The study demonstrates that the exercise pressor reflex overactivity manifest in hypertension is attenuated by dynamic exercise training. Moreover, training normalizes the dysfunction of the mechanically and chemically sensitive components of the reflex. These findings identify a novel mechanism by which exercise training is beneficial in the treatment of hypertension.
Keywords: exercise pressor reflex, blood pressure, sympathetic nerve activity, hypertension, exercise training
Abstract
Cardiovascular responses to exercise are exaggerated in hypertension. We previously demonstrated that this heightened cardiovascular response to exercise is mediated by an abnormal skeletal muscle exercise pressor reflex (EPR) with important contributions from its mechanically and chemically sensitive components. Exercise training attenuates exercise pressor reflex function in healthy subjects as well as in heart failure rats. However, whether exercise training has similar physiological benefits in hypertension remains to be elucidated. Thus we tested the hypothesis that the EPR overactivity manifest in hypertension is mitigated by exercise training. Changes in mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) in response to muscle contraction, passive muscle stretch, and hindlimb intra-arterial capsaicin administration were examined in untrained normotensive Wistar-Kyoto rats (WKYUT; n = 6), exercise-trained WKY (WKYET; n = 7), untrained spontaneously hypertensive rats (SHRUT; n = 8), and exercise-trained SHR (SHRET; n = 7). Baseline MAP after decerebration was significantly decreased by 3 mo of wheel running in SHRET (104 ± 9 mmHg) compared with SHRUT (125 ± 10 mmHg). As previously reported, the pressor and renal sympathetic responses to muscle contraction, stretch, and capsaicin administration were significantly higher in SHRUT than WKYUT. Exercise training significantly attenuated the enhanced contraction-induced elevations in MAP (SHRUT: 53 ± 11 mmHg; SHRET: 19 ± 3 mmHg) and RSNA (SHRUT: 145 ± 32%; SHRET: 57 ± 11%). Training produced similar attenuating effects in SHR during passive stretch and capsaicin administration. These data demonstrate that the abnormally exaggerated EPR function that develops in hypertensive rats is significantly diminished by exercise training.
NEW & NOTEWORTHY
The study demonstrates that the exercise pressor reflex overactivity manifest in hypertension is attenuated by dynamic exercise training. Moreover, training normalizes the dysfunction of the mechanically and chemically sensitive components of the reflex. These findings identify a novel mechanism by which exercise training is beneficial in the treatment of hypertension.
the sympathetically mediated blood pressure response to exercise is abnormally accentuated in patients with hypertension compared with normal healthy subjects (7, 8, 30, 66). The exercise pressor reflex (EPR), whose sensory arm transmits afferent signals from working skeletal muscle that serve as an important source of neural input to the brain stem during physical activity, contributes significantly to the regulation of the cardiovascular system during exercise (1, 6, 32, 36, 38). Our previous studies suggested that the exaggerated pressor response to exercise in hypertension is mediated, at least in part, by an abnormal EPR (26, 41–44, 47, 60).
An increasing body of evidence suggests that exercise training is beneficial for the treatment of hypertension (51). However, the mechanisms underlying the benefits of training are largely undetermined. For example, the effects of exercise training on EPR overactivity in this disease are unknown. It has been reported that exercise training attenuates EPR-mediated cardiovascular responsiveness to physical activity in healthy humans (13, 46, 54). In healthy individuals, forearm training has been demonstrated to decrease sympathetic responses during nonfatiguing rhythmic handgrip, suggesting reductions in mechanically sensitive muscle afferent discharge (54). Furthermore, handgrip exercise training has been shown to reduce metabolite production during ischemic exercise resulting in an attenuated skeletal muscle metaboreceptor-mediated increase in blood pressure (46). More specific to cardiovascular disease, exercise training has been shown to ameliorate exaggerations in EPR activity in rats with chronic heart failure (CHF) (70). Companion studies demonstrated that exercise training prevents the abnormal sensitization of muscle afferents in CHF rats (69). Taken together, it is logical to suggest that exercise training may attenuate the heightened EPR-mediated sympathetic and cardiovascular responses to physical activity in hypertension.
Based on these previous reports, it was hypothesized that the EPR overactivity manifest in hypertension is mitigated by exercise training. To test this hypothesis, we examined the cardiovascular and sympathetic responses to stimulation of the EPR (via electrically induced static muscle contraction) as well as the mechanically (via passive muscle stretch), and chemically (via intra-arterial capsaicin administration in the hindlimb) sensitive afferent fibers associated with the reflex in untrained normotensive Wistar-Kyoto (WKYUT), exercise-trained Wistar-Kyoto (WKYET), untrained spontaneously hypertensive (SHRUT), and exercise-trained spontaneously hypertensive (SHRET) rats.
METHODS
Ethical Approval
The procedures outlined were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center at Dallas. All studies were conducted in accordance with the US Department of Health and Human Services NIH Guide for the Care and Use of Laboratory Animals.
Exercise Training
Age-matched male WKY (n = 13) and SHR (n = 15) rats were kept in individual cages and randomly divided into untrained (WKYUT, n = 6; SHRUT, n = 8) and exercise-trained (WKYET, n = 7; SHRUT, n = 7) groups at 21 days of age. The exercise-trained group was provided with a running wheel of 106 cm in circumference (Tecniplast) in their cage. These animals were allowed to exercise spontaneously. The total number of wheel rotations was recorded daily for the duration of the training period. The total distance run by each animal was then calculated from the total number of wheel rotations. The training period was 85–95 days in total duration. Animals were maintained in a temperature-controlled environment, fed ad libitum, and kept on a 12:12-h light-dark cycle.
Measurement of Peak Oxygen Uptake
Peak oxygen uptake was assessed in both the untrained groups (WKYUT, SHRUT) and the exercise-trained groups (WKYET, SHRET after 68–70 days of the 85- to 95-day training period). Four to 6 days before beginning oxygen uptake testing, animals were given two familiarization trials on the treadmill apparatus to allow adaptation to the testing environment (4). Each familiarization trial lasted 7 min and the trials were conducted on nonconsecutive days. The speed during the first familiarization trial increased progressively from 10 to 15 m/min throughout the 7-min duration. The speed during the second familiarization trial increased progressively from 10 to 20 m/min. Peak oxygen uptake (V̇o2) was determined for WKYUT, WKYET, SHRUT, and SHRET according to previously established methods (18). Briefly, the test began with a 3-min warm-up at a treadmill grade and speed of 0% and 15 m/min, respectively. The treadmill speed and/or grade was increased every 3 min. Peak V̇o2 was defined as the point at which the V̇o2 did not increase with further increases in workload or when the rat was unable or unwilling to continue running. Confirmation that peak V̇o2 was truly attained in each animal was demonstrated by having each rat perform a subsequent maximal exercise test after 48 h of recovery from the initial test.
General Acute Surgical Procedures
Rats were initially anesthetized with isoflurane gas (2–4% in 100% oxygen) and intubated for mechanical ventilation (model 683, Harvard). A pressure transducer (MLT0380/D, ADInstruments) connected to a left carotid arterial catheter was used to measure arterial blood pressure (ABP) continuously. Fluid-filled catheters were placed within the right jugular vein for the administration of solutions. Needle electrodes were placed on the back of the animal to obtain electrocardiograph (ECG) recordings. Heart rate (HR) was calculated from the time between successive R waves in the ECG recording. The renal nerve was exposed and attached to a pair of stainless steel wire electrodes. The nerve and electrodes were covered with silicone glue for insulation and fixation. To quantify renal sympathetic nerve activity (RSNA), the preamplified nerve signal was band-pass filtered at 150-1,000 Hz then full-wave rectified and low-pass filtered with a cutoff frequency of 30 Hz. A laminectomy exposing the lower lumber portions of the spinal cord (L2–L6) was performed as described previously (58). The L4 and L5 ventral roots were carefully isolated and sectioned. The cut peripheral ends of the roots were placed on bipolar electrodes. The gastrocnemius and soleus muscle of the right hindlimb were isolated. The calcaneal bone of the right hindlimb was cut and the Achilles tendon connected to a force transducer (FT-10, Grass Instruments) for the measurement of muscle tension. To allow the injection of chemicals into the arterial supply of the right leg, the circulation of the hindlimb was surgically isolated as described previously. Briefly, a catheter was placed in the left common iliac artery with the catheter tip advanced to the bifurcation of the abdominal aorta. In addition, a reversible ligature was placed around the common iliac vein emptying the right hindlimb. Animals were held in a stereotaxic head unit, and a precollicular decerebration was performed rendering the animals insentient. Immediately following the decerebrate procedure, gas anesthesia was discontinued. To stabilize fluid balance and maintain baseline arterial blood pressure, a continuous infusion (2 ml 1 M NaHCO3 and 10 ml 5% dextrose in 38 ml Ringer solution) was established via the jugular vein (3–5 ml·h−1·kg−1). A minimum recovery period of 1.25 h was employed after all surgeries before beginning any experimental protocol.
Experimental Protocols
Stimulation of the EPR.
The EPR was stimulated in trained and untrained animals by contracting the gastrocnemius and soleus muscles of the right hindlimb for 30 s via electrical stimulation of the isolated L4 and L5 ventral roots (58). Constant current stimulation was used at a 3 times motor threshold (i.e., the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. Muscles were initially stretched to 70–100 g of tension prior to each perturbation. Both the mechanically and metabolically sensitive components of the EPR are stimulated concomitantly by contracting hindlimb skeletal muscle in this manner.
Stimulation of the mechanically sensitive afferent fibers.
To selectively activate the mechanically sensitive component of the EPR, hindlimb muscles were passively stretched in trained and untrained animals using a calibrated 9.5-mm rack and pinion system (Harvard Apparatus). To evoke a mechanical stimulus similar to that elicited during muscle contraction, care was taken to generate the same pattern and magnitude of muscle tension developed during static contractions. Muscles were initially stretched to 70–100 g of tension prior to each perturbation. Passively stretching hindlimb skeletal muscle does not appreciably increase muscle metabolism and, therefore, is commonly employed to selectively activate the mechanically sensitive component of the EPR (17, 58, 62).
Stimulation of the chemically sensitive afferent fibers.
Selective activation of chemically sensitive afferent fibers innervating skeletal muscle was achieved by administering capsaicin into the arterial supply of the hindlimb (0.1, 0.3, and 1.0 μg/100 μl). Capsaicin was administered into the right common iliac artery via bolus injection while the reversible ligature placed around the right common iliac vein was occluded for 2 min. Capsaicin has been shown to preferentially stimulate receptors associated with chemically sensitive afferent fibers (20, 61).
A minimum of ∼10 min elapsed between trials in all reflex testing. At the conclusion of all experiments, an intravenous infusion of hexamethonium bromide (60 mg/kg) was used to abolish SNA signals confirming they were recorded from postganglionic renal sympathetic fibers. Animals were humanely killed by intravenous injection of saturated potassium chloride (4 M, 2 ml/kg iv). The heart and lungs were excised and weighed. Additionally, the tibia was harvested, weighed, and measured.
Data Acquisition and Statistical Analyses
ABP, HR, RSNA, and muscle tension were recorded with data-acquisition software (LabChart, ADInstruments) and stored in a computer hard drive. Tension-time index (TTI, kg·s) was calculated by integrating the developed tension (integrated total tension minus integrated baseline tension prior to the maneuver) during the muscle contraction period. Baseline values for mean arterial pressure (MAP, mmHg), HR (beats/min), and tension (kg) were determined by evaluating 30 s of recorded data before a muscle contraction, stretch, or capsaicin administration. The peak response of each variable was defined as the greatest change from baseline elicited by muscle contraction, stretch, or capsaicin administration. To quantify RSNA responses, basal measurements were obtained by taking the mean value of 30 s of baseline data immediately prior to the maneuver. This mean was considered 100% of basal RSNA. Subsequently, relative changes in RSNA (%) from this baseline were evaluated.
Data were analyzed using two-way ANOVA (rat group × exercise training). If significant interaction and main effects were observed with ANOVA, a post hoc Fisher's PLSD test was used to identify differences between specific group means. The significance level was set at P < 0.05. Results are presented as means ± SE.
RESULTS
Morphometric characteristics, peak V̇o2, TTI, and baseline hemodynamics are summarized in Table 1. Heart weight-to-body weight ratio as well as heart weight-to-tibial length ratio were significantly greater in SHR than WKY as previously reported (42, 43, 47, 60). Further, exercise training significantly increased these ratios in both WKY and SHR. The lung weight-to-body weight ratio was not different between groups. There was no significant difference in TTI between groups. Three months of voluntary wheel running significantly increased peak V̇o2 in both WKY and SHR. Baseline MAP and HR under 1% isoflurane anesthesia were significantly higher in SHR than WKY. No effect of exercise training was observed in baseline hemodynamics as well as RSNA under anesthesia. Although MAP after decerebration in SHR was significantly greater than WKY, exercise training significantly decreased MAP in SHRET compared with SHRUT. There were no significant differences in HR or RSNA after decerebration between groups. Although daily running distance in SHRET was significantly greater than WKYET in the 8th week of training (9,774 ± 373 m vs. 4,271 ± 892 m, respectively), wheel activity at the beginning as well as the end of training was comparable among groups (4th week: 4,651 ± 532 vs. 4,806 ± 677 m; 12th week: 3,769 ± 424 vs. 5,606 ± 861 m, respectively).
Table 1.
Morphometric characteristics, peak V̇o2, TTI, and baseline hemodynamics
| WKYUT (n = 6) | WKYET (n = 7) | SHRUT (n = 8) | SHRET (n = 7) | Rats | Exercise Training | Interaction | |
|---|---|---|---|---|---|---|---|
| Body weight, g | 330 ± 9 | 353 ± 10 | 360 ± 9 | 373 ± 9 | P = 0.0129 | P = 0.0647 | P = 0.6135 |
| Heart weight/body weight, mg/g | 2.75 ± 0.06 | 3.19 ± 0.15 | 3.31 ± 0.08 | 3.51 ± 0.13 | P = 0.0006 | P = 0.0082 | P = 0.2911 |
| Heart weight/tibial length, mg/mm | 23.2 ± 0.7 | 28.4 ± 1.0 | 31.0 ± 0.7 | 33.7 ± 1.5 | P < 0.0001 | P = 0.0007 | P = 0.2400 |
| Lung weight/body weight, mg/g | 5.28 ± 0.11 | 5.23 ± 0.23 | 5.16 ± 0.24 | 4.88 ± 0.43 | P = 0.4216 | P = 0.5787 | P = 0.6937 |
| Peak V̇o2, ml·kg−1·min−1 | 51 ± 2 | 61 ± 3 | 59 ± 1 | 63 ± 2 | P = 0.0437 | P = 0.0066 | P = 0.2682 |
| TTI, kg·s | 21 ± 1 | 20 ± 3 | 17 ± 1 | 18 ± 2 | P = 0.2151 | P = 0.9319 | P = 0.6922 |
| 1% Isoflurane anesthesia | |||||||
| MAP, mmHg | 90 ± 5 | 97 ± 6 | 125 ± 10 | 104 ± 9 | P = 0.0152 | P = 0.4234 | P = 0.0975 |
| HR, beats/min | 349 ± 12 | 371 ± 12 | 391 ± 9 | 376 ± 8 | P = 0.0281 | P = 0.7101 | P = 0.0846 |
| Signal-to-noise ratio for RSNA | 4.3 ± 0.9 | 2.9 ± 0.9 | 5.0 ± 1.0 | 5.0 ± 1.6 | P = 0.2457 | P = 0.5578 | P = 0.5509 |
| After decerebration | |||||||
| MAP, mmHg | 85 ± 8 | 88 ± 6 | 121 ± 7* | 92 ± 3† | P = 0.0052 | P = 0.0550 | P = 0.0198 |
| HR, beats/min | 479 ± 17 | 455 ± 24 | 458 ± 12 | 452 ± 7 | P = 0.4787 | P = 0.3723 | P = 0.5743 |
| Signal-to-noise ratio for RSNA | 6.4 ± 2.4 | 4.9 ± 0.6 | 7.2 ± 1.9 | 9.4 ± 2.8 | P = 0.2358 | P = 0.8570 | P = 0.3987 |
Values are means ± SE. V̇o2, oxygen uptake; MAP, mean arterial pressure; HR, heart rate; RSNA, renal sympathetic nerve activity; TTI, tension-time index; WKYET, exercise-trained Wistar-Kyoto rats; WKYUT, untrained WKY; SHRET, exercise-trained spontaneously hypertensive rats; SHRUT, untrained SHR.
P < 0.05 compared with WKYUT.
P < 0.05 compared with SHRUT.
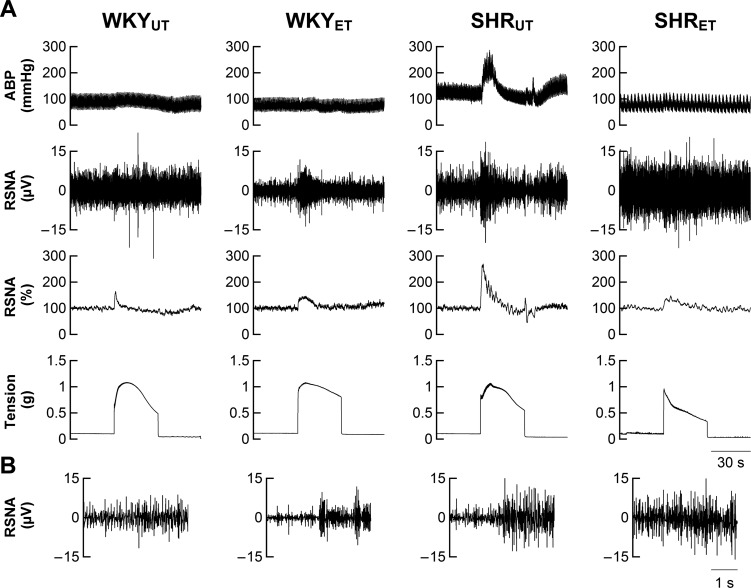
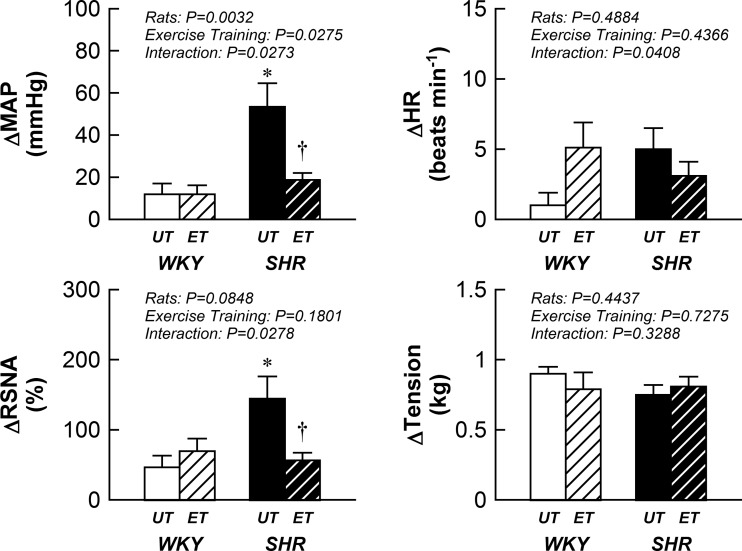
Representative tracings of ABP, RSNA, and tension developed during electrically induced static muscle contraction are shown in Fig. 1. In response to approximately the same amount of tension development, activation of the EPR elicited larger elevations in MAP and RSNA in SHRUT compared with WKYUT (Fig. 2). Notably, exercise training significantly attenuated changes in pressor and sympathetic response in SHRET but not in WKYET.
Fig. 1.
A: cardiovascular and sympathetic responses to exercise pressor reflex (EPR) activation in representative untrained Wistar-Kyoto rats (WKYUT), exercise-trained WKY (WKYET), untrained spontaneously hypertensive rats (SHRUT), and exercise-trained SHR (SHRET). ABP, arterial blood pressure. B: the magnified data of renal sympathetic nerve activity (RSNA) for 2 s before and during muscle contraction.
Fig. 2.
Cardiovascular and sympathetic responses to activation of the EPR in WKYUT, WKYET, SHRUT, and SHRET. MAP, mean arterial pressure; HR, heart rate. *P < 0.05 compared with WKYUT. †P < 0.05 compared with SHRUT.
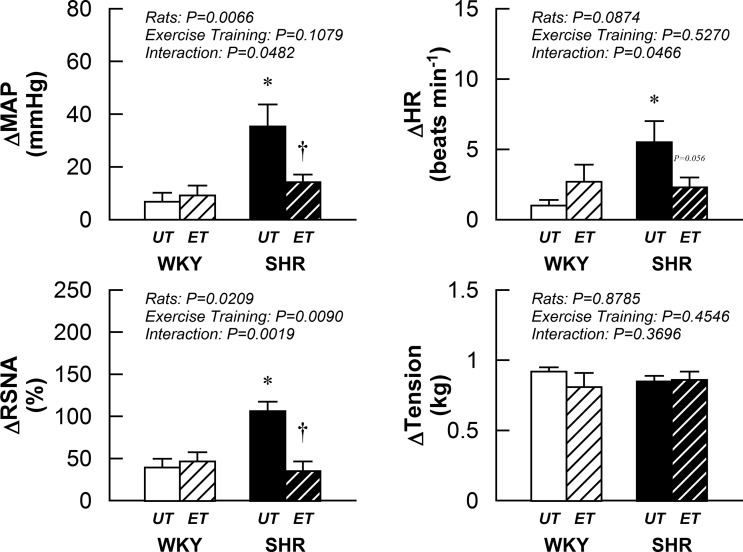
Consistent with previous reports, the cardiovascular and sympathetic responses to activation of the mechanically sensitive component of the EPR via passive muscle stretch were exaggerated in SHRUT compared with WKYUT (Fig. 3). More importantly, voluntary wheel running significantly decreased changes in MAP, HR, and RSNA responses to stimulation of muscle mechanoreflex in SHRET compared with SHRUT. No significant effect of exercise training was observed in any variables in WKY.
Fig. 3.
Cardiovascular and sympathetic responses to passive stretch of the gastrocnemius and soleus muscles in WKYUT, WKYET, SHRUT, and SHRET. *P < 0.05 compared with WKYUT. †P < 0.05 compared with SHRUT.
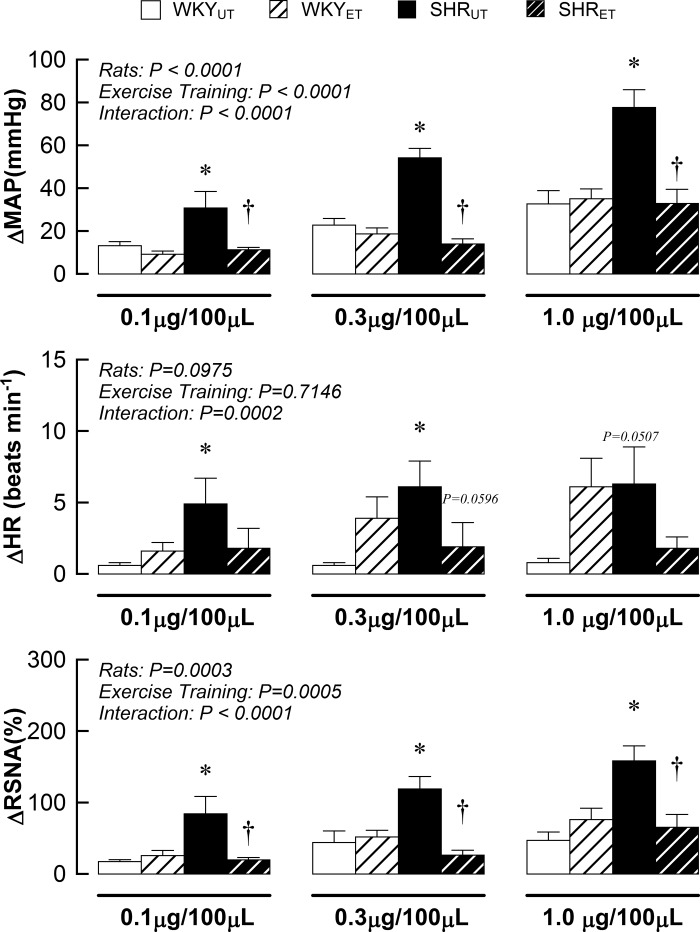
The cardiovascular and sympathetic responses to administration of capsaicin were heightened in SHRUT compared with WKYUT (Fig. 4). Exercise training did not affect MAP, HR, and SNA in WKY. In contrast, MAP, HR, and SNA responses to activation of chemically sensitive afferent fibers were significantly attenuated by voluntary wheel running in SHR.
Fig. 4.
Cardiovascular and sympathetic responses to administration of capsaicin into the arterial supply of the hindlimb in WKYUT, WKYET, SHRUT, and SHRET. *P < 0.05 compared with WKYUT. †P < 0.05 compared with SHRUT.
DISCUSSION
The major findings from this investigation were 1) the cardiovascular and sympathetic responses to EPR stimulation during muscle contraction in SHRET were significantly lower than SHRUT; 2) the MAP, HR, and RSNA responses to passive muscle stretch in SHRET were significantly less than SHRUT; and 3) exercise training by voluntary wheel running in SHRET produced significantly diminished pressor, tachycardia, and sympathoactivation responses to intra-arterial capsaicin administration in the hindlimb compared with SHRUT. Collectively, these findings support the hypothesis that the EPR overactivity manifest in hypertension is mitigated by exercise training. Moreover, the investigation suggests that attenuating EPR function is a mechanism by which exercise training is beneficial for the treatment of hypertension.
Exercise Training Attenuates EPR Function in SHR
Consistent with our previous studies, EPR function was abnormally heightened in untrained SHR compared with WKY (26, 42, 43, 47, 60). This characteristic EPR overactivity has been shown to manifest not only in SHR but also in other models of the disease such as prenatally programmed hypertension (41, 44) and angiotensin II-induced hypertension (24). In SHR, 3 mo of voluntary wheel running attenuated the cardiovascular and sympathetic responses to EPR activation. To the best our knowledge, this is the first evidence that exercise training improves EPR activity in SHR. Given that EPR overactivity is expressed in multiple forms of the disease, it is probable that exercise training likewise improves muscle reflex function in other forms of hypertension as well.
Unlike hypertensive animals, exercise training did not affect EPR function in WKY in the present investigation. This agrees with previous studies in which 8–10 wk of exercise training had no significant effect on the activity of the EPR, mechanoreflex, and metaboreflex in normotensive control Sprague-Dawley rats (69, 70). In contrast, studies in healthy humans have demonstrated that exercise training reduces the cardiovascular and sympathetic responses to handgrip exercise (46, 54). However, in those studies, training-induced alterations in other neural mechanisms known to contribute importantly to cardiovascular control during exercise, such as central command and the arterial baroreflex, could not be excluded. That being stated, Fisher and White (13) demonstrated significant alterations in central command's ability to regulate the pressor response to exercise after training with smaller adaptations occurring in peripheral reflex components (i.e., EPR). Thus it appears in healthy humans that exercise training may indeed alter EPR function although the magnitude of the effect may be relatively small. This might explain our, and others, inability to demonstrate an appreciable effect of training on EPR function in normotensive control rats.
It should be noted that exercise training has been shown to have beneficial effects on other mechanisms contributing to blood pressure control during physical activity. For example, functional sympatholysis (the local attenuation of sympathetic vasoconstriction in exercising muscle) optimizes blood flow to active muscle while concomitantly preventing excessive increases in blood pressure during physical exertion (53). Studies have shown that functional sympatholysis is impaired in hypertension (40, 71). We recently demonstrated in hypertensive rats that these impairments are normalized by chronic exercise training (40). In relation to the current study, it is probable that training-induced restoration of normal functional sympatholysis contributed to the reduced pressor response to activation of the EPR in trained hypertensive animals.
Exercise Training Attenuates Responses to Activation of Mechanically Sensitive Afferent Fibers in SHR
Similar to reports in normotensive animals (17), we previously demonstrated that blockade of skeletal muscle mechanoreceptors with gadolinium significantly attenuated the cardiovascular and sympathetic responses to muscle stretch in SHR. This finding suggested that accentuated EPR activity in hypertensive rats is mediated, in part, by stimulation of the muscle mechanoreflex (43). Similar to hypertension, it has been reported that muscle mechanoreflex activity is exaggerated in CHF (59). Wang et al. (68) demonstrated that the responsiveness of group III mechanically sensitive skeletal muscle afferents (most closely associated with the mechanoreflex) to passive stretch was markedly greater in CHF rats compared with controls. Further, pyridoxal phosphate-6-azophenyl-2,4-disulfonic acid, a purinergic 2X receptor (P2X) antagonist, attenuated the responsiveness of group III afferents to contraction and stretch to a greater extent in CHF animals compared with controls. They also indicated that P2X receptors were significantly upregulated in the dorsal root ganglia (DRG) of CHF rats (68). Moreover, exercise training prevented the sensitization of group III afferent responsiveness to passive stretch in CHF animals being associated with a downregulation of P2X receptors in the DRG (69). Speculatively, muscle mechanoreflex overactivity in hypertension may likewise be attenuated by exercise training via similar mechanisms.
Interestingly, a recent study by Stone et al. (64) demonstrated that 87% of group III afferents that respond to stretch likewise respond to muscle contraction in decerebrated rats. Additional analysis of the data obtained in the present study determined that the peak RSNA response appeared within 2 s of muscle contraction in 100% of WKYUT rats (6 of 6 animals), 71% of WKYET rats (5 of 7 animals), 75% of SHRUT rats (6 of 8 animals), and 71% of SHRET rats (5 of 7 animals). Based on the rapidity at which peak RSNA was reached, the data agree with the concept that the training-induced attenuation in EPR function in hypertensive animals may be heavily influenced by significant reductions in muscle mechanoreflex activity.
Exercise Training Attenuates Responses to Activation of Chemically Sensitive Afferent Fibers in SHR
In skeletal muscle, the transient receptor potential vanilloid 1 receptor (TRPv1) has been shown to be primarily localized to group IV chemically sensitive afferent fibers (most closely associated with the metaboreflex) (20). It has been demonstrated that TRPv1 contributes significantly to activation of the EPR in normotensive rats (57). We previously demonstrated that TRPv1 protein expression within the DRG is significantly greater in SHR than WKY. Importantly, blockade of the TRPv1 receptor partially corrects metaboreflex overactivity in SHR (42). As such, it is tempting to suggest that improvements in metaboreflex function were induced by exercise training in the current study and may be due, in part, to a downregulation of TRPv1 expression to more normal levels. Alternatively, it has been reported that pyridoxal-5-phosphate, a purinergic P2 receptor (P2) antagonist, partially attenuates muscle SNA during postexercise muscle ischemia in moderately hypertensive adults (16). This finding suggests a role for P2 receptors in evoking the exaggerated sympathetic response to activation of chemically sensitive afferent fibers in hypertension (16). Like the possibility suggested for TRPv1, exercise training may normalize P2 expression reducing metaboreflex overactivity in hypertension. Clearly, further research is needed to definitively determine whether exercise training normalizes metaboreflex function in hypertension.
Possible Central Mechanisms Mediating Muscle Reflex Improvements with Training
Changes in centrally located neural control mechanisms could potentially contribute to the training-induced improvements in EPR function in hypertensive animals. For example, neuroplastic changes are known to occur in the neuronal dendritic fields of central sympathoexcitatory sites with exercise training that may reduce sympathetic responsiveness to reflex activation (48). As another example, sensory information generated by stimulation of the EPR is processed within the medulla oblongata. Within the medulla, the production of nitric oxide (NO) within the nucleus tractus solitarius (NTS), the site most critical for processing EPR sensory signals, buffers reflex-induced increases in SNA (56). It has been demonstrated that expression of neuronal NO synthase (NOS), necessary for the production of NO, is reduced throughout a large portion of the NTS in hypertensive rats (47). This could compromise NO production within the NTS of hypertensive animals reducing its sympathetic buffering capacity. Moreover, increased generation of superoxide, an NO scavenger, via an angiotensin-II mechanism may also reduce the NO available for biological activity within the NTS. Collectively, it is plausible that exercise training normalizes EPR function in hypertension by rescuing NO production within the NTS. In support of this contention, it has been shown that exercise training reduces angiotensinogen mRNA expression within NTS of SHR (10).
In addition to the NTS, the rostral ventrolateral medulla (RVLM) is known to play an important role in the control of the cardiovascular system during exercise (49). It has been reported that 4 wk of swim training attenuates the pressor response to microinjection of l-glutamate within RVLM in conscious normotensive rats (34). This finding suggests that RVLM neurons are desensitized after exercise training. Further, it has been demonstrated that exercise training inhibits SNA via reductions in oxidative stress within the RVLM of stroke-prone SHR (22). Although speculative, it is possible that training-induced improvements in EPR activity are likewise mediated by neural adaptations within the RVLM.
Analytical and Methodological Considerations
It is acknowledged that daily running distance differed between WKYET and SHRET in the middle of the training session while being similar at the beginning and end of training. Reports of differences in voluntary running activity between WKY and SHR have been inconsistent with some studies demonstrating no difference between the groups while others showed differences similar to those in this investigation (11, 21, 52). In addition, although WKYUT exhibited lower peak V̇o2 compared with SHRUT, this is consistent with an earlier study (2). Regardless, in the present study 3 mo of voluntary wheel running significantly increased peak V̇o2 in both WKY and SHR (Table 1). As such, it is unlikely that the differences in daily running distance noted impacted the findings and conclusions of the investigation significantly. Other forms of training (e.g., treadmill exercise) in which the dose and intensity of exercise can be more readily controlled may alleviate these concerns in future studies.
It has been demonstrated that the cardiovascular response to stimulation of the EPR is accentuated in barodenervated normotensive animals (67). This finding suggests that the baroreflex buffers EPR-mediated increases in blood pressure. Baroreflex sensitivity is reduced in hypertension (25, 37, 45). It has been reported that exercise training improves this impairment in baroreflex function (3, 35, 63). Therefore, it is possible that the training-induced normalization of EPR activity in SHR is partly due to an increase in the buffering capacity of the baroreflex. That being stated, it has been shown previously that the baroreflex maintains its ability to buffer the EPR in hypertensive rats (60). As such, the findings reported in this study would likely be affected by training-induced alterations in baroreflex function only minimally.
Baseline MAP under isoflurane anesthesia in SHRET did not differ from SHRUT (Table 1). In contrast, baseline blood pressure after decerebration in trained hypertensive rats was significantly lower than in untrained SHR. These findings agree with a previous report in which training-induced reductions in baseline blood pressure observed in conscious SHR were absent after anesthetization (5). Further, this suggests that the baseline hypotensive effects of exercise training are mediated, at least in part, within the brain stem since animals were decerebrated at the precollicular level in the present study.
As previously reported (42, 43), the methods used to evaluate sympathetic activity in the current study did not detect differences in baseline RSNA between normotensive or hypertensive animals under either isoflurane anesthesia or after decerebration (Table 1). It should be noted, however, that in the conscious state it has been reported that basal levels of RSNA are higher in SHR than WKY (31). Although not detected, if this were the case in the current study, our use of relative changes in RSNA from baseline may have underestimated the true change in RSNA in response to EPR activation in SHR. In future investigations, surrogate measures expressing the sympathetic responses to stimulation of the EPR as a percent of maximum RSNA, as performed in previous studies (70), could address this limitation. It is likewise acknowledged that there are limitations to using absolute values of RSNA (μV) to establish baseline sympathetic activity. For example, the accuracy of such measurements may be affected by various factors such as nerve size, electrical conductance, and amplifier parameters. Each of these limitations should be taken into consideration when interpreting results.
Finally, in addition to activating skeletal muscle afferent neurons, injection of capsaicin into the right common iliac artery has been shown to stimulate thin fiber afferents in the joints and skin (14). Therefore, use of this technique may not be completely indicative of metaboreflex function. As such, further research is warranted before the impact of exercise training on muscle metaboreflex function in hypertension can be clearly discerned.
Perspectives and Clinical Significance
The blood pressure response to exercise is exaggerated in hypertension (7, 8, 24, 30, 41–44, 60, 66). This augmented pressor response to physical activity is associated with an increased risk for adverse cardiovascular and/or cerebrovascular events during or immediately following a bout of exercise (19, 39). Excessive blood pressure elevation during physical exertion has been shown to contribute to impaired exercise tolerance in hypertensive patients even in the absence of coronary artery disease or left ventricular dysfunction (9, 29, 33, 50). Furthermore, numerous epidemiological studies have demonstrated that exercise blood pressure predicts the pathogenesis of left ventricular hypertrophy, stroke, myocardial infarction, and death (12, 23, 27, 28, 65) independent of resting ABP. Accordingly, current safety guidelines prohibit exercise in hypertensive patients if exertional ABP reaches or exceeds 220/105 (15, 51). As a result, exercise prescription is often limited in hypertensive patients (55). This is unfortunate as exercise training has been shown to improve cardiovascular health in these patients on numerous occasions. Understanding not only the mechanisms underlying abnormal cardiovascular control during exercise but also the mechanisms by which circulatory regulation is improved by training may be a key to overcoming these barriers. To this end, the current investigation suggests that an improvement in EPR function is a mechanism by which exercise training is beneficial for the treatment of hypertension. As such, it is tempting to suggest that pretreatment of EPR dysfunction prior to beginning an exercise regimen would allow the prescription of activity at higher intensities for longer durations maximizing the benefits and increasing the safety of training.
GRANTS
This research was supported by grants from the National Heart, Lung, and Blood Institute (HL-088422 to S. A. Smith) and the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to J. H. Mitchell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M., J.H.M., and S.A.S. conception and design of research; M.M. and G.A.I. performed experiments; M.M. and G.A.I. analyzed data; M.M., G.A.I., W.V., J.H.M., and S.A.S. interpreted results of experiments; M.M. prepared figures; M.M. drafted manuscript; M.M., G.A.I., W.V., J.H.M., and S.A.S. edited and revised manuscript; M.M., G.A.I., W.V., J.H.M., and S.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Romero and J. Lamar, Jr., for expert technical assistance.
REFERENCES
- 1.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol 47: 1278–1283, 1979. [DOI] [PubMed] [Google Scholar]
- 3.Bertagnolli M, Campos C, Schenkel PC, de Oliveira VL, De Angelis K, Bello-Klein A, Rigatto K, Irigoyen MC. Baroreflex sensitivity improvement is associated with decreased oxidative stress in trained spontaneously hypertensive rat. J Hypertens 24: 2437–2443, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, White TP. Determination of metabolic and heart rate responses of rats to treadmill exercise. J Appl Physiol 45: 1009–1015, 1978. [DOI] [PubMed] [Google Scholar]
- 5.Brum PC, Da Silva GJ, Moreira ED, Ida F, Negrao CE, Krieger EM. Exercise training increases baroreceptor gain sensitivity in normal and hypertensive rats. Hypertension 36: 1018–1022, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Champlain J, Petrovich M, Gonzalez M, Lebeau R, Nadeau R. Abnormal cardiovascular reactivity in borderline and mild essential hypertension. Hypertension 17: III22–III28, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagard R, Staessen J, Amery A. Maximal aerobic power in essential hypertension. J Hypertens 6: 859–865, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Felix JV, Michelini LC. Training-induced pressure fall in spontaneously hypertensive rats is associated with reduced angiotensinogen mRNA expression within the nucleus tractus solitarii. Hypertension 50: 780–785, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson SA, Cada AM. A longitudinal study of short- and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav Neurosci 117: 271–282, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Filipovsky J, Ducimetiere P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension 20: 333–339, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Fisher WJ, White MJ. Training-induced adaptations in the central command and peripheral reflex components of the pressor response to isometric exercise of the human triceps surae. J Physiol 520: 621–628, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster RW, Ramage AG. The action of some chemical irritants on somatosensory receptors of the cat. Neuropharmacology 20: 191–198, 1981. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O'Reilly MG, Winters WL Jr, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 106: 1883–1892, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Helwig B, Schreurs KM, Hansen J, Hageman KS, Zbreski MG, McAllister RM, Mitchell KE, Musch TI. Training-induced changes in skeletal muscle Na+-K+ pump number and isoform expression in rats with chronic heart failure. J Appl Physiol 94: 2225–2236, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol 65: 583–589, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain 110: 149–157, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kingwell BA, Arnold PJ, Jennings GL, Dart AM. The effects of voluntary running on cardiac mass and aortic compliance in Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens 16: 181–185, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Kishi T, Hirooka Y, Katsuki M, Ogawa K, Shinohara K, Isegawa K, Sunagawa K. Exercise training causes sympathoinhibition through antioxidant effect in the rostral ventrolateral medulla of hypertensive rats. Clin Exp Hypertens 34: 278–283, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens 19: 1343–1348, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Koba S, Watanabe R, Kano N, Watanabe T. Oxidative stress exaggerates skeletal muscle contraction-evoked reflex sympathoexcitation in rats with hypertension induced by angiotensin II. Am J Physiol Heart Circ Physiol 304: H142–H153, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol 283: R815–R826, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O'Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol 101: 1614–1620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim PO, Donnan PT, MacDonald TM. Blood pressure determinants of left ventricular wall thickness and mass index in hypertension: comparing office, ambulatory and exercise blood pressures. J Hum Hypertens 15: 627–633, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Lim PO, MacFadyen RJ, Clarkson PB, MacDonald TM. Impaired exercise tolerance in hypertensive patients. Ann Intern Med 124: 41–55, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Lund-Johansen P. Twenty-year follow-up of hemodynamics in essential hypertension during rest and exercise. Hypertension 18: III54–III61, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Lundin S, Ricksten SE, Thoren P. Interaction between “mental stress” and baroreceptor reflexes concerning effects on heart rate, mean arterial pressure and renal sympathetic activity in conscious spontaneously hypertensive rats. Acta Physiol Scand 120: 273–281, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. [DOI] [PubMed] [Google Scholar]
- 33.Marraccini P, Palombo C, Giaconi S, Michelassi C, Genovesi-Ebert A, Marabotti C, Fommei E, Ghione S, L'Abbate A. Reduced cardiovascular efficiency and increased reactivity during exercise in borderline and established hypertension. Am J Hypertens 2: 913–916, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Martins-Pinge MC, Becker LK, Garcia MR, Zoccal DB, Neto RV, Basso LS, de Souza HC, Lopes OU. Attenuated pressor responses to amino acids in the rostral ventrolateral medulla after swimming training in conscious rats. Auton Neurosci 122: 21–28, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Masson GS, Costa TS, Yshii L, Fernandes DC, Soares PP, Laurindo FR, Scavone C, Michelini LC. Time-dependent effects of training on cardiovascular control in spontaneously hypertensive rats: role for brain oxidative stress and inflammation and baroreflex sensitivity. PLoS One 9: e94927, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minami N, Yoshikawa T, Kataoka H, Mori N, Nagasaka M, Kurosawa H, Kanazawa M, Kohzuki M. Effects of exercise and beta-blocker on blood pressure and baroreflexes in spontaneously hypertensive rats. Am J Hypertens 16: 966–972, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 39.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med 329: 1677–1683, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno M, Iwamoto GA, Vongpatanasin W, Mitchell JH, Smith SA. Exercise training improves functional sympatholysis in spontaneously hypertensive rats through a nitric oxide-dependent mechanism. Am J Physiol Heart Circ Physiol 307: H242–H251, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuno M, Lozano G, Siddique K, Baum M, Smith SA. Enalapril attenuates the exaggerated sympathetic response to physical stress in prenatally programmed hypertensive rats. Hypertension 63: 324–329, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension 61: 180–186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreira ED, Ida F, Oliveira VL, Krieger EM. Early depression of the baroreceptor sensitivity during onset of hypertension. Hypertension 19: II198–II201, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Mostoufi-Moab S, Widmaier EJ, Cornett JA, Gray K, Sinoway LI. Forearm training reduces the exercise pressor reflex during ischemic rhythmic handgrip. J Appl Physiol 84: 277–283, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Murphy MN, Mizuno M, Downey RM, Squiers JJ, Squiers KE, Smith SA. Neuronal nitric oxide synthase expression is lower in areas of the nucleus tractus solitarius excited by skeletal muscle reflexes in hypertensive rats. Am J Physiol Heart Circ Physiol 304: H1547–H1557, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson AJ, Juraska JM, Musch TI, Iwamoto GA. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. J Appl Physiol 99: 2312–2322, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Nolan PC, Waldrop TG. Integrative role of medullary neurons of the cat during exercise. Exp Physiol 82: 547–558, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Olsen MH, Wachtell K, Hermann KL, Bella JN, Andersen UB, Dige-Petersen H, Rokkedal J, Ibsen H. Maximal exercise capacity is related to cardiovascular structure in patients with longstanding hypertension. A LIFE substudy Losartan Intervention For Endpoint-Reduction in Hypertension. Am J Hypertens 14: 1205–1210, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 36: 533–553, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Peters RV, Zoeller RT, Hennessey AC, Stopa EG, Anderson G, Albers HE. The control of circadian rhythms and the levels of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus are altered in spontaneously hypertensive rats. Brain Res 639: 217–227, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. [DOI] [PubMed] [Google Scholar]
- 54.Sinoway L, Shenberger J, Leaman G, Zelis R, Gray K, Baily R, Leuenberger U. Forearm training attenuates sympathetic responses to prolonged rhythmic forearm exercise. J Appl Physiol 81: 1778–1784, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Smith SA. Exercise in hypertension: Do skeletal muscle reflexes make this a dangerous proposition? Am J Physiol Heart Circ Physiol 299: H1302–H1303, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Smith SA, Leal AK, Murphy MN, Downey RM, Mizuno M. Muscle mechanoreflex overactivity in hypertension: a role for centrally-derived nitric oxide. Auton Neurosci 188: 58–63, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988. [DOI] [PubMed] [Google Scholar]
- 63.Stern JE, Sonner PM, Son SJ, Silva FC, Jackson K, Michelini LC. Exercise training normalizes an increased neuronal excitability of NTS-projecting neurons of the hypothalamic paraventricular nucleus in hypertensive rats. J Neurophysiol 107: 2912–2921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone AJ, Copp SW, McCord JL, Kaufman MP. Femoral artery ligation increases the responses of thin fiber muscle afferents to contraction. J Neurophysiol 113: 3961–3966, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sung J, Ouyang P, Silber HA, Bacher AC, Turner KL, DeRegis JR, Hees PS, Shapiro EP, Stewart KJ. Exercise blood pressure response is related to left ventricular mass. J Hum Hypertens 17: 333–338, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol 589: 1209–1220, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldrop T, Mitchell J. Effects of barodenervation on cardiovascular responses to muscle contraction. Am J Physiol Heart Circ Physiol 249: H710–H714, 1985. [DOI] [PubMed] [Google Scholar]
- 68.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang HJ, Li YL, Zucker IH, Wang W. Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol 302: R1260–R1270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang HJ, Pan YX, Wang WZ, Gao L, Zimmerman MC, Zucker IH, Wang W. Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J Appl Physiol 108: 1365–1375, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W, Thomas GD. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension 48: 637–643, 2006. [DOI] [PubMed] [Google Scholar]