Abstract
We previously identified aldo-keto reductase 1b7 (AKR1B7) as a marker for juxtaglomerular renin cells in the adult mouse kidney. However, the distribution of renin cells varies dynamically, and it was unknown whether AKR1B7 maintains coexpression with renin in response to different developmental, physiological, and pathological situations, and furthermore, whether similar factor(s) simultaneously regulate both proteins. We show here that throughout kidney development, AKR1B7 expression—together with renin—is progressively restricted in the kidney arteries toward the glomerulus. Subsequently, when formerly renin-expressing cells reacquire renin expression, AKR1B7 is reexpressed as well. This pattern of coexpression persists in extreme pathological situations, such as deletion of the genes for aldosterone synthase or Dicer. However, the two proteins do not colocalize within the same organelles: renin is found in the secretory granules, whereas AKR1B7 localizes to the endoplasmic reticulum. Interestingly, upon deletion of the renin gene, AKR1B7 expression is maintained in a pattern mimicking the embryonic expression of renin, while ablation of renin cells resulted in complete abolition of AKR1B7 expression. Finally, we demonstrate that AKR1B7 transcription is controlled by cAMP. Cultured cells of the renin lineage reacquire the ability to express both renin and AKR1B7 upon elevation of intracellular cAMP. In vivo, deleting elements of the cAMP-response pathway (CBP/P300) results in a stark decrease in AKR1B7- and renin-positive cells. In summary, AKR1B7 is expressed within the renin cell throughout development and perturbations to homeostasis, and AKR1B7 is regulated by cAMP levels within the renin cell.
Keywords: aldosterone synthase, renin, juxtaglomerular cell, cyclic adenosine monophosphate, Dicer
the renin-angiotensin system (RAS) is responsible for maintaining blood pressure and fluid-electrolyte homeostasis. A key point in the regulation of RAS is control of the synthesis and secretion of the hormone renin within highly specialized juxtaglomerular (JG) renin cells in the kidney. Throughout embryonic development and into adult life, renin-producing cells show a remarkable plasticity, and their location and number change in a dynamic but stereotyped manner. Initially, renin cells are found along large arteries of the developing kidney, but as the animal matures, renin expression is progressively restricted downstream toward the glomerulus, until renin is confined to the adult JG location (8, 9, 30). When homeostasis is threatened, adult animals are capable of recruiting renin-expressing cells along the arterioles. This process does not involve cellular migration or proliferation, but instead dedifferentiation of formerly renin-expressing cells from a subset of smooth muscle cells lining the renal arteries (29). A similar phenomenon occurs with a subset of glomerular mesangial cells derived from cells that expressed renin earlier in development (29). Interestingly, these cells retain the capability to reexpress renin in response to physiological stress that necessitates an increase in renin production, such as hypotension, blood loss, or sodium depletion (29). This remarkable ability to switch phenotypes has long been an area of interest, as identifying the molecules that establish and regulate the identity of the renin cell may have important implications, not only in the study of homeostatic regulation, but also for our understanding of cell memory and/or plasticity.
One barrier to the study of the renin cell has been the lack of a reliable and independent marker for renin cells aside from renin itself, as manipulations that directly affect the expression of renin (and may not otherwise modify the renin cell) will prevent identification of the cell. Using microarray analysis, we have previously identified one enzyme, aldo-keto reductase family 1, subfamily B member 7 (AKR1B7), which was highly enriched in the adult renin cell of mice (2). AKR1B7 belongs to an enzymatic superfamily of more than 100 aldo-keto reductases whose members can be found in many organ systems throughout a multitude of vertebrate and invertebrate animal species. Within the mouse, AKR1B7 expression has previously been described in the vas deferens and the adrenal glands (1, 21). However, little is known about the suitability of AKR1B7 as a marker for renin cells, particularly under developmental, physiological, and pathological situations that modify renin expression and renin cell number.
In addition to studying the expression pattern of AKR1B7, we explored whether factors already known to regulate renin expression also regulate AKR1B7, as identification of molecular pathways that coregulate AKR1B7 and renin could give insight into an overarching program responsible for regulating the phenotype of the renin cell. One potential candidate is cAMP. Our laboratory and others have previously shown that cAMP upregulates renin expression in vitro and in vivo, and that deletion of elements of the cAMP-response pathway blunts the organism's expression of renin and its ability to respond to homeostatic challenge (10, 22, 24). Therefore, we hypothesize that cAMP regulates AKR1B7 within the kidney as well.
MATERIALS AND METHODS
Immunohistochemistry for renin and AKR1B7.
To study the ontogeny of AKR1B7, kidneys were harvested from fetal, newborn, and adult mice and fixed and prepared for immunostaining, as previously described (30). Antigen retrieval was conducted by boiling for 10 min in 10 mM sodium citrate, 0.5% Tween-20, and pH 6.0 before exposure to our antibody against renin [rabbit polyclonal 1:500 dilution, (29)] or AKR1B7 [goat polyclonal, 1:200 dilution, Santa Cruz Biotechnology (2)]. Visualization was performed using the appropriate Vectastain Elite ABC kit (Vector Laboratories), and sections were counterstained with hematoxylin. Kidney sections were mounted and examined under a microscope (Leica DFC 480) and imaged with a digital camera (Leica DFC310 FX).
Intracellular localization of AKR1B7.
To define the intracellular localization of AKR1B7, we performed immunofluorescence for AKR1B7, renin, and markers of lysosomes and endoplasmic reticulum. Kidneys were fixed, embedded in paraffin, and sectioned, as previously described. Sections were deparaffinized and rehydrated using Histo-clear (National Diagnostics) and a succession of alcohols. Antigen retrieval was accomplished by boiling the sections in a solution of 5 mM EDTA and 1 mM Tris·HCl (pH 6.0). Kidney sections were incubated overnight at 4°C with primary antibody against renin, AKR1B7, protein disulfide isomerase (PDI, a marker for the endoplasmic reticulum; 1:500; Sigma-Aldrich), or lysosome-associated membrane protein 1 (LAMP1, 1:500; Abcam), a marker for lysosomes. Sections were exposed to Alexa Fluor-488 donkey anti-goat and Alexa Fluor-568 donkey anti-rabbit (1:500 dilution) secondary antibodies and counterstained with DAPI (Invitrogen). Slides were mounted using Permount (Fischer Scientific) and imaged using a Deltavision confocal microscope. Images were taken at 0.12-μm thickness and processed using Softwerx deconvolution software.
Animals.
To understand the expression and regulation of AKR1B7 and renin, we studied mice with manipulations known to affect renin expression. Mice studied included wild-type C57BL6 mice (Jackson Laboratories) and several genetically modified mouse strains, including deletion of aldosterone synthase (AS−/−), which displays a prominent increase in the number of renin cells along the arterioles (14), deletion of the micro-RNA-processing enzyme Dicer in renin cells (Dicerflox/del; Ren1dcre/+), which results in a severe decrease in renin cells (31); deletion of the renin gene (Ren1c-KO) (32); homozygous deletion of histone acetyl transferases CBP and P300 in renin cells (CBPfl/fl × P300fl/fl × Ren1dcre/+), which shows a marked diminution of renin expression (10); ablation of renin cells with diphtheria toxin (Ren1d-DTA) (23); and expression of YFP driven by the Ren1c promoter (Ren1c-YFP), which reports activity of the renin promoter (24). A minimum of three animals per group were examined. Physiological challenge to induce increased renin expression was done using 7-day treatment with captopril and administering a low-sodium diet as previously described (3). All animals were handled in accordance with the National Institutes of Health guidelines for the care and use of experimental animals, and the study was approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Isolation of renin cells.
Renin-expressing cells were isolated from kidney cortex of 1-mo-old animals using FACS sorting, as previously described (3), utilizing our Ren1c-YFP reporter mice. RNA was isolated using the RNAqueous-Micro kit (Ambion) and was reverse transcribed and amplified as described previously (24).
Quantitative PCR.
To measure mRNA for renin and GAPDH in cell culture, we conducted quantitative PCR (qPCR), as described previously (24), by reverse transcribing mRNA isolated from cells and amplified using the following primers: GAPDH [5′-AAC TTT GGC ATT GTG GAA GGG CTC-3′ (forward) and 5′-ACC AGT GGA TGC AGG GAT GAT GTT-3′ (reverse)], and AKR1B7 [5′-CAG ATT GAG AGC CAC CCT TA-3′ (forward) and 5′-TGG GAA TCT CCA TTA CTA CG-3′ (reverse)].
Cell culture.
To examine expression and regulation of AKR1B7 in vitro, we isolated and cultured CFP-expressing arterial smooth muscle cells of the renin lineage, which also carried a YFP-expressing renin promoter (CFP/YFP cells), as previously described (24). Cells were exposed to either 10 mM forskolin to stimulate cAMP production or DMSO vehicle, as previously described (24), every 24 h until harvesting after 2 days, with an additional treatment 1 h before harvest. mRNA was isolated, reverse-transcribed to cDNA, and quantified using qPCR, as earlier described (10).
Statistical analysis.
qPCR experiments were performed in triplicate, and qPCR measurements were conducted three times per replicate. All results are presented as means ± SE. Statistical significance between two groups was determined by unpaired Student's t-test. A P value ≤0.05 was considered significant.
RESULTS
Ontogeny of AKR1B7 expression.
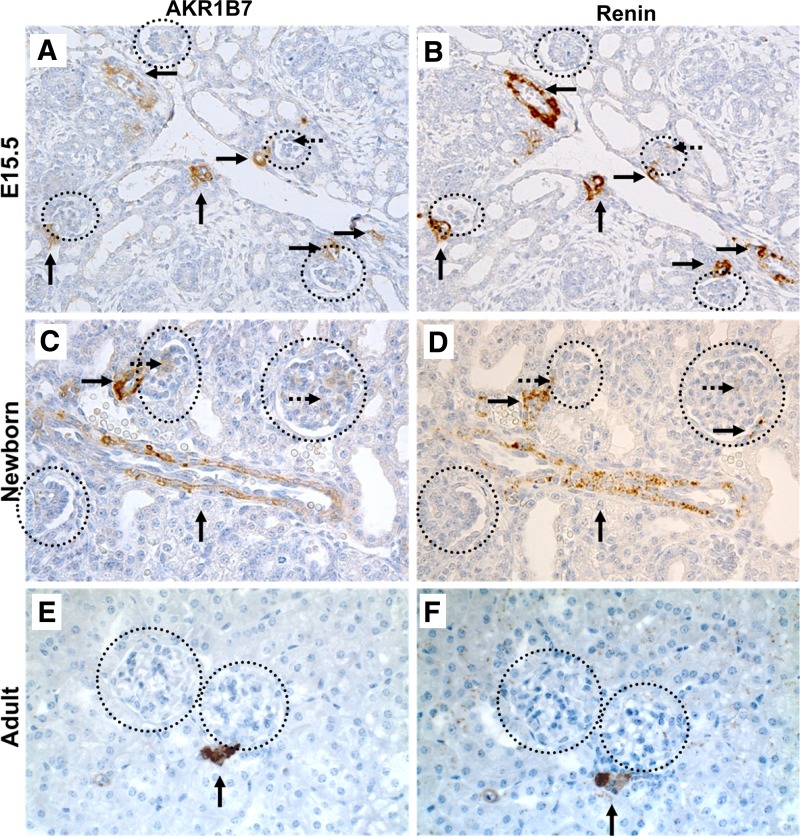
Previous work done in our laboratory using microarray analysis and immunostaining showed expression of AKR1B7 in the renin cells of adult mice (2). In the present study, we examined the pattern of AKR1B7 expression during kidney maturation and whether coexpression of AKR1B7 with renin was maintained throughout the dynamic changes of renin cell localization that occur during renal development. Immunostaining for AKR1B7 at various ages showed a pattern of regressing staining along the arterioles that exactly duplicated the well-established developmental distribution of renin. Within the embryonic kidney at embryonic day 15.5, AKR1B7 expression was primarily found along the large arteries of the kidney (Fig. 1A). Staining was also seen near mature glomeruli outside the nephrogenic zone, and occasionally in the glomerular mesangium. In the newborn mouse (postnatal day 1), AKR1B7 expression was segregated to developing afferent arterioles and smaller arteries, along with JG areas (Fig. 1C), while in adulthood, AKR1B7 was confined to the few cells adjacent to the mature glomerulus that form the JG apparatus (Fig. 1E). We confirmed the colocalization of AKR1B7 and renin using staining in consecutive kidney sections. AKR1B7 staining was always accompanied by renin staining in the corresponding region (Fig. 1, B, D, F), demonstrating a consistent association between the expression patterns of the two proteins throughout renal development.
Fig. 1.
Immunostaining for AKR1B7 and renin in consecutive sections at various ages. A and B: AKR1B7 and renin staining in embryonic day 15.5 (E15.5) kidneys. Staining is found along large vessels (arrows) and in the glomerular mesangium (dashed arrows). C and D: AKR1B7 and renin staining in kidneys from newborn animals. Staining is found in arteries (arrows) and juxtaglomerular (JG) areas. E and F: AKR1B7 and renin staining in adult (postnatal day 45) kidneys. Staining is limited primarily to JG areas (arrow). At all stages, AKR1B7 staining colocalizes to the same areas as renin. Circles indicate glomeruli.
Expression of AKR1B7 following pharmacological or genetic manipulation.
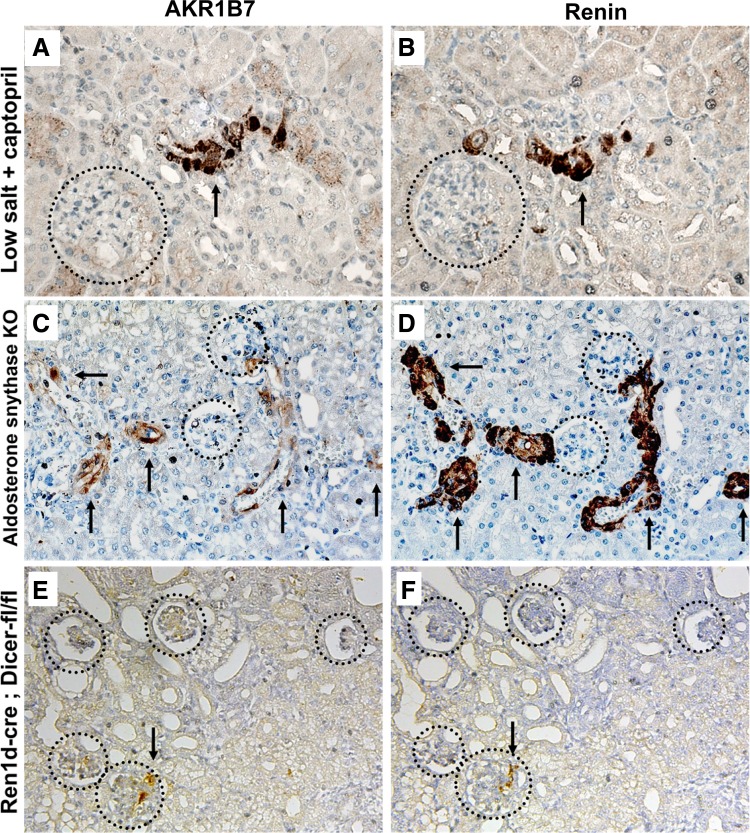
The colocalization of renin and AKR1B7 throughout kidney development suggests that the two proteins may respond similarly to manipulations known to affect renin synthesis and the distribution of renin cells. We first examined AKR1B7 expression using a well-established method of physiological and pharmacological manipulation that increases renin levels: mice treated with the angiotensin-converting enzyme inhibitor captopril and fed a low-salt diet show reexpression of renin along the renal arterioles to compensate for diminished blood pressure and electrolyte-fluid depletion (11, 25). Immunostaining for AKR1B7 in treated mice showed a concomitant increase in the number of AKR1B7-positive cells (Fig. 2A). Expression of AKR1B7 extended along the afferent arteriole and could be found occasionally within the cells of the glomerular mesangium, in a pattern reminiscent of embryonic life. Staining with renin in consecutive sections showed coincident expression along the renal arterioles and mesangial cells (Fig. 2B), confirming that AKR1B7 expresses alongside renin in conditions of homeostatic stress, as well as basal state. The reverse result was obtained when examining animals fed a high-salt diet. The decrease in renin-positive cells and fewer renin-positive juxtaglomerular regions was accompanied by a decrease in the number of AKR1B7-positive cells (not shown).
Fig. 2.
Immunohistochemistry for AKR1B7 and renin in consecutive sections under manipulations known to increase renin expression. Circles indicate glomeruli, and arrows indicate staining in arterioles. A and B: the domain of AKR1B7 and renin expression expands in animals treated with captopril and fed a low-salt diet, in a pattern reminiscent of that observed during embryonic development. C and D: AKR1B7 and renin in kidneys from mice homozygous for aldosterone synthase deletion. While the number of labeled cells increases significantly along arteries and arterioles, the increase in intensity of renin expression is not matched by AKR1B7. E and F: AKR1B7 and renin in kidneys from mice with conditional deletion of Dicer in renin cells. The number of renin- and AKR1B7-positive cells is markedly reduced.
We also investigated the expression of both proteins under pathological conditions previously shown to severely alter renin expression levels. Mice in which the gene for aldosterone synthase had been deleted, exhibit a dramatic expansion of renin expression along the kidney arteries (14, 18) to a degree far greater than sodium depletion and captopril. The number of renin-positive cells and the amount of renin in each cell increase dramatically, with renin expression extended along almost the entirety of the afferent arteriole and interlobular arteries. Immunostaining in consecutive sections showed that these renin-positive cells also express AKR1B7 (Fig. 2, C and D), with many AKR1B7-positive cells lining the arteries and arterioles. Although not directly comparable, we note that the increase in the intensity of AKR1B7 staining within each individual cell was not as dramatic as that of renin. The opposite result was found in mice with conditional deletion of the micro-RNA-processing enzyme Dicer in renin cells, which have significantly fewer renin-positive JG cells and lower plasma renin levels. The decrease in renin expression is accompanied by a reduction in the domain of AKR1B7 staining, with fewer AKR1B7-positive cells present in the knockout kidneys. However, AKR1B7 still coincides specifically with renin in the few remaining renin-positive JG areas (Fig. 2, E and F).
AKR1B7 localizes primarily to the plasma membrane and endoplasmic reticulum.
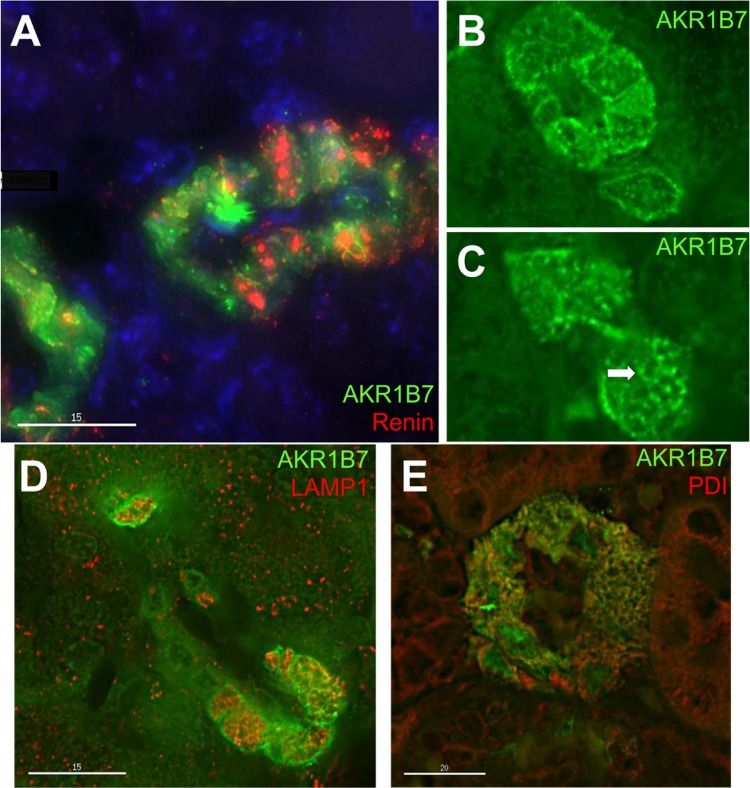
To confirm coexpression of renin and AKR1B7 in the same cell and to characterize the subcellular localization of AKR1B7, we conducted dual immunofluorescence and confocal microscopy of adult mouse kidney sections. Both proteins were consistently found in the same cells of the juxtaglomerular apparatus (Fig. 3A), with renin localized to round bodies in the cytoplasm, corresponding to the secretory granules of JG cells. AKR1B7, however, was not associated with the same subcellular structures as renin, as AKR1B7 staining was found in distinct regions within the cytoplasm (Fig. 3B), and occasionally near the plasma membrane. The subcellular localization of AKR1B7 did not change when examining mice at earlier developmental timepoints (newborn, not shown) or mice treated with captopril and fed a low-sodium diet (not shown).
Fig. 3.
Confocal immunofluorescence for AKR1B7, renin, and organelle markers in JG cells. A: immunofluorescence for AKR1B7 (green) and renin (red), with counterstaining with DAPI (blue). The two proteins are found in the same cells, but seldom coincide subcellularly (yellow). B and C: immunofluorescence for AKR1B7 (green). AKR1B7 can be found in or near the plasma membrane (B), and also in lattice-shaped structures in the cytoplasm (C, arrow). D: Immunostaining for AKR1B7 (green) and LAMP1 (red), a marker for lysosomes. The two only show occasional colocalization. E: AKR1B7 (green) and protein disulfide isomerase (red), a marker for endoplasmic reticulum. The two show extensive colocalization (yellow).
Interestingly, we frequently observed AKR1B7 in net-like, latticework structures (Fig. 3C), suggestive of association with a particular subcellular structure. To investigate AKR1B7 localization to a specific organelle, we conducted costaining with subcellular markers. JG cells have prominent lysosomes, and it is widely thought that the renin granules derive from lysosomes (7, 33). However, costaining with LAMP1, a lysosomal membrane protein, showed only occasional colocalization with AKR1B7 (Fig. 3D). By contrast, costaining AKR1B7 with PDI, an endoplasmic reticulum (ER) resident protein, demonstrated strong colocalization with AKR1B7 (Fig. 3E), with coincident staining in the previously described net-like pattern, indicating that AKR1B7 also localizes to the ER in renin cells.
AKR1B7 is a renin-independent marker for renin cells.
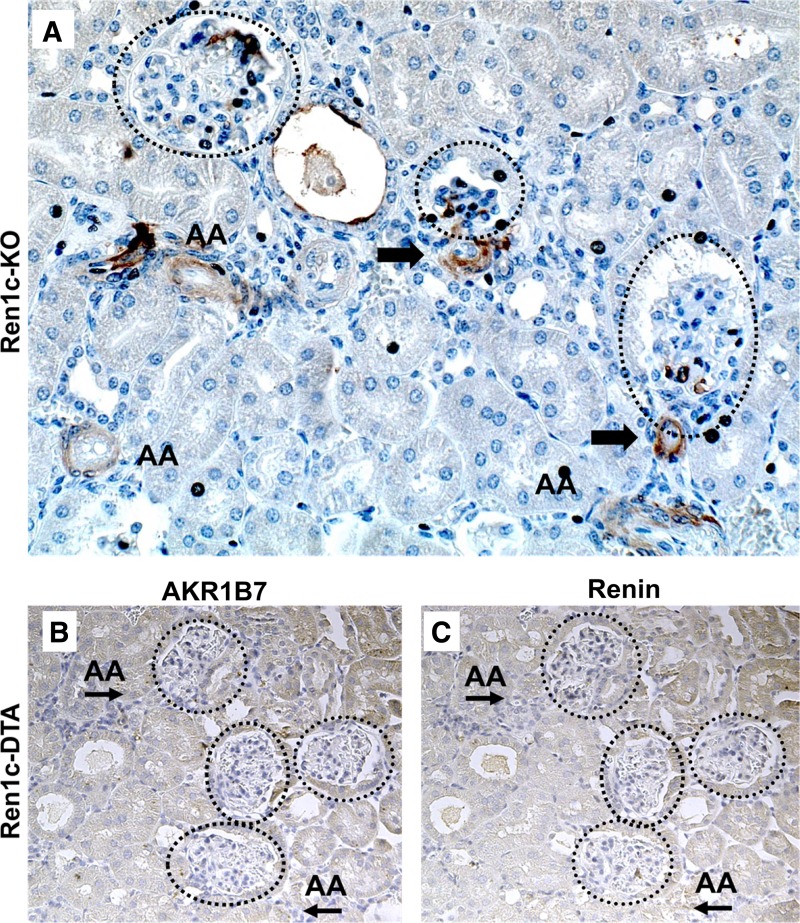
As mentioned earlier, studies of renin cells have been complicated by the fact that the only reliable marker for cells of the renin phenotype was renin itself. To test whether AKR1B7 would label cells in the absence of renin expression, we immunostained for AKR1B7 in kidneys from mice with deletion of the renin gene. Global renin knockout mice have kidneys with prominent morphological defects, including vascular thickening and a complete absence of renin staining (32). However, AKR1B7 staining is preserved in cells adjacent to the glomerulus (Fig. 4A, arrows). In addition, we found significant extension of AKR1B7 expression along afferent arterioles (AA) and occasional staining of the mesangial cells in glomeruli (circles). Interestingly, this pattern is strongly reminiscent of the distribution of renin cells during conditions that acutely or chronically stimulate renin production (see Fig. 2, A–D). We conclude that the renin cell persists in renin knockout mice and continues to express the marker protein AKR1B7, despite an inability to produce renin.
Fig. 4.
AKR1B7 expression is independent of renin and is only absent upon deletion of renin cells. A: immunostaining for AKR1B7 in Ren1c KO mice. Mice homozygous for deletion of renin show extensive staining for AKR1B7 in JG cells (arrow), cells in the wall of the afferent arterioles (AA) of the kidney, and in mesangial cells of the glomeruli (circles). B and C: AKR1B7 and renin in kidneys from mice where renin cells have been ablated by the conditional expression of diphtheria toxin A (DTA). No positive staining can be found near glomeruli (circles) or in afferent arterioles (AA).
A contrasting observation was made in mice where renin cells have been ablated using expression of diphtheria toxin chain A (DTA) under control of the renin promoter [Ren1d-DTA, (23)]. Kidneys from those animals lack renin cells, leading to a disruption of vascular and tubular patterning that is not seen in renin knockout animals. We detected no AKR1B7 in Ren1d-DTA kidneys (Fig. 4, B and C), and AKR1B7 signal was absent from any of the cell types that typically arise from renin cells and might be induced to reexpress renin, such as JG areas, arterial smooth muscle cells, or mesangial cells. Thus, while AKR1B7 expression persisted in the absence of the renin gene, deletion of the renin cell itself resulted in the complete abrogation of AKR1B7 expression within the kidney, emphasizing that AKR1B7 marks cells specifically programmed to express renin.
cAMP regulates the expression of AKR1B7.
We have shown here that AKR1B7 and renin are consistently expressed together, raising the important possibility that the same mechanisms that regulate renin levels also regulate AKR1B7. To examine the regulation of AKR1B7 on a cellular level, we utilized our previously described CFP/YFP cell culture system (24). Briefly, CFP/YFP cells are derived from isolated kidney arteriolar smooth muscle cells of the renin lineage that were labeled permanently with CFP using the Cre/Lox system. When grown in culture, these cells do not express renin at a basal state, but upon appropriate stimulation, reactivate renin expression and report renin promoter activity through YFP expression [Ren1c-YFP, (24)]. In this manner, CFP/YFP cells mimic the in vivo ability of smooth muscle cells of the renin lineage (CFP+) to reacquire the ability to express renin (acquiring YFP+), and are an appropriate platform to study renin regulation.
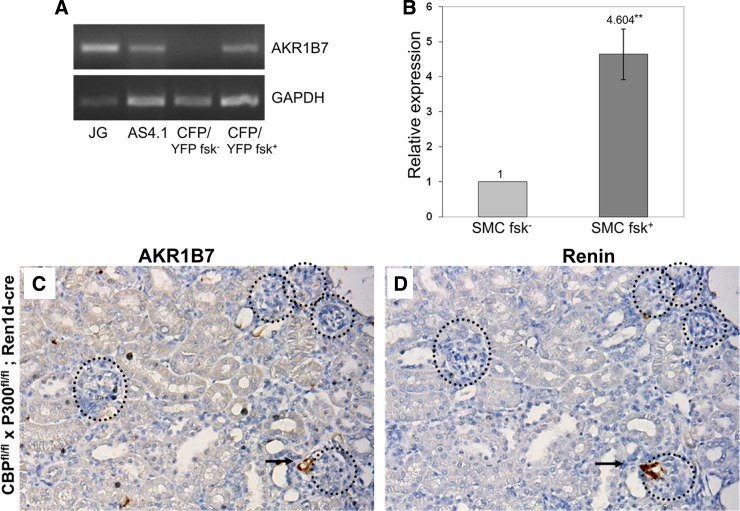
As we have previously shown that treatments that increase intracellular cAMP, such as incubation with forskolin (27), cause CFP/YFP cells to reacquire renin expression and become YFP-positive (24), we tested whether stimulation of the cAMP pathway would also increase AKR1B7 expression in our cell culture model. Figure 5A shows that unstimulated CFP/YFP cells expressed only low amounts of AKR1B7 (“CFP/YFP fsk−”). However, forskolin-treated CFP/YFP cells, in addition to activating the renin promoter (24), also increased AKR1B7 message levels (“CFP/YFP fsk+”). As positive controls, we observed that FACS-sorted juxtaglomerular renin cells (isolated from Ren1c-YFP animals), contained significant amounts of AKR1B7 message (“JG”). Interestingly, we find that As4.1 cells (“As4.1”), a kidney tumor cell line that constitutively expresses renin (34), also contained AKR1B7 mRNA. To quantify the increase in expression, we conducted qPCR on RNA isolated from control and forskolin-treated CFP/YFP cells and found a more than four-fold increase in AKR1B7 message (Fig. 5B) after 48 h of forskolin treatment. This confirmed that in cells of the renin lineage, control of AKR1B7 expression, like renin, is cAMP-sensitive.
Fig. 5.
AKR1B7 expression is regulated by the cAMP signaling pathway. A: PCR products for AKR1B7 from mRNA isolated from JG cells. We detect AKR1B7 mRNA in JG cells (lane 1) and AS4.1 cells (lane 2), a kidney tumor cell line that expresses renin. AKR1B7 message is nearly absent from unstimulated cells of the renin lineage (lane 3), but appears in cells of the renin lineage stimulated with forskolin (lane 4). B: AKR1B7 mRNA levels as measured with qPCR increase nearly five-fold in cells of the renin lineage after treatment with forskolin compared with treatment with DMSO vehicle (anchored to a value of one), P < 0.005. C and D: significantly fewer AKR1B7- and renin-positive glomeruli are found in mice where two elements of cAMP-response, the genes CBP and p300, have been deleted. Circles indicate glomeruli, and arrows indicate areas of coincident staining.
To investigate this effect in vivo, we examined animals that had key elements of the cAMP response pathway knocked out. Animals with conditional deletion (in renin cells) of two important mediators of cAMP response, p300, and CREB-binding protein (CBP) [CBPfl/fl × P300fl/fl; Ren1dcre/+, (10)], have markedly fewer renin-positive JG cells. Similarly, those animals had significantly fewer AKR1B7-positive cells (Fig. 5C), all found within JG areas. Staining in consecutive sections showed that AKR1B7 was still segregated specifically to those cells that still expressed renin (likely due to incomplete deletion) (Fig. 5D). Thus, the data indicate that the cAMP pathway controls expression of both AKR1B7 and renin in vivo and in vitro.
DISCUSSION
The present series of experiments demonstrate that AKR1B7 is expressed in the same cells as renin throughout the dynamic changes in renin cell distribution during kidney development, and in response to pharmacological and pathological manipulations that increase or decrease the number of cells expressing renin. Moreover, we show that AKR1B7 is a renin-independent marker for cells attempting to make renin and that only complete ablation of renin cells using DTA resulted in an absence of AKR1B7 protein. Finally, we demonstrated the key role of the cAMP signaling pathway in regulating the expression of AKR1B7 both in vitro and in vivo.
AKR1B7 expresses specifically in renin cells throughout a variety of manipulations.
We have previously shown that AKR1B7 was highly enriched in renin-producing cells of the adult mouse, by means of both microarray study and immunostaining (2). In this article, we show using immunostaining that coexpression of renin and AKR1B7 occurs throughout all stages of fetal and postnatal kidney development. Confocal microscopy combined with coimmunofluoresence of AKR1B7 and renin confirmed definitively that the two proteins are coexpressed in the same cell. Thus, AKR1B7 labels renin cells throughout renal development and can serve as a marker for renin cells when assaying directly for renin is not possible or practical. We also show that the coexpression of AKR1B7 and renin is maintained under physiological and pharmacological manipulations of renin levels. In the same way that smooth muscle cells along the afferent arteriole reacquire the ability to express renin (29) in response to a low-salt diet and administration of captopril, they also reacquire the ability to express AKR1B7, demonstrating that AKR1B7 is part of a larger genetic program reactivated by former renin-expressing cells in response to a need to increase renin levels. This idea was reinforced by the observation that when the domain of renin cells contracts in response to a high-salt diet, AKR1B7 is likewise restricted to the reduced-in-number renin-positive cells. Thus, when cells cease the renin-expressing program, they also stop synthesizing AKR1B7.
We also examined AKR1B7 expression under genetic manipulations, which have been previously demonstrated to cause widespread pathological alterations of renin expression. Global deletion of the aldosterone synthase gene results in hypotension, abnormal fluid and electrolyte homeostasis, and significantly elevated renin production accompanied by an increase in renin cells. We show that these cells are also positive for AKR1B7, indicating the coordinated response of AKR1B7 to this extreme homeostatic need. By contrast, conditional deletion of the microRNA-processing enzyme Dicer within renin cells results in fewer renin cells and lower plasma renin levels, in addition to lower blood pressure, vascular abnormalities, and renal fibrosis. Here, we demonstrate that the few remaining renin-positive cells are still positive for AKR1B7. This finding also implicates a possible role for micro-RNAs to regulate AKR1B7, although the identity of the micro-RNAs that regulate renin and AKR1B7 in the renin cell is a matter of ongoing study (20).
Regardless of the manipulation, the result was the same: as the expression domain of renin expands, AKR1B7 is also found in the cells that acquire the ability to express renin. When renin expression decreases and cells cease expressing renin, those cells also stop expressing AKR1B7. Only complete ablation of renin cells using diphtheria toxin (Ren1c-DTA) results in abolition of AKR1B7 expression within the kidney, emphasizing the specificity of AKR1B7 in the kidney for renin cells.
The specific coexpression of AKR1B7 with renin raises the question of AKR1B7 function in the renin cell. One possibility is that the two proteins exert direct regulatory effects on each other and are in the same direct molecular pathway upstream or downstream from each other. However, data generated in this study and in other laboratories' studies suggest that this is not the case. Confocal microscopy shows that AKR1B7 and renin are localized to different organelles (the endoplasmic reticulum and lysosomes, respectively) and, thus, are unlikely to directly interact, although this would need to be confirmed using other assays such as coimmunoprecipitation or FRET. We also note that, interestingly, the amino acid sequence of AKR1B7 lacks the canonical NH2-terminal ER localization motif lysine-aspartic acid-glutamic acid-leucine (KDEL), and it remains to be determined what mechanism causes the localization of AKR1B7 to the ER. The absence of cross-regulation between AKR1B7 and renin was further supported by our data, showing the persistence of AKR1B7 expression after deletion of the renin gene. Conversely, Machura et al. (17) show that systemic deletion of AKR1B7 does not alter the levels of renin transcription and expression. Thus, it is likely that AKR1B7 plays a role in parallel to renin as part of a larger genetic program of establishing the renin cell phenotype but that AKR1B7 does not directly interact with renin.
Other alternatives exist for a role for AKR1B7 in the kidney. Endocrine cells may be particularly vulnerable to oxidative stress due to their increased levels of protein synthesis. Interestingly, AKR1B7 has been shown to possess a protective role in adrenal cells by catalyzing the reduction of isocaproaldehyde, a by-product of hormone synthesis associated with oxidative stress, into less toxic forms (19). In addition, analysis of adrenal tumors showed that tumoral cells had blunted AKR1B7 expression (15), with oxidative stress being a hallmark of tumorigenesis. It is possible that AKR1B7 functions in a similar role in renin secretory cells to protect against accumulated oxidative stress involved in the renin cell's primary duties of renin synthesis and granulopoeisis. This hypothesis would be supported by AKR1B7s localization to the ER, the primary site of protein synthesis in the cell, although studies of oxidative stress in renin cells are currently sparse.
Alternatively, AKR1B7 could play a role in prostaglandin synthesis; prostaglandins have long been thought to play a role in the regulation of renin (6, 26, 35). It was previously found that AKR1B7 was capable of catalyzing the synthesis of prostaglandin F2α, both biochemically (12) and in vitro (13). As prostaglandin F2α has also been shown to stimulate renin secretion (36, 37), it is possible that AKR1B7 is responsible for regulating renin secretion through prostaglandin F2α synthesis. This hypothesis is supported by the fact that AKR1B7 was described as changing localization to the plasma membrane when synthesizing prostaglandin F2α (13), confirming our observation that AKR1B7 localizes to the plasma membrane of the renin cell. However, studies of renin release will need to be performed to answer this question.
AKR1B7 marks cells attempting to synthesize renin, independent of the cell's actual production of renin.
Study of renin cells has long been complicated by the fact that the only reliable marker for renin cells was renin itself. In situations where renin expression was blunted or absent, a separate marker for renin cells would be necessary to identify cells attempting to produce renin. As mentioned earlier, we show in vivo that AKR1B7 expression persists in the kidney, even when the gene for renin is deleted, thus establishing AKR1B7's independence of renin expression. Intriguingly, we also observe that the staining pattern of AKR1B7 in renin knockout mice replicates that of renin cells under conditions that stimulate renin production, such as the earlier described phenotype following sodium depletion and captopril treatment. This suggests that there is an attempt by cells in the renin knockout kidney to synthesize renin, likely in response to systemic hypotension and sodium depletion and that AKR1B7 is a marker for those renin-less cells of the renin phenotype. The AKR1B7-marked cells, thus, represent a system in which the genes for the renin cell phenotype and the renin promoter itself [which is intact in the renin knockout animal used here (32)] would be chronically stimulated, as those cells attempt to compensate for a lack of renin. Isolating these cells could provide an important platform for the study of renin regulation, as studies in renin cell plasticity have been primarily conducted under conditions of acute stimulation.
Mechanisms of AKR1B7 regulation.
The degree of coexpression between AKR1B7 and renin, and a lack of evidence that suggests that the two enzymes directly regulate each other, presents the highly interesting possibility that both genes are regulated in a similar manner through an overarching genetic program that controls renin cell fate. Various inputs are incorporated to control the identity and subsequent production and secretion of renin by the renin cell (for a review, see Ref. 28), but the exact mechanism(s) that regulates the renin gene is not fully understood. The AKR1B7 gene and its associated promoter could provide an important tool in that search, as having an additional promoter to look at in silico, in vitro, and in vivo adds a degree of power not previously available. In addition, identifying transcription factors and other regulatory molecules that affect AKR1B7 could provide candidates that potentially regulate renin as well.
One possibility examined here is that cAMP plays an important role in the simultaneous regulation of both proteins. Our laboratory and others have previously identified the cAMP pathway as an activator of renin expression (5, 10). In addition, there exists a cAMP response element in the AKR1B7 promoter that is key to its regulation, and cAMP has been demonstrated in vitro and in vivo to play a key role in the control of AKR1B7 transcription in the adrenal glands (1, 15). In this study, we show that cAMP plays a similar role in regulating AKR1B7 in the kidney, using multiple approaches. In vitro, increasing intracellular cAMP levels in cultured cells of the renin lineage via forskolin causes an increase in AKR1B7 expression. In vivo, deletion of elements of the renin cell's cAMP response pathway—the histone acetyl-transferases p300 and CBP—significantly blunts the expression of AKR1B7 in the murine kidney. Taken together, these experiments demonstrate that cAMP plays a significant role in coregulating both AKR1B7 and renin in the kidney. Previously, we have also shown that deletion of recombination signal binding protein for the immunoglobulin κJ region, the final transcriptional mediator of the Notch signaling pathway, resulted in a significant decrease in renin- and AKR1B7-positive cells (4, 16). Whether and how the Notch pathway and cAMP signaling interact to regulate renin and AKR1B7 expression, potentially as part of an overarching genetic program controlling renin cell identity, remains to be determined.
Perspectives and Significance
AKR1B7 is coexpressed and regulated by the same factors that regulate the renin gene, suggesting the possibility of an overarching program controlling the renin phenotype. Interestingly, when the renin gene is mutated, this program continues to operate, and AKR1B7 permits the identification of those cells that were programmed to express renin but cannot do so. Under these circumstances, the renin-less mutated cells do not disappear; they can be identified as AKR1B7+ cells, which expand along and around the arterioles of the kidney, possibly participating in the concentric obstructive vessel hypertrophy commonly seen in animals with mutations of the renin gene. Thus, AKR1B7 adds a new degree of power when investigating the biology of the renin cell.
GRANTS
National Institutes of Health Grants R37 HL-066242 and R01 HL-096735 to R. A. Gomez and R01 DK-091330 to M. L. Sequeira-Lopez,P50DK096373 to R. A. Gomez and M. L. Sequeira-Lopez, and the American Heart Association Pre-Doctoral Grant 13PRE16920043 to E. E. Lin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.E.L., M.L.S.S.-L., and R.A.G. conception and design of research; E.E.L. performed experiments; E.E.L., E.S.P., M.L.S.S.-L., and R.A.G. analyzed data; E.E.L., E.S.P., M.L.S.S.-L., and R.A.G. interpreted results of experiments; E.E.L. prepared figures; E.E.L. drafted manuscript; E.E.L., E.S.P., M.L.S.S.-L., and R.A.G. edited and revised manuscript; E.E.L., M.L.S.S.-L., and R.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully recognize the assistance of Dr. Brian Belyea in helping to prepare this manuscript. We thank LaToya Roker for help with confocal microscopy, and Michelle Ko for assistance with statistical analysis. We are grateful to Dr. Oliver Smithies for the Ren1c and aldosterone synthase mice.
REFERENCES
- 1.Aigueperse C, Martinez A, Lefrancois-Martinez AM, Veyssiere G, Jean CI. Cyclic AMP regulates expression of the gene coding for a mouse vas deferens protein related to the aldo-keto reductase superfamily in human and murine adrenocortical cells. J Endocrinol 160: 147–154, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA. Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellanos-Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez ML, Gomez RA. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics 43: 1021–1028, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellanos-Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, Sequeira-Lopez ML, Gomez RA. Recombination signal binding protein for Ig-κJ region regulates juxtaglomerular cell phenotype by activating the myo-endocrine program and suppressing ectopic gene expression. J Am Soc Nephrol 26: 67–80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Faulhaber-Walter R, Wen Y, Huang Y, Mizel D, Chen M, Sequeira Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Renal failure in mice with gsalpha deletion in juxtaglomerular cells. Am J Nephrol 32: 83–94, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier RL, Carey RM, Kaiser DL. Endogenous prostaglandins modulate autoregulation of renal blood flow in young rats. Am J Physiol Renal Fluid Electrolyte Physiol 253: F66–F75, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Fransen JA. Lysosomal characteristics of human renin-containing granules. Lab Invest 56: 124, 1987. [PubMed] [Google Scholar]
- 8.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 259: F660–F665, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, Peach MJ. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 254: F582–F587, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira-Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol 296: H1255–H1262, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez RA, Robillard JE. Developmental aspects of the renal responses to hemorrhage during converting-enzyme inhibition in fetal lambs. Circ Res 54: 301–312, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Kabututu Z, Manin M, Pointud JC, Maruyama T, Nagata N, Lambert S, Lefrancois-Martinez AM, Martinez A, Urade Y. Prostaglandin F2alpha synthase activities of aldo-keto reductase 1B1, 1B3 and 1B7. J Biochem 145: 161–168, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Lambert-Langlais S, Pointud JC, Lefrancois-Martinez AM, Volat F, Manin M, Coudore F, Val P, Sahut-Barnola I, Ragazzon B, Louiset E, Delarue C, Lefebvre H, Urade Y, Martinez A. Aldo keto reductase 1B7 and prostaglandin F2alpha are regulators of adrenal endocrine functions. PLoS ONE 4: e7309, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee G, Makhanova N, Caron K, Lopez ML, Gomez RA, Smithies O, Kim HS. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology 146: 2650–2656, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Lefrancois-Martinez AM, Bertherat J, Val P, Tournaire C, Gallo-Payet N, Hyndman D, Veyssiere G, Bertagna X, Jean C, Martinez A. Decreased expression of cyclic adenosine monophosphate-regulated aldose reductase (AKR1B1) is associated with malignancy in human sporadic adrenocortical tumors. J Clin Endocrinol Metab 89: 3010–3019, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Lin EE, Sequeira-Lopez ML, Gomez RA. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am J Physiol Renal Physiol 306: F249–F258, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machura K, Iankilevitch E, Neubauer B, Theuring F, Kurtz A. The aldo-keto reductase AKR1B7 coexpresses with renin without influencing renin production and secretion. Am J Physiol Renal Physiol 304: F578–F584, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Makhanova N, Sequeira-Lopez ML, Gomez RA, Kim HS, Smithies O. Disturbed homeostasis in sodium-restricted mice heterozygous and homozygous for aldosterone synthase gene disruption. Hypertension 48: 1151–1159, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A, Aigueperse C, Val P, Dussault M, Tournaire C, Berger M, Veyssiere G, Jean C, Lefrancois MA. Physiological functions and hormonal regulation of mouse vas deferens protein (AKR1B7) in steroidogenic tissues. Chem Biol Interact 130–132: 903–917, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Medrano S, MCM, Sequeira-Lopez MLS, Pentz ES, Gomez RA. Two microRNAs -miR-330 and miR-125b-5p- mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol Renal Physiol 302: F29–F37, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pailhoux E, Martinez A, Jean-Faucher C, Veyssiere G, Jean C. Lack of expression of the mRNA encoding a major protein of the mouse vas deferens after neonatal exposure to oestrogens. J Mol Endocrinol 7: 63–69, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Pentz ES, Cordaillat M, Carretero OA, Tucker AE, Sequeira Lopez ML, Gomez RA. Histone acetyl transferases CBP and p300 are necessary for maintenance of renin cell identity and transformation of smooth muscle cells to the renin phenotype. Am J Physiol Heart Circ Physiol 302: H2545–H2552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol 286: R474–R483, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Pentz ES, Sequeira Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: An in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol 294: H699–H707, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Robillard JE, Weismann DN, Gomez RA, Ayres NA, Lawton WJ, VanOrden DE. Renal and adrenal responses to converting-enzyme inhibition in fetal and newborn life. Am J Physiol Regul Integr Comp Physiol 244: R249–R256, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Schweda F, Kurtz A. Cellular mechanism of renin release. Acta Physiol Scand 181: 383–390, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Seamon KB, Daly JW. Forskolin: A unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res 7: 201–224, 1981. [PubMed] [Google Scholar]
- 28.Sequeira Lopez ML, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep 12: 26–32, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol 21: 460–467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi N, Lopez ML, Cowhig JE Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Taugner R, Metz R. Development and fate of the secretory granules of juxtaglomerular epithelioid cells. Cell Tissue Res 246: 595–606, 1986. [DOI] [PubMed] [Google Scholar]
- 34.Thompson HA, Burson JM, Lang JA, Gross KW, Sigmund CD. Tissue-specific expression of novel messenger ribonucleic acids cloned from a renin-expressing kidney tumor cell line (As4.1). Endocrinology 136: 3037–3045, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Vander AJ. Direct effects of prostaglandin on renal function and renin release in anesthetized dog. Am J Physiol 214: 218–221, 1968. [DOI] [PubMed] [Google Scholar]
- 36.Whorton AR, Lazar JD, Smigel MD, Oates JA. Prostaglandins and renin release: III. Effects of PGE1, E2 F2 alpha and D2 on renin release from rabbit renal cortical slices. Prostaglandins 22: 455–468, 1981. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Lucitt MB, Stubbe J, Cheng Y, Friis UG, Hansen PB, Jensen BL, Smyth EM, FitzGerald GA. Prostaglandin F2alpha elevates blood pressure and promotes atherosclerosis. Proc Natl Acad Sci USA 106: 7985–7990, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]