Abstract
All photosynthetic organisms, with the exception of several species of photosynthetic bacteria, are thought to contain the sulfolipid 6-sulfo-alpha-D-quinovosyldiacylglycerol. The association of this lipid with photosynthetic membranes has led to the assumption that it plays some role in photosynthesis. Stable null mutants of the photosynthetic bacterium Rhodobacter sphaeroides completely lacking sulfolipid were obtained by disruption of the sqdB gene. The ratios of the various components of the photosynthetic electron transport chain, as well as the electron transfer rates during cyclic electron transport, were not altered in the mutants, when grown under optimal conditions. Growth rates of wild type and mutants were identical under a variety of growth conditions, with the exception of phosphate limitation, which resulted in reduced growth of the mutants. Phosphate limitation of the wild type caused a significant reduction in the amount of all phospholipids and an increased amount of sulfolipid. By contrast, the sulfolipid-deficient mutant had reduced levels of phosphatidylcholine and phosphatidylethanolamine but maintained a normal level of phosphatidylglycerol. In addition, two unidentified lipids lacking phosphorus accumulated in the membranes of both wild-type and mutant strains under phosphate limitation. We conclude that sulfolipid plays no significant unique role in photoheterotrophic growth or photosynthetic electron transport in R. sphaeroides but may function as a surrogate for phospholipids, particularly phosphatidylglycerol, under phosphate-limiting conditions.
Full text
PDF

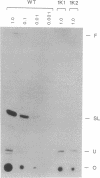
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENSON A. A. THE PLANT SULFOLIPID. Adv Lipid Res. 1963;1:387–394. doi: 10.1016/b978-1-4831-9937-5.50016-8. [DOI] [PubMed] [Google Scholar]
- Benning C., Somerville C. R. Identification of an operon involved in sulfolipid biosynthesis in Rhodobacter sphaeroides. J Bacteriol. 1992 Oct;174(20):6479–6487. doi: 10.1128/jb.174.20.6479-6487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C., Somerville C. R. Isolation and genetic complementation of a sulfolipid-deficient mutant of Rhodobacter sphaeroides. J Bacteriol. 1992 Apr;174(7):2352–2360. doi: 10.1128/jb.174.7.2352-2360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. A., Daniel H., Wiser R. A SULFOLIPID IN PLANTS. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1582–1587. doi: 10.1073/pnas.45.11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Davidson E., Prince R. C., Haith C. E., Daldal F. The cytochrome bc1 complex of Rhodobacter sphaeroides can restore cytochrome c2-independent photosynthetic growth to a Rhodobacter capsulatus mutant lacking cytochrome bc1. J Bacteriol. 1989 Nov;171(11):6059–6068. doi: 10.1128/jb.171.11.6059-6068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Petty K. M., Bonner H. S., Morse S. D. Cytochrome c2 and reaction center of Rhodospeudomonas spheroides Ga. membranes. Extinction coefficients, content, half-reduction potentials, kinetics and electric field alterations. Biochim Biophys Acta. 1975 Jun 17;387(3):536–556. doi: 10.1016/0005-2728(75)90092-4. [DOI] [PubMed] [Google Scholar]
- Haines T. H. Anionic lipid headgroups as a proton-conducting pathway along the surface of membranes: a hypothesis. Proc Natl Acad Sci U S A. 1983 Jan;80(1):160–164. doi: 10.1073/pnas.80.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff J. F., Kushner D. J., Kushwaha S. C., Kates M. Polar lipids in phototrophic bacteria of the Rhodospirillaceae and Chromatiaceae families. J Bacteriol. 1982 Jun;150(3):1192–1201. doi: 10.1128/jb.150.3.1192-1201.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. Replacement of acidic phosphates by acidic glycolipids in Pseudomonas diminuta. Nature. 1974 May 17;249(454):268–269. doi: 10.1038/249268a0. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Poirier Y., Thoma S., Somerville C., Schiefelbein J. Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 1991 Nov;97(3):1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radunz A. Uber das Sulfochinovosyl-diacylglycerin aus höheren Pflanzen, Algen und Purpurbakterien. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):411–417. [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:607–616. doi: 10.1099/00221287-28-4-607. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Specific deficit in the synthesis of 6-sulfoquinovsyl diglyceride in Chlorella pyrenoidosa. J Bacteriol. 1977 Jan;129(1):516–524. doi: 10.1128/jb.129.1.516-524.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wellington C. L., Taggart A. K., Beatty J. T. Functional significance of overlapping transcripts of crtEF, bchCA, and puf photosynthesis gene operons in Rhodobacter capsulatus. J Bacteriol. 1991 May;173(9):2954–2961. doi: 10.1128/jb.173.9.2954-2961.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilsel J., Lilburn T. G., Beatty J. T. Formation of functional inter-species hybrid photosynthetic complexes in Rhodobacter capsulatus. FEBS Lett. 1989 Aug 14;253(1-2):247–252. doi: 10.1016/0014-5793(89)80969-x. [DOI] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]