This research has identified the ability of antioxidants to increase skeletal muscle blood flow and O2 consumption during exercise in patients with chronic obstructive pulmonary disease. Thus, targeting oxidative stress may have important therapeutic implications for improving O2 transport and utilization, and therefore exercise tolerance, in patients with chronic obstructive pulmonary disease.
Keywords: chronic bronchitis, emphysema, oxidative stress, exercise, oxygen transport, chronic obstructive pulmonary disease
Abstract
The consequence of elevated oxidative stress on exercising skeletal muscle blood flow as well as the transport and utilization of O2 in patients with chronic obstructive pulmonary disease (COPD) is not well understood. The present study examined the impact of an oral antioxidant cocktail (AOC) on leg blood flow (LBF) and O2 consumption during dynamic exercise in 16 patients with COPD and 16 healthy subjects. Subjects performed submaximal (3, 6, and 9 W) single-leg knee extensor exercise while LBF (Doppler ultrasound), mean arterial blood pressure, leg vascular conductance, arterial O2 saturation, leg arterial-venous O2 difference, and leg O2 consumption (direct Fick) were evaluated under control conditions and after AOC administration. AOC administration increased LBF (3 W: 1,604 ± 100 vs. 1,798 ± 128 ml/min, 6 W: 1,832 ± 109 vs. 1,992 ± 120 ml/min, and 9W: 2,035 ± 114 vs. 2,187 ± 136 ml/min, P < 0.05, control vs. AOC, respectively), leg vascular conductance, and leg O2 consumption (3 W: 173 ± 12 vs. 210 ± 15 ml O2/min, 6 W: 217 ± 14 vs. 237 ± 15 ml O2/min, and 9 W: 244 ± 16 vs 260 ± 18 ml O2/min, P < 0.05, control vs. AOC, respectively) during exercise in COPD, whereas no effect was observed in healthy subjects. In addition, the AOC afforded a small, but significant, improvement in arterial O2 saturation only in patients with COPD. Thus, these data demonstrate a novel beneficial role of AOC administration on exercising LBF, O2 consumption, and arterial O2 saturation in patients with COPD, implicating oxidative stress as a potential therapeutic target for impaired exercise capacity in this population.
NEW & NOTEWORTHY
This research has identified the ability of antioxidants to increase skeletal muscle blood flow and O2 consumption during exercise in patients with chronic obstructive pulmonary disease. Thus, targeting oxidative stress may have important therapeutic implications for improving O2 transport and utilization, and therefore exercise tolerance, in patients with chronic obstructive pulmonary disease.
chronic obstructive pulmonary disease (COPD) is a proinflammatory condition that primarily impacts the lungs, resulting in diminished pulmonary function (29). Other detrimental sequelae of this condition include mitochondrial (35) and skeletal muscle (31) dysfunction, and, consequently, exercise intolerance and decreased fatigue resistance are recognized hallmarks of patients with COPD (3). Interestingly, peripheral vascular function is also impaired in these patients (24). Through the release of autocrine and paracrine factors that modulate vascular tone, the vasculature plays a critical role in regulating skeletal muscle blood flow (37) and therefore the delivery of O2 and nutrients. As such, poor vascular function also has the potential to influence exercise capacity and fatigability in patients with COPD (39). However, the mechanistic link among COPD, vascular dysfunction, and exercising skeletal muscle blood flow remains to be elucidated.
Peripheral vascular dysfunction in COPD has been attributed to numerous factors, including systemic inflammation and oxidative stress (20, 24). Indeed, relative to age-matched healthy subjects, both elevated systemic inflammation and oxidative stress have been well documented in patients with COPD (6, 24, 32). Specific to oxidative stress, our group has recently demonstrated the beneficial effects of an acutely administered oral antioxidant cocktail (AOC) on vascular function, as assessed by flow-mediated dilation, in patients with COPD (24). The AOC and the dosing strategy used have been previously documented to reduce O2 and carbon-centered free radicals in patients with COPD (40). Thus, the improvement in vascular function after AOC administration in the prior work was attributed to the free radical scavenging ability of the AOC, restoration of the redox balance in the patients, and, potentially, a subsequent improvement in nitric oxide (NO) bioavailability (24). Based on these findings, targeting oxidative stress with an acute AOC appears to represent a viable mechanism for improving peripheral vascular function in patients with COPD.
Oxidative stress may impair vascular function and potentially adversely impact vascular control during exercise by enhancing endothelin-1 activity (19, 43), potentiating angiotensin II-mediated vasoconstriction (17), augmenting sympathetically mediated vascular tone (42), and suppressing cyclooxygenase-2-mediated dilation (21), in addition to, among these and other mechanisms, directly reducing the bioavailability of the vasodilator NO. With regard to NO, Crecelius et al. (16) documented an improvement in forearm blood flow in older individuals during rhythmic handgrip exercise with an intra-arterial infusion of ascorbate, and this effect was abrogated when ascorbate was coinfused with a NO synthase inhibitor. In addition, Wray et al. (46) documented elevated end plantar flexion exercise muscle perfusion, as assessed by nuclear magnetic resonance spectroscopy, after AOC administration in older individuals. Collectively, these studies suggest that there are numerous mechanisms by which oxidative stress may adversely impact vascular control during exercise and implicate antioxidant administration and free radical scavenging as strategies to improve exercise hyperemia, potentially through an improvement in NO bioavailability.
Therefore, the purpose of the present study was to examine the impact of an acutely administered oral AOC on leg blood flow (LBF) and O2 transport in the exercising skeletal muscle of patients with COPD and healthy subjects. We tested the following two hypotheses. First, administration of the AOC would improve exercising LBF in patients with COPD, with less of an effect in healthy subjects. Second, the increase in LBF after AOC administration would be accompanied by an improvement in redox balance, as assessed by blood markers of antioxidant status relative to markers of oxidative stress.
METHODS
Subjects.
A total of 32 subjects, 16 patients with COPD and 16 age- and sex-matched healthy subjects, completed this study. Subjects were included in the study based on spirometric evidence of either COPD (patients with COPD) or normal lung function (healthy subjects) and sedentary to low physical activity levels (assessment described below). Exclusion criteria for the study included: hypertension or other overt cardiovascular disease, diabetes, neuromuscular disease, known cancer, and obesity. All subjects performed standard pulmonary function tests during an initial visit to the laboratory. General morphometric characteristics and peak knee extension (KE) exercise work rate were also determined during this visit. The Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center approved all protocol, and written informed consent was obtained from all participants before their inclusion in the study.
Physical activity level.
Before participation in the study, physical activity was assessed by a subjective modified physical activity level recall questionnaire (41) in all subjects, with the goal of matching physical activity levels between patients with COPD and healthy subjects. After this initial data collection, physical activity was further evaluated to confirm similar physical activity levels between groups by objective accelerometry in a representative subset of 20 subjects (10 subjects/group). The physical activity questionnaire included items regarding the average type, frequency, intensity, and duration of physical activity in any given week (23). For the accelerometry, 10 patients with COPD and 10 healthy subjects wore accelerometers (GT1M, Actigraph, Pensacola, FL) for 7 consecutive days after receiving standardized operating instructions. Average total daily physical activity was expressed as average steps per day and, to better characterize the intensity of physical activity, average total accelerometer counts per minute for each subject was parsed into sedentary, low-, moderate-, and high-intensity activity using device-specific software (Actilife, Actigraph, Pensacola, FL). Previous research has documented the validity and reliability of the Actigraph GT1M in the estimation of daily physical activity (1, 44).
Exercise protocols and general procedures.
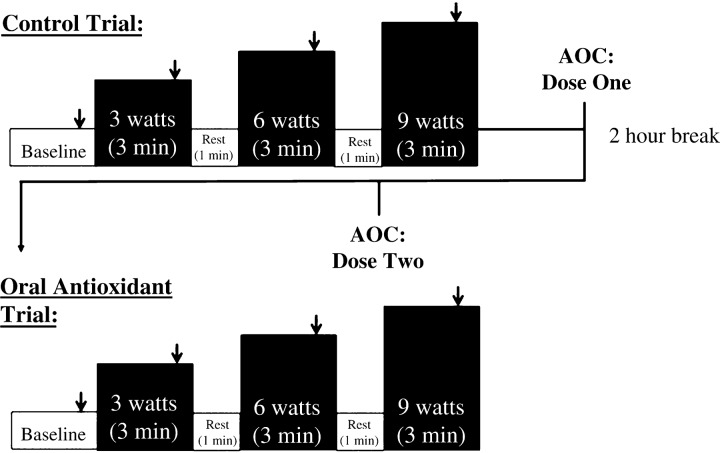
Before data collection, all subjects were familiarized with the KE exercise during one to three laboratory visits, during which subjects practiced performing the KE workloads and durations required for the study. On the experimental day, subjects reported to the laboratory after a 12-h fast and rested for ∼30 min before all procedures. Subsequently, catheters were placed in the femoral artery and vein using sterile techniques, as previously described (4). After catheter placement, subjects rested for an additional 30 min before beginning KE exercise, as shown in Fig. 1. KE exercise was performed at 60 rpm on a cycle erogmeter (Monark) modified to allow KE exercise (38). Briefly, this exercise modality recruits the quadriceps muscle group for active leg extension from 90 to ∼170° before a lever arm attached to a flywheel passively returns the leg to 90°. Due to the potentially long-lasting effects of the AOC, the AOC trial was always performed after the control condition. In addition, the invasive nature of the study and subject risk associated with catheter placement precluded separation of the study into 2 separate days. However, of note, our group has previously documented the reproducibility of hemodynamic measurements achieved with this serial exercise testing experimental design, without an intervention, across a range of exercise intensities (8). Each workload (3, 6, and 9 W) was sustained for 3 min. One minute of rest was allowed between each stage. LBF, mean arterial pressure (MAP), leg O2 consumption (V̇o2), and heart rate were assessed during the last minute of baseline and each exercise stage. Blood samples were taken anaerobically during the last minute of both baseline and each exercise stage.
Fig. 1.
Experimental protocol. Arrows indicate points at which leg blood flow (LBF) was recorded and arterial and venous blood samples were obtained. AOC, oral antioxidant cocktail.
Antioxidant supplementation.
All subjects were instructed to refrain from vitamin supplementation for at least 5 days before data collection. On the experimental day, the AOC was administered in a split dose, consumed 2 and 1.5 h before the second exercise bout, to improve absorption and maximize the time of antioxidant efficacy. The first dose consisted of 300 mg α-lipoic acid, 500 mg vitamin C, and 200 IU of vitamin E, and the second dose consisted of the same amounts of α-lipoic acid and vitamin C and 400 IU vitamin E. This AOC and the dosing strategy used have been previously documented to lower carbon- and O2-centered free radical levels, as measured by electron paramagnetic resonance spectroscopy, and improve vascular function in patients with COPD (24, 40).
Central cardiovascular responses.
Arterial blood pressure measurements were collected continuously from an indwelling catheter placed in the common femoral artery, with the pressure transducer at the level of the catheter (Transpac IV, Abbott Laboratories). MAP (in mmHg) was calculated as follows: MAP = diastolic arterial pressure + (arterial pulse pressure × 0.33). Heart rate (HR) was monitored from a standard three-lead ECG, a component of the data-acquisition device (Biopac, Goleta, CA).
LBF and leg vascular conductance.
Measurements of femoral artery blood velocity and vessel diameter in the leg being studied were performed at rest and during the last minute of each exercise stage using a Logic 7 ultrasound system (General Electric Medical Systems) as previously described (9). The Logic 7 ultrasound system was equipped with a linear array transducer operating at an imaging frequency of 9 MHz. The blood velocity profile was obtained with the same transducer with a Doppler frequency of 5 MHz operated in the high-pulsed repetition frequency mode. LBF was calculated as follows: LBF = (mean femoral artery blood velocity) × π × (femoral artery vessel diameter/2)2 × 60. Leg vascular conductance (LVC) was calculated as follows: LVC = LBF/arterial catheter-derived MAP.
Blood analysis.
A standard lipid panel was obtained for all subjects. At rest and in the last 15 s of each exercise stage, femoral arterial and venous blood samples (1–2 ml) were collected, and 1 ml of each sample was presented to a GEM 4000 combined blood gas analyzer and cooximeter (Instrumentation Laboratories, Bedford, MA). Arterial and venous blood hemoglobin (Hb) concentration as well Hb O2 saturation were measured by cooximetry. The GEM 4000 also contains different polymer film electrochemical sensors specific for relevant substrates, which were used to measure Po2 and Pco2 as well as lactate and pH. Arterial and venous blood O2 content (in ml/dl) were calculated as follows: blood O2 content = 1.39(Hb) × (O2 saturation/100) + 0.003 × Po2. Leg V̇o2 (in ml/min) was calculated as follows: V̇o2 = (arterial O2 content − venous O2 content) × LBF.
Oxidative stress and inflammation.
Venous blood samples taken at rest were centrifuged to collect plasma, and plasma samples were stored at −80°C until analysis. Lipid peroxidation, a marker of oxidant damage, was assessed by plasma malondialdehyde levels (Bioxytech LPO-586, Foster City, CA). Total antioxidant capacity was evaluated by determining the ferric reducing ability of plasma (FRAP) using the method previously described by Benzie and Strain (10). The efficacy of the AOC specific to plasma ascorbate levels was assayed as previously described (CosmoBio, Carlsbad, CA) (11). Endogenous antioxidant activity was assessed by catalase activity in the plasma (Cayman Chemical, Ann Arbor, MI) (45). Plasma C-reactive protein levels, an index of systemic inflammation, were determined by high-sensitivity ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis.
Independent-sample t-tests were used to determine differences in subject characteristics, including physical activity-related variables. A 2 × 2 mixed-design ANOVA was used to evaluate the effect of the AOC on indexes of antioxidant status, inflammation, and oxidative stress. When a significant effect was detected, differences were identified using paired t-tests for the within-subject factor (AOC) and independent t-tests for the between-subject factor (COPD patients vs. healthy subjects). A 2 × 3 (subject × workload) ANOVA was used to determine differences between patents with COPD and healthy control subjects (subject factor) on physiological variables measured during exercise (workload: 3, 6, and 9 W), and a 2 × 3 (AOC × workload) repeated-measures ANOVA was used to identify significant changes in measured variables due to AOC administration within healthy subjects and COPD patients. Tukey's post hoc analysis was used if a significant main effect was found. Statistical significance was set at α = 0.05 for all tests. All data are expressed as means ± SE.
RESULTS
Subject characteristics.
Subject characteristics are shown in Table 1. Patients with COPD exhibited reduced pulmonary function relative to the healthy subjects, and blood gas characteristics consistent with COPD (Table 1). Apart from pulmonary function, arterial blood gases, and pulmonary disease medications, the healthy subjects were well matched with the patients with COPD (Table 1). By experimental design, in general, physical activity levels were relatively similar between the healthy subjects and the patient group, resulting in similar peak KE work rates between groups (Table 1). Two of the patients with COPD were current smokers, who refrained from the use of tobacco products for 12 h before all data collection. Four patients qualified for supplemental O2; only one of these patients, however, required the use of supplemental O2 during exercise (the blood gas data for the individual using supplemental O2 was excluded from the analyses).
Table 1.
Subject characteristics
| Healthy Subjects | COPD Patients | |
|---|---|---|
| Age, yr | 68 ± 2 | 62 ± 3 |
| Men/women | 13/3 | 13/3 |
| Height, m | 1.73 ± 0.02 | 1.74 ± 0.02 |
| Weight, kg | 75 ± 4 | 85 ± 4 |
| Body mass index, kg/m2 | 25 ± 1 | 28 ± 1 |
| Peak knee-extensor work rate, W | 31 ± 3 | 26 ± 3 |
| Glucose, mg/dl | 84 ± 4 | 84 ± 3 |
| Cholesterol, mg/dl | 201 ± 14 | 202 ± 16 |
| High-density lipoprotein, mg/dl | 52 ± 3 | 61 ± 5 |
| Low-density lipoprotein, mg/dl | 132 ± 10 | 120 ± 13 |
| Triglycerides, mg/dl | 94 ± 16 | 117 ± 14 |
| Pulmonary function | ||
| Forced vital capacity, liters (%predicted) | 4.7 ± 0.3 (113 ± 5) | 4.1 ± 0.3* (94 ± 5*) |
| FEV1, l/s (%predicted) | 3.4 ± 0.2 (115 ± 4) | 1.8 ± 0.2* (54 ± 6*) |
| FEV1/forced vital capacity, % | 75 ± 1 | 48 ± 4* |
| Resting arterial blood gases | ||
| Oxyhemoglobin, % | 94.4 ± 0.5 | 91.7 ± 1.2* |
| Po2, mmHg | 87 ± 4 | 71 ± 3* |
| Pco2, mmHg | 30 ± 1 | 35 ± 1* |
| Bicarbonate, mmol/l | 19.7 ± 0.5 | 23.4 ± 0.8* |
| pH | 7.43 ± 0.01 | 7.43 ± 0.01 |
| Medications (%group) | ||
| Long-acting β-Agonists | 0 | 26 |
| Short-acting β-Agonists | 0 | 80 |
| Acetylcholine antagonists | 0 | 53 |
| Inhaled corticosteroids | 15 | 33 |
| Physical activity (n = 10 subjects/group) | ||
| Sedentary, min/day | 1,215 ± 17 | 1,156 ± 75 |
| Light, min/day | 153 ± 9 | 127 ± 20 |
| Moderate, min/day | 26 ± 5 | 15 ± 6 |
| High, min/day | 0.4 ± 0.2 | 0.1 ± 0.01* |
| Steps, counts/day | 5,101 ± 671 | 3,798 ± 728 |
Values are means ± SE. COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.
Significantly different from healthy subjects.
Antioxidants, oxidative stress, and inflammation.
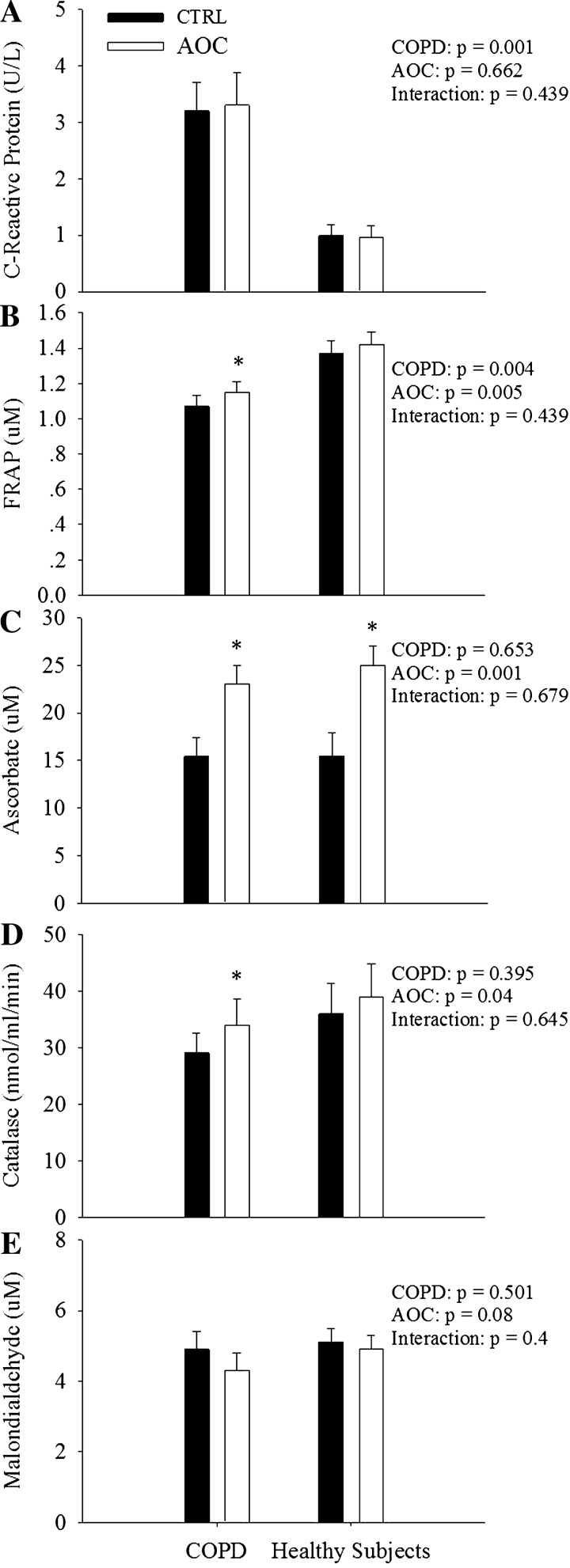
Baseline plasma ascorbate was not different between patients with COPD and healthy subjects (Fig. 2C). Relative to healthy subjects, patients with COPD exhibited a reduced antioxidant capacity, as assessed by FRAP (Fig. 2B). In addition, C-reactive protein, a marker of systemic inflammation, was elevated in patients with COPD compared with healthy subjects (Fig. 2A). Plasma catalase activity and malondialdehyde levels were not different between patients with COPD and healthy subjects (Fig. 2, D and E). Administration of the AOC increased plasma ascorbate levels similarly in both groups (Fig. 2A). Interestingly, however, both FRAP (Fig. 2B) and catalase activity (Fig. 2D) were only increased in patients with COPD as a consequence of ingestion of the AOC.
Fig. 2.
Impact of an AOC on indexes of oxidative stress, antioxidant capacity, and inflammation in patients with chronic obstructive pulmonary disease (COPD) and healthy subjects. A: C-reactive protein. B: ferric reducing ability of plasma (FRAP). C: ascorbate. D: catalase. E: malondialdehyde. Values are means ± SE. *Significantly different from the control (CTRL) condition (P < 0.05); #significantly different from healthy subjects (P < 0.05).
Resting responses.
In patients with COPD at rest, administration of the AOC did not impact MAP, LBF, LVC, HR, arterial O2 content, leg arterial-venous O2 difference, net lactate release, or venous pH (Table 2); however, arterial O2 saturation was elevated slightly (91.7 ± 1.3% vs. 92.2 ± 1.1% for control vs. AOC, respectively) but significantly. In contrast, in healthy subjects, LBF and LVC were elevated after AOC consumption, whereas the other measured variables were unchanged (Table 2).
Table 2.
Impact of AOC administration on selected physiological variables at rest and during exercise
| Work Rate |
||||
|---|---|---|---|---|
| Rest | 3 W | 6 W | 9 W | |
| Healthy subjects | ||||
| Mean arterial pressure, mmHg | ||||
| CTRL | 119 ± 2 | 127 ± 3 | 125 ± 2 | 127 ± 3 |
| AOC | 117 ± 2 | 124 ± 2 | 125 ± 3 | 127 ± 2 |
| Heart rate, beats/min | ||||
| CTRL | 64 ± 2 | 76 ± 4 | 78 ± 4 | 81 ± 5 |
| AOC | 64 ± 2 | 77 ± 4 | 79 ± 4 | 83 ± 5 |
| Leg O2 delivery, l/min | ||||
| CTRL | 0.06±.01 | 0.31 ± 0.03 | 0.36 ± 0.02 | 0.41 ± 0.02 |
| AOC | 0.06 ± 0.01 | 0.32 ± 0.02 | 0.38 ± 0.02 | 0.41 ± 0.02 |
| Hemoglobin, g/dl | ||||
| CTRL | 15 ± 0.3 | 14.8 ± 0.2 | 14.8 ± 0.2 | 14.8 ± 0.2 |
| AOC | 14.8 ± 0.4 | 15.0 ± 0.3 | 14.8 ± 0.2 | 14.8 ± 0.2 |
| Arterial O2 content, ml/dl | ||||
| CTRL | 19.9 ± 0.3 | 19.6 ± 0.2 | 19.6 ± 0.2 | 19.7 ± 0.3 |
| AOC | 19.6 ± 0.3 | 19.8 ± 0.3 | 19.8 ± 0.3 | 19.7 ± 0.3 |
| Net lactate release, mmol/min | ||||
| CTRL | 8 ± 18 | 338 ± 82 | 417 ± 113 | 975 ± 179 |
| AOC | 34 ± 10 | 343 ± 99 | 571 ± 184 | 799 ± 212 |
| Venous pH | ||||
| CTRL | 7.41 ± 0.01 | 7.31 ± 0.01 | 7.30 ± 0.01 | 7.29 ± 0.01 |
| AOC | 7.38 ± 0.01 | 7.29 ± 0.02 | 7.28 ± 0.02 | 7.27 ± 0.02 |
| COPD patients | ||||
| Mean arterial pressure, mmHg | ||||
| CTRL | 129 ± 4† | 137 ± 4† | 137 ± 4† | 138 ± 4† |
| AOC | 131 ± 4† | 138 ± 4† | 136 ± 4† | 140 ± 4† |
| Heart rate, beats/min | ||||
| CTRL | 72 ± 4 | 87 ± 4 | 90 ± 4 | 92 ± 4 |
| AOC | 72 ± 4 | 85 ± 4 | 87 ± 4 | 90 ± 4 |
| Leg O2 delivery, l/min | ||||
| CTRL | 0.05 ± 0.01 | 0.3 ± 0.02 | 0.34 ± 0.02 | 0.38 ± 0.02 |
| AOC | 0.06 ± 0.01 | 0.32 ± 0.02 | 0.36 ± 0.02 | 0.39 ± 0.03 |
| Hemoglobin, g/dl | ||||
| CTRL | 14.0 ± 0.4 | 14.3 ± 0.4 | 14.1 ± 0.4 | 14.3 ± 0.4 |
| AOC | 13.9 ± 0.4 | 13.8 ± 0.4* | 14.0 ± 0.4* | 13.8 ± 0.4* |
| Arterial O2 content, ml/dl | ||||
| CTRL | 18.1 ± 0.4† | 18.5 ± 0.4† | 18.2 ± 0.4† | 18.5 ± 0.5† |
| AOC | 18.0 ± 0.4† | 17.9 ± 0.4† | 18.3 ± 0.4† | 18.0 ± 0.4† |
| Net lactate release, mmol/min | ||||
| CTRL | 48 ± 7 | 1,777 ± 373† | 1,968 ± 369† | 2,083 ± 442† |
| AOC | 70 ± 11 | 1,606 ± 346† | 1,596 ± 298† | 2,258 ± 461† |
| Femoral venous pH | ||||
| CTRL | 7.38 ± 0.01 | 7.29 ± 0.02 | 7.28 ± 0.02 | 7.27 ± 0.02 |
| AOC | 7.38 ± 0.01 | 7.31 ± 0.02 | 7.29 ± 0.02 | 7.28 ± 0.02 |
Values are means ± SE. AOC, oral antioxidant cocktail.
Significantly different from control (CTRL) conditions;
significantly different from healthy subjects.
Exercise responses.
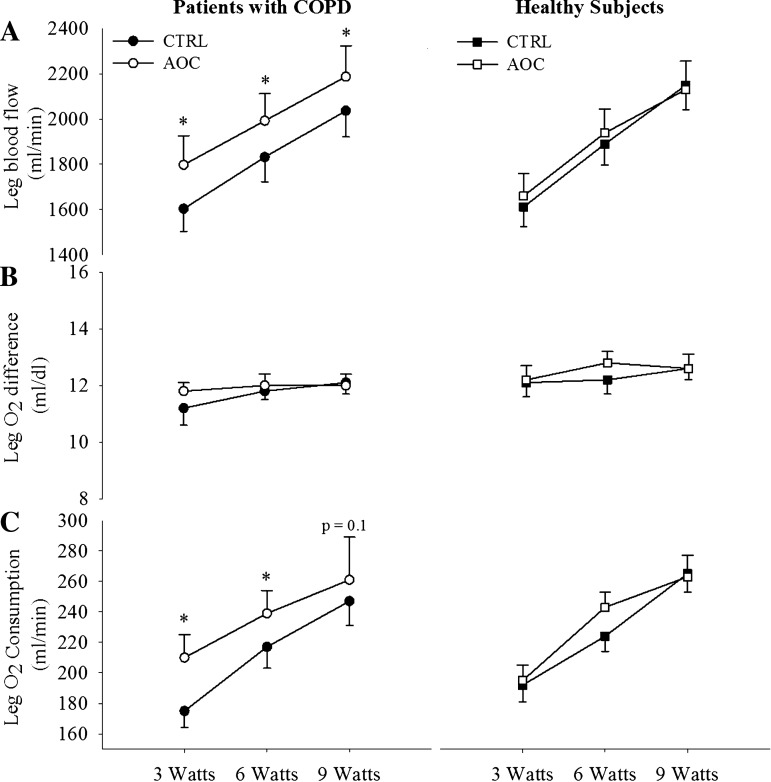
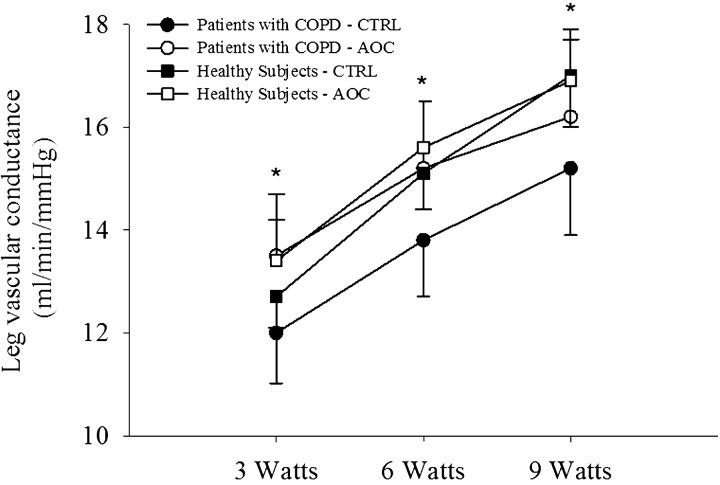
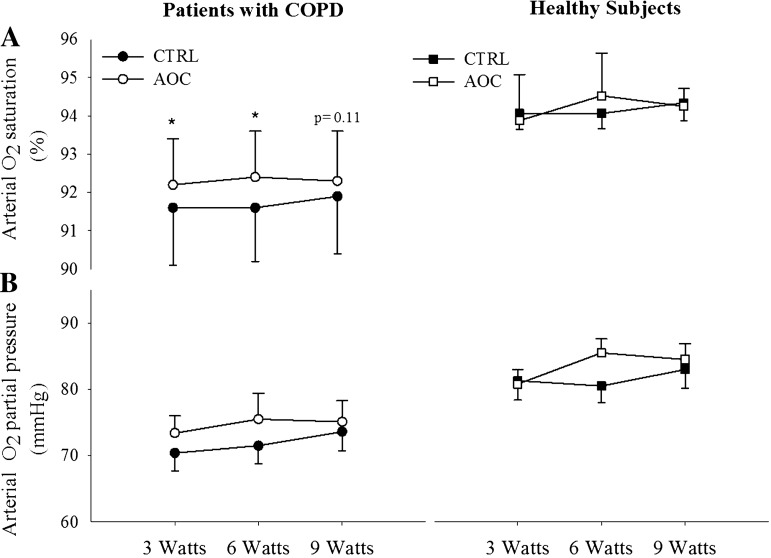
During exercise at 3, 6, and 9 W, LBF was elevated relative to the control condition after AOC consumption in patients with COPD (Fig. 3A). The elevated LBF, in combination with an unaltered arterial-venous O2 difference, resulted in an increase in leg V̇o2 in the AOC condition in patients with COPD (Fig. 3). In healthy subjects, LBF, arterial-venous O2 difference, and leg V̇o2 were unaltered by AOC administration (Fig. 3, A–C). In addition, LVC was elevated with the AOC over the control condition in patients with COPD but not in healthy subjects (Fig. 4). Arterial O2 saturation as also elevated in the AOC trial compared with control conditions only in patents with COPD (Fig. 5A), whereas arterial Po2 was unchanged (Fig. 5B). Neither arterial O2 saturation nor arterial Po2 were impacted by the AOC in healthy subjects (Fig. 5, A and B). Arterial O2 content was not different between conditions in either group, because in the patient group the elevated arterial O2 saturation was offset by a small, but significant, decrease in Hb concentration during exercise in the AOC condition (Table 2).
Fig. 3.
Impact of an AOC on exercising leg blood flow (A), O2 extraction (B), and O2 consumption (C) in patients with COPD and healthy subjects. Values are means ± SE. *Main effect of the AOC (P < 0.05).
Fig. 4.
Impact of an AOC on leg vascular conductance during exercise in patients with COPD and healthy subjects. Values are means ± SE. *Significantly different from the CTRL condition within patients with COPD.
Fig. 5.
Impact of an AOC on arterial O2 saturation (A) and arterial Po2 (B) during exercise in patients with COPD and healthy subjects. Values are means ± SE. *Main effect of the AOC (P < 0.05).
DISCUSSION
The present study examined the impact of an acutely administered oral AOC with previously documented efficacy on exercise-induced skeletal muscle blood flow, vasodilation, O2 transport, and O2 utilization during small muscle mass exercise in patients with COPD and age- and sex-matched healthy subjects. Patients with COPD exhibited basal evidence of elevated inflammation and reduced antioxidant capacity. AOC consumption improved antioxidant status in patients with COPD, and these alterations led to favorable changes in central and peripheral cardiorespiratory responses to exercise. Specifically, LBF and LVC during single-leg KE exercise were augmented in patients with COPD after AOC consumption, whereas no changes were observed in healthy subjects. The elevation in LBF, in combination with an unaltered arterial-venous O2 difference from control conditions, led to increased V̇o2 during exercise in patients with COPD. In addition, arterial O2 saturation was improved, at rest and during exercise, in patients with COPD with the AOC, whereas there was no apparent effect in healthy subjects. These data demonstrate beneficial effects of antioxidant administration on exercise-induced hemodynamics and skeletal muscle metabolism in patients with COPD and indicate that impaired O2 transport, as a consequence of elevated oxidative stress, may represent a novel mechanistic link between oxidative stress and exercise intolerance in this population.
Oxidative stress and exercise.
Oxidative stress has previously been documented to be elevated in patients with COPD relative to age-matched healthy subjects at rest (6, 24, 32). In addition, indexes of oxidative stress, such as protein carbonyls, have been inversely correlated with disease severity (6). Exercise appears to especially augment oxidative stress in patients with COPD, and, as such, markers of oxidative damage are typically elevated in patients with COPD compared with healthy subjects after exercise (32). Interestingly, this amplified oxidant production during exercise in COPD occurs after exercise that minimally taxes the pulmonary system, such as isolated quadriceps exercise (14, 15), implying that organs beyond the lung may be contributing to the free radical production. Increased oxidative stress in patients with COPD has been attributed to mitochondrial electron transport chain dysfunction (35) and the systemic inflammation (29) accompanying COPD, among other factors (36).
The current data support the concept of elevated oxidative stress and inflammation in patients with COPD. Specifically, decreased plasma antioxidant capacity and increased C-reactive protein levels were documented in patients with COPD relative to healthy subjects (Fig. 2). Administration of the AOC partially corrected the pro-/antioxidant imbalance only in the patient group, increasing both FRAP and catalase activity, with minimal effects in healthy subjects (Fig. 2). Although not examined in the present study, these data are in accordance with the previously observed free radical diminishing effects of the AOC in patients with COPD using ex vivo spin-trapping and electron paramagnetic resonance spectroscopy (24, 40). In the present study, the absence of a change in antioxidant or oxidant status in healthy subjects, despite an equal increase in plasma ascorbate (Fig. 2A), is likely due to the absence of a substantial redox imbalance in these healthy individuals. Thus, these data further document elevated oxidative stress in patients with COPD as well as beneficial effects of antioxidant administration on redox balance in patients with COPD, which may be useful in the face of the elevated free radical production during exercise in this population.
Exercise hyperemia and oxidative stress.
Independent of COPD, aging itself is characterized by a proinflammatory, prooxidant phenotype, which has been implicated as a causative factor in the age-associated decline in vascular function (18). As such, previous research has suggested that the increase in oxidative stress with age may impair resting limb blood flow and exercise hyperemia (12, 13, 16, 46). There are numerous mechanisms by which oxidative stress may impair vascular control and limb blood flow, including the amplification of vasoconstrictor activity (17, 19, 42, 43) or by diminishing the bioactivity of vasodilating agents, such as cyclooxygenase-2 (21) and NO (16). Accordingly, antioxidant administration has been documented to augment resting blood flow and exercise-induced hyperemia in older individuals (16, 25, 46), although this is not a universal observation (34). Likewise, an abnormal pro-/antioxidant balance has repeatedly been documented in patients with COPD (6, 24, 30), as observed in the present study (Fig. 2). In addition, our group has previously observed beneficial effects of AOC administration on vascular function in patients with COPD (24). Collectively, these observations support the possibility that oxidative stress may negatively impact skeletal muscle blood flow in this population.
Similar to previous research in older individuals, the present data, for the first time, demonstrate an augmented exercise hyperemia after AOC administration in patients with COPD (Fig. 3A). The increase in LBF in patients with COPD was observed across all submaximal workloads (Fig. 3) and can be attributed to an increase in LVC (Fig. 4), as MAP was unaffected by AOC administration. These data imply an increase in leg vasodilation during exercise in patients with COPD, providing a novel mechanism to target with the goal of improving O2 transport in this population.
O2 transport and utilization.
In the presence of increased LBF after AOC administration and no change in the arterial-venous O2 difference, exercising skeletal muscle V̇o2 was elevated in patients with COPD (Fig. 3). Although the typical response to augmented O2 delivery is to decrease O2 extraction and thereby maintain V̇o2, according to the Fick principle (22), there is growing evidence that skeletal muscle can increase or decrease V̇o2 at a given workload when O2 availability is altered (4, 5, 7, 28, 33), and these changes in V̇o2 do not always result in compensatory changes in glycolytic metabolism. Accordingly, our group (4) and others (33) have previously observed lower levels of V̇o2 during exercise and unchanged lactate production with interventions that result in decreased skeletal muscle blood flow. Similarly, enhanced blood flow has led to augmented skeletal muscle V̇o2 without a corresponding decrease in lactate production (5, 8). Collectively, these data suggest that the total energy consumed by the muscle at a given workload may vary with changes in blood flow and O2 delivery. The potential functional consequences of these changes in V̇o2 have been examined by Amann et al. (2, 5), who demonstrated that augmented LBF during submaximal KE exercise in patients with heart failure resulted in an increase in skeletal muscle V̇o2, which attenuated exercise-induced skeletal muscle fatigue.
The data from the present study support the notion that augmented exercise hyperemia may enhance skeletal muscle V̇o2 during exercise, as observed after AOC administration in patients with COPD (Fig. 3). In line with previous research (4, 8, 33), the changes in V̇o2 in patients with COPD were not accompanied by changes in glycolytic metabolism, as evidenced by a lack of an effect of the AOC on lactate release (Table 2). As enhanced muscle V̇o2 has been suggested to lead to greater fatigue resistance during exercise (2, 5), it is tempting to speculate that increased V̇o2 during exercise may enhance fatigue resistance in COPD.
Central responses.
Secondary to a significant ventilation-perfusion mismatch in their diseased lungs (26), patients with COPD are typically characterized by depressed arterial O2 saturation and reduced arterial Po2. In addition, the degree of hypoxemia is inversely related to exercise capacity, and hypoxemia exacerbates oxidative stress in patients with COPD (27). As expected, patients with COPD in the present study exhibited reduced arterial Po2 and arterial O2 saturation relative to healthy subjects at rest (Table 1) and during exercise (Fig. 5). Interestingly, after AOC administration, a small, but significant, increase in arterial O2 saturation, but no change in arterial Po2, was observed in patients with COPD, both at rest and during exercise, whereas no impact of the AOC was observed in healthy subjects (Fig. 5, A and B). These data indicate that at a similar arterial Po2 as control conditions, arterial O2 saturation was elevated after AOC administration in patients with COPD. Although it is difficult to elucidate the precise mechanisms responsible for the increase in arterial O2 saturation, it may have been due to a direct effect of AOC administration at the lung or an increase in Hb affinity for O2. The increased Hb O2 affinity may have been related to a decrease in metabolic perturbation during exercise as a consequence of the increase in aerobic metabolism afforded by the AOC (Fig. 3C), although no measurable changes in lactate or pH were observed (Table 2). Although the practical significance of the ∼1% increase in saturation is questionable, as this increase was accomplished with a relatively small antioxidant dose, the impact on saturation of further decreases in oxidative stress with a more potent antioxidant intervention potentially deserves further examination. In addition, an increase in arterial O2 saturation has the potential to improve arterial O2 content and O2 delivery, which would likely have beneficial effects for exercising skeletal muscle (2). Arterial O2 content, however, was not altered in the patients in the present study because of the small, but significant, decrease in Hb concentration that offset the increased arterial O2 saturation and maintained arterial O2 content (Table 2). These data do, however, suggest that reducing oxidative stress in patients with COPD has the potential to attenuate arterial hypoxemia, which may improve exercise tolerance in this population.
Experimental considerations.
It is important to acknowledge potential limitations of the present study. Specifically, the AOC trial was always performed after the control trial, which does not exclude the possibility of an order effect. However, due to the invasive nature of catheter placement, the control and AOC trials had to be performed on the same day. In addition, because of the potential long-lasting effects of the AOC, the AOC trial always had to be performed last. Importantly, our group has previously documented the reproducibility of hemodynamic measurements achieved with sequential exercise bouts, and no intervention, across a range of exercise intensities (8). Thus, we are confident that the observed changes are most likely due to the intervention and the favorable impact of decreasing oxidative stress in patients with COPD. Additionally, and somewhat surprisingly, despite minimalistic blood sampling to avoid a change in Hb, Hb levels were lower in patients with COPD in the second AOC trial. As there was no evidence of greater bleeding in patients with COPD, this could simply have been the effect of random variation in the measurements (i.e., a type 1 error). Importantly, arterial O2 content, which actually has the ability to affect blood flow, was not different between trials (Table 2).
Summary and conclusions.
The purpose of the present study was to examine the impact of an AOC on oxidative stress and antioxidant capacity and, subsequently, exercise hemodynamics during small muscle mass exercise in patients with COPD and healthy subjects. Patients with COPD exhibited evidence of reduced antioxidant capacity relative to healthy subjects. Administration of the AOC improved the redox balance in patients with COPD, with little effect in healthy subjects. These favorable changes in redox balance were accompanied by improved LBF and LVC as well as increased skeletal muscle V̇o2 during submaximal KE exercise in patients with COPD, whereas minimal effects were observed in healthy subjects. In addition, arterial O2 saturation was improved at rest and during exercise in patients with COPD after AOC administration. Collectively, these data illustrate the role of oxidative stress in the integration of O2 transport and utilization during exercise in this population and further implicate oxidative stress in the systemic pathophysiological consequences of COPD.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant PO1-HL-09830, HL-103786, and HL-116579 and by Veterans Affairs Merit Grant E6910R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.R., J.D.C., and R.S.R. conception and design of research; M.J.R., J.D.T., R.S.G., S.J.I., J.D.C., M.A.H.W., A.D.B., D.E.M., S.R., V.R.R., M.A., D.W.W., and R.S.R. performed experiments; M.J.R., J.D.T., R.S.G., S.J.I., Z.B.-O., and J.Z. analyzed data; M.J.R., J.D.T., R.S.G., and R.S.R. interpreted results of experiments; M.J.R. and R.S.R. prepared figures; M.J.R. and R.S.R. drafted manuscript; M.J.R., J.D.T., M.A.H.W., and R.S.R. edited and revised manuscript; M.J.R., J.D.T., R.S.G., S.J.I., J.D.C., Z.B.-O., M.A.H.W., A.D.B., D.E.M., S.R., V.R.R., J.Z., M.A., D.W.W., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Abel MG, Hannon JC, Sell K, Lillie T, Conlin G, Anderson D. Validation of the Kenz Lifecorder EX and ActiGraph GT1M accelerometers for walking and running in adults. Appl Physiol Nutr Metab 33: 1155–1164, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol 104: 861–870, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299: R314–R324, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Runnels S, Morgan DE, Trinity J, Fjeldstad A, Wray DW, Reese VR, Richardson RS. On the Contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J, Richardson RS. Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174: 368–375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreiro E, de la Puente B, Minguella J, Corominas JM, Serrano S, Hussain SN, Gea J. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 1116–1124, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-O'Keefe Z, Helgerud J, Wagner PD, Richardson RS. Maximal strength training and increased work efficiency: contribution from the trained muscle bed. J Appl Physiol 113: 1846–1851, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Endothelin-A-mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 70: 554–565, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett-O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS, Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304: H162–H169, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239: 70–76, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Bradley DW, Emery G, Maynard JE. Vitamin C in plasma: a comparative study of the vitamin stabilized with trichloroacetic acid or metaphosphoric acid and the effects of storage at −70 degrees, −20 degrees, 4 degrees, and 25 degrees on the stabilized vitamin. Clin Chim Acta 44: 47–52, 1973. [DOI] [PubMed] [Google Scholar]
- 12.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couillard A, Koechlin C, Cristol JP, Varray A, Prefaut C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 20: 1123–1129, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Couillard A, Maltais F, Saey D, Debigare R, Michaud A, Koechlin C, LeBlanc P, Prefaut C. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167: 1664–1669, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Groot AA, van Zwieten PA, Peters SL. Involvement of reactive oxygen species in angiotensin II-induced vasoconstriction. J Cardiovasc Pharmacol 43: 154–159, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178: 1211–1218, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307: H1754–H1763, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–H619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, Wray DW, Richardson RS. Vascular dysfunction and chronic obstructive pulmonary disease: the role of redox balance. Hypertension 63: 459–467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 103: 1715–1721, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chronic Obstruct Pulm Dis 6: 199–208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koechlin C, Maltais F, Saey D, Michaud A, LeBlanc P, Hayot M, Prefaut C. Hypoxaemia enhances peripheral muscle oxidative stress in chronic obstructive pulmonary disease. Thorax 60: 834–841, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13: 149–159, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Maclay JD, MacNee W. Cardiovascular disease in COPD: mechanisms. Chest 143: 798–807, 2013. [DOI] [PubMed] [Google Scholar]
- 30.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 50–60, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R, Dekhuijzen PN, Franssen F, Gayan-Ramirez G, Gea J, Gosker HR, Gosselink R, Hayot M, Hussain SN, Janssens W, Polkey MI, Roca J, Saey D, Schols AM, Spruit MA, Steiner M, Taivassalo T, Troosters T, Vogiatzis I, Wagner PD; ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 189: e15–62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172: 994–1001, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590: 5361–5370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puente-Maestu L, Tejedor A, Lazaro A, de Miguel J, Alvarez-Sala L, Gonzalez-Aragoneses F, Simon C, Agusti A. Site of mitochondrial reactive oxygen species production in skeletal muscle of chronic obstructive pulmonary disease and its relationship with exercise oxidative stress. Am J Respir Cell Mol Biol 47: 358–362, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Rahman I. The role of oxidative stress in the pathogenesis of COPD: implications for therapy. Treatments Respir Med 4: 175–200, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Richardson RS. Oxygen transport and utilization: an integration of the muscle systems. Adv Physiol Educ 27: 183–191, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Richardson RS, Frank LR, Haseler LJ. Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. Int J Sports Med 19: 182–187, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Rossman MJ, Garten RS, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Ascorbate infusion increases skeletal muscle fatigue resistance in patients with chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol 305: R1163–R1170, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossman MJ, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Oxidative stress and COPD: the effect of oral antioxidants on skeletal muscle fatigue. Med Sci Sports Exerc 45: 1235–1243, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31: 741–755, 1978. [DOI] [PubMed] [Google Scholar]
- 42.Thomas GD, Zhang W, Victor RG. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: role of oxidative stress. Circ Res 88: 816–823, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Welk GJ, Blair SN, Wood K, Jones S, Thompson RW. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc 32: S489–497, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184: 193–199, 1990. [DOI] [PubMed] [Google Scholar]
- 46.Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol 297: H1870–H1875, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]