Abstract
Chronic myeloid leukemia (CML) accounts for approximately 15% of adult leukemias. Forty percent of patients with CML are asymptomatic, in whom the disease is detected solely based on laboratory abnormalities. Since the introduction of tyrosine kinase inhibitor therapy in 2001, CML has become a chronic disease for the majority of patients. Primary care physicians may be the first to recognize a new diagnosis of CML. In patients with known CML, the primary care physician may be the first to detect disease progression or adverse effects to therapy. This article provides an overview of the clinical presentation, diagnostic approach, and treatment considerations of CML.
Keywords: Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Primary Health Care
INTRODUCTION
Chronic myeloid leukemia (CML) accounts for approximately 15% of adult leukemias, with an estimated 6,660 new cases of CML diagnosed in the United States in 2015.1) Although the median age of diagnosis is 67 years, CML may present at any age.2) The advent of tyrosine kinase inhibitor (TKI) therapy has transformed CML from a fatal disease into a chronic disease for the majority of patients. Prior to 1983, the 8-year survival of CML was less than 15%. The 8-year survival improved from 42% to 65% from 1983 to 2000 with the use of interferon-α-based therapy and allogeneic hematopoietic stem cell transplant (HSCT) therapy. With the introduction of TKI therapy in 2001, the 8-year survival is now 87% and continues to improve with the use of second- and third-generation TKI therapy.3) Given the dramatic decrease in the number of deaths in CML patients and the stable incidence, the prevalence of CML continues to increase. The estimated prevalence of CML in the United States was approximately 70,000 in 2010 and is expected to increase to approximately 112,000 in 2020.4)
PATHOGENESIS
CML is a clonal myeloproliferative disorder characterized by the presence of a balanced genetic translocation of chromosomes 22 and 9, termed the Philadelphia (Ph) chromosome (Figure 1). The resulting breakpoint cluster region-Abelson murine leukemia (BCR-ABL) fusion oncogene is translated into the BCR-ABL oncoprotein.5) BCR-ABL is a constitutively active tyrosine kinase that activates a number of signal-transduction pathways that affect the growth and survival of hematopoietic cells.6)
Figure 1. The Philadelphia chromosome. ABL, Abelson murine leukemia; BCR, breakpoint cluster region.
CLINICAL MANIFESTATIONS AND STAGING
CML is classified into three different phases: chronic, accelerated, and blast (Table 1).7,8,9) The natural history of CML is a chronic phase for three to five years followed by rapid progression to the fatal blast phase. In two-thirds of patients, the blast phase is proceeded by an accelerated phase.
Table 1. Stages of chronic myeloid leukemia.
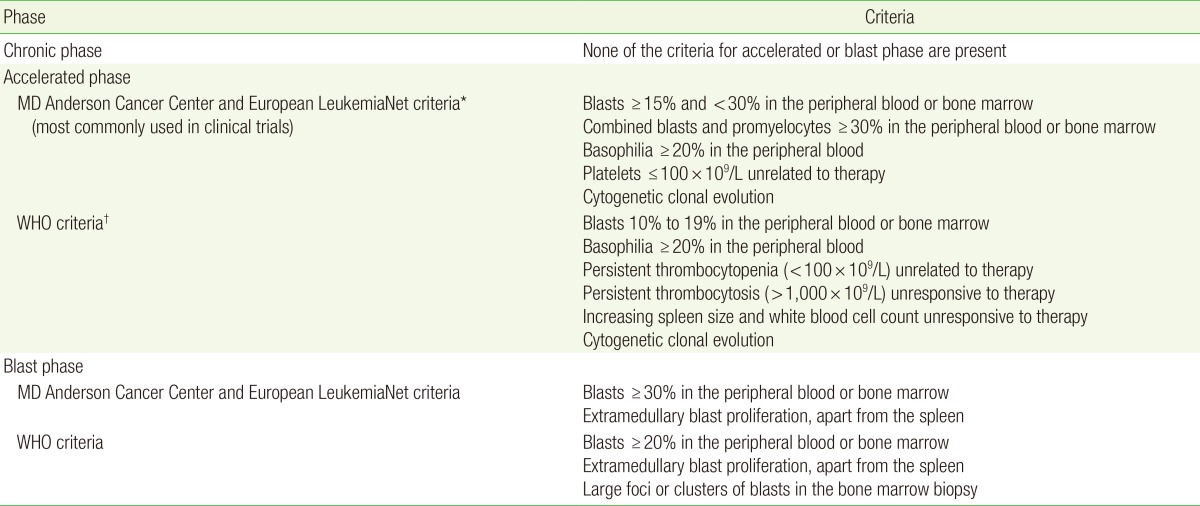
WHO, World Health Organization.
*Most commonly used in clinical trials. †Most commonly used by pathologists.
Approximately 85% of patients with CML are diagnosed in the chronic phase.10) Forty percent of patients with chronic phase CML are asymptomatic with the diagnosis made solely based on an abnormal blood count.6) Among the patients who have symptoms, complaints are usually related to anemia and splenomegaly; these include fatigue, weight loss, anorexia, early satiety, and left upper quadrant pain or fullness. Other less common symptoms include thrombosis or bleeding from thrombocytopenia or platelet dysfunction. Splenomegaly is the most common finding on physical exam and is present in over half of patients.11)
DIAGNOSIS
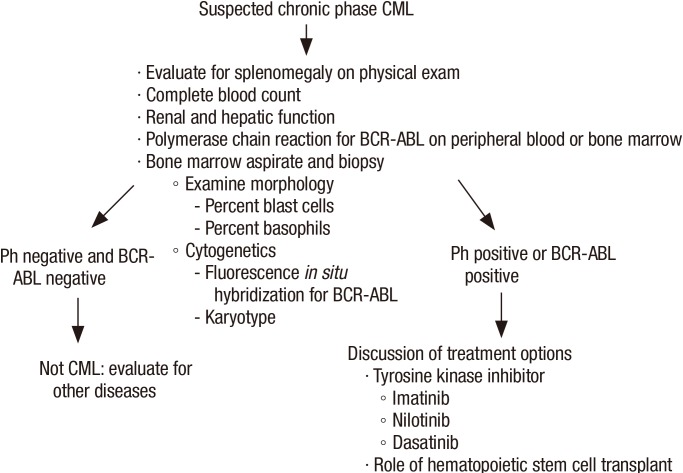
Unexplained leukocytosis with left shift (immature myeloid cells including myelocytes, promyelocytes or blasts), basophilia, and splenomegaly are suggestive of CML (Figure 2). The differential diagnosis includes leukemoid reaction (due to infection or inflammation), Ph negative myeloproliferative disorder, chronic myelomonocytic leukemia, and proliferative myelodysplastic syndrome. On occasion, CML may present as an isolated thrombocytosis. The diagnosis of CML may be confirmed with fluorescence in situ hybridization (FISH) or reverse transcriptase polymerase chain reaction (RT-PCR) for BCR-ABL performed on the peripheral blood. However, bone marrow aspiration with cytogenetic analysis (karyotype) is required to appropriately stage as the chronic phase, accelerated phase, or blast phase and to identify chromosomal abnormalities that are not detectable with FISH for BCR-ABL (Figures 3, 4).12)
Figure 2. Peripheral blood of chronic phase chronic myeloid leukemia showing leukocytosis with circulating immature myeloid cells (Wright-Giemsa stain, ×4).
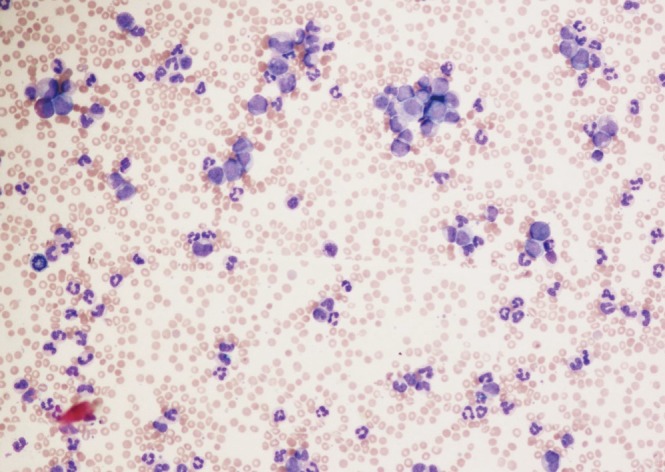
Figure 3. Bone marrow aspirate of chronic phase chronic myeloid leukemia showing a spectrum of immature myeloid cells including blasts and promyelocytes (Wright-Giemsa stain, ×20).
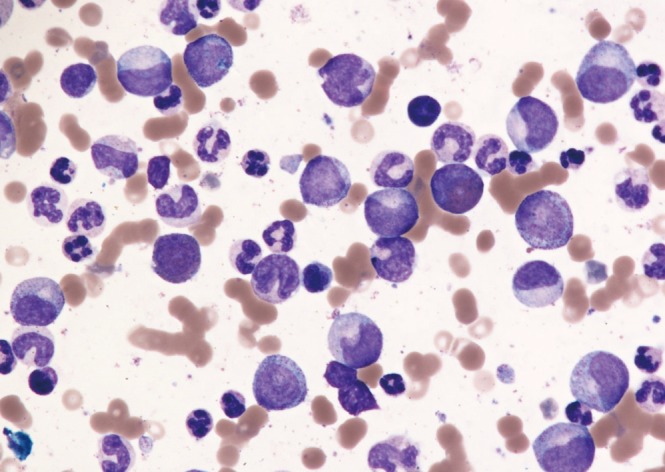
Figure 4. Evaluation of suspected CML. CML, chronic myeloid leukemia; BCR, breakpoint cluster region; ABL, Abelson murine leukemia.
TREATMENT
TKI therapy has transformed the outcomes of patients with CML over the last 15 years. TKIs interfere with the interaction between the BCR-ABL oncoprotein and adenosine triphosphate, thereby blocking proliferation of the malignant clone. There are currently three TKIs approved by the Food and Drug Administration for the first-line treatment of chronic phase CML: imatinib, dasatinib, and nilotinib. Imatinib was the first TKI to be approved in 2001. In the landmark study comparing imatinib to combination interferon and cytarabine therapy, imatinib had superior tolerability, hematologic and cytogenetic responses, and decreased likelihood of progression to accelerated phase or blast phase CML.13) The choice of first-line therapy depends on the Sokal or Hasford risk stratification score, patient age, ability to tolerate therapy, and medical comorbidities. The Sokal score includes age, spleen size, platelet count, and blast percentage.14) The Hasford score also incorporates the percent of eosinophils and basophils.15) Compared to imatinib, dasatinib and nilotinib have improved efficacy and may be preferred in intermediate- and high-risk patients based on the Sokal or Hasford risk stratification scores.16,17,18,19)
In patients who are refractory or intolerant to first-line TKI therapy, second-line options include second-generation TKIs, dasatinib, nilotinib, and bosutinib. Ponatinib is a third-generation TKI and is the only TKI that is effective in patients who harbor the threonine-to-isoleucine mutation at position 315 (T315I). Previously, ponatinib was considered a third-line treatment option. However, due to the associated risk of arterial and venous thromboembolism, its use is generally reserved for patients harboring the T315I mutation.20,21)
All TKI agents are administered orally. The initial dosing and common and notable adverse effects are summarized in Table 2.22,23) The management of toxicities associated with TKI therapy is discussed elsewhere.12,24) Patients with chronic phase CML are continued on TKI therapy indefinitely. Although discontinuation of TKI therapy with close molecular monitoring may be possible in selected patients, the discontinuation of TKI therapy should only been considered in the context of a clinical trial.12,25,26)
Table 2. Tyrosine kinase inhibitors available for treatment of chronic phase chronic myeloid leukemia.
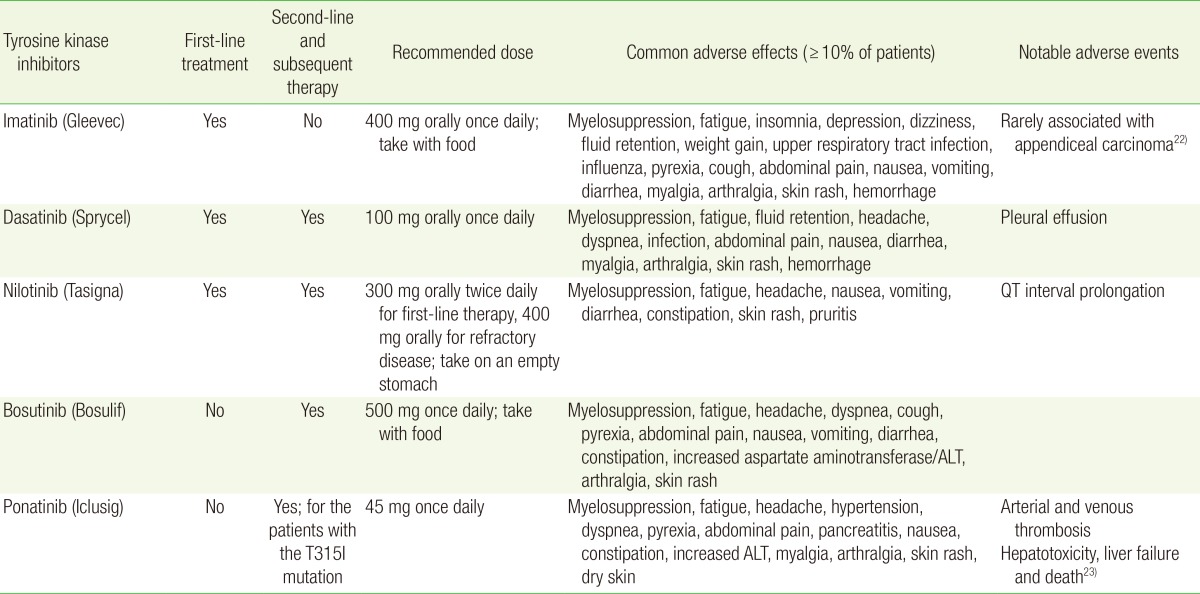
ALT, alanine aminotransferase.
The response to therapy is classified based on hematological, cytogenetic, and molecular responses (Table 3).12) Optimal responses to first-line TKI therapy include complete hematologic response with BCR-ABL1 transcript ≤10% (RT-PCR) and/or Ph positive cells ≤35% (bone marrow cytogenetics) at 3 months, BCR-ABL1 transcript <1% and/or no detectable Ph positive cells at 6 months, and BCR-ABL1 transcript ≤0.1% at 12 months. Failure of first-line TKI therapy is defined as failure to achieve a complete hematologic response and/or Ph positive cells >95% at 3 months, BCR-ABL1 transcript >10% and/or Ph positive cells >35% at 6 months, and BCR-ABL1 transcript >1% and/or Ph positive cells at 12 months. Loss of complete hematologic response, complete cytogenetic response, or major molecular response or presence of mutations or clonal evolution are also considered as treatment failure.8)
Table 3. Assessment response to tyrosine kinase inhibitor therapy.
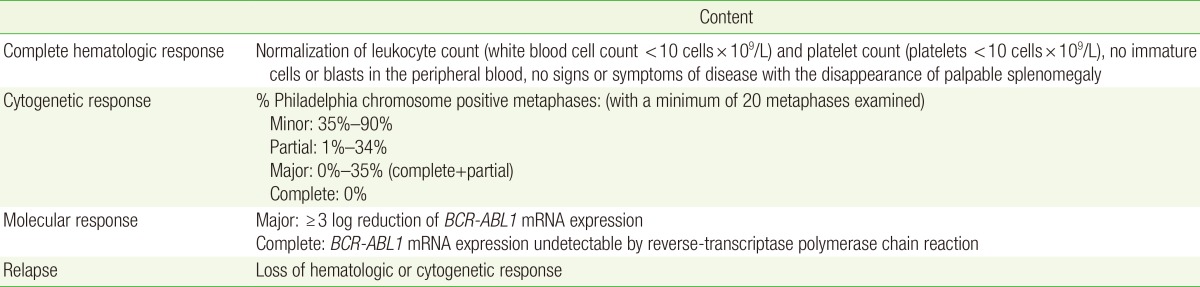
BCR, breakpoint cluster region; ABL, Abelson murine leukemia; mRNA, messenger ribonucleic acid.
Administration of the protein synthesis inhibitor omacetaxine is a treatment option for patients who have failure or intolerance to two or more TKIs, including patients who harbor the T315I mutation.27,28) The accelerated or blast phases may be treated with an alternative TKI as a bridge to HSCT. HSCT is a potentially curative treatment in patients with CML, and HSCT evaluation is recommended for patients with the T315I mutation, failure or intolerance to two or more TKIs, or those with accelerated or blast phase CML.12)
PREGNANCY
Although most pregnancy outcomes are normal in patients exposed to imatinib, there is still a risk for serious fetal malformation with imatinib exposure.29) For this reason, discontinuation of TKI therapy is generally advised during pregnancy.12) For women who desire pregnancy, a planned pregnancy is preferred. Once a woman is in at least a major molecular response, then a two to three month washout period of TKI therapy is advised prior to conception. TKI therapy should be held during pregnancy and resumed immediately after birth. When treatment is needed during pregnancy due to a very high white blood cell (WBC) count, leukapheresis is preferred.30) Interferon may be used safely in pregnancy. However, its use is limited by toxicity and slow time to response.31,32,33) The use of hydroxyurea also appears to be safe in pregnancy, but its use is typically reserved for pulse dosing to control very high WBC counts.30,34,35,36,37,38,39) Although the use of TKI appears to be safe in men fathering a child, patients should be advised that the data in this setting is limited.40,41,42)
ROLE OF THE PRIMARY CARE PHYSICIAN
Primary care physicians may be the first to detect CML since 40% patients present only with an abnormal blood count.6) Thus, it is important for primary care physicians to be aware of the appropriate initial evaluation of patients with suspected CML.
During the routine follow-up of other medical comorbidities, the primary care physician may be the first to detect new adverse effects to TKI therapy, treatment failure, or progression of disease. With recognition of the common and serious adverse effects of TKI therapy, the primary care physician may provide appropriate counseling or arrange for earlier follow-up with the patient's hematologist/oncologist for management. With the recognition of possible treatment failure or progression of disease from the chronic phase to the accelerated or blast phases (i.e., increasing WBC count), the primary care physician should arrange for the patient to been seen by his/her hematologist/oncologist promptly for further evaluation.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER cancer statistics factsheets: chronic myeloid leukemia [Internet] Bethesda (MD): National Cancer Institute; 2014. [cited 2015 Apr 20]. Available from: http://seer.cancer.gov/statfacts/html/cmyl.html. [Google Scholar]
- 3.Kantarjian H, O'Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119:1981–1987. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123–3127. doi: 10.1002/cncr.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 6.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Keating MJ, Smith TL, Talpaz M, McCredie KB. Proposal for a simple synthesis prognostic staging system in chronic myelogenous leukemia. Am J Med. 1990;88:1–8. doi: 10.1016/0002-9343(90)90119-x. [DOI] [PubMed] [Google Scholar]
- 8.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013 Aug 8;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer Press; 2008. [Google Scholar]
- 10.Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 11.Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96:111–116. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien S, Radich JP, Abboud CN, Akhtari M, Altman JK, Berman E, et al. Chronic myelogenous leukemia, version 1.2015. J Natl Compr Canc Netw. 2014;12:1590–1610. doi: 10.6004/jnccn.2014.0159. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 14.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- 15.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. Writing Committee for the Collaborative CML Prognostic Factors Project Group. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. J Natl Cancer Inst. 1998;90:850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boque C, Chuah C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 19.Larson RA, Kim DW, Jootar S, Pasquini R, Clark RE, Lobo C, et al. ENESTnd 5-year (y) update: long-term outcomes of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib (NIL) versus imatinib (IM) J Clin Oncol. 2014;32(15_suppl):7073. [Google Scholar]
- 20.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. FDA drug safety communication: FDA investigating leukemia drug Iclusig (ponatinib) after increased reports of serious blood clots in arteries and veins. Silver Spring (MD): Food and Drug Administration; 2013. [Google Scholar]
- 22.Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 23.ARIAD Pharmaceuticals Inc. Ponatinib [Internet] Cambridge (MA): ARIAD Pharmaceuticals Inc.; 2012. [cited 2015 Apr 25]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203469lbl.pdf. [Google Scholar]
- 24.Akhtari M, Barber NA. Practical approach to the hematologic adverse effects of imatinib. In: Akhtari M, El-Helmaidi I, editors. Imatinib: chemical structure, pharmacology and adverse effects. Hauppauge (NY): Nova Science Publishers Inc.; 2013. pp. 65–80. [Google Scholar]
- 25.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 26.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24:1719–1724. doi: 10.1038/leu.2010.185. [DOI] [PubMed] [Google Scholar]
- 27.Cortes JE, Nicolini FE, Wetzler M, Lipton JH, Akard L, Craig A, et al. Subcutaneous omacetaxine mepesuccinate in patients with chronic-phase chronic myeloid leukemia previously treated with 2 or more tyrosine kinase inhibitors including imatinib. Clin Lymphoma Myeloma Leuk. 2013;13:584–591. doi: 10.1016/j.clml.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–2580. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pye SM, Cortes J, Ault P, Hatfield A, Kantarjian H, Pilot R, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111:5505–5508. doi: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390–1397. doi: 10.1182/blood-2012-03-378919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Hemaidi I, Robinson SE. Management of haematological malignancy in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2012;26:149–160. doi: 10.1016/j.bpobgyn.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Baer MR, Ozer H, Foon KA. Interferon-alpha therapy during pregnancy in chronic myelogenous leukaemia and hairy cell leukaemia. Br J Haematol. 1992;81:167–169. doi: 10.1111/j.1365-2141.1992.tb08202.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuroiwa M, Gondo H, Ashida K, Kamimura T, Miyamoto T, Niho Y, et al. Interferon-alpha therapy for chronic myelogenous leukemia during pregnancy. Am J Hematol. 1998;59:101–102. doi: 10.1002/(sici)1096-8652(199809)59:1<101::aid-ajh23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 34.Delmer A, Rio B, Bauduer F, Ajchenbaum F, Marie JP, Zittoun R. Pregnancy during myelosuppressive treatment for chronic myelogenous leukemia. Br J Haematol. 1992;82:783–784. doi: 10.1111/j.1365-2141.1992.tb06968.x. [DOI] [PubMed] [Google Scholar]
- 35.Patel M, Dukes IA, Hull JC. Use of hydroxyurea in chronic myeloid leukemia during pregnancy: a case report. Am J Obstet Gynecol. 1991;165:565–566. doi: 10.1016/0002-9378(91)90285-y. [DOI] [PubMed] [Google Scholar]
- 36.Tertian G, Tchernia G, Papiernik E, Elefant E. Hydroxyurea and pregnancy. Am J Obstet Gynecol. 1992;166(6 Pt 1):1868. doi: 10.1016/0002-9378(92)91590-7. [DOI] [PubMed] [Google Scholar]
- 37.Jackson N, Shukri A, Ali K. Hydroxyurea treatment for chronic myeloid leukaemia during pregnancy. Br J Haematol. 1993;85:203–204. doi: 10.1111/j.1365-2141.1993.tb08672.x. [DOI] [PubMed] [Google Scholar]
- 38.Baykal C, Zengin N, Coskun F, Guler N, Ayhan A. Use of hydroxyurea and alpha-interferon in chronic myeloid leukemia during pregnancy: a case report. Eur J Gynaecol Oncol. 2000;21:89–90. [PubMed] [Google Scholar]
- 39.Celiloglu M, Altunyurt S, Undar B. Hydroxyurea treatment for chronic myeloid leukemia during pregnancy. Acta Obstet Gynecol Scand. 2000;79:803–804. [PubMed] [Google Scholar]
- 40.Ault P, Kantarjian H, O'Brien S, Faderl S, Beran M, Rios MB, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24:1204–1208. doi: 10.1200/JCO.2005.04.6557. [DOI] [PubMed] [Google Scholar]
- 41.Ramasamy K, Hayden J, Lim Z, Mufti GJ, Ho AY. Successful pregnancies involving men with chronic myeloid leukaemia on imatinib therapy. Br J Haematol. 2007;137:374–375. doi: 10.1111/j.1365-2141.2007.06542.x. [DOI] [PubMed] [Google Scholar]
- 42.Cortes J, O'Brien S, Ault P, Borthakur G, Jabbour E, Bradley-Garelik B, et al. Pregnancy outcomes among patients with chronic myeloid leukemia treated with dasatinib. Blood. 2008;112:Abstract 3230. [Google Scholar]