Passive leg movement (PLM) is a novel method of assessing predominantly nitric oxide mediated vascular function. PLM-induced vasodilation is attenuated with age in men; however, PLM has never before been used in the female population. Our data demonstrate that aging impairs PLM-induced vasodilation in women, thus expanding the utility of the PLM model.
Keywords: passive leg movement, vascular function, posture, endothelium
Abstract
Passive leg movement (PLM), an assessment of predominantly nitric oxide-dependent vasodilation, is decreased with age and cannot be augmented by posture-induced increases in femoral perfusion pressure in older men. However, this novel method of assessing vascular function has yet to be used to evaluate alterations in nitric oxide-dependent vasodilation with age in females. PLM was performed in 10 young (20 ± 1 yr) and 10 old (73 ± 2 yr) women in both the supine and upright-seated postures, whereas central and peripheral hemodynamic measurements were acquired second by second using noninvasive techniques (finger photoplethysmography and Doppler ultrasound, respectively). The heart rate response to PLM was attenuated in the old compared with the young in both the supine (young, 10 ± 1; and old, 5 ± 1 beats/min; P < 0.05) and upright-seated posture (young, 10 ± 2; and old, 5 ± 1 beats/min; P < 0.05), leading to a blunted cardiac output response in the old in the upright-seated posture (young, 1.0 ± 0.2; and old, 0.3 ± 0.1 l/min; P < 0.05). The PLM-induced peak change in leg vascular conductance was lower in the old compared with the young in both postures (young supine, 5.7 ± 0.5; old supine, 2.6 ± 0.3; young upright, 9.2 ± 0.7; and old upright, 2.2 ± 0.4 ml·min−1·mmHg−1; P < 0.05) and was significantly augmented by the upright-seated posture in the young only, revealing a vasodilatory reserve capacity in the young (3.5 ± 0.6 ml·min−1·mmHg−1, P < 0.05) that was absent in the old (−0.5 ± 0.3 ml·min−1·mmHg−1, P = 0.18). These data support previous literature demonstrating attenuated PLM-induced vasodilation with age and extend these findings to include the female population, thus bolstering the utility of PLM as a novel assessment of vascular function across the life span in humans.
NEW & NOTEWORTHY
Passive leg movement (PLM) is a novel method of assessing predominantly nitric oxide mediated vascular function. PLM-induced vasodilation is attenuated with age in men; however, PLM has never before been used in the female population. Our data demonstrate that aging impairs PLM-induced vasodilation in women, thus expanding the utility of the PLM model.
recently, passive leg movement (PLM), a novel method for assessing vascular function, has been gaining recognition. In the supine posture, PLM initiates a vasodilatory response in young men that has been documented to be attenuated in old men (28, 39), is predominantly nitric oxide (NO) mediated, and therefore likely reflects endothelium-dependent vascular function (28, 39). Interestingly, when femoral perfusion pressure (FPP) is increased by moving from the supine to the upright-seated posture, young men exhibit an NO-mediated vasodilatory reserve capacity, whereas old men, due to diminished NO bioavailability, do not (10, 11). Likewise, the initial vasodilatory response to PLM (∼9 s) differs between young and old men, with the old demonstrating a slower onset (10, 11, 38). Due to the powerful antiatherogenic properties of NO (18, 34), and the importance of intact vascular function in cardiovascular disease (CVD) prevention, an assessment such as the response to PLM, which has the potential to be used across the life span, may be of great clinical importance. However, this novel method of assessing vascular function has yet to be used to evaluate alterations in endothelium-dependent vasodilation with age in females.
Premenopausal women appear to have a reduced risk of CVD compared with both men and postmenopausal women (21). This has led to the belief that young women possess an inherent cardioprotection, which may be related to estrogen. Estrogen, which declines postmenopause, is recognized to have powerful effects on the vasculature. As a steroid hormone, estrogen is able to diffuse easily across endothelial and vascular smooth muscle cell membranes, resulting in vascular-specific genomic modifications, such as increased endothelial NO synthase (eNOS) transcription and ultimately eNOS protein expression (29) and nongenomic effects, such as eNOS activation and NADPH oxidase inhibition (29). Thus postmenopausal women would be expected to have decreased NO-mediated vascular function compared with young women, and, indeed, this has been the conclusion of studies using the brachial artery flow-mediated dilation (FMD) technique (4, 13). However, due to procedural complexities that have limited the clinical usefulness of FMD and recent questions regarding the contribution of NO to FMD (31, 45), there is a growing need to develop a clinically relevant tool that can be used to track the changes in vascular function of women across the life span.
Consequently, the purpose of this investigation was to assess vascular function in young and old women using the novel PLM test. Specifically, we hypothesized that 1) old women would exhibit an attenuated PLM-induced vasodilation compared with young women, 2) increasing FPP by assuming an upright-seated posture would augment the vasodilatory response in young women only, such that 3) the vasodilatory reserve capacity would be greater in the young women than in the old women and 4) increased FPP would augment the rapid vasodilation in the young women, with no change in the old women. If proven to be correct, such findings would support the potential clinical relevance of the PLM test for assessing vascular function in women across the life span.
METHODS
Subjects
Twenty healthy women (10 young and 10 old) participated in this research study. Subjects were included based on a lack of overt cardiovascular or metabolic disease and aged 18–25 yr for the young and >65 yr for the old. All procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center, and written informed consent was obtained from each participant before inclusion in the study. The study conformed to the standards set by the Declaration of Helsinki.
Experimental Protocol
Each subject reported to the laboratory for both a familiarization and experimental trial. Upon arrival for the familiarization trial, anthropometric measurements were performed, followed by instrumentation and PLM, both to acquaint participants with the experimental procedure and to ensure their ability to remain relaxed during a PLM protocol.
Subjects arrived on the experimental day fasted and having refrained from caffeine for 12 h prior, and exercise for 24 h before the initiation of data collection. To control for variations in circulating hormones, young females were included only if they were not taking pharmaceutical contraceptives and were then studied within the first 7 days (follicular phase) of the menstrual cycle. The old women were postmenopausal and not on hormone replacement therapy. Blood was collected from the antecubital vein to assess blood lipids, fasting glucose, C-reactive protein (CRP), ferric-reducing ability of plasma (FRAP), protein carbonyl, and hemoglobin and to perform a complete blood cell count. Subjects were then assigned to either the supine or upright-seated posture using a counterbalanced design. After instrumentation, participants rested for at least 20 min in the assigned posture before the start of data collection.
Hemodynamic measurements were collected during 1 min of baseline with the leg held at a 180° knee joint angle, followed by 2 min of passive knee flexion-extension through a 90° range of motion (180-90°) at 1 Hz. Throughout the protocol, the contralateral leg remained supported and motionless with the knee joint extended (180°). PLM was achieved by a member of the research team with real-time feedback provided by a position sensor and digital display to ensure full range of motion. A metronome, initiated before the start of baseline data collection, was used to maintain cadence. Before the start and throughout the protocol participants were encouraged to remain passive and to resist the urge to help or hinder the passive movement. To avoid the startle reflex, participants were made aware that the PLM would begin in ∼1 min but were not told exactly when the movement would begin to reduce the chance of an anticipatory response (42). The protocol was repeated in the opposing body posture (supine or upright seated) after a rest period of at least 20 min in the new posture.
Baseline for all variables was determined using the average of the data for the 60 s before the initiation of PLM. Data collected during the first minute of PLM were analyzed second by second and smoothed using a 3-s rolling average before final data analysis, with 12 s-averages used for the second minute of movement.
Measurements
Central hemodynamics.
Heart rate (HR) was determined using an electrocardiogram (ECG), and mean arterial pressure (MAP) was determined by finger photoplethysmography with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) positioned at heart level. Stroke volume (SV) was calculated using the Modelflow method (Beatscope, version 1.1; Finapres Medical Systems), with cardiac output (CO) calculated as the product of SV and HR. Throughout each protocol, ECG, SV, CO, and MAP signals underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) using commercially available data acquisition software (AcqKnowledge, Biopac Systems, Goleta, CA).
Peripheral hemodynamics.
Measurements of blood velocity in the common femoral artery (CFA) and vessel diameter were performed in the passively moved leg distal to the inguinal ligament and proximal to the bifurcation of the superficial and deep femoral artery using a Logic 7 ultrasound system (General Electric Medical Systems, Milwaukee, WI). The Logic 7 was equipped with a linear array transducer operating at an imaging frequency of 14 MHz. CFA diameter was determined at a perpendicular angle along the central axis of the scanned area, and blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. With the use of CFA diameter and mean velocity (Vmean; angle corrected and intensity weighted) leg blood flow (LBF) was automatically calculated by commercially available software (Logic 7) as Vmeanπ(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. Leg vascular conductance (LVC) was calculated as LBF divided by MAP. The peak changes in LBF and LVC (ΔLBFpeak and ΔLVCpeak, respectively) were calculated as peak minus baseline, cumulative area under the curve (LBFAUC and LVCAUC) was calculated as ∑{yi[x(i+1) − xi] + (1/2)[y(i+1) − yi][x(i+1) − xi]}, and vasodilatory reserve capacity was calculated as the upright-seated ΔLVCpeak minus the supine ΔLVCpeak.
Blood assays.
Glucose, lipids, and complete blood cell count were determined using standard clinical techniques. Plasma and serum samples were stored at −80° until analysis. The FRAP assay was performed to assess total antioxidant capacity, using the method described by Benzie and Strain (3), whereas oxidative stress was assessed by measuring protein carbonyl levels (Northwest Life Science Specialties, Vancouver, WA). Systemic inflammation was assessed by measuring CRP levels (R&D Systems, Minneapolis, MN). All assays were performed in duplicate.
Knee joint angle.
During each protocol, knee joint angle of the passively moved leg was continuously recorded using a Vishay Spectrol 360 degree Smart Position Sensor (Vishay Intertechnology, Malvern, PA) mounted on a BREG X2K knee brace (BREG, Vista, CA) worn by the participants.
Anthropometrics.
Body mass and height were recorded and used to calculate body mass index (BMI) as BMI = body mass × height2, where body mass is measured in kilograms and height is measured in meters. Thigh volume of the passively moved leg was calculated, as previously described (19), using three measurements of thigh circumference (proximal, middle, and distal), thigh length, and skinfold measurements.
Physical activity level.
Physical activity level (PAL) was assessed using both a subjective PAL recall questionnaire and objective accelerometer data. The PAL questionnaire included items regarding the average type, frequency, intensity, and duration of physical activity in any given week. After receiving standardized operating instructions, subjects wore an accelerometer (GT1M; Actigraph, Pensacola, FL) for a minimum of a continuous 7 days, with adherence automatically assessed by the device. Average total daily physical activity was expressed as both average steps per day, and average total accelerometer as counts per minute. The latter assessment was separated into sedentary, low-, moderate-, high-, and very high-intensity categories using device-specific software (Actilife, Pensacola, FL). Previous research has documented the validity and reliability of the Actigraph GT1M in terms of the estimation of daily physical activity with both steps per day and accelerometer counts per minute (1, 43). Classification of the subjects' level of physical activity, as determined by steps per day, was based on a validated scale (sedentary, <5,000; low active, 5,000–7,499; somewhat active, 7,500–9,999; active, 10,000–12,499; and highly active, ≥12,500 steps/day) (33, 40).
Statistical Analysis
Two-way, repeated-measures ANOVA were used to determine significant differences in baseline and the absolute change from baseline to peak for HR, SV, CO, MAP, LBF and LVC, as well as AUC for LBF and LVC. Student's t-tests were used to compare subject characteristics. Pearson's correlations were employed to assess the strength of relationships between variables. Significance was set at an α-level of 0.05, and data are presented as means ± SE.
RESULTS
Subjects
In addition to an average age difference of over 50 yr, the old women had significantly greater body mass (∼19%), BMI (∼18%), total cholesterol (∼30%), protein carbonyl (∼52%), CRP (∼393%), and larger CFA diameter (∼13%) (Table 1). The groups exhibited similar total daily physical activity, as assessed by both questionnaire and accelerometry, and all subjects were categorized within the sedentary to low physical activity ranges based on the step-determined scale (Table 1) (33, 40).
Table 1.
Subject characteristics
| Young | Old | |
|---|---|---|
| n | 10 | 10 |
| Age, yr | 20 ± 1 | 73 ± 2# |
| Height, cm | 162 ± 2 | 163 ± 1 |
| Weight, kg | 58 ± 2 | 69 ± 3# |
| Body mass index, kg/m2 | 22 ± 1 | 26 ± 1# |
| Thigh volume, dl | 42 ± 3 | 44 ± 3 |
| CFA diameter, cm | 0.71 ± 0.01 | 0.80 ± 0.02# |
| Glucose, mg/dl | 71 ± 2 | 75 ± 2 |
| Cholesterol, mg/dl | 171 ± 12 | 221 ± 11# |
| Triglycerides, mg/dl | 80 ± 9 | 126 ± 18 |
| HDL, mg/dl | 57 ± 3 | 62 ± 2 |
| LDL, mg/dl | 99 ± 10 | 127 ± 9 |
| Hemoglobin, g/dl | 14.1 ± 0.3 | 14.3 ± 0.1 |
| White blood cell, k/μl | 5.4 ± 0.4 | 5.4 ± 0.5 |
| Neutrophil, k/μl | 2.8 ± 0.3 | 3.0 ± 0.3 |
| Lymphocyte, k/μl | 2.0 ± 0.2 | 1.8 ± 0.1 |
| Monocyte, k/μl | 0.46 ± 0.02 | 0.42 ± 0.03 |
| FRAP, mM/ml | 0.90 ± 0.01 | 0.88 ± 0.04 |
| Protein carbonyls, ηm/mg | 0.089 ± 0.004 | 0.135 ± 0.010# |
| C-reactive protein, ηg/ml | 317 ± 59 | 1,563 ± 249# |
| Activity counts/min | 156 ± 21 | 119 ± 15 |
| Steps/day | 6,734 ± 797 | 5,012 ± 563 |
Values are means ± SE; n, number of subjects.
CFA, common femoral artery; FRAP, ferric-reducing ability of plasmaprotein.
P < 0.05, significantly different from young women.
Central Hemodynamics
Resting and peak changes in central hemodynamics are displayed in table 2. At rest there was no difference in SV, HR, or CO between groups in either body posture. While all subjects were normotensive (<140/90 mmHg), resting MAP was significantly higher in the old compared with the young women in both body postures (P < 0.05). In the young, the upright-seated posture elicited a significant decrease in SV (P < 0.05) and an increase in HR (P < 0.05) that maintained CO, whereas the same posture alterations had no effect on central hemodynamics at rest in the old.
Table 2.
Central and peripheral hemodynamics
| Supine |
Upright Seated |
|||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Rest | ||||
| MAP, mmHg | 86 ± 2 | 104 ± 7# | 81 ± 3 | 106 ± 6# |
| CO, l/min | 4.9 ± 0.3 | 4.7 ± 0.5 | 4.9 ± 0.2 | 4.1 ± 0.4 |
| SV, ml/beat | 75 ± 4 | 80 ± 8 | 69 ± 2* | 73 ± 8 |
| HR, beats/min | 66 ± 4 | 63 ± 1 | 71 ± 4* | 64 ± 2 |
| LBF, ml/min | 351 ± 44 | 284 ± 29 | 350 ± 44 | 229 ± 21#* |
| LVC, ml·min−1·mmHg−1 | 4.0 ± 0.5 | 2.9 ± 0.4 | 4.3 ± 0.5 | 2.2 ± 0.2# |
| Passive leg movement | ||||
| ΔMAPpeak, mmHg | −11 ± 1 | −14 ± 2 | −12 ± 1 | −17 ± 2 |
| ΔCOpeak, l/min | 0.7 ± 0.1 | 1.2 ± 0.5 | 1.0 ± 0.2 | 0.3 ± 0.1# |
| ΔSVpeak, ml/beat | 4 ± 1 | 14 ± 6 | 10 ± 2* | 6 ± 1 |
| ΔHRpeak, beats/min | 10 ± 1 | 5 ± 1# | 10 ± 2 | 5 ± 1# |
| ΔLBFpeak, ml/min | 490 ± 34 | 250 ± 33# | 713 ± 59* | 224 ± 40# |
| LBFAUC, ml/min | 200 ± 23 | 79 ± 22# | 284 ± 45* | 67 ± 17# |
| ΔLVCpeak, ml·min−1·mmHg−1 | 5.7 ± 0.5 | 2.6 ± 0.3# | 9.2 ± 0.7* | 2.2 ± 0.4# |
| LVCAUC, ml·min−1·mmHg−1 | 2.3 ± 0.3 | 0.8 ± 0.2# | 3.3 ± 0.5* | 0.6 ± 0.2# |
Values are means ± SE; n = 10 old and 10 young women.
MAP, mean arterial pressure; CO, cardiac output; SV, stroke volume; HR, heart rate; LBF, leg blood flow; LVC, leg vascular conductance; Δpeak, peak change from baseline; AUC, area under the curve.
P < 0.05, significantly different from supine posture;
P < 0.05, significantly different from young women.
Both groups demonstrated significant increases in SV, HR, and CO and decreases in MAP (P < 0.05) as a consequence of PLM. However, the ΔHRpeak was significantly lower in the old compared with the young in both body postures, and the ΔSVpeak was greater in the young with the upright-seated posture, while tending to be lower in the old with this posture (P = 0.19), resulting in an attenuated ΔCOpeak in the old women while in the upright-seated posture (P < 0.01). Decreases in MAP (ΔMAPpeak) during PLM were similar between groups and postures.
Leg Blood Flow and Leg Vascular Conductance
Resting, peak change, and AUC data for peripheral hemodynamics are displayed in Table 2. Resting LBF and LVC were not different between groups in the supine posture. With the upright-seated posture, resting LBF was reduced in the old, such that both LBF and LVC were significantly lower in old compared with young women.
PLM in the supine and upright-seated postures resulted in a significant hyperemic response in both the young and old women (P < 0.05; Table 2, Figs. 1 and 2). In the supine posture, ΔLBFpeak and LBFAUC were attenuated in the old compared with the young women (49 and 60%, respectively; Fig. 1, B and C), resulting in a similarly attenuated ΔLVCpeak and LVCAUC (54 and 66%, respectively; Fig. 2, B and C) in the old. The upright-seated posture significantly increased the LBF (ΔLBFpeak = 46%, and LBFAUC = 42%; Fig. 1, B and C) and LVC (ΔLVCpeak = 61%, and LVCAUC = 43%; Fig. 2, B and C) response to PLM in the young, with no effect of posture in the old. This posture-induced increase in ΔLVCpeak in response to PLM in the young women revealed a significant vasodilatory reserve capacity (3.5 ± 0.6 ml·min−1·mmHg−1; P < 0.001) that was absent in the old (−0.5 ± 0.3 ml·min−1·mmHg−1; P = 0.18) women (Fig. 3).
Fig. 1.
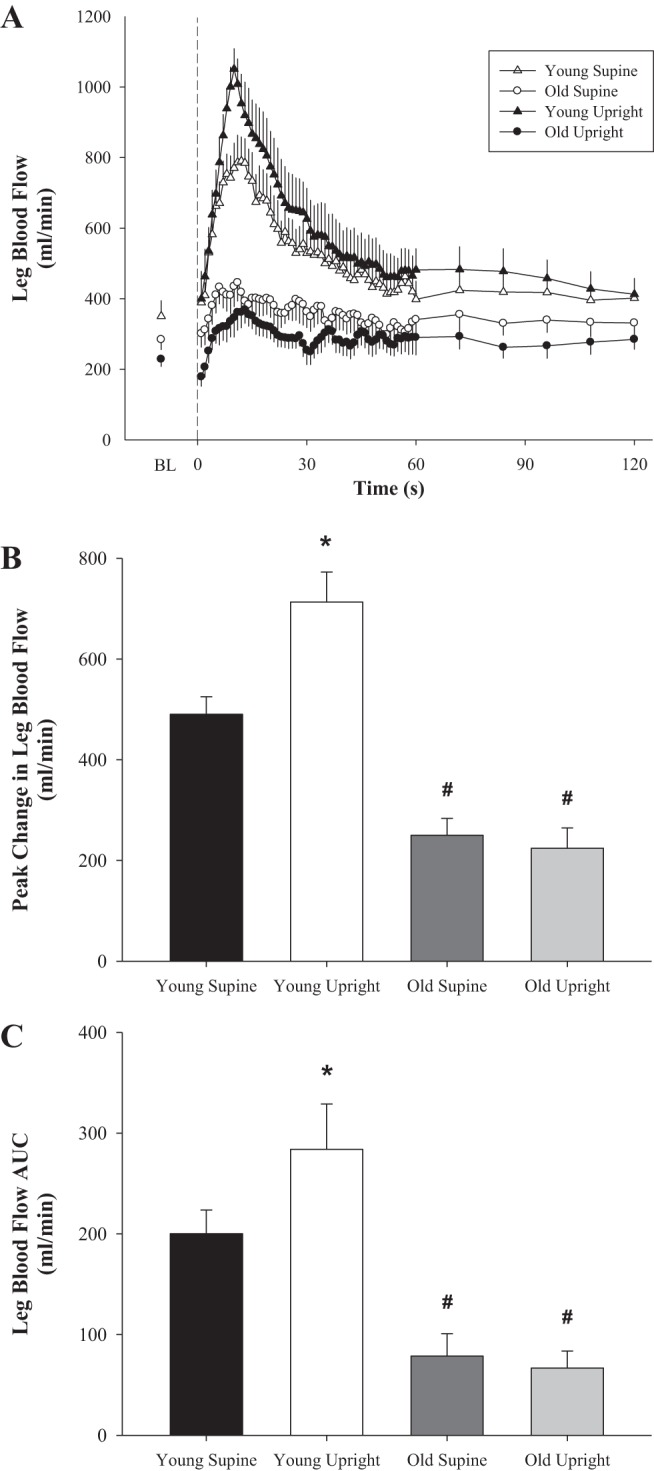
Passive leg movement (PLM)-induced changes in leg blood flow (LBF) with age in women. A: second-by-second tracing of LBF (ml/min) at baseline (BL) and throughout 2 min of PLM. B: peak change in LBF from BL (ΔLBFpeak, ml/min); C: LBF area under the curve (AUC) for the first 60 s of PLM (LBFAUC, ml/min). Young, n = 10; old, n = 10. BL indicates the average of the 60 s just before initiation of PLM. Dashed line at 0 s indicates the start of 2 min of PLM. *P < 0.05, significantly different from supine posture; #P < 0.05; significantly different from young women. Values are means ± SE.
Fig. 2.
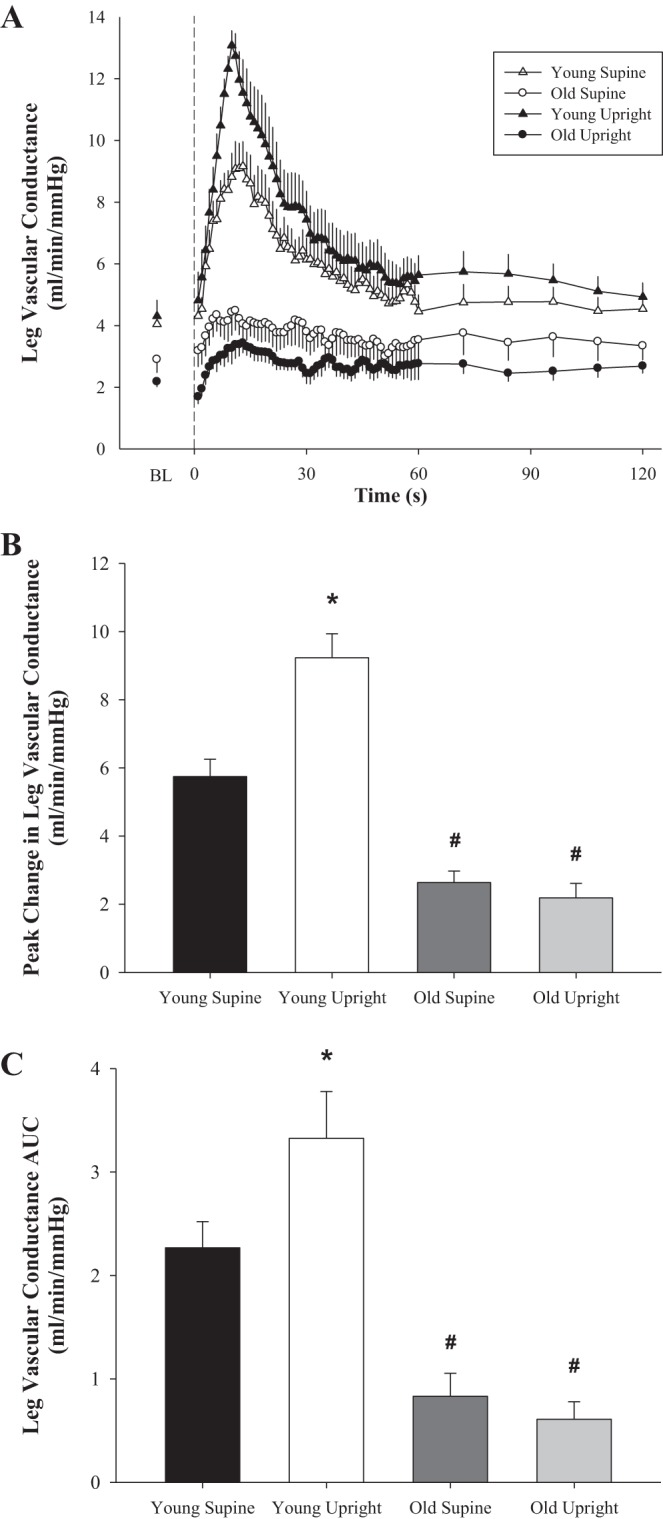
PLM-induced changes in leg vascular conductance (LVC) with age in women. A: second-by-second tracing of LVC (ml·min−1·mmHg−1) at baseline and throughout 2 min of PLM. B: peak change in LVC from BL (ΔLVCpeak, ml·min−1·mmHg−1). C: LVC AUC for the first 60 s of PLM (LVCAUC, ml·min−1·mmHg−1). Young, n = 10; old, n = 10. BL indicates the average of the 60 s just before initiation of PLM. Dashed line at 0 s indicates the start of 2 min of PLM. *P < 0.05, significantly different from supine posture; #P < 0.05, significantly different from young women. Values are means ± SE.
Fig. 3.
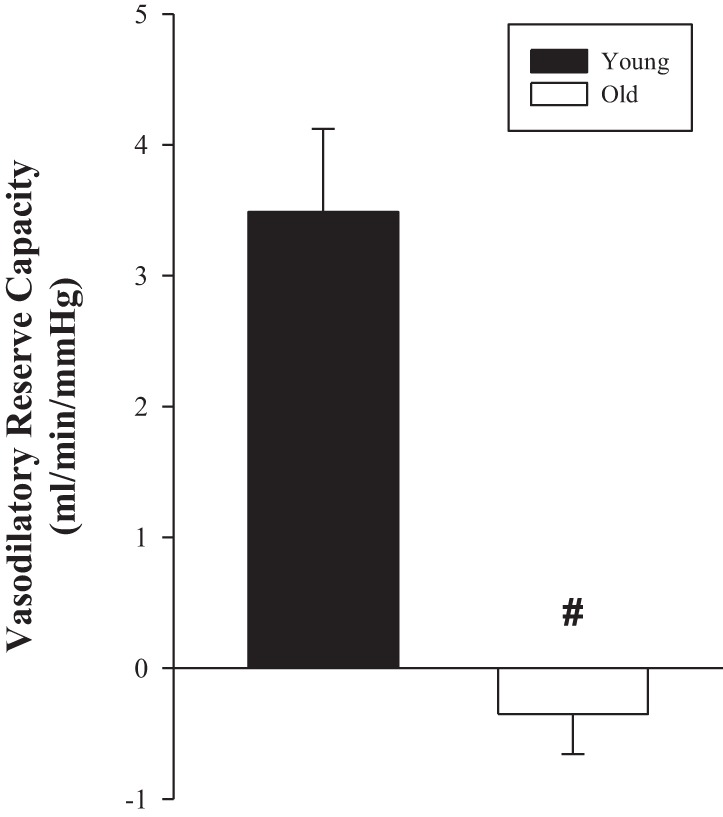
PLM-induced vasodilatory reserve capacity with age in women. The difference in the PLM-induced ΔLVCpeak (ml·min−1·mmHg−1) between the supine and upright-seated posture (vasodilatory reserve capacity). Young, n = 10; old, n = 10. #P < 0.05, significantly different from young women. Values are means ± SE.
In terms of total antioxidant capacity, oxidative stress, and inflammation, neither FRAP nor PC was significantly related to either ΔLVCpeak or LVCAUC. CRP displayed a significant negative relationship to ΔLVCpeak in the supine and upright-seated posture (r = 0.56, P < 0.01; and r = 0.70, P < 0.001, respectively), as well as with the vasodilatory reserve capacity (r = 0.65, P < 0.01). Similarly, CRP was negatively related to both supine and upright-seated LVCAUC (r = 0.56, P < 0.05; and r = 0.65, P < 0.01, respectively).
The immediate vasodilatory response (slopes of increasing LVC over time, for the first 9 s) to PLM was significantly reduced with age in both the supine (old, 0.15 ± 0.04; and young: 0.55 ± 0.04; P < 0.001) and the upright-seated (old, 0.16 ± 0.06; and young, 0.93 ± 0.09; P < 0.001) postures and was significantly elevated in the upright-seated posture, compared with supine, in the young only (P < 0.001; Fig. 4).
Fig. 4.
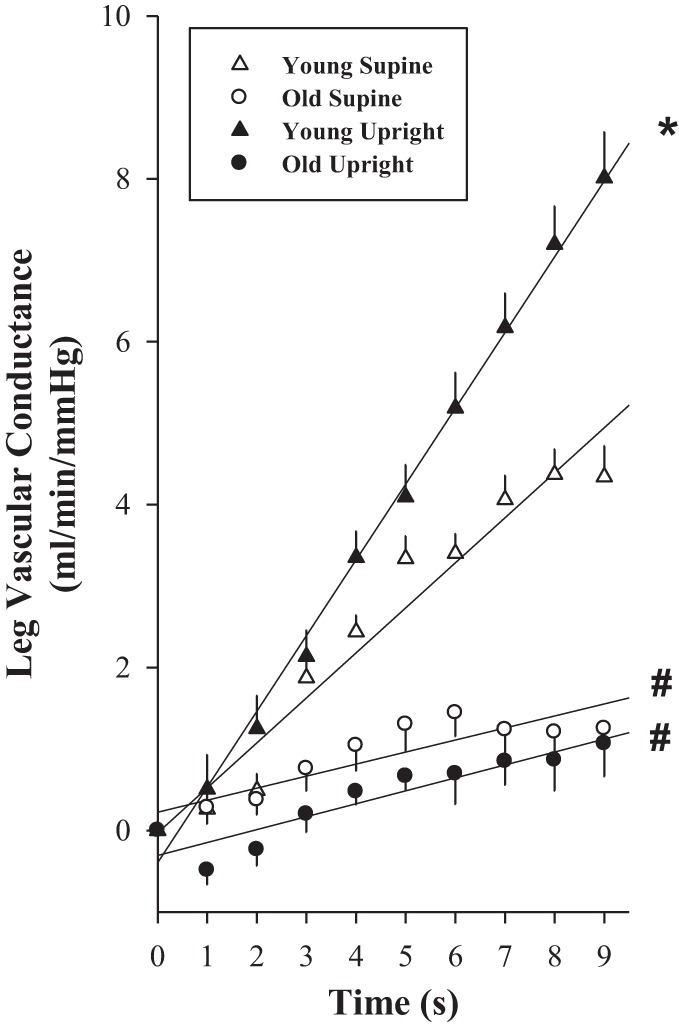
PLM-induced rapid vasodilation with age in women. The rate of increase in the LVC (ml·min−1·mmHg−1) over time was determined for the first 9 s of PLM. Young, n = 10; old, n = 10. *P < 0.05, significantly different from supine posture; #P < 0.05, significantly different from young women. Values are means ± SE.
DISCUSSION
The purpose of this investigation was to increase the generalizability of the PLM model by extending the previous literature to include the female population. Similar to findings in men, older women demonstrated an attenuated vasodilatory response to PLM in the supine posture compared with the young. The upright-seated posture, which significantly augmented the PLM-induced vasodilation in young women, had no effect on the vasodilatory response in the old. Similarly, in the old, the rapid vasodilatory (first 9 s) response to PLM in the supine posture was attenuated compared with the young women, and unlike in the young where the response was accelerated, the rapid vasodilatory response in the old women remained unchanged with increased FPP, thus resulting in a greater age difference in the upright-seated posture. Finally, the augmented PLM-induced ΔLVCpeak due to the upright-seated posture revealed a significant vasodilatory reserve capacity in young women that was absent in the old. These findings demonstrate that vascular function, assessed by the PLM model, declines with age in women. This expands the use of PLM to a wider population and bolsters the utility of PLM as a vascular function assessment across the human life span.
Age and Supine PLM-Induced Vasodilation in Women
PLM is an experimental model whereby changes in quadriceps and hamstring muscle length, without increases in metabolism, cause a significant increase in LBF and LVC. This PLM-induced hyperemia has previously been reported in both young and old men; however, the magnitude of the vasodilatory response to PLM is significantly attenuated with age (11, 23). Recent investigations into the mechanisms responsible for the PLM response conclude that PLM-induced vasodilation is predominantly NO mediated in young men, as evidenced by an ∼80–90% reduction in the hyperemic response during intra-arterial infusion of the NO synthase (NOS) inhibitor NG-monomethyl-l-argenine (10, 28, 39). Furthermore, by once again using NOS inhibition, both Trinity et al. (38) and Groot et al. (10) reported that the attenuated PLM-induced vasodilation described in older men was a consequence of reduced NO bioavailability with age. However, before this investigation, the PLM model had not been used to examine alterations in vascular function with age in women.
Results from the current investigation, which are strikingly similar to those previously reported in young and old men (11, 23), reveal a significant age-associated reduction in the PLM-induced ΔLBFpeak and ΔLVCpeak in the supine posture (∼50%) in old women compared with young, likely because of decreased NO bioavailability (Table 2, and Figs. 1B and 2B). Of note, when individual responses were examined, the PLM-induced hyperemia was lower in all 10 of the older women compared with all, but one, of the young subjects. It should be noted that the old women had less of a HR response to PLM than the young; however, given that the PLM-induced ΔMAPpeak was similar between young and old, it is unlikely that this central hemodynamic difference contributed to the peripheral hemodynamic findings. This supposition is supported by the LVC data, which take MAP into account (Fig. 2). Additionally, the rapid vasodilatory response (slope of the increasing LVC over time for the first 9 s) to PLM, previously determined to be NO-independent in men in the supine posture (10, 38), was also significantly attenuated with age in women (∼ 70%; Fig. 4). At the initiation of PLM, mechanical deformation of the vascular beds of the thigh may release vasodilators that act independently of the NO pathway (6, 17, 24). However, despite differences in this initial non-NO-dependent portion of the PLM response, subsequent mechanotransduction of wall shear forces likely contribute to the vasodilatory cascade making NO the dominant vasodilator for the remainder of PLM (14, 25, 30, 35).
Age and Upright-Seated PLM-Induced Vasodilation in Women
The upright-seated posture, which similarly increases FPP in young and old men (11), significantly augmented the PLM response in the young women, while having no effect on the old (Table 2, and Figs. 1 and 2). Of note, examination of the individual responses revealed that the PLM-induced hyperemia was augmented by the upright-seated posture in all ten young women, whereas the response in the old women was mixed. This posture-induced increase in vasodilation during PLM in the young enhanced the rapid vasodilation (Fig. 4) and revealed a vasodilatory reserve capacity that was absent in the old (Fig. 3).
Groot et al. (10) demonstrated that the increased rapid hyperemia with the upright-seated posture in young men could be attenuated to similar levels as the supine posture with NOS inhibition, but this rapid hyperemia was unaffected by posture in old men. This indicates that the onset of NO-mediated vasodilation during PLM is accelerated by increased FPP in the young, but not the old, and lends additional utility to the PLM model as an assessment of NO-mediated vasodilation. Similarly, whereas the upright-seated posture significantly augmented the PLM-induced rapid vasodilation in young women, the same postural alteration had no effect on the old (Table 2, and Figs. 1 and 2). In light of previous evidence that the increase in rapid vasodilation at the onset of PLM is reliant on NO, it can be inferred that this lack of an effect of the upright-seated posture on the rapid vasodilation in the old women is likely due to reduced NO bioavailability with age.
The ability for the ΔLVCpeak to increase when moving from the supine to upright-seated posture has been recognized to represent a vasodilatory reserve capacity (11). The upright-seated posture elicits a similar (∼7 mmHg) increase in FPP in both young and old men, as measured directly in the CFA and vein (11). This augmented driving force of blood across the vascular beds of the thigh has the potential to increase endothelial shear forces at the onset of PLM, thereby resulting in a greater NO-mediated vasodilation. This was indeed the case in young men whose ΔLVCpeak nearly doubled in the upright-seated posture, revealing a vasodilatory reserve capacity that was nearly entirely due to NO (10). In contrast, because of reduced NO bioavailability with age, similar increases in FPP in old men did not augment the ΔLVCpeak in the upright-seated posture, thus a vasodilatory reserve capacity was absent in the old (10). In support of these previous findings, the young women displayed a significant vasodilatory reserve capacity, whereas the old women, who already possessed an attenuated PLM response compared with the young in the supine posture, failed to increase their ΔLVCpeak in the upright-seated posture and therefore lacked any vasodilatory reserve capacity (Fig. 3). Given that the vasodilatory reserve is an NO-mediated phenomena, its absence in older women provides additional evidence that NO bioavailability, as measured by PLM, is decreased with age.
Age and NO Bioavailability in Women
While it is clear that PLM-induced vasodilation is attenuated in older humans, the mechanisms leading to this age-associated decrease in NO bioavailability remain unclear. However, previous reports implicate an age-associated increase in oxidative stress and, specifically in women, the postmenopausal fall in circulating estrogen as likely causes of impaired vascular function and NO bioavailability with age.
Oxidative stress is the result of free radicals, molecules with unpaired electrons that lead to oxidizing reactions, which interfere with cellular processes resulting in damage, dysfunction, and/or apoptosis. Many of these free radicals are found in the vasculature and can interfere with NO signaling, thus diminishing NO bioavailability (5, 44). Indeed, markers of free radical production are elevated in old subjects in both endothelial cells and circulating plasma (8, 12, 41, 44) and are associated with decreased vascular function (8, 44). Interventions aimed at acutely decreasing oxidative stress, such as the infusion of the antioxidant ascorbic acid, result in improved vascular function and NO bioavailability in old subjects (9, 36, 37). In the present investigation, the older women, while not exhibiting an attenuated total antioxidant capacity, as assessed by FRAP, did have significantly elevated levels of inflammation and oxidative stress (Table 1). Additionally, although FRAP and protein carbonyls were unrelated to the PLM response, CRP was significantly related to measures of PLM-induced vasodilation (r = 0.56–0.70), suggesting that inflammation may play a role in the attenuated PLM response with age in women. However, further investigations into the possible effects of oxidative stress on PLM-induced vasodilation with age are necessary.
Estrogen has powerful effects on NO production, and this phenomenon is likely, at least in part, responsible for the observation that premenopausal women have decreased CVD risk compared with men and postmenopausal women. Estrogen can cause genomic modifications, such as increased transcription and ultimately the expression of eNOS protein (26, 29), As well as nongenomic effects, such as increased eNOS activation (16, 20, 26, 29). Furthermore, estrogen provides additional antioxidant effects by inhibiting NADPH oxidase and augmenting superoxide dismutase, thereby decreasing oxidative stress in the vasculature (26, 29). By these mechanisms, estrogen can decrease free radical production and NO scavenging and improve eNOS coupling and NO synthesis, resulting in greater NO bioavailability. Therefore, drastic reductions in estrogen after menopause likely contribute to decreased NO-mediated vasodilation and impaired vascular function in older women. Support for this supposition can be found by a direct comparison of the current investigation to previously published PLM results in men which suggest that women may have a greater age related impairment in vascular function (11, 23). Indeed, the magnitude of attenuation in the PLM response with age in women is 10–20% greater than that reported in men and may be potentially due to the postmenopausal fall in estrogen. Furthermore, previous investigations examining endothelium-dependent vasodilation with age in women have revealed reduced vasodilation in postmenopausal women compared with young women (4, 13) and in ovariectomized rats compared with intact animals (20, 27). These reductions in vascular function can be rescued with hormone replacement therapy (7, 15, 20, 27, 32). A direct assessment of the potential link between estrogen levels and PLM-induced hyperemia warrants further investigation.
Experimental Considerations
Because of the minimally invasive nature of this study, the direct measurement of femoral arterial, venous, and the subsequent calculation of actual perfusion pressure was not performed. Therefore, while we cannot rule out the possibility of differential FPP responses to the upright-seated posture in young and old women, a similar study performed in men reported identical posture-induced increases in FPP between the young and old subjects (11). Furthermore, while older women display some differences in orthostatic coping strategies compared with men, the responsiveness of peripheral resistance vessels appears to be similar (2), suggesting that FPP responses to the upright-seated posture are likely comparable to those previously reported in young and old men. Additionally, because infusion of a NOS inhibitor was not performed, we cannot rule out the possibility that the mechanisms for the PLM-induced vasodilation may differ between men and women. However, Mannacio et al. (22) reported that compared with young controls and age-matched men, older women displayed attenuated eNOS mRNA and a reduction in internal mammary artery relaxation in response to acetylcholine. This would suggest that attenuated NO bioavailability with age not only is an important factor in the impaired vascular function observed in older women but may play an even greater role than that observed in older men. However, future investigations into the direct role of NO in the PLM-induced vasodilation in women are needed. Additionally, as with any study focused on aging, there is often some difficulty in discerning age-related changes, such as increases in BMI and total cholesterol, from aging, per se, and that is also the case in this investigation. Finally, the relatively small sample size may have limited our ability to detect changes in certain variables (e.g., triglycerides); therefore, future studies using PLM may benefit from a larger cohort.
Conclusion
The results of the current investigation support previous findings that PLM-induced vasodilation is attenuated with age and extend these observations to include women. Additionally, increasing FPP by moving from the supine to the upright-seated posture, which magnifies the role of NO in both the rapid and overall vasodilatory response, revealed a vasodilatory reserve capacity in young, but not old women. These findings imply reduced NO bioavailability with age in women and add additional support to the utility of the PLM model as an assessment of NO-mediated vascular function across the human life span.
GRANTS
The work was supported by National Heart, Lung, and Blood Institute Grant P01-H1-091830 (to R. S. Richardson); Veterans Affairs Rehabilitation Research and Development Service Merit Grant E6910-R (to R. S. Richardson), Merit Grant E1697-R (to R. S. Richardson), and CDA2 1IK2RX001215 (to J. D. Trinity); SPiRe Grant E1433-P (to R. S. Richardson), American Heart Association Grant 14SDG1850039 (to J. D. Trinity); and an Advanced Fellowship in Geriatrics from the Department of Veterans Affairs (to S. J. Ives).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.J.G., J.D.T., and R.S.R. conception and design of research; H.J.G., M.J.R., J.D.T., G.L., and S.J.I. performed experiments; H.J.G. and M.J.R. analyzed data; H.J.G. and R.S.R. interpreted results of experiments; H.J.G. prepared figures; H.J.G. drafted manuscript; H.J.G., M.J.R., J.D.T., G.L., S.J.I., and R.S.R. edited and revised manuscript; H.J.G., M.J.R., J.D.T., G.L., S.J.I., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects who partook in this research and Van Reese and Jia Zhao for performing the blood assays.
REFERENCES
- 1.Abel MG, Hannon JC, Sell K, Lillie T, Conlin G, Anderson D. Validation of the Kenz Lifecorder EX and ActiGraph GT1M accelerometers for walking and running in adults. Appl Physiol Nutr Metab 33: 1155–1164, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, Burgdorf C, Pramstaller PP, Schunkert H, Bonnemeier H. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol 19: 1296–1303, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239: 70–76, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AF, Chen DD, Daiber A, Faraci FM, Li H, Rembold CM, Laher I. Free radical biology of the cardiovascular system. Clin Sci (Lond) 123: 73–91, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Kleijn MJ, Wilmink HW, Bots ML, Bak AA, van der Schouw YT, Planellas J, Engelen S, Banga JD, Grobbee DE. Hormone replacement therapy and endothelial function. Results of a randomized controlled trial in healthy postmenopausal women. Atherosclerosis 159: 357–365, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilation with age: insight from alterations in femoral perfusion pressure. J Physiol. Jun 24. doi: 10.1113/JP270195 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–H619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med 250: 29–36, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Kawano H, Motoyama T, Kugiyama K, Hirashima O, Ohgushi M, Fujii H, Ogawa H, Yasue H. Gender difference in improvement of endothelium-dependent vasodilation after estrogen supplementation. J Am Coll Cardiol 30: 914–919, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids 73: 864–869, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 297: R1713–R1723, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Mannacio V, Di Tommaso L, Antignano A, De Amicis V, Stassano P, Pinna GB, Vosa C. Endothelial nitric oxide synthase expression in postmenopausal women: a sex-specific risk factor in coronary surgery. Ann Thorac Surg 94: 1934–1939, 2012. [DOI] [PubMed] [Google Scholar]
- 23.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol 112: 560–565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momoi H, Ikomi F, Ohhashi T. Estrogen-induced augmentation of endothelium-dependent nitric oxide-mediated vasodilation in isolated rat cerebral small arteries. Jpn J Physiol 53: 193–203, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, D'Anna R, Lasco A, Squadrito G, Gaudio A, Cancellieri F, Arcoraci V, Squadrito F. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol 21: 1512–1519, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt MD, Cleland VJ, Shaw K, Dwyer T, Venn AJ. Cardiometabolic risk in younger and older adults across an index of ambulatory activity. Am J Prev Med 37: 278–284, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Shechter M, Marai I, Marai S, Sherer Y, Sela BA, Feinberg MS, Rubinstein A, Shoenfeld Y. The association of endothelial dysfunction and cardiovascular events in healthy subjects and patients with cardiovascular disease. Isr Med Assoc J 9: 271–276, 2007. [PubMed] [Google Scholar]
- 35.Sun D, Huang A, Recchia FA, Cui Y, Messina EJ, Koller A, Kaley G. Nitric oxide-mediated arteriolar dilation after endothelial deformation. Am J Physiol Heart Circ Physiol 280: H714–H721, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tudor-Locke C, Bassett DR Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 34: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Vasilaki A, Mansouri A, Van Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5: 109–117, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302: H333–H339, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welk GJ, Blair SN, Wood K, Jones S, Thompson RW. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc 32: S489–S497, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 62: 345–351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


