting occlusion and task failure is significantly different from baseline (P < 0.001). No significant differences were detected between conditions at baseline, end of resting occlusion, or task failure.
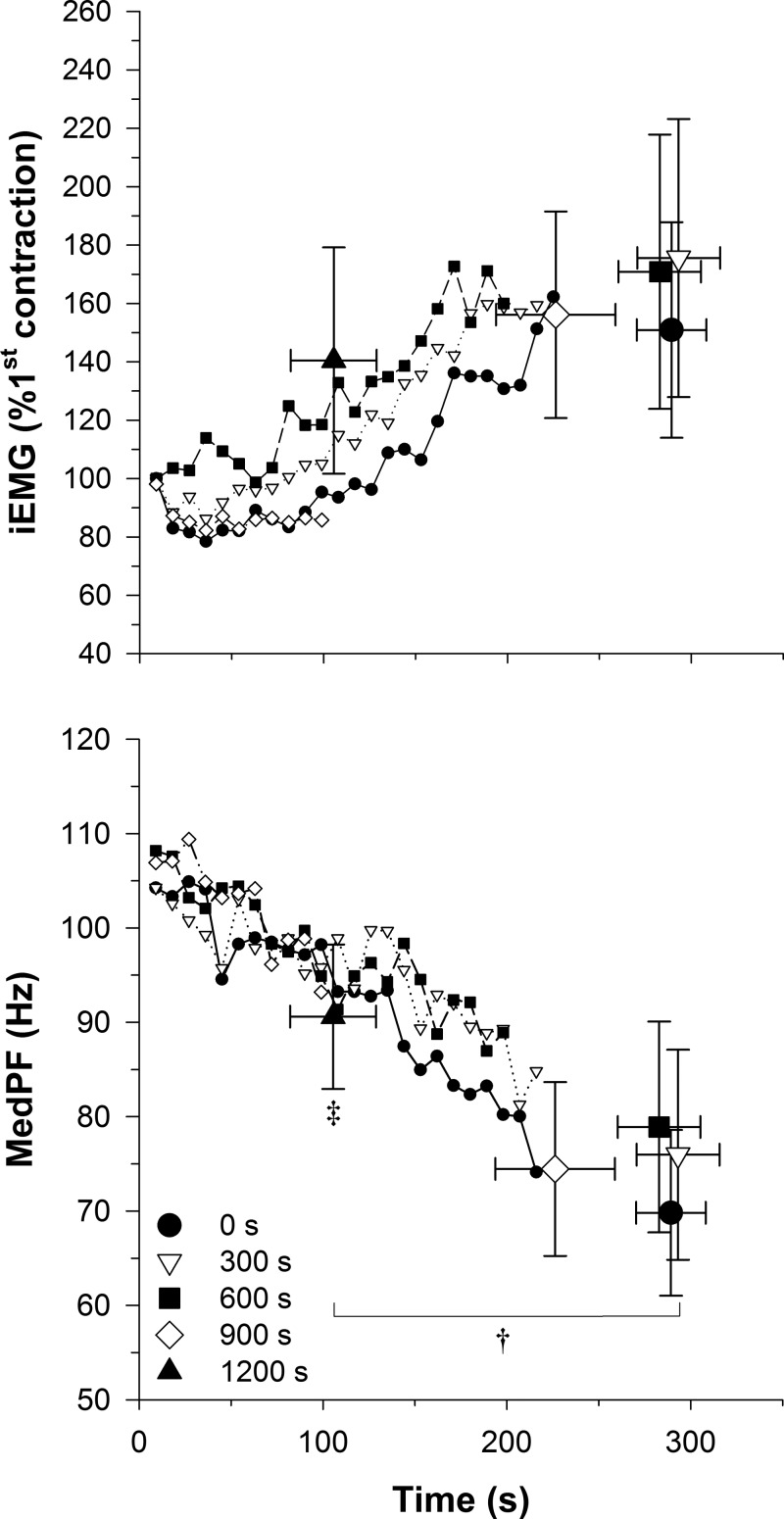
There were no main effects detected for iEMG or MedPF at the first contraction or at task failure. A significant main effect was detected for MedPF at task failure compared with the first contraction (P = 0.03), while no significant main effect was detected for iEMG (P = 0.08). The MedPF at task failure was significantly greater for the 1,200-s condition compared with the 0-, 300-, 600-, and 900-s conditions (P = 0.04) (Fig. 4).
Fig. 4.
Mean EMG data for each condition. Integrated EMG (iEMG) and median power frequency (MedPF) exercise data for each condition. †Significant main effect for task failure compared with the first contraction. ‡Significantly different from 0-, 300-, 600-, and 900-s conditions.
DISCUSSION
The current investigation empirically assessed the extent to which W′ may be utilized during resting blood flow occlusion. Consistent with our first hypothesis, W′ utilization in six of the seven subjects did not begin until ∼761 s into resting blood flow occlusion. However, Subject 1 demonstrated a linear W′ utilization across all of the resting blood flow occlusion conditions. There were not apparent differences with the other measured responses of Subject 1 compared with the other subjects. After ∩W′, W′ was utilized progressively more with longer resting blood flow occlusion durations, despite no mechanical work being performed. In contrast to our second hypothesis, the onset of W′ utilization during resting blood flow occlusion occurred after the attainment of the nadir in muscle oxygenation. The present investigation provides empirical evidence that the utilization of W′ is not exclusive to exercise and further supports that W′ may be dissociated from a constant amount of mechanical work performed above CP.
Influence of resting blood flow occlusion on W′.
A primary novel finding of the current investigation was that W′ (as the amount of work that can be performed above CP) was reduced following a sufficient duration of resting blood flow occlusion. This occurred despite no mechanical work being performed during the resting blood flow occlusion. This is consistent with our previous postulation that W′ may be utilized in its entirety if the duration of resting blood flow occlusion is sufficient, even though mechanical work is not being performed (1). Furthermore, Subject 5 in the current investigation was not able to complete a single contraction at the required power output following 1,200 s of resting blood flow occlusion, which was consistent with the predicted time for complete W′ utilization during resting blood flow occlusion for this subject. Previous findings suggest that resting metabolism is maintained via aerobic energy production over the initial several minutes of resting blood flow occlusion, after which resting metabolism is maintained via supplemental anaerobic energy production (16, 18). Consistent with this, W′ was not initially utilized during resting blood flow occlusion. However, prolonged resting blood flow occlusion did result in the utilization of W′, and the W′ utilization occurred at a rate of ∼3.1 ml O2/min, which is similar to previous direct measurements of resting metabolic rate obtained from the forearm (27). Thus, it appears that W′ may be utilized without muscle contraction and at a rate similar to resting metabolism.
It has previously been demonstrated that muscle oxygenation decreases over the initial several minutes of resting blood flow occlusion until a nadir is attained, after which [PCr] is progressively depleted (16). Consistent with this, muscle oxygenation decreased in a monoexponential fashion after a 19-s TD to a nadir at ∼490 s in the current investigation. Therefore, it is likely that the onset of [PCr] depletion occurred in close proximity to this time point. However, the onset of W′ utilization did not occur until later during resting blood flow occlusion. These findings may suggest that W′ is not solely dependent on [PCr], as the onset in [PCr] depletion likely occurred prior to the onset of W′ utilization. Consistent with this, exercise can be maintained for several minutes after the attainment of a nadir in [PCr] (30). These data suggest that additional mechanisms, such as the depletion of glycogen and O2 stores, as well as the accumulation of [Pi] and [H+] contribute to the determination of the W′. Conversely, these findings may suggest that the sensitivity for detecting the onset of W′ utilization may be less than the sensitivity for detecting the onset in [PCr] depletion. The standard error of W′ estimation is ∼13% for handgrip exercise, as measured in the current study and our previous work (2). During 600 s of resting blood flow occlusion, there is an approximate 9.0% depletion in [PCr], which represents ∼10% of the total [PCr] depletion that occurs during ischemic handgrip exercise (16, 18). Therefore, the relatively small decrease in [PCr] over this duration may not be sufficient to measurably reduce the amount of work performed during exercise. Moreover, the alterations occurring in O2 and glycogen stores and [Pi] and [H+] may also not be sufficient to reduce the amount of work performed. The current findings cannot distinguish the role of energy store depletion and fatigue-inducing metabolite accumulation in determining W′, but they do demonstrate that W′ utilization does not occur initially during resting blood flow occlusion. In toto, these findings suggest that work may not be the determinant of W′. Rather, it appears that other mechanisms associated with anaerobic metabolism determine W′, which then constrain the amount of work that can be performed above CP.
Muscle oxygenation and EMG characteristics.
During resting blood flow occlusion, muscle deoxygenation progressively increased up to a plateau, as oxygen was utilized without replenishment. There was no further change in muscle deoxygenation with the onset of muscle contraction and the %Sat remained elevated above zero throughout each protocol. These results further support that muscle oxygenation decreases until the capillary-mitochondria Po2 gradient attains equilibrium with the inherent resistance to diffusion (ḊO2−1) (1), so that complete deoxygenation is not attained in the microvasculature (1, 8, 18, 28, 29) during blood flow occlusion.
The pattern of change was similar across each exercise bout for the iEMG and MedPF. However, MedPF at task failure was greater for the 1,200-s condition than the other conditions. These results suggest that the rate of change in the EMG variables was not affected by resting blood flow occlusion. Rather, longer durations of resting blood flow occlusion appear to result in task failure prior to the full expression of the EMG characteristics. Consistent with previous findings (1), attainment of a constant EMG value is not requisite for task failure across exercise conditions.
Methodological considerations.
In the current study, the NIRS and EMG measurements were obtained in separate limbs due to the limited surface area over the flexor digitorum superficialis muscle relative to the size of the instruments. Thus, the measurements obtained from each limb may differ. The NIRS field of interrogation relative to the active muscle mass must also be considered when interpreting NIRS-derived measurements. For large-muscle mass (e.g., quadriceps) exercise, the NIRS field of interrogation is relatively small compared with the active muscle mass. However, an advantage to the current exercise model is that the NIRS field of interrogation is more closely matched to the depth and length of the active muscle mass, allowing for more adequate characterization of the entire muscle. Another experimental consideration is the use of the two-segment linear model for characterizing the W′ remaining after five durations of resting blood flow occlusion. The data were also well fit with an exponential model. The onset of W′ utilization determined with the exponential model was shifted earlier in three subjects and later in three subjects and as such, was not significantly different from ∩W′ determined with the two-segment linear model (761 ± 98 vs. 755 ± 380 s, P = 0.98). Therefore, the two-segment linear model was utilized, as it provided the best fit to the data and is consistent with previous [PCr] time course data during resting blood flow occlusion (16, 18). Additional testing may be needed to better distinguish the most appropriate model for characterizing resting W′ utilization. Moreover, intramuscular metabolic measurements were not measured during the resting blood flow occlusion tests. Therefore, we cannot be certain of the time of the onset in [PCr] depletion. However, Hamaoka et al. (16) demonstrated that the attainment of a nadir in muscle oxygenation occurred at approximately the same time as the onset in [PCr] depletion with resting blood flow occlusion. Therefore, we utilized the time at which muscle oxygenation attained this nadir as an approximation of the onset of [PCr] depletion. Therefore, the current data cannot specifically determine how much each potential mechanism contributed to the utilization of W′. However, this was not the aim of the current study. Rather, the study was designed to determine whether W′ (as the amount of work performed above CP) was altered with resting blood flow occlusion.
Conclusions.
In the present investigation, resting blood flow occlusion for durations up to 600 s did not result in the utilization of W′. However, the amount of W′ available to be utilized for severe-intensity exercise was progressively reduced following 900 and 1,200 s of resting blood flow occlusion, suggesting that W′ was utilized during these durations of resting blood flow occlusion, despite no mechanical work being performed. In addition, a nadir in muscle oxygenation was attained prior to the onset of W′ utilization during resting blood flow occlusion. The time course of muscle oxygenation and W′ utilization, as well as the rate of W′ utilization during resting blood flow occlusion, suggest that metabolism is initially maintained via aerobic energy production, utilizing tissue oxygen stores, which does not utilize W′. The progressive contribution of anaerobic energy production with prolonged resting blood flow occlusion results in the progressive utilization of W′. The current findings demonstrate that W′ utilization is not exclusive to exercise and may be dissociated from a constant amount of mechanical work performed above CP, supporting other mechanisms that are anaerobic in nature as determinants of W′.
GRANTS
This work was supported by the National Aeronautics and Space Administration (NASA) under Grant NNX10AK60G awarded to T. J. Barstow and by NASA Experimental Program to Stimulate Competitive Research (EPSCoR) under Grant NNX11AM05A supporting R. M. Broxterman.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.M.B., J.C.C., C.J.A., S.L.W., and T.J.B. conception and design of research; R.M.B., J.C.C., and S.L.W. performed experiments; R.M.B. and T.J.B. analyzed data; R.M.B., J.C.C., C.J.A., S.L.W., and T.J.B. interpreted results of experiments; R.M.B. prepared figures; R.M.B. drafted manuscript; R.M.B., J.C.C., C.J.A., S.L.W., and T.J.B. edited and revised manuscript; R.M.B., J.C.C., C.J.A., S.L.W., and T.J.B. approved final version of manuscript.
REFERENCES
- 1.Broxterman RM, Ade CJ, Craig JC, Wilcox SL, Schlup SJ, Barstow TJ. Influence of blood flow occlusion on muscle deoxygenation characteristics and the parameters of the power-duration relationship. J Appl Physiol 118: 880–889, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Broxterman RM, Ade CJ, Wilcox SL, Schlup SJ, Craig JC, Barstow TJ. Influence of duty cycle on the power-duration relationship: Observations and potential mechanisms. Respir Physiol Neurobiol 192: 102–111, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Broxterman RM, Craig JC, Smith JR, Wilcox SL, Jia C, Warren S, Barstow TJ. Influence of blood flow occlusion on the development of peripheral and central fatigue during small muscle mass handgrip exercise. J Physiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnley M, Jones AM. Oxygen uptake kinetics as a determinant of sports performance. Eur J Sport Sci 7: 63–79, 2007. [Google Scholar]
- 5.Coats EM, Rossiter HB, Day JR, Miura A, Fukuba Y, Whipp BJ. Intensity-dependent tolerance to exercise after attaining V̇o2 max in humans. J Appl Physiol 95: 483–490, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Copp SW, Hirai DM, Musch TI, Poole DC. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588: 5077, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Blasi RA, Cope M, Elwell C, Safoue F, Ferrari M. Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. Eur J Appl Physiol Occup Physiol 67: 20–25, 1993. [DOI] [PubMed] [Google Scholar]
- 8.De Blasi RA, Cope M, Ferrari M. Oxygen consumption of human skeletal muscle by near-infrared spectroscopy during tourniquet-induced ischemia in maximal voluntary contraction. Adv Exp Med Biol 317: 771–777, 1992. [DOI] [PubMed] [Google Scholar]
- 9.DeLorey DS, Kowalchuk JM, Paterson DH. Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate intensity exercise. J Appl Physiol 95: 113–120, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson CS, Whipp BJ, Cathcart AJ, Rossiter HB, Turner AP, Ward SA. Effects of prior very-heavy intensity exercise on indices of aerobic function and high-intensity exercise tolerance. J Appl Physiol 103: 812–822, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari M, Binzoni T, Quaresima V. Oxidative metabolism in muscle. Philos Trans R Soc Biol Sci 352: 677–683, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Effects of pedal frequency on estimated muscle microvascular O2 extraction. J Appl Physiol 96: 558–563, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Fukuba Y, Miura A, Endo M, Kan A, Yanagawa K, Whipp BJ. The curvature constant parameter of the power-duration curve for varied-power exercise. Med Sci Sports Exerc 35: 1413–1418, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P. Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95: 149–158, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gratton E, Fantini S, Franceschini MA, Gratton G, Fabiani M. Measurements of scattering and absorption changes in muscle and brain. Philos Trans R Soc Biol Sci 352: 727–735, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaoka T, Iwane H, Shimomitsu T, Katsumura T, Murase N, Nishio S, Osada T, Kurosawa Y, Chance B. Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. J Appl Physiol 81: 1410–1417, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Jones AM, Wilkerson DP, DiMenna FJ, Fulford J, Poole DC. Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294: R585–R593, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Lanza IR, Wigmore DM, Befroy DE, and Kent-Braun JA. In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577: 353–367, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura A, Kino F, Kajitani S, Sato H, Sato H, Fukuba Y. The effect of oral creatine supplementation on the curvature constant parameter of the power-duration curve for cycle ergometry in humans. Jpn J Physiol 49: 169–174, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Miura A, Sato H, Sato H, Whipp BJ, Fukuba Y. The effect of glycogen depletion on the curvature constant parameter of the power-duration curve for cycle ergometry. Ergonomics 43: 133–141, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Monod H, Scherrer J. The work capacity of a synergic muscular group. Ergonomics 8: 329–338, 1965. [Google Scholar]
- 22.Moritani T, Nagata A, DeVries HA, Muro M. Critical power as a measure of physical work capacity and anaerobic threshold. Ergonomics 24: 339–350, 1981. [DOI] [PubMed] [Google Scholar]
- 23.Murgatroyd SR, Ferguson CS, Ward SA, Whipp BJ, Rossiter HB. Pulmonary O2 uptake kinetics as a determinant of high-intensity exercise tolerance in humans. J Appl Physiol 110: 1598–1606, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Poole DC. Resolving the determinants of high-intensity exercise performance. Exp Physiol 94: 197–198, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Poole DC, Barstow TJ. The critical power framework provides novel insights into fatigue mechanisms. Exerc Sport Sci Rev 43: 65–66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31: 1265–1279, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Richards JC, Crecelius AR, Kirby BS, Larson DG, Dinenno FA. Muscle contraction duration and fibre recruitment influence blood flow and oxygen consumption independent of contractile work during steady-state exercise in humans. Exp Physiol 97: 750–761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roca J, Agusti AGN, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at V̇o2 max. J Appl Physiol 73: 1067–1076, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD. Evidence for tissue diffusion limitation of V̇o2 max in normal humans. J Appl Physiol 67: 291–299, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration work relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol 95: 528–540, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Vanhatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs. all-out sprint exercise. Am J Physiol Regul Integr Comp Physiol 300: R700–R707, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]